Abstract
Background/Objectives
Pregnancy triggers a physiological change in weight status. Postpartum weight retention in the childbearing years can substantially alter a woman’s weight gain trajectory, with several potential contributing factors identified. Most research has relied on women’s recall of pre-pregnancy weight during pregnancy or later, and not considered risk factors in combination. Using measured pre-pregnancy weight, this study aimed to examine the associations of maternal postpartum weight retention with parity, pre-pregnancy BMI, excessive gestational weight gain (GWG), maternal serum vitamin D concentration and dietary Glycaemic Index in early and late pregnancy and breastfeeding duration, including analysis of the combined impact of potentially modifiable risk factors.
Subjects/Methods
Prospective cohort study of 12,583 non-pregnant women aged 20-34 years in Southampton (UK) who were assessed prior to pregnancy, with those who subsequently became pregnant followed up in early and late gestation, and after delivery (n=2,559 in the final sample). Linear regression models examined potential predictors of weight retention in adjusted individual and multivariate analyses, and as a risk factor score.
Results
Compared with pre-pregnancy weight, 73% of women retained some weight at six months postpartum [mean (SD): 3.5 (6.2) kg]. In the adjusted multivariate model, women who were primiparous, had a lower pre-pregnancy BMI, excessive GWG, a lower early pregnancy vitamin D concentration and breastfed for <6 months had greater weight retention six months postpartum (p<0.05 for all variables). For each additional modifiable risk factor (excessive GWG, low vitamin D concentration in early pregnancy and short breastfeeding duration; scale 0-3), women retained an additional 2.49 kg (95%CI: 2.16, 2.82; p<0.001).
Conclusions
Having a greater number of modifiable risk factors was associated with greater weight retention six months postpartum. Initiatives supporting women to target these risk factors in the years prior to, during and after pregnancy could impact on their weight gain trajectory and later risk of adverse weight related outcomes.
Keywords: gestational weight gain, pre-gravid BMI, breastfeeding, vitamin D, overweight, obesity, pregnancy
Introduction
Pregnancy triggers physiological changes in body composition and weight cycling 1. UK National Institute for Health and Clinical Excellence (NICE) Guidelines identified pregnancy and the first year postpartum as key periods when women are vulnerable to excess weight gain and long term weight retention 2. Postpartum weight retention is highly variable, with women on average retaining 1-5.5 kg at 6-12 months postpartum 3. Importantly, weight retention during the child bearing years can substantially alter a woman’s lifetime weight gain trajectory 1, 4.
Factors associated with greater postpartum weight retention include excess gestational weight gain (GWG) 3, 5, with varied findings for parity 6, 7, pre-pregnancy BMI 8–10, and short breastfeeding duration 11–13. An inverse association between obesity and serum vitamin D concentrations is well established 9; with evidence that vitamin D metabolism, storage, and action both influence, and are influenced by, adiposity1. Low vitamin D concentration during pregnancy has been associated with pregnancy complications 6, 7 and poor child health outcomes 6, 7, although it has not previously been examined as a determinant of postpartum weight retention. Observational evidence has shown that a high Glycaemic Index (GI) diet is associated with obesity 16, with a review of Randomised Control Trials (RCTs) also concluding that a low GI diet promoted weight loss 17. Few studies have directly investigated the association between postpartum weight retention and maternal dietary GI or Glycaemic Load (GL) in pregnancy, although one study has demonstrated that high GL diet in pregnancy may be related to excessive GWG and greater postpartum weight retention for overweight or obese women 18. Gaining insight into associations between potential contributing factors and postpartum weight retention would enable the development of more targeted behaviour change interventions.
A limitation of postpartum weight retention research is use of self-report pre-pregnancy weight or estimates from first trimester measurements 16, 17. Pregnancy cohorts relying on maternal recall may introduce bias as such weights are ~1kg lower than measured pre-pregnancy weight; the underestimation being greater with increasing BMI (~5kg underreported by women in higher BMI groups) 18–21. Underestimation of pre-pregnancy weight not only inflates estimates of postpartum weight retention, but also overestimates GWG, impacting on the validity of findings. Some evidence suggests that first trimester GWG is more strongly linked with postpartum weight retention than second or third trimester GWG 19, therefore key variability in weight retention data may be lost if early pregnancy weight measures are used. Research investigating postpartum weight retention using measured pre-pregnancy weight is needed.
Using data from a UK mother-offspring cohort with measurements of pre-pregnancy weight, we examined the role of six factors in maternal weight retention; parity, pre-pregnancy BMI, GWG, serum vitamin D concentration and GI in early and late pregnancy, and duration of breastfeeding. Additionally, we sought to gain insight into the combined impact of modifiable risk factors on weight retention.
Materials and Methods
The Southampton Women’s Survey (SWS) is a prospective preconception mother-offspring cohort of 12,583 non-pregnant women aged 20-34 years, residing in Southampton (UK) and recruited through their general practitioners 20. At recruitment (between April 1998 and December 2002), SWS research nurses visited the women in their homes and lifestyle, anthropometry, and dietary assessments were undertaken. SWS women who subsequently became pregnant were followed up in early and late pregnancy at the SWS Ultrasound Unit at the Princess Anne Hospital in Southampton, and postpartum in their homes. SWS women who became pregnant, had term live singleton births and were assessed six months postpartum were considered in this study.
A total of 3,158 women had live singleton births in the SWS, of which 205 women gave birth <37 weeks gestation, leaving 2,953 women with term live singleton births. Of these, 100 women were excluded from this analysis as they were ‘possibly already pregnant’ at the time of pre-pregnancy assessments (<38 weeks between pre-pregnancy measures and expected date of delivery for term births). A further 294 had missing pre-pregnancy or six month postpartum weight measures, leaving 2,559 in the final sample.
Ethical approval was obtained from the Southampton and South West Hampshire Local Research Ethics Committee (approval number: 06/Q1702/104) and the study carried out according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained.
Measures
Outcome measure
Pre-pregnancy weight was measured (removing shoes and heavy items of clothing/jewellery to 0.1kg) using a portable scale (Seca, Hamburg, Germany), and height measured (to 0.1cm) using a portable stadiometer (Harpenden, CMS Weighing Equipment Ltd., London, UK). Pre-pregnancy data were collected a median (1st and 3rd quartile) of 1.8 (0.8; 3.2) years before conception. Maternal weight (kg) was re-measured at six months postpartum. Postpartum weight retention was calculated by subtracting pre-pregnancy weight from six month postpartum weight.
Exposures
Maternal data on parity was collected prior to pregnancy and analysed as a binary variable; i) primiparous or ii) multiparous. Pre-pregnancy maternal BMI was calculated using measured pre-pregnancy weight and height (see above), and BMI categories (underweight, healthy weight, overweight and obese) defined according to World Health Organisation (WHO) cut-points 11. GWG was calculated as the difference between measured pre-pregnancy weight and measured weight at 34 weeks gestation, with weekly gains used to estimate GWG on the day of birth 12. Women were categorised as gaining inadequate, adequate, or excessive weight, according to the 2009 Institute of Medicine (IOM) recommendations 10. Maternal serum 25-hydroxy-vitamin D concentration (nmol/l) in early pregnancy was analysed by liquid-liquid extraction followed by HPLC/MS/MS and in late pregnancy by radioimmunoassay (Liaison, Diasorin, Stillwater, Minnesota, USA). Maternal diet during pregnancy was measured using an interviewer-administered, 100-item Food Frequency Questionnaire (FFQ) assessing habitual intake over the previous three months 13. GI values were assigned to each food item in the FFQ according to the GI database published by the Medical Research Council Human Nutrition Research (MRC NHR), Cambridge, United Kingdom 14. Each participant’s average dietary GI in early and late pregnancy was calculated by determining their GL and dividing their GL by their total daily intake of available carbohydrates 15. Direct GI matches were provided for 83.5% of foods assessed, and the remaining foods were assigned GI values of similar foods (13.5%) or an arbitrary value of 70 (3.3%) 15, according to the procedure developed by the MRC HNR 14. For infants who were breastfed, the date of the last breastfeed was recorded at interview at six, 12 and 24 months postpartum. Breastfeeding duration was analysed as a binary variable; <6 months and ≥6 months.
Possible confounders
Potential confounding variables included gestational age at birth, maternal age, pre-pregnancy walking speed and diet quality, and postpartum maternal smoking. Gestational age at birth was determined using a computerised algorithm based on menstrual and early ultrasound data 29. Maternal age was ascertained at birth, and smoking status (yes/no) assessed at six months postpartum. Pre-pregnancy walking speed and diet quality were included as proxies of postpartum physical activity and dietary behaviour. Women were asked to describe their pre-pregnancy walking speed according to five categories (very slow, stroll at an easy pace, normal speed, fairly brisk and fast) as an indication of usual physical activity level. Maternal diet before pregnancy was measured using the same FFQ as used to measure maternal diet during pregnancy 27. Principal Component Analysis (PCA) was performed on reported frequencies of consumption of the 48 foods and food groups from the FFQ data, based on the correlation matrix to adjust for unequal variances of the original variables score 30. The first principal component identified a ‘prudent’ pattern that was consistent with UK dietary recommendations 31, 32. From this pattern ‘prudent’ diet scores before pregnancy were calculated by multiplying the coefficients from the PCA by each woman’s standardised reported frequencies of pre-pregnancy consumption and were interpreted as a measure of diet quality 30.
Statistical analysis
Statistical analyses were performed using Stata version 14.1 (Statacorp LP, College Station, TX, USA). All data were assessed for normality; the mean and standard deviation were reported for normally distributed variables, the median and 1st and 3rd quartile for non-normally distributed data. Data were transformed using log transformations where possible, and z-scores of the transformed variable calculated to express the value as a standard deviation from the mean. T-tests (for normally distributed variables), Mann-Whitney U-Tests (for non-normally distributed variable) and chi-squared tests (for categorical variables) were used to compare differences in sociodemographic, anthropometric and breastfeeding variables of mothers included, with those not in the study.
Parity, pre-pregnancy BMI, GWG categories, serum vitamin D concentration, GI in early and late pregnancy, and breastfeeding duration were examined as predictors of weight retention at six months using linear regression models. Gestational age at birth, maternal age at birth, pre-pregnancy diet quality, pre-conception walking speed and postpartum maternal smoking were all included as confounding variables. The combined effects of the predictor variables were considered by including those that were significantly associated with weight retention at 6 months (as determined by backward elimination stepwise regression) in one linear regression model, adjusting for the same confounding variables. A p-value of <0.05 was considered statistically significant. A sensitivity analysis restricted to participants who fell pregnant within one year of their pre-pregnancy weight measurement was conducted to reduce the likelihood of large fluctuations in weight prior to conception affecting the analysis.
Modifiable risk factor analysis
Modifiable risk factors found to be independently associated with weight retention at six months in the mutually-adjusted multivariate model were examined as a risk factor score. Binary variables were derived for each risk factor, and these were summed to given an integer risk factor score, ranging from zero to three. Linear regression models were fitted with the risk factor score as a categorical predictor (zero as reference category), with and without adjustment for potential confounders. The same models were also fitted with the risk factor score as a continuous variable to assess trends in relation to the number of risk factors.
Results
Study population
Table 1 summarises the baseline characteristics of the 2,559 women included in this analysis. Women with term live singleton births who were excluded from the analysis were more likely to have a lower education level, slower walking speed, poorer pre-pregnancy prudent diet score, lower mean dietary GI index in early pregnancy, lower serum vitamin D concentration in late pregnancy, to have gained excess weight according to the IOM guidelines and retained less weight at six months postpartum compared to women included in the analysis (Table 1).
Table 1.
Characteristics of mothers and children included in the study compared with term live singleton births not in the study.
| Characteristic | In study |
||||
|---|---|---|---|---|---|
| Yes | No | p-value | |||
| n | Value | n | Value | ||
| Mother | |||||
| Primiparous, n (%) | 2557 | 1299 (51%) | 393 | 189 (48%) | 0.32 |
| Education, n (%) | 2552 | 392 | 0.04 | ||
| No academic qualification | 64 (3%) | 24 (6%) | |||
| GCSE grade D or below | 241 (9%) | 30 (8%) | |||
| GCSE grade C or above | 726 (28%) | 121 (31%) | |||
| A-level or equivalent | 781 (31%) | 117 (30%) | |||
| HNDs or equivalent | 168 (7%) | 20 (5%) | |||
| Degree | 572 (22%) | 80 (20%) | |||
| Pre-pregnancy BMI, median (1st & 3rd quartiles) | 2557 | 24.2 (21.9-27.3) | 369 | 24.0 (21.6-27.4) | 0.49 |
| Pre-pregnancy walking speed, n (%) | 2558 | 393 | 0.04 | ||
| Very slow | 7 (0.3%) | 4 (1%) | |||
| Stroll at an easy pace | 163 (6%) | 33 (8%) | |||
| Normal speed | 1039 (41%) | 167 (42%) | |||
| Fairly brisk | 1222 (48%) | 169 (43%) | |||
| Fast | 127 (5%) | 20 (5%) | |||
| Pre-pregnancy prudent diet score, mean (SD) | 2558 | 0.05 (0.96) | 393 | -0.06 (0.98) | 0.03 |
| Glycaemic Index 11-weeks, mean (SD) | 1852 | 59.5 (2.9) | 231 | 59.1 (2.8) | 0.03 |
| Glycaemic Index 34-weeks, mean (SD) | 2262 | 58.8 (2.8) | 280 | 58.6 (2.8) | 0.24 |
| Serum Vitamin D 11-weeks, nmol/l, mean (SD) | 1693 | 62.3 (25.4) | 201 | 62.9 (26.4) | 0.74 |
| Serum Vitamin D 34-weeks, nmol/l, median (1st & 3rd quartiles) | 1996 | 59.0 (41.0-84.5) | 243 | 55 (36.1-80.0) | 0.01 |
| GWG, kg, mean (SD) | 2216 | 12.1 (6.2) | 153 | 13.0 (6.2) | 0.08 |
| GWG according to IOM categories, n (%) | 2214 | 153 | 0.04 | ||
| Inadequate | 499 (23%) | 26 (17%) | |||
| Adequate | 657 (30%) | 41 (27%) | |||
| Excessive | 1058 (48%) | 86 (56%) | |||
| Age at child’s birth, years, mean (SD) | 2559 | 30.7 (3.8) | 392 | 30.4 (3.9) | 0.11 |
| Smoking at 6 months postpartum, n (%) | 2553 | 471 (18%) | 224 | 44 (20%) | 0.66 |
| Pre-pregnancy weight, kg, median (1st & 3rd quartiles) | 2559 | 64.8 (58.1-73.3) | 370 | 64.1 (57.6-72.8) | 0.43 |
| Postpartum weight 6 months, kg, median (1st & 3rd quartiles) | 2559 | 68.2 (60.4, 78.6) | 112 | 67.6 (59.6, 80.9) | 0.69 |
| Postpartum weight retention 6 months, kg, mean (SD) | 2559 | 3.5 (6.2) | 92 | 2.2 (5.6) | 0.04 |
| Child | |||||
| Gestational age at birth, weeks, mean (SD) | 2559 | 40.1 (1.2) | 392 | 40.0 (1.3) | 0.44 |
| Duration of breastfeeding, <6 months, n (%) | 2473 | 1811 (73%) | 233 | 160 (69%) | 0.14 |
Abbreviations: n – number; GCSE – General Certificate of Secondary Education (compulsory, ages 15-16 years); A-level – school leaving qualification (ages 17-18 years); HNDs – Higher National Diplomas; BMI – Body Mass Index; SD – standard deviation; GWG – Gestational Weight Gain; IOM – Institute of Medicine.
Weight trajectories and characteristics of postpartum weight retention
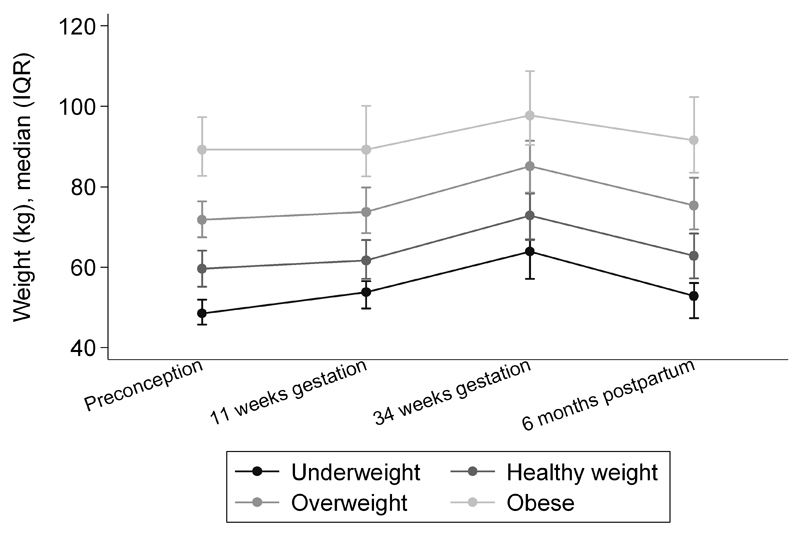
Figure 1 shows weight change trajectories across pregnancy to six months postpartum according to pre-pregnancy BMI. At six months postpartum, 73% of women had retained weight (mean (SD): 3.5 (6.2) kg); 35% retained >5 kg. Women who were underweight (n=36) or of healthy weight (n=1460) in pre-pregnancy retained 3.6 (3.9) kg and 3.5 (5.2) kg above their pre-pregnancy weight, respectively. Women who were overweight (n=717) or obese (n=344) pre-pregnancy retained 4.1 (7.1) and 2.4 (7.8) kg, respectively.
Figure 1.
Weight (kg) trajectories at pre-pregnancy, 11-weeks gestation, 34-weeks gestation and 6 months postpartum according to pre-pregnancy BMI group.
Women who gained excess weight according to the IOM GWG guidelines retained an additional 4.4 (95%CI: 3.9, 4.9) kg at six months postpartum compared with women who gained weight within the recommendations (p<0.001). Overweight women were most likely to exceed the IOM GWG guidelines (64%), followed by obese (51%), healthy weight (39%) and underweight women (28%). Primiparous women were also more likely to exceed the guidelines than multiparous women (52% and 44%, respectively; p<0.001), although their pre-pregnancy BMI was lower (24.6 (4.2) vs 25.9 (5.0) kg/m2); p<0.001).
Predictors of postpartum weight retention: individual and multivariate analysis
The individual and mutually-adjusted multivariate analyses found similar predictors of postpartum weight retention (Table 2). The final multivariate analysis included 1564 women as many did not provide an early pregnancy sample for serum vitamin D analyses. Primiparous women, women who had a lower pre-pregnancy BMI, gained excess GWG, had lower vitamin D concentration in early pregnancy and breastfed for less than six months retained more weight six months postpartum. The effect sizes were slightly greater for pre-pregnancy BMI and smaller for breastfeeding duration in the multivariate analyses. There were no associations between GI in early or late pregnancy and postpartum weight retention. The sensitivity analysis in women who fell pregnant within one year of their pre-pregnancy measurements produced similar coefficients to the main multivariate analysis, although the coefficients were greater for pre-pregnancy BMI and lower for excess GWG in the sensitivity analysis (Supplementary File 1).
Table 2.
Individual and mutually-adjusted multivariate associations examining the predictors of postpartum weight retention (kg) at six months.
| Predictor | Individual models* | Mutually-adjusted multivariate model* (n=1564) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value | ||
| Parity | 1 | 2550 | reference | reference | ||||
| 2 + | -1.45 | -1.94, -0.96 | <0.001 | -1.06 | -1.60, -0.53 | <0.001 | ||
| Pre-pregnancy BMI | (kg/m2, standardised)^ | 2550 | -0.25 | -0.50, -0.01 | 0.04 | -0.53 | -0.81, -0.26 | <0.001 |
| GWG | Inadequate | -3.00 | -3.61, -2.39 | <0.001 | -2.75 | -3.47, -2.04 | <0.001 | |
| Adequate | 2207 | reference | reference | |||||
| Excessive | 4.42 | 3.91, 4.93 | <0.001 | 4.45 | 3.85, 5.06 | <0.001 | ||
| Serum Vitamin D | Early pregnancy (nmol/l) | 1687 | -0.01 | -0.03, -0.003 | 0.01 | -0.01 | -0.02, -0.001 | 0.03 |
| Late pregnancy (nmol/l)^ | 1989 | -0.20 | -0.46,0.06 | 0.14 | ||||
| Glycaemic Index | Early pregnancy | 1846 | 0.08 | -0.02, 0.19 | 0.12 | |||
| Late pregnancy | 2255 | 0.01 | -0.09, 0.11 | 0.83 | ||||
| Breastfeeding duration | < 6 months | 2466 | reference | reference | ||||
| ≥ 6 months | -1.01 | -1.59, -0.43 | 0.001 | -0.66 | -1.26, -0.05 | 0.04 | ||
Adjusted for gestational age at birth, maternal age at conception, pre-pregnancy diet quality, pre-conception walking speed, and postpartum maternal smoking.
log transformed and standardised with units in SDs
Modifiable risk factor analysis
Three potentially modifiable risk factors identified as independently associated with weight retention at six months postpartum in the mutually-adjusted multivariate analyses were examined. The factors were dichotomised and defined as: excess GWG according to IOM guidelines 26, low 25-hydroxy-vitamin D concentration in early pregnancy (<64 nmol/L) as used in the SWS population previously 33, and less than six months breastfeeding duration according to the WHO recommendations 34 (Table 3). Primiparity and a lower pre-pregnancy BMI, although associated with postpartum weight retention, were not considered modifiable risk factors since parity cannot be modified through a behaviour change intervention, and it would be counterintuitive to recommend that women with a healthy weight BMI gain weight prior to pregnancy to reduce their overall risk of postpartum weight retention. Parity and pre-pregnancy BMI were tested as interaction factors in the model, and the interaction between i) parity - risk factor score and ii) pre-pregnancy BMI – risk factor score, were both found to be non-significant (p=0.07 and p=0.33, respectively).
Table 3.
Definition and prevalence of modifiable risk factors in the SWS.
| Risk factor | Definition | Total N | Prevalence |
|
|---|---|---|---|---|
| n | % | |||
| Excess GWG | According to IOM GWG guidelines (1) | 2214 | 1058 | 48% |
| Low vitamin D level in early pregnancy | Serum Vitamin D concentration in early pregnancy < 64 nmol/L (3) | 1693 | 923 | 55% |
| Short duration of breastfeeding | < 6 months duration of breastfeeding according to the WHO recommendations (2) | 2473 | 1811 | 73% |
Of the 1571 women, 117 (7%) had no early-life risk factors, 477 (30%) had one, 655 (42%) had two, and 322 (21%) had three. Table 4 shows weight retention six months postpartum according to the number of modifiable risk factors. Taking account of potential confounding influences, there was a graded increase in weight retention at six months postpartum with an increasing number of risk factors. For each additional risk factor, weight retention increased by 2.49 kg (95% CI: 2.16, 2.82; p<0.001) six months postpartum, adjusted for confounders.
Table 4.
Weight retention at six months postpartum in women according to the number of behavioural risk factors.
| Number of modifiable risk factors | Unadjusted (n=1571) | Adjusted* (n=1565) | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value | |
| 0 | reference | - | - | reference | - | - |
| 1 | 1.68 | 0.52, 2.83 | 0.004 | 1.86 | 0.71, 3.01 | 0.002 |
| 2 | 3.82 | 2.70, 4.94 | <0.001 | 4.22 | 3.09, 5.35 | <0.001 |
| 3 | 6.81 | 5.60, 8.01 | <0.001 | 7.20 | 5.98, 8.41 | <0.001 |
| β-trend1 | 2.35 | 2.03, 2.68 | <0.001 | 2.49 | 2.16, 2.82 | <0.001 |
Adjusted for gestational age at birth, maternal age at conception, pre-pregnancy diet quality, pre-conception walking speed and postpartum maternal smoking.
P-trend was determined by linear regression models of weight retention using a continuous risk factor score.
Discussion
The current study examined six factors in relation to maternal weight retention six months postpartum using measured pre-pregnancy weight, with a focus on modifiable risk factors for postpartum weight retention. Women who were: i) primparous, ii) underweight or of a healthy weight prior to pregnancy, iii) had excess GWG, iv) lower serum 25-hydroxy-vitamin D concentrations in early pregnancy and v) breastfed for less than six months, retained more weight at six months postpartum. Having more of the three modifiable risk factors (excess GWG, lower serum Vitamin D concentration in early pregnancy, and breastfeeding < 6 months) was associated with greater weight retention; for each additional modifiable risk factor women retained an extra 2.49 kg six months postpartum. To our knowledge, this is the first study to examine the factors associated with weight retention using measured pre-pregnancy weight, and to evaluate the combined effects of modifiable risk factors on postpartum weight retention.
The mean weight retention of 3.5 kg in the SWS is consistent with previous studies using self-report weight which indicate that, on average, women retain 1-5.5 kg at 6-12 months postpartum 18, and considering that women underestimate their self-report pregnancy weight by ~1kg 18–21. Given the 6 month follow-up period, it is not surprising that 73% of SWS women still retained some weight. While some of the risk factors identified here (i.e. lower pre-pregnancy BMI 8 and excess GWG 3, 5, 35) are consistent with the findings from studies that used self-report pre-pregnancy weight measures and monitored weight retention over a longer term from one to three years, it will be important to extend the findings on early pregnancy serum vitamin D concentration in future studies over a longer follow-up time.
Greater GWG has consistently been associated with postpartum weight retention 3, 5, 35. From a public health perspective, we found that women who gained adequate weight according to IOM GWG guidelines 26, retained 4.45 kg less than women who gained excess weight. These findings are higher than other pregnancy cohorts that used self-report pre-pregnancy weight and measured weight retention up to one year postpartum, where women who exceeded the guidelines retained 2.98 kg (95% CI: 1.77, 4.18) more weight 3. Almost half of SWS women exceeded the IOM GWG guidelines, indicating the magnitude of the issue. This highlights the importance of adequate weight gain during pregnancy to minimise postpartum weight retention and prevent overweight and obesity in childbearing women. Greater efforts are needed to support women in terms of optimal GWG, but monitoring and managing weight gain in pregnancy is challenging 36.
A lower pre-pregnancy BMI was associated with greater weight retention. These findings are consistent with a recent meta-analysis of 10 studies (n=116 735 women) which found a linear association between pre-pregnancy BMI and weight retention, where overweight and obese women retained -0.81 kg and -2.34 kg less weight respectively, compared with healthy weight women 8. Weight retention was assessed at varying time points between 1 month and 15 years, although there were no differences between the short and long term effects of BMI on weight retention 8. Evidence from SWS and other studies 8 suggests that underweight and healthy weight women experience greater weight retention. However, it is important to note that categorising GWG according to the IOM GWG guidelines somewhat adjusts for pre-pregnancy BMI as the GWG guidelines differ according to BMI groups. Considering that overweight women were most likely to exceed the IOM GWG guidelines (64%) followed by obese women (51%), continuing to intervene and prevent excess GWG in these higher BMI groups remains a high priority.
On average SWS women who maintained ‘any’ intensity of breastfeeding to six months retained 0.66 kg less than those who did not. There is inconsistent evidence that reduced breastfeeding is associated with greater weight retention 12. A recent systematic review 12 of prospective and retrospective observational studies found little or no association between duration of breastfeeding and weight loss (n=27/43), or change in body composition (n=16/18) in the majority of studies, but the heterogeneous nature of the study designs precluded the synthesis of findings 12. Some evidence of a time-dependent association was evident in the systematic review 12 and also in a study published since 37, such that breastfeeding duration of less than three months tended to have little impact, whereas continued breastfeeding to six months was associated with lower weight retention, although this was not consistent across all studies. Theoretically, the increased energy cost of lactation (approximately 2.62 MJ/d 38) should lead to weight loss 39, but the multifactorial nature of weight retention and the contextual factors associated with breastfeeding means that this may not be generalisable to all women. If a longer duration of breastfeeding is protective against weight retention as well as its many other benefits 40–42, it is concerning that only one third of UK women 43, or 27% of SWS women, breastfed to six months 12.
To our knowledge, this is the first data linking serum vitamin D concentration in early pregnancy with postpartum weight retention. This may be because, for practical reasons, many pregnancy cohorts have recruited women mid-pregnancy (~16-20 weeks gestation), and were unable to explore early pregnancy factors. For each 10nmol/l reduction in vitamin D concentration, women retained an additional 100g of weight. While statistically significant, this is unlikely to be clinically significant for individuals. One explanation for the relationship between vitamin D and weight retention could be an association between adiposity and lower circulating 25-hydroxyvitamin D [25(OH)D] levels 23. Since vitamin D is stored in fatty tissues, obese individuals have a larger capacity to store vitamin D, which may lower circulating 25(OH)D levels, or 25(OH)D levels may influence fat accumulation 1, 2. A bi-directional Mendelian randomization analysis 1 provided evidence for obesity as a causal factor in vitamin D deficiency, but no evidence of reverse causation. In SWS, both pre-pregnancy BMI and early pregnancy vitamin D concentration were associated with weight retention in the multivariate model, suggesting that higher adiposity levels do not solely explain the variation in postpartum weight retention. Further research is needed to replicate these findings and explore the potential causal mechanisms of vitamin D on weight retention, particularly in regards to timing during early gestation.
Few studies have examined GI or GL in relation to postpartum weight retention; the majority of studies have focused on other pregnancy outcomes including gestational diabetes mellitus and small-for-gestational age 49. In a study of 47,003 women from the Danish National Birth Cohort, GL during pregnancy, with diet assessed using a validated FFQ, was associated with postpartum weight retention at 18 months 15. The association was greater for women who were obese prior to conception, with an increase in weight retention of 1.3 kg from the lowest to higher GL quintile 15. Given the limited evidence, further research is needed to examine if there is an association between GI and postpartum weight retention, particularly in regard to pre-pregnancy BMI and timing during gestation.
Strengths and weaknesses
The SWS provides data from a large contemporary cohort of women recruited from the general population regardless of whether they were planning a pregnancy. Women were characterised before pregnancy, and in early and late pregnancy for those who became pregnant, making the SWS cohort a rare preconception study. The prevalence of the risk factors examined (e.g. BMI, excess GWG and breastfeeding rates) and amount of weight retention are similar to other countries in the western world, increasing the generalisability of the study findings. Measured pre-pregnancy weight was used to calculate weight retention, pre-pregnancy BMI and GWG, reducing the risk of misreporting and maternal recall bias associated with self-report and estimated pre-pregnancy weight. Women’s behaviours through the pre-pregnancy, pregnancy and early postpartum periods were characterised in detail, enabling comprehensive analysis of risk factors and accounting for potential confounding influences.
Limitations to our study include that pre-pregnancy weight may have been measured some years prior to conception (median (1st and 3rd quartile) = 1.8 (0.8; 3.2) years) and fluctuations in weight during the study pre-pregnancy period may have occurred. However, a sensitivity analysis assessing only women who became pregnant within one year of their pre-pregnancy measures identified similar coefficients for the predictors of weight retention to the main multivariate analysis. While 2559 women were followed-up six months postpartum, the final multivariate analysis included 1564 women (many did not provide an early pregnancy sample for serum vitamin D analyses). However, individual models (with larger sample sizes) identified the same predictors of weight retention. Despite adjusting for potential confounding factors, some confounders may have been omitted. Pre-pregnancy diet and walking speed were included as proxies of postpartum diet and physical activity behaviour due to a lack of diet and physical activity data collected on women between birth and six months.
Recommendations for research and practice
Modifiable early life risk factors have previously been identified for adiposity and overweight in SWS children; including maternal obesity, GWG above the IOM guidelines, low pregnancy vitamin D concentration, short breastfeeding duration and smoking in pregnancy 50. Adjusting for potential confounders, the risk of being overweight or obese for children who had four or five of these early life risk factors was 3.99 at four years and 4.65 at six years, compared with children with no risk factors 50. Behaviour change interventions that address these common modifiable risk factors that are associated with both postpartum weight retention in women and adiposity in their offspring, may offer an opportunity to simultaneously optimise health, and prevent overweight and obesity, in two generations. Behavioural support spanning the entire period from pre-pregnancy through to postpartum is warranted, a strategy supported by the IOM GWG guidelines 26.
In comparison to primiparous women, multiparous women retained 1.06 kg less weight six months postpartum. These findings contrast with other studies which found that weight retention is greater in multiparous women 13, 51; however, a recent study indicated that while multiparous women had a higher pre-pregnancy BMI, primiparous women reported greater GWG 52, consistent with the SWS.
Excess GWG has consistently been associated with greater weight retention 3, 5. However, GWG is not regularly monitored in prenatal care, or its implications on maternal and child health risk discussed 53. There have been calls to formally integrate weight management and monitoring into routine prenatal care through practice-level policies 53. Health practitioners, particularly obstetricians and midwives, have frequent contact with women during pregnancy and are well placed to support women to manage GWG 54, or refer women to dietitians if further dietary support is required. Additional health professional training on the importance of adequate GWG and how to facilitate sensitive conversations on weight management may be needed 54, 55.
Conclusions
Most women in this study retained weight six months postpartum, with 35% retaining more than 5 kg. Having more modifiable risk factors was associated with large differences in postpartum weight retention; women who had three risk factors retained 7.20 kg more than those without any. Primary prevention initiatives that target these risk factors and support women of child bearing age prior to, during, and after pregnancy could alter their weight trajectory, reducing the risk of overweight and obesity across a woman’s life time and having benefits for their children.
Supplementary Material
Acknowledgements
We are grateful to the women of Southampton and their children who gave their time to take part in these studies, and to the research nurses and other staff who collected and processed the data.
Financial Support
This work was supported by grants from the UK Medical Research Council, the British Heart Foundation, UK Foods Standard Agency, the Dunhill Medical Trust, the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre, the European Union's Seventh Framework Programme (FP7/2007-2013), and projects EarlyNutrition and ODIN (under grant agreement numbers 289346 and 613977). JLH received an Endeavour Research Fellowship funded by the Australian Government (Department of Education and Training). CEC is supported by a National health and Medical research Council of Australia Senior Research Fellowship.
Footnotes
Conflicts of Interest
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products; KMG, HMI and CC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. None of the other authors had any potential conflicts of interest.
Authorship
All authors were responsible for the design of the study and formulated the research question. JLH conducted the statistical analysis under the guidance of SRC. JLH completed the literature review and drafted the initial paper. All authors were responsible for revising the manuscript and have approved the final version.
Data Availability
The study data are not freely available due to ethical restrictions. The SWS team will provide the data on request, subject to appropriate approvals. For further information contact the corresponding author: Professor Siân Robinson. 1) Details of ethical restrictions behind these data: Local institutional ethics committee approval may be required. Data would be anonymised. 2) The procedure to obtain the data by the interested parties: Bona fide researchers would need to make a formal application to the SWS Steering Committee and possibly to the Ethics Committee through the cohort PI (Prof HM Inskip). The cohort investigators will provide the data to any interested party/parties once the approvals have been obtained. 3) Any other restrictions to these data: There are no further restrictions applicable to these data.
References
- 1.Gunderson E, Murtaugh M, Lewis C, Quesenberry C, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) International Journal of Obesity. 2004;28(4):525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. NICE public health guidance 27: weight management before, during and after pregnancy. London, United Kingdom: NICE; 2010. [Google Scholar]
- 3.Mannan M, Doi SA, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71(6):343–352. doi: 10.1111/nure.12034. [DOI] [PubMed] [Google Scholar]
- 4.Harris H, Ellison G, Clement S. Do the psychosocial and behavioral changes that accompany motherhood influence the impact of pregnancy on long-term weight gain'. J Psychosom Obstet Gynaecol. 1999;20(2):65–79. doi: 10.3109/01674829909075579. [DOI] [PubMed] [Google Scholar]
- 5.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94(5):1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 6.Hill B, McPhie S, Skouteris H. The Role of Parity in Gestational Weight Gain and Postpartum Weight Retention. WHI. 2016;26(1):123–129. doi: 10.1016/j.whi.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Maddah M, Nikooyeh B. Weight retention from early pregnancy to three years postpartum: a study in Iranian women. Midwifery. 2009;25(6):731–737. doi: 10.1016/j.midw.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Rong K, Yu K, Han X, Szeto IM, Qin X, Wang J, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutrition. 2015;18(12):2172–2182. doi: 10.1017/S1368980014002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity. 2010;18(10):1996–2003. doi: 10.1038/oby.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abebe DS, Von Soest T, Von Holle A, Zerwas SC, Torgersen L, Bulik CM. Developmental trajectories of postpartum weight 3 years after birth: Norwegian mother and child cohort study. Maternal and Child Health Journal. 2015;19(4):917–925. doi: 10.1007/s10995-014-1593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Zhu M, Hu C, Tao X, Li Y, Wang Q, et al. Breast-feeding and postpartum weight retention: a systematic review and meta-analysis. Public Health Nutrition. 2015;18(18):3308–3316. doi: 10.1017/S1368980015000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neville C, McKinley M, Holmes V, Spence D, Woodside J. The relationship between breastfeeding and postpartum weight change—a systematic review and critical evaluation. International Journal of Obesity. 2014;38(4):577–590. doi: 10.1038/ijo.2013.132. [DOI] [PubMed] [Google Scholar]
- 13.Martin JE, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. Predictors of post-partum weight retention in a prospective longitudinal study. Maternal and Child Nutrition. 2014;10(4):496–509. doi: 10.1111/j.1740-8709.2012.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obesity Reviews. 2015;16(4):341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen VK, Heitmann BL, Halldorsson TI, Sørensen TI, Olsen SF. Maternal dietary glycaemic load during pregnancy and gestational weight gain, birth weight and postpartum weight retention: a study within the Danish National Birth Cohort. Br J Nutr. 2013;109(08):1471–1478. doi: 10.1017/S0007114512003443. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson EP. Obesity During Pregnancy in Clinical Practice. Springer; 2014. Epidemiologic Trends and Maternal Risk Factors Predicting Postpartum Weight Retention; pp. 77–97. [Google Scholar]
- 17.Schmitt N, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity–results of a systematic review and meta-analysis on the natural history of postpartum weight retention. International Journal of Obesity. 2007;31(11):1642–1651. doi: 10.1038/sj.ijo.0803655. [DOI] [PubMed] [Google Scholar]
- 18.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317–332. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens-Simon C, Roghmann KJ, McAnarney ER. Relationship of self-reported prepregnant weight and weight gain during pregnancy to maternal body habitus and age. J Am Diet Assoc. 1992;92(1):85–87. [PubMed] [Google Scholar]
- 20.Shin D, Chung H, Weatherspoon L, Song WO. Validity of Prepregnancy Weight Status Estimated from Self-reported Height and Weight. Maternal and Child Health Journal. 2013;18(7):1667–1674. doi: 10.1007/s10995-013-1407-6. [DOI] [PubMed] [Google Scholar]
- 21.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of Self-Reported Pre-Pregnancy Weight and Body Mass Index Classification in an Integrated Health Care Delivery System. Paediatr Perinat Epidemiol. 2016;30(4):314–319. doi: 10.1111/ppe.12286. [DOI] [PubMed] [Google Scholar]
- 22.Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol. 2015;212(4):499.e491–412. doi: 10.1016/j.ajog.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: the Southampton women's survey. Int J Epidemiol. 2006;35(1):42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organization; 2000. Contract No.: 894. [PubMed] [Google Scholar]
- 25.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91(6):1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IOM (Institute of Medicine) and NRC (National Research Council) Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 27.Robinson S, Godfrey K, Osmond C, Cox V, Barker D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50(5):302–308. [PubMed] [Google Scholar]
- 28.Okubo H, Crozier SR, Harvey NC, Godfrey KM, Inskip HM, Cooper C, et al. Maternal dietary glycemic index and glycemic load in early pregnancy are associated with offspring adiposity in childhood: the Southampton Women's Survey. Am J Clin Nutr. 2014;100(2):676–683. doi: 10.3945/ajcn.114.084905. [DOI] [PubMed] [Google Scholar]
- 29.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101:368–385. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crozier SR, Robinson SM, Borland SE, Inskip HM. Dietary patterns in the Southampton Women's Survey. Eur J Clin Nutr. 2006;60(12):1391–1399. doi: 10.1038/sj.ejcn.1602469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Department of Health Committee on Medical Aspects of Food Policy. Nutritional aspects of cardiovascular disease. London: HMSO; 1994. [Google Scholar]
- 32.Department of Health Committee on Medical Aspects of Food Policy. Nutritional aspects of the development of cancer. London: The Stationery Office; 1998. [Google Scholar]
- 33.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women's Survey. Am J Clin Nutr. 2012;96(1):57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. The Cochrane Database of Systematic Reviews. 2002;1:CD003517. doi: 10.1002/14651858.CD003517. [DOI] [PubMed] [Google Scholar]
- 35.Siega-Riz AM, Viswanathan M, Moos M-K, Deierlein A, Mumford S, Knaack J, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201(4):339. e331–339. e314. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Gilmore LA, Redman LM. Weight gain in pregnancy and application of the 2009 IOM guidelines: toward a uniform approach. Obesity. 2015;23(3):507–511. doi: 10.1002/oby.20951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandhagen M, Lissner L, Brantsaeter AL, Meltzer HM, Häggkvist A-P, Haugen M, et al. Breast-feeding in relation to weight retention up to 36 months postpartum in the Norwegian Mother and Child Cohort Study: modification by socio-economic status? Public Health Nutrition. 2014;17(07):1514–1523. doi: 10.1017/S1368980013001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutrition. 2005;8(7a):1010–1027. doi: 10.1079/phn2005793. [DOI] [PubMed] [Google Scholar]
- 39.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 41.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014:kwu072. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 42.Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 43.McAndrew F, Thompson J, Fellows L, Large A, Speed M, Renfrew MJ. Infant feeding survey 2010. Leeds: Health and Social Care Information Centre; 2012. [Google Scholar]
- 44.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:1–14. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 45.Wei S-Q, Qi H-P, Luo Z-C, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26(9):889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 46.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obesity Reviews. 2013;14(5):393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 47.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Medicine. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Earthman C, Beckman L, Masodkar K, Sibley S. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. International Journal of Obesity. 2012;36(3):387–396. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 49.Louie JCY, Brand-Miller JC, Markovic TP, Ross GP, Moses RG. Glycemic index and pregnancy: a systematic literature review. Journal of Nutrition and Metabolism. 2011;2010:1–8. doi: 10.1155/2010/282464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101(2):368–375. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanotti J, Capp E, Wender MCO. Factors associated with postpartum weight retention in a Brazilian cohort. Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(4):164–171. doi: 10.1590/SO100-720320150005186. [DOI] [PubMed] [Google Scholar]
- 52.Paulino DS, Surita FG, Peres GB, do Nascimento SL, Morais SS. Association between parity, pre-pregnancy body mass index and gestational weight gain. J Matern Fetal Neonatal Med. 2016;29(6):880–884. doi: 10.3109/14767058.2015.1021674. [DOI] [PubMed] [Google Scholar]
- 53.Scott C, Andersen CT, Valdez N, Mardones F, Nohr EA, Poston L, et al. No global consensus: a cross-sectional survey of maternal weight policies. BMC pregnancy and childbirth. 2014;14(1):1. doi: 10.1186/1471-2393-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heslehurst N, Newham J, Maniatopoulos G, Fleetwood C, Robalino S, Rankin J. Implementation of pregnancy weight management and obesity guidelines: a meta-synthesis of healthcare professionals' barriers and facilitators using the Theoretical Domains Framework. Obesity Reviews. 2014;15(6):462–486. doi: 10.1111/obr.12160. [DOI] [PubMed] [Google Scholar]
- 55.Heslehurst N, Crowe L, Robalino S, Sniehotta FF, McColl E, Rankin J. Interventions to change maternity healthcare professionals’ behaviours to promote weight-related support for obese pregnant women: a systematic review. Implementation Science. 2014;9(1):1. doi: 10.1186/s13012-014-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.