Abstract
BACKGROUND
A central tenet of patient-centered health care advocated by the Institute of Medicine and the American Medical Association is to enhance informed decision-making in a way that incorporates patient values, knowledge and beliefs. Achievement of this goal is constrained by a lack of validated measures of patients’ knowledge needs.
METHODS
In this study we present a comprehensive and valid methodology for developing a clinically informed and patient centered measure of knowledge about left ventricular assist device (LVAD) therapy to facilitate discussion and measure candidate understanding of treatment options. Using structured interviews with patients, caregivers, candidates for LVAD treatment (New York Heart Association Class III and IV) and expert clinicians (n = 71), we identified top patient decisional needs and perspectives on essential knowledge needs for informed decision-making. From this list, we generated 20 knowledge scale question items to refine in cognitive interviews (n = 5) with patients and patient consultants.
RESULTS
Good internal consistency and reliability of the knowledge scale (Cronbach’s α = 0.81) was seen in 30 LVAD patients and candidates. Knowledge was higher among patients currently with LVADs than candidates, regardless of receiving standard education (with education: 69.9 vs 50.1, adjusted p = 0.02; without education: 69.9 vs 37.6, adjusted p < 0.001).
CONCLUSION
The LVAD knowledge scale may be useful in clinical settings to identify gaps in knowledge among patient candidates considering LVAD treatment, and to better tailor education and discussion with patients and their caregivers, and to enhance informed decision-making before treatment decisions are made.
Keywords: cardiology, cardiomyopathy, CV surgery, heart failure, transplantation, ventricular assistance
Left ventricular assist devices (LVADs) have become an increasingly effective treatment for advanced heart failure (HF), with the number of potential LVAD candidates estimated at about 250,000 to 300,000 individuals per year in the United States alone.1 Although LVAD treatment can be a promising option for improving longevity and quality of life, many LVAD candidates have made decisions about their LVAD treatment quickly and reflexively, often before education is complete.2,3 Some even make decisions to decline LVAD treatment before receiving full education about potential lifestyle changes and complications.4 According to international mechanical circulatory support guidelines,5 patients should also be trained in proper self-care as part of their education. Nevertheless, a large percentage of patients receiving treatment for HF have no documentation of having received adequate education about factors affecting outcomes and risk for rehospitalization,6 showing a lack of emphasis on patient education. Perhaps of greater concern, patients report a lack of clarity about how LVADs impact (or do not impact) the likelihood of receiving a heart transplant, suggesting a lack of understanding or appreciation of key aspects related to LVAD decision-making.2
Although most LVAD programs have patient education programs in place during the time of consent, no standardized tools yet exist for ensuring informed decision-making among candidates for LVAD treatment.7 One noteworthy LVAD knowledge measure has been developed by Edlund et al8 to explore patients’ understanding of LVAD therapy. However, their open-ended question format makes it difficult to unambiguously gauge the degree of knowledge comprehension necessary to make an informed decision about LVAD surgery.
Rizzieri et al7 called for more consistent discussions and assessments among LVAD candidates to ensure understanding of treatment options for advanced HF, including anticipated device-related complications and long-term health risks (e.g., bleeding, stroke, infection); lifestyle changes among patients and caregivers (drive-line maintenance behaviors, leisure and travel limitations, etc.); potential financial burdens; and alternative options, such as medical management, and comfort-directed therapies, such as palliative care. Greater knowledge about treatment options has been shown to reduce anxiety and improve decision-making.9 However, so far, no robust measures have been developed to facilitate discussion and measure LVAD candidate understanding, a key component of the informed consent process. We used a patient-centered approach with current LVAD patients and candidates for LVAD treatment to develop and refine a measure of patient knowledge about LVAD therapy. Patients may use this tool to gauge their understanding of treatment options, and health-care providers (e.g., cardiologists, cardiothoracic surgeons and clinic coordinators) may use this scale as a tool to help identify strengths and gaps in knowledge during the course of patient decision-making (pre-implant) about LVAD and alternative therapies. The scale can also help providers to tailor education and discussion with patients and their caregivers, and well to enhance informed consent before decisions for treatment are made.
Methods
Framework
Our methodology for scale development and validation is based on best practices outlined by Brod et al10 for inductive development of scale items using qualitative methods that involves interview research, developing the interview discussion guide, reaching saturation, analysis of data, developing a theoretical model and generating question items. Based on these guidelines, we used the Ottawa Decision Support Framework (ODSF) as a guiding theoretical model, an evidence-based, mid-range theory for guiding patients to make health decisions, incorporating insights on decision-making from general psychology,11 social psychology,12 decision analysis,13 decisional conflict,14 social support15,16 and economic concepts of expectations and values.17 The framework emphasizes the centrality of assessing client and practitioner determinants of decisions to identify decision support needs, while providing decision support tailored to client needs. This model was used because of the importance of identifying decision support needs as a basis for constructing a knowledge measure. Our methodological approach combined inductive qualitative methods (open-ended interviews, free-listing and ranking exercises, described in what follows) with deductive quantitative validation of scale items through cognitive testing and psychometric analysis to assess content and face validity.
Sample and setting
Each phase of this research was conducted with a purposive sample of participants at a partnering site chosen to provide a wide range of variation and to reflect demographics of the larger LVAD population according to the latest data from the Interagency Registry for Mechanical Circulatory Support (INTERMACS).18 For all phases of this research, patient eligibility criteria included LVAD patients and candidates with advanced heart failure (New York Heart Association [NYHA] Class III and IV), age range of 30 to 80 years (this range was selected to enhance our ability to capture a wide range of patient perspectives), and intact decision-making capacity, with an acceptable surgical risk-benefit ratio for LVAD implantation. Patients were also screened by a transplant social worker for good psychosocial support, coping mechanisms and sufficient financial resources. Patients were also administered the Montreal Cognitive Assessment (MOCA)19 test as a screening measure to detect cognitive impairments, including “vascular dementia,” or an overall lack in decision-making capacity that may interfere with individuals’ ability to participate in the study. This scale has been validated for use among 30- to 80-year-olds, and has excellent reliability. For each of the phases, we took a separate sub-sample from the overall participant population described earlier.
In-depth interviews and ranking exercises to generate items
In-depth interviews were conducted with: LVAD patients; eligible candidates for LVAD treatment; caregivers; decliners of LVAD treatment; and leading LVAD clinical providers from 2 different hospitals, including cardiologists, cardiothoracic surgeons, LVAD (nurse and physician assistant) clinical coordinators, hospital financial advisors, lead clinical social workers and clinical bioethicists. Consistent with the definition provided by the Centers for Medicare and Medicaid Services, caregivers are defined as family members, friends or neighbors who provide unpaid assistance to a person with a chronic illness or disabling condition. We add that these caregivers are identified by the patient as a primary resource for daily, post-implant assistance with lifestyle changes and device management. Structured guides for in-depth interviews were developed from the authors’ prior knowledge of domains and areas of interest, literature review of decisional needs for LVAD patients, and expert opinion.3,20,21 These interview guides were devised separately for each participant group and are available elsewhere.2 The purpose of these interviews was to identify knowledge needs from both patient and provider perspectives that could inform question items of the knowledge scale. All interviewed patients were to have already received education about the LVAD from clinic coordinators and be in the process of making a decision about implantation. The intention of selecting already-educated patients among this group was to identify remaining gaps in patient-preferred knowledge after having received standard education. The purpose of interviewing clinicians was to identify clinical perspectives on knowledge needs considered essential for providing informed consent for LVAD treatment.
Qualitative analysis of knowledge needs
Patients’ knowledge needs were identified from the interviews. All interviews were audio-recorded and transcribed verbatim with identifiers removed. They were analyzed using ATLAS.ti (http://www.atlasti.com/index.html),22 a well-known computer-assisted qualitative analysis software program, to identify themes and common knowledge needs, separately for patients and providers. Common knowledge needs were organized by emergent domains (e.g., Lifestyle, Complications, Medication, etc.) and frequency counts of patient knowledge needs allowed us to identify top needs among patients within each domain and overall. These top knowledge needs were then confirmed in a ranking exercise with 3 patient consultants working closely with the research team.
Free-listing to further generate knowledge need items
Top knowledge needs among providers were similarly identified by asking providers to free-list knowledge needs they consider essential for patient provision of informed consent for LVAD treatment. They provided at least 5 items in each of 7 domains generated by one of our expert cardiothoracic surgeons and endorsed by other members of our expert panel. These domains included: (1) device-specific knowledge and competencies; (2) what patients need to know about communicating with coordinators and hospital staff; (3) what patients need to know about medical management; (4) what patients need to know about program protocols and surveillance specifics (e.g., How often do patients need to come back to the clinic?); (5) what patients need to know about outcomes, prognosis and expected quality of life, specifically regarding adverse events (peri-operative and long-term); (6) what patients need to know about outcomes and likelihoods dealing with efficacy and survival (e.g., cost-benefit ratios, short vs long-term survival statistics, etc.); and (7) what patients need to know about misconceptions and mispredictions about LVAD therapy.
Together, high-frequency knowledge needs for patients (identified via interviews) and providers (identified via interviews and confirmed in free-listing exercise) were combined into a finalized list, examined for overlap (refer to Table 2) and translated into question items for the LVAD knowledge scale. Answer sets were generated via consultations with expert cardiologists and other members of the LVAD clinic team, reflecting common misconceptions or understandings, and included “I don’t know” as an answer choice to discourage guessing.
Table 2.
Top-ranked Informational Needs Among Patients and Caregivers, Patient Consultants and Providers
| Rank | Patients and caregivers | Patient consultants | Providers |
|---|---|---|---|
| 1 | Lifestyle: Mobility | Lifestyle | Patients should know who to contact on the VAD team in emergencies |
| 2 | Lifestyle: Spending time with family/friends | Life expectancy without LVAD | Patients need to know their risk of re-hospitalization |
| 3 | Lifestyle: Overall feeling well-being | Caregiver lifestyle: Degree of impact and involvement, QoL | Patients understand the timelines of recovery process |
| 4 | Lifestyle: Maintaining activities and hobbies | Lifestyle: Mobility | Patients should know how the device functions |
| 5 | Technical: Effort needed for care of driveline | Overall feeling of well-being/QoL | Post-operative recovery is variable among patients |
| 6 | Lifestyle: Psychosocial/cognitive changes | Chances of rehospitalization due to driveline dysfunction | There is a risk of stroke |
| 7 | Complications: Likelihood of infections (average number, type, frequency, consequences) | Risks/complications | There is a risk of bleeding |
| 8 | Lifestyle: Overall activity restrictions | Possibility of heart repairing itself from VAD | There is a risk of infection |
| 9 | Lifestyle: Independence/control | Comparison of VAD to transplant | Patient should know the names of VAD coordinators, cardiology and surgical staff |
| 10 | Lifestyle: Medication (type, frequency) | Possibility of heart continuing to beat if VAD stops | Patients want survival data |
| 11 | Caregiver lifestyle: Degree of impact, involvement, quality of life | Changes in work life | Patients need to know about out-of-pocket expenses |
| 12 | Impacts on travel | Lifestyle: Maintaining activities and hobbies | Doctors and coordinators must deliver a unified message to patients |
| 13 | Prospect of being a burden | Travel | Patients should know what equipment they are responsible for |
| 14 | Difficulty/burden of carrying battery pack | Prospect of being a burden | Patients should be aware of lifestyle changes that come with the LVAD (e.g., dietary restrictions, activity limitations, traveling, avoidance of power tools, diuretics) |
| 15 | Surgery: Probability of death | Water activities | Patients need to be able to identify critical alarms |
Overlapping informational needs indicated in bold. VAD, ventricular assist device.
Validity testing
Cognitive testing of items
Cognitive testing of these 20 items was conducted among 2 patients and 3 candidates and involved face-to-face “think-aloud” exercises to gauge participants’ perceptions of readability and comprehensibility, to confirm that instructions, question items and response sets are clear, understandable and relevant, and that the format is acceptable. These aspects contribute to the measure’s content validity. This method has been shown to effectively reveal respondents’ thought processes and improve questionnaire interpretability.23 We also recorded patients’ suggestions for alternative phrasing for greater comprehensibility. All results were used to refine questions to meet users’ level of comprehension.
Cognitive testing results were used to modify the items iteratively. After the first 3 cognitive interviews, answers on each subsequent interview were compared with earlier interviews to examine consensus and plan for potential interviews beyond the initial 5, with lower consensus (i.e., greater variability) in responses signaling a need to collect additional perspectives. Based on results from cognitive testing, question items were finalized and reviewed by clinical experts to ensure further content validity.
Group differences in LVAD knowledge
Construct validity was tested by looking for evidence of significant differences in performance on the questionnaire among 3 different participant groups, including existing LVAD patients, eligible candidates for LVAD treatment who have received standard education from LVAD clinic coordinators, and eligible candidates who have not yet received standard education. Standard education in this hospital setting takes ~45 minutes and covers basic information about LVAD therapy through educational videos and an informational packet from the device manufacturer (Thoratec), as well as a packet prepared by the site hospital. Together, these materials include information about the average likelihood of survival with and without LVAD treatment; what to expect post-implant in terms of lifestyle changes, improvements and potential complications; and the importance of caregiver support. There is no separate session for caregivers, who are encouraged to attend the session with the patient. The different participant types were included with the intention of observing a range of knowledge scores, with the assumption that patients who have personal experience living with the LVAD will exhibit greater knowledge than either LVAD candidates who have received standard education or LVAD candidates who have not yet received education. Preliminary data suggest that the standard deviation of scores would be about 15 points. A sample size of 10 subjects per group was selected to provide 80% power to detect a 20-point difference between pairwise groups using an independent, 2-sample t-test, assuming a common standard deviation of 15 points and α = 0.05. This is equivalent to a standard effect size of 1.33, which is generally considered to be a large effect size that likely yields important differences between groups.24,25
Patient participants were recruited from the DeBakey Heart and Vascular Center at Methodist Hospital in Houston, Texas. A roster was compiled once every week from December 2014 to April 2015 by the LVAD clinical coordinator, listing patients who were eligible for participation in the study. Participants were approached before or during their appointments at the LVAD clinic or as hospital inpatients, without interfering with clinic flow, and written consent was obtained. Patients were given a pencil-and-paper version of the test to fill out in the waiting area or, if they preferred, in a private, adjacent room.
Supplementary questions
Convergent validity was assessed by examining knowledge scale scores with a set of 6 supplementary questions assessing patient perceptions about: (1) whether the LVAD team prepared the patient enough to comfortably manage life with an LVAD; (2) comfort level in interacting with their doctors; (3) comfort level in interacting with other members of the LVAD clinic team; (4) whether they received conflicting messages during the process of education; (5) whether they had met with any other LVAD patients; and (6) the need for caregiver involvement. Validity would be demonstrated by correlation of knowledge scores with more positive educational experiences, with the assumption that patients with greater knowledge would have experienced more extensive and positive educational experiences (i.e., greater comfort with LVAD clinic team interactions, fewer conflicting messages, greater comfort in managing life with an LVAD). Responses to supplementary questions were compared among patient types using the Kruskal-Wallis test or Fisher’s exact test (Question 5). Overall, comparisons were adjusted for multiple hypothesis testing using Holm’s step-down Bonferroni correction. If the overall test was significant at the adjusted 0.05 level, then all pairwise comparisons were assessed using similar adjustments. Correlations between knowledge scores and each supplemental question were estimated using Spearman’s rank.
Scoring and distribution
The overall score was computed as the summation of possible (100) points awarded, with higher scores indicating increased knowledge. Summary statistics were computed for the overall sample and stratified by participant type (patient, candidates with standard education, candidates without standard education). A general linear model was used to compare knowledge scores between participant types. All pairwise differences were estimated and p-values were adjusted using Tukey’s method. The proportion of correct responses for each question was compared between participant types using Fisher’s exact test. Given that validity testing was conducted in LVAD patients and in candidates both with and without education, those with LVADs were anticipated to have the highest scores due to first-hand experience and learning.
Consistency reliability
Cronbach’s α coefficient was used as a measure of internal consistency reliability. Item-specific correlations with overall scores were computed and the effect of item deletion on α coefficients was estimated.
Results
In-depth interviews and ranking exercise to generate items
A total of 71 in-depth interviews (~45 minutes each) were conducted with patients, including existing LVAD patients (n = 15), eligible candidates for LVAD treatment (n = 15), caregivers (n = 15) and decliners of LVAD treatment (n = 15) (Table 1). Interviews were also conducted with leading health-care providers (total n = 11), including cardiologists (n = 3), cardiothoracic surgeons (n = 2), lead program coordinators (n = 2), hospital financial advisors (n = 1), lead clinical social workers (n = 2) and a clinical bioethicist (n = 1), from 2 different hospitals. A free-listing exercise was also conducted with a subset (n = 6) of these same providers (see Methods).
Table 1.
Formative In-depth Interview Demographics (n = 60)a
| Subject type | Participants (n) | Average age (range) (years) | M/F ratio | White, black, Hispanic (%) | BTT, DT (n) |
|---|---|---|---|---|---|
| LVAD patients | 15 | 60 (33–74) | 11M/4F | 47% white, 40% black, 13% Hispanic | 2 BTT, 13 DT |
| LVAD candidates | 15 | 61 (33–77) | 13M/2F | 40% white, 20% black, 40% Hispanic | NA |
| LVAD caregivers | 15 | 59 (36–74) | 5M/10F | 60% white, 33% black, 7% Hispanic | 1 BTT, 14 DTb |
| LVAD decliners | 15 | 61 (45–82) | 10M/5F | 47% white, 47% black, 6% Hispanic | NA |
BTT, bridge to transplant; DT, destination therapy; F, female; LVAD left ventricular assist device; M, male; NA, not applicable.
Does not include provider interviews.
Status of the caregivers’ charge.
The highest-ranked considerations among patients and patient consultants during their decision whether or not to get an LVAD are presented in Table 2 along with top considerations ranked most important among providers for patients’ demonstration of informed consent. Patient knowledge needs centered on issues of mobility and lifestyle, whereas provider considerations were related to patients’ understanding of risk, outcomes and factors contributing to rehospitalization. High-frequency and overlapping items, as in addition to other items designated as salient by patients specifically, were taken from these rankings to form question items for the LVAD knowledge scale.
Cognitive testing of items
Cognitive testing of these items was conducted among a total of 5 patients (3 patients and 2 candidates) (Table 3). They judged a majority of question items to be clearly stated and at an appropriate reading level. Table 4 includes a list of question items in the knowledge scale, and a full version of the scale with answer sets is available at http://www.lvaddecisionaid.com in the “For Clinicians” tab.
Table 3.
Combined Cognitive Testing (n = 5) and Validity Testing (n = 30) Demographics
| Participants (n) | Average age (range) (years) | M/F ratio | White, black, Hispanic | Ratio of BTT to DT | |
|---|---|---|---|---|---|
| Cognitive testing (n = 5) | |||||
| LVAD patients | 3 | 49 (44–57) | 3M/0F | 70% white, 30% black, 0% Hispanic | 0 BTT, 3 DT |
| LVAD candidates | 2 | 60 (53–66) | 1M/1F | 100% white, 0% black, 0% Hispanic | NA |
| Validity testing (n = 30) | |||||
| LVAD patients | 10 | 59 (26–73) | 9M/1F | 70% white 20% black, 10% Hispanic | 4 BTT, 6 DT |
| LVAD candidates w/education | 10 | 61 (33–77) | 10M/0F | 30% white 40% black, 30% Hispanic | NA |
| LVAD candidates w/o education | 10 | 54 (32–61) | 8M/2F | 30% white, 70% black, 0% Hispanic | NA |
See Table 1 for abbreviations.
Table 4.
Knowledge Scale
| LVAD knowledge questionnaire stems |
|---|
|
Item answers and response sets may be requested from the corresponding author or found at lvaddecisionaid.com/. LVAD, left ventricular assist device.
Validity and reliability testing
A total of 30 patients completed the knowledge scale, including 10 existing LVAD patients, 10 eligible candidates for LVAD treatment who received standard education, and 10 candidates who had not yet received education (Table 3). This sample size was selected to be able to detect a best-guess of a 15-point difference between groups with 80% power using a t-test, with a large effect size of 1.3. Overall, the mean score was 52.5 (SD = 20). At least 80% of all participants correctly answered Questions 1 (regarding the purpose of LVAD), Question 15 (regarding extent of caregiver support needed post-implantation) and Question 18 (regarding the need for blood thinners) (see Table 4). Less than 25% of the participants correctly answered Question 2 (regarding factors affecting recovery time), Question 8 (regarding percentage of patients still living after 2 years), Question 13 (regarding average likelihood of 1-year mortality if LVAD is declined), Question 14 (regarding projected physical and lifestyle improvements from LVAD therapy) and Question 19 (regarding what could happen if the LVAD ceases to function).
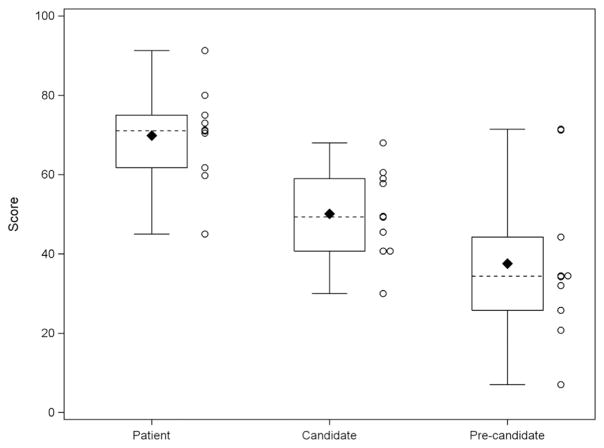
The average time to complete the questionnaire was 30 minutes, which included supplemental questions. On average, patients scored significantly higher than candidates who received standard education (69.9 vs 50.1, adjusted p = 0.02) and candidates without education (69.9 vs 37.6, adjusted p < 0.001) (Figure 1). There was no significant difference between candidates with education and candidates without education (adjusted p = 0.18); however, candidates with education did score about 12.5 points higher than candidates without education.
Figure 1.
LVAD knowledge scale scores by study participant type.
There were significant differences in the proportion of correct responses to 5 of the questions, including those regarding factors affecting recovery, likelihood of rehospitalization, equipment necessary for leaving the house on a day trip, how often to clean the drive-line, and the role of blood thinners after implantation. Although not significant at the 0.05 level, patients also tended to be more likely to provide correct answers to questions regarding: necessarily lifestyle changes post-implant; average likelihood of 1-year mortality if LVAD is declined; whether LVAD affects future eligibility for heart transplant; what will happen if the LVAD ceases to function; and pro-recovery behaviors. For these and most other question items, the proportion of correct responses tended to be highest among patients, followed by candidates with education, and then candidates without education. Surprisingly, 1 of the LVAD patients (10%) did not correctly answer Question 1, which is intended to determine whether or not the patient understands the function of the device. Overall, there was significant variation on each question (none consistently correct or incorrect across all 3 groups) to warrant not eliminating any of the items.
Cronbach’s α was 0.81, suggesting good internal consistency reliability. Removing individual questions did not markedly decrease α for any of the questions. The lowest α for single-item deletion was 0.78.
Supplemental questions were completed by all study participants. There were significant differences between groups regarding whether or not the participant has met with at least 1 other patient with an LVAD (adjusted p = 0.01). Investigating pairwise differences, patients were significantly more likely to have met with at least 1 other patient with an LVAD compared with candidates without education (90% vs 10%, adjusted p = 0.003). Knowledge scores were higher among study participants who had met with at least 1 other patient with an LVAD (63 vs 42, p = 0.002). However, after adjusting for participant type in a multiple regression model, the association was no longer significant (p = 0.37).
Otherwise, there were no significant differences in responses to the supplemental questions between groups (adjusted p ≥0.23). Knowledge scores correlated positively with supplementary questions on preparedness to manage life with LVAD (r = 0.21, p = 0.25), comfort with doctors from the LVAD team (r = 0.06, p = 0.75), comfort with other members of the LVAD team such as coordinators and nurses (r = 0.25, p = 0.18), and meetings with at least 1 other patient with an LVAD (r = 0.50, p = 0.005). However, scores correlated negatively with receipt of conflicting messages (r = −0.29, p = 0.12) and the magnitude of caregiver involvement (r = −0.15, p = 0.44).
Discussion
This study responds to the call for further insight into how patients understand treatment options for advanced heart failure.7 Patients often consent to treatment without fully comprehending or reading the information contained in consent forms.26 Likewise, an examination of patient charts reveals a dearth of documentation of discussions occurring between patients and providers about potential complications involved in LVAD treatment.6 The often urgent circumstances under which treatment decisions are made2 exacerbates the patient’s likelihood of making decisions quickly and reflexively, without fully considering the range of alternatives, and further necessitates a quick and efficient tool for assessing pre-implant patient and caregiver knowledge about essential aspects of LVAD treatment.
The scale presented in this study efficiently tests for knowledge needs valued by patients and caregivers considering LVAD treatment, as well as knowledge considered necessary by clinical experts for providing informed consent. The inductive methodology outlined was selected to ensure that the measure is both patient-centered and clinically robust for use in shared decision-making practices during patient evaluation for LVAD candidacy. That the largest group differences were observed between patients currently living with the device and candidates with no standard education about LVAD treatment suggests that the LVAD knowledge scale is an accurate gauge of patient knowledge about LVAD treatment.
The average LVAD patient scored 70% on the knowledge scale, suggesting that even experienced patients struggle to understand aspects of LVAD therapy and outcomes that are judged to be subjectively valuable and clinically important by patients, caregivers and providers. Patients and candidates seemed to understand the overall reason for getting an LVAD, but demonstrated less expertise about specific functions, maintenance and expected outcomes of the device. LVAD candidates who received standard education answered only half of the questions correctly, on average, indicating substantial gaps in standard education for providing adequate information for informed consent. We believe that this finding highlights an urgent need for additional educational support for candidates considering LVAD therapy, as well as opportunities for continuing education for LVAD patients after implantation. Other approaches, such as “teach back methodology,” or multi-format tools, such as the decision-aid described here (paper, video and online supplement), may help to ensure learning and retention. Cognitive testing before administering decisional and educational tools—for example, using the MOCA19 test as we did, or similar measures—could also help avoid lack of comprehension due to neurophysiologic conditions.
Variability and lack of standardization in current educational processes may contribute to differences or gaps in knowledge. Anecdotal evidence suggests that standard education varies across hospitals with regard to when patients receive education (i.e., before or after medical review for candidacy), who participates in educational discussions (typically, a variety of clinical team members meet with patients to answer questions in informal visits outside of standard education), and whether patients seek information on their own through LVAD- or transplant-related websites or social media.27 More research and guidelines are needed to ensure that standard education is consistent across settings and optimally designed to meet patient and provider needs for informed consent.
In addition, participants scored higher on the knowledge scale if they met with at least 1 other LVAD patient already living with the device. However, these participants were also more likely to be patients themselves, potentially confounding the observed benefits of patient-to-patient education. A further exploration of including patient-to-patient education in future education procedures may serve to enhance knowledge acquisition and sharing.
This measure of LVAD knowledge will be effective in the clinic setting for ensuring that pre-implant patients and caregivers can understand the full range of treatment options for advanced heart failure, such as medical management and comfort-related therapies; anticipate device-related complications and long-term health risks; and prepare for lifestyle changes. We intend for this measure to be used in the context of standard education offered by LVAD clinics and/or alongside enhanced educational procedures, such as the use of decision aids. The LVAD knowledge scale can be accessed and printed for clinical use from our LVAD decision aid website, http://www.lvaddecisionaid.com/!for-clinicians/cael/.
Strengths and weaknesses
The primary strength of this knowledge scale is that it is based on extensive formative, inductive research into the knowledge needs of patients from both a clinical perspective and a subjective perspective, and is recommended by medical anthropologists,28–30 making it a user-generated and relevant measure. The use of cognitive interviewing further improved construct clarification, question item wording and interpretability by users.33 Weaknesses include a potential lack of generalizability to other patient samples with different knowledge needs, although the diversity of Houston’s patient population suggests that our findings likely encompass a variety of LVAD patient samples throughout the United States.32 A majority of those we interviewed were destination therapy patients, which could potentially bias knowledge needs as well as patients’ focus during education. In terms of generalizability of the measure, hospitals may also have different patient care recommendations and center-specific outcomes that vary from the national outcomes reflected in our scale. Some of the items in the scale may also reflect device- and device-generation-specific information. Further, because the primary supplier of devices for these patients is Thoratec, patient and provider experiences that informed the scale construction may overlook nuances of devices from other manufacturers. However, all other data (e.g., INTERMACS) used for scale construction encompasses the full range of current-generation LVADs, and efforts were made to include providers’ experiences with the other 2 most common devices presently in use (HeartMate II and HeartWare HVAD). The scale may also need to be updated as technology progresses.
In conclusion, the LVAD knowledge scale presented here is based on information generated from provider-recommended as well as patient-centered considerations, representing common knowledge needs expressed by patients in their decisions surrounding LVAD treatment. The scale can be used as an accurate gauge of LVAD knowledge in clinical settings to identify any gaps in knowledge among patient candidates considering LVAD treatment, to better tailor education and discussion with patients and their caregivers, and to enhance informed consent before treatment decisions are made.
Acknowledgments
The authors thank our clinician partners, Brian Bruckner, MD, Linda Pham, LCSW, Donna Martinez, RN, and Sherry Grogan, APRN, MSN, and our patient partners, Kenneth and Becky Mitchell, Mary Yorgensen and Brenda Mays, for their contributions to this project.
Footnotes
Disclosure statement
J.D.E. and M.L. receive consultant and research support from Thoratec Corporation. The remaining authors have no conflicts of interest to disclose. This research was supported by a grant (CDR-1306-01769) from the Patient Centered Outcomes Research Institute (PCORI; ClinicalTrials.gov Identifier: NCT02248974). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. The corporation had no role in the funding or writing of this article.
References
- 1.Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol. 2013;61:1209–21. doi: 10.1016/j.jacc.2012.08.1029. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal-Barby J, Kostick KM, Delgado ED, et al. Assessment of patients’ and caregivers’ informational and decisional needs for left ventricular assist device placement: implications for informed consent and shared decision making. J Heart Lung Transplant. 2015 doi: 10.1016/j.healun.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIlvennan CK, Allen LA, Nowels C, et al. Decision making for destination therapy left ventricular assist devices “there was no choice” versus “I thought about it an awful lot. Circ Cardiovasc Qual Outcomes. 2014;7:374–80. doi: 10.1161/CIRCOUTCOMES.113.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtenay R, Bruce J, Kostick K, et al. Reasons Why Eligible Candidates Decline Left Ventricular Assist Device Placement. J Card Fail. 2015;21(10):835–9. doi: 10.1016/j.cardfail.2015.06.008. http//dx.doi.org/10.1016/j.cardfail.2015.06.008Epub June 22, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Niewald A, Broxterman J, Rosell T, et al. Documented consent process for implantable cardioverter-defibrillators and implications for end-of-life care in older adults. J Med Ethics. 2013;39:94–7. doi: 10.1136/medethics-2012-100613. [DOI] [PubMed] [Google Scholar]
- 7.Rizzieri AG, Verheijde JL, Rady MY, et al. Ethical challenges with the left ventricular assist device as a destination therapy. Phil Ethics Hum Med. 2008;3:20. doi: 10.1186/1747-5341-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund JE, Edlund AE, Carey MG. Patient understanding of potential risk and benefit with informed consent in a left ventricular assist device population. J Cardiovasc Nurs. 2015;30:435–9. doi: 10.1097/JCN.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 9.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10:10. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res. 2009;18:1263–78. doi: 10.1007/s11136-009-9540-9. [DOI] [PubMed] [Google Scholar]
- 11.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Sci. 1981;211:453–8. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 12.Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Engelwood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- 13.Keeney RL, Raiffa H. Decisions with multiple objectives: preferences and value trade-offs. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 14.Janis IL, Mann L. Decision making. New York: Free Press; 1977. [Google Scholar]
- 15.Norbeck JS. Social support. Annu Rev Nurs Res. 1988;6:85. [PubMed] [Google Scholar]
- 16.Orem DE, Vardiman EM. Orem’s nursing theory and positive mental health: practical considerations. Nurs Sci Q. 1995;8:165–73. doi: 10.1177/089431849500800407. [DOI] [PubMed] [Google Scholar]
- 17.Feather NT, Newton JW. Values, expectations, and the prediction of social action: an expectancy-valence analysis. Motiv Emotion. 1982;6:217–44. [Google Scholar]
- 18.Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIlvennan CK, Allen LA, Nowels C, et al. Decisional needs in patients considering left ventricular assist device as destination therapy: contrast between acceptors and decliners. Circ Cardiovasc Qual Outcomes. 2013;6(suppl):A15. [Google Scholar]
- 22.Muhr T, Friese S. User’s manual for ATLAS.ti 5.0. Berlin: ATLAS.ti Scientific Software Development; 2004. [Google Scholar]
- 23.Ryan K, Gannon-Slater N, Culbertson MJ. Improving survey methods with cognitive interviews in small-and medium-scale evaluations. Am J Eval. 2012;33:414–30. [Google Scholar]
- 24.Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Social Psychol. 1962;65:145–53. doi: 10.1037/h0045186. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4:279–82. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Sympt Manage. 2007;33:737–44. doi: 10.1016/j.jpainsymman.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Kostick KM, Blumenthal-Barby JS, Wilhelms LA, et al. Content analysis of social media related to left ventricular assist devices. Circ Cardiovasc Qual Outcomes. 2015;8:517–23. doi: 10.1161/CIRCOUTCOMES.115.002032. [DOI] [PubMed] [Google Scholar]
- 28.Fabrega H. Disease and social behavior: an interdisciplinary perspective. Cambridge, MA: MIT Press; 1974. [Google Scholar]
- 29.Goodenough WH, editor. Essays in honour of George Peter Murdock. New York: McGraw-Hill; 1964. Explorations in cultural anthropology. [Google Scholar]
- 30.Lynch LR. The cross-cultural approach to health behavior. Madison, NJ: Fairleigh Dickinson University Press; 1969. [Google Scholar]
- 31.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks, CA: Sage Publications; 2004. [Google Scholar]
- 32.Emerson MO, Bratter J, Howell J, et al. Houston region grows more racially/ethnically diverse, with small declines in segregation: a joint report analyzing census data from 1990, 2000, and 2010. Houston, TX: Kinder Institute for Urban Research & Hobby Center for the Study of Texas; 2014. [Google Scholar]