Abstract
Background
Obesity and cardiometabolic dysfunction are associated with increased risk of heart failure and other cardiovascular diseases. We sought to examine the association of cardiometabolic traits with left ventricular (LV) cardiac mechanics. We hypothesized that specific obesity-related phenotypes are associated with distinct aspects of LV strain.
Methods and Results
We evaluated the associations of obesity-related phenotypes, including central adiposity, diabetes mellitus, insulin resistance, and circulating adipokine concentrations with echocardiographic measures of LV mechanical function among participants of the Framingham Heart Study Offspring and Third Generation cohorts. Among 6231 participants, the mean age was 51±16 years and 54% were women. Greater body-mass index (BMI) was associated with worse LV longitudinal strain, radial strain (apical view), and longitudinal synchrony (multivariable-adjusted P<0.0001). After accounting for BMI, we found that central adiposity as measured by waist circumference, was associated with worse global longitudinal strain and synchrony (P≤0.006). Measures of insulin resistance, dyslipidemia, and diabetes mellitus also were associated with distinct aspects of LV mechanical function. Circulating leptin concentrations were associated with global longitudinal and radial strain (apical view, P<0.0001), whereas no such association was found with leptin receptor, adiponectin, or C-reactive protein.
Conclusions
Our findings highlight the association of central obesity and related cardiometabolic phenotypes above and beyond BMI with subclinical measures of LV mechanical function. Interestingly, obesity-related traits were associated with distinct aspects of LV mechanics, underscoring potential differential effects along specific LV planes of deformation. These findings may shed light onto obesity-related cardiac remodeling and heart failure.
Keywords: epidemiology, obesity, strain, metabolic syndrome
More than one in four adults are obese in the United States, and over half of the population is projected to be obese by 2030.1 Obesity and metabolic disease are closely related, and both represent major risk factors for cardiovascular disease, including heart failure.2-5 Moreover, subclinical changes in cardiac structure and function are apparent in cardiometabolic disease, and may precede the future development of heart failure among obese individuals.6-8 The underlying mechanisms driving cardiac remodeling in obesity are likely multifaceted, and include systemic inflammation and cardiac fibrosis.9 Obesity may also have direct myocardial effects via circulating adipose-derived hormones such as adiponectin and leptin, which in turn have been linked to clinical heart failure.10,11
Advanced echocardiographic imaging techniques have enabled the assessment of subclinical cardiac dysfunction. Specifically, echocardiographic strain measures have allowed for sensitive quantification of myocardial deformation and mechanics; these measures have been associated with clinical outcomes not only among patients with heart failure, but also among asymptomatic community-based populations.12,13 Notably, individual components of left ventricular (LV) deformation may reflect processes affecting specific layers of myofibers oriented anatomically in distinct planes. For example, shortening of the LV long axis as assessed by global longitudinal strain is thought to be a marker of predominantly subendocardial dysfunction, whereas decrease in circumferential strain around the LV short axis is considered a greater reflection of mesomyocardial dysfunction.13,14 Accordingly, changes in longitudinal strain can occur early along the disease progression of high afterload states, when circumferential and radial strain may initially remain preserved.15 How adiposity affects LV function along different planes is not well understood. We therefore sought to evaluate obesity traits in relation to distinct components of LV systolic mechanics.
Importantly, it is clear that there are many different facets to obesity that are manifest in phenotypes such as central adiposity, diabetes mellitus, insulin resistance, dyslipidemia, and abnormal fasting glucose. Given their complementary nature, we hypothesized that specific obesity-related phenotypes are associated with distinct aspects of LV strain. We therefore investigated the association of each of these cardiometabolic traits as well as circulating adipokine levels with LV mechanics in a large community-based sample of middle-aged and older adults free of overt cardiovascular disease.
Methods
Study sample
Participants of the Framingham Heart Study (FHS) Offspring cohort attending the eighth examination (2005-2008) and Third Generation attending the first examination (2002-2005) were included in this analysis. Details of the cohorts have previously been published.16,17 In brief, the FHS original cohort was started in 1948 with the overall objective to identify common factors that contribute to cardiovascular disease. In 1971, the offspring cohort was recruited, and included 5,124 of the original participant's adult children and their spouses. The Third generation cohort in turn included the grandchildren of the original cohort. Of 7714 eligible participants, 883 were excluded (107 due to offsite exam, 183 with prevalent myocardial infarction, 44 with heart failure, 110 with valvular heart disease, 42 with atrial fibrillation, 227 with no strain measures available, 170 with missing covariates), leaving 6231 participants for analysis.
Clinical assessment
Participants underwent medical history, anthropometrics, assessment of resting blood pressure, and fasting bloodwork. Diabetes mellitus was defined as a fasting serum glucose level ≥126 mg/dL and/or anti-diabetic therapy. Metabolic syndrome was defined as meeting 3 or more of 5 accepted criteria:18 (a) increased waist circumference (≥102 cm in men; ≥88 cm in women); (b) increased fasting triglyceride (≥150 mg/dL); (c) high blood pressure (≥130/85 mmHg) or treatment for hypertension; (d) decreased HDL-C (<40 mg/dL in men; <50 mg/dL in women); (e) impaired fasting glucose (≥100 mg/dL). All participants provided informed consent, and the study was approved by the Boston University Medical Center Institutional Review Board.
Laboratory assessment
Fasting blood samples were obtained at the baseline examination and frozen at -80°C until assayed. Glucose and insulin assays have previously been described.19 The homeostasis model for insulin resistance was calculated as follows: HOMA-IR = (fasting glucose [mmol/l] * fasting insulin [μU/ml]/22.5.20 Plasma C-reactive protein (CRP) concentration was measured with Dade Behring BN100 nephelometer (Dade Behring, Deerfield, IL) with intra-assay coefficient of variation (CV) of 2.8%.
On samples from Third Generation participants included in this study (n=3683), plasma leptin, soluble leptin receptor, and total adiponectin concentrations were measured by enzyme-linked immunosorbent assay (Quantikine, R&D Systems, Minneapolis, MN). Intra-assay CV's were 2.9% for leptin, 6.9% for leptin receptor, and 2.2% for adiponectin. Serum aldosterone concentration was measured via radioimmunoassay (Quest Diagnostics), and plasma renin activity was measured using the GammaCoat Plasma Renin Activity RIA Kit (DiaSorin), with inter-assay CV of 12.6%.
Echocardiography
All participants underwent standard two-dimensional transthoracic echocardiograms using an HP Sonos 5500 ultrasound machine (Phillips Medical Systems, Andover, Massachusetts, USA). Digital echocardiographic data were analyzed offline, and conventional LV measures obtained according to standardized techniques by readers blinded to clinical data. Fractional shortening was calculated as: [(LV end-diastolic dimension (LVDD)-LV end-systolic dimension)/ LVDD] *100, and LV ejection fraction was derived using the Teichholz method.21 LV mass was calculated as: 0.8{1.04[(LVDD+LV posterior wall thickness+LV septal wall thickness)3-LVDD3]} +0.6.22 LV hypertrophy was defined as LV mass >95 g/m2 in women and >115 g/m2 in men. Concentric hypertrophy was defined as LVH and relative wall thickness >0.42, and concentric remodeling as no LVH and relative wall thickness >0.42.
Speckle-tracking-based analyses of LV function were performed offline with excellent reproducibility as previously described (2D Cardiac Performance Analysis v1.1, TomTec Imaging Systems, Unterschleissheim, Germany).23,24 Primary measures obtained included global longitudinal strain (apical two- and four-chamber views), global circumferential strain (mid-LV short axis view), and longitudinal and radial (apical) segmental synchrony, calculated as the standard deviation of time-to-peak strain.14 Secondary measures included global radial strain (short axis view), and global radial strain (apical two- and four-chamber views). The interobserver within-subject coefficients of variation for longitudinal and circumferential strain measures ranged from 3.0-4.0%, and from 4.7-7.3% for radial strain (short axis and apical views) as described previously.24
Statistical analysis
Baseline clinical and echocardiographic characteristics were summarized by sex. Continuous variables that displayed skewed distributions were natural log-transformed (triglyceride concentrations, HOMA-IR, biomarkers, measures of synchrony). The associations of cardiometabolic traits and echocardiographic measures of LV strain were evaluated using multivariable linear regression models, with a strata term used to indicate cohort. Models were adjusted for age, sex, heart rate, systolic blood pressure, hypertension treatment, BMI, diabetes mellitus, HDL cholesterol, and triglyceride concentrations. Primary analyses examined associations of four cardiometabolic traits (BMI, triglyceride levels, HOMA-IR, and CRP) with longitudinal and circumferential strain. Results were deemed significant using a Bonferroni-corrected P-value threshold of 0.05/8 = 0.00625.
For significant multivariable-adjusted associations, secondary analyses additionally adjusted for echocardiographic measures (left atrial size, LV mass, and LV fractional shortening). We also examined other clinical traits including waist circumference, fasting glucose, HDL cholesterol, diabetes mellitus, metabolic syndrome, adiponectin, leptin, leptin receptor, and the aldosterone-to-renin ratio in secondary analyses. In an exploratory analysis, we examined age- and sex-adjusted least squares means of strain parameters grouped by number of metabolic syndrome risk factors present (ranging from 0 to 5), and assessed between-group differences using ANCOVA. In additional analyses, we examined Pearson partial correlation coefficients between traditional echocardiographic measures and speckle tracking measures after adjusting for age- and sex. In secondary analyses, we fitted generalized linear models to further adjust primary models for sibling correlations using PROC GENMOD. All analyses were performed using SAS v9.2.
Results
Baseline clinical and echocardiographic characteristics of 2843 men (mean age 50±15 years) and 3388 women (mean age 51±16 years) are displayed in Table 1. Among men, the mean BMI was 28.2±4.6 kg/m2 and 8% had diabetes mellitus. Women had mean BMI of 26.6±6.0 kg/m2, 5% had diabetes. Baseline echocardiographic characteristics included normal mean LV ejection fraction and wall thicknesses, with 3% of the sample meeting LV hypertrophy criteria, and 3% with left atrial enlargement. Mean global longitudinal strain was -19±3% in men and -21±3% in women.
Table 1. Clinical, laboratory, and echocardiographic characteristics of sample by sex.
| Men | Women | |
|---|---|---|
| n=2843 | n=3388 | |
| Clinical characteristics | ||
| Age, years | 50 (15) | 51 (16) |
| Systolic blood pressure, mmHg | 124 (15) | 119 (17) |
| Body mass index, kg/m2 | 28.2 (4.6) | 26.6 (6.0) |
| Waist circumference, cm | 100 (13) | 92 (16) |
| Heart rate, bpm | 61 (10) | 63 (10) |
| Diabetes mellitus, n (%) | 224 (8) | 169 (5) |
| Antihypertensive medications, n (%) | 736 (26) | 776 (23) |
| Metabolic syndrome, n (%) | 1286 (45) | 1102 (33) |
| Laboratory characteristics | ||
| HDL cholesterol, mg/dl | 48 (13) | 62 (17) |
| Triglycerides, mg/dl | 129 (97) | 104 (62) |
| C-reactive protein, mg/L | 2.2 (4.5) | 3.2 (6.5) |
| HOMA-IR | 1.9 (2.0) | 1.6 (1.7) |
| Fasting glucose, mg/dl | 103 (21) | 96 (19) |
| Echocardiographic characteristics | ||
| Left atrial diameter, cm | 4.0 (0.5) | 3.6 (0.5) |
| Left atrial enlargement, n (%) | 85 (3) | 87 (3) |
| Left ventricular end-diastolic dimension, cm | 5.1 (0.4) | 4.7 (0.3) |
| Interventricular septal thickness, cm | 1.0 (0.1) | 0.9 (0.1) |
| Posterior wall thickness, cm | 1.0 (0.1) | 0.9 (0.1) |
| Relative wall thickness | 0.39 (0.05) | 0.37 (0.05) |
| Left ventricular mass, g | 192 (38) | 135 (29) |
| Left ventricular hypertrophy, n (%) | 244 (9) | 321 (10) |
| Concentric remodeling, n (%) | 494 (19) | 288 (9) |
| Concentric hypertrophy, n (%) | 109 (4) | 100 (3) |
| Fractional shortening, % | 36 (4) | 37 (4) |
| Left ventricular ejection fraction, % | 64 (5) | 66 (5) |
| Mitral tissue Doppler e', cm/s | 11.3 (2.8) | 11.3 (3.0) |
| Mitral inflow E/e' ratio | 6.0 (1.6) | 6.5 (2.0) |
| Strain measures | ||
| Longitudinal strain, % | -19 (3) | -21 (3) |
| Circumferential strain, % | -29 (5) | -30 (5) |
| Radial strain (short axis), % | 45 (17) | 47 (17) |
| Radial strain (apical), % | 29 (7) | 30 (7) |
| Longitudinal synchrony, msec | 94 (38) | 90 (36) |
| Radial synchrony, msec | 124 (49) | 121 (48) |
Data are means and standard deviations for continuous variables, unless otherwise indicated.
Central adiposity and other cardiometabolic traits are associated with LV strain
We found that higher BMI was associated with worse longitudinal and radial strain (apical). Specifically, each 1-standard deviation (SD) increase in BMI was associated with a 0.37% higher longitudinal strain, and a 0.52% lower radial strain (apical) after accounting for potential confounders, including age, sex, heart rate, HDL cholesterol, triglyceride concentrations, diabetes mellitus, systolic blood pressure, and antihypertensive therapy (multivariable-adjusted P<0.0001 for both, Table 2). A similar association with worse longitudinal and radial strain (apical) was observed with lower HDL cholesterol (P<0.001 for both).
Table 2. Association of cardiometabolic traits and strain measures.
| Longitudinal strain | Circumferential strain | Radial strain (short axis) | Radial strain (apical) | |||||
|---|---|---|---|---|---|---|---|---|
| estimate (s.e.) | P | estimate (s.e.) | P | estimate (s.e.) | P | estimate (s.e.) | P | |
| Primary traits | ||||||||
| Body mass index | 0.37 (0.04) | <0.0001 | -0.12 (0.07) | 0.08 | -0.04 (0.26) | 0.87 | -0.52 (0.10) | <0.0001 |
| Triglycerides | 0.20 (0.04) | <0.0001 | -0.06 (0.07) | 0.39 | 0.12 (0.27) | 0.66 | -0.05 (0.11) | 0.63 |
| HOMA-IR | 0.36 (0.06) | <0.0001 | -0.18 (0.11) | 0.09 | -0.33 (0.38) | 0.39 | -0.29 (0.15) | 0.06 |
| C-reactive protein | 0.03 (0.04) | 0.51 | -0.11 (0.07) | 0.11 | 0.24 (0.25) | 0.35 | -0.13 (0.10) | 0.20 |
| Secondary traits | ||||||||
| Waist circumference | 0.52 (0.10) | <0.0001 | -0.41 (0.17) | 0.01 | 0.68 (0.61) | 0.26 | -0.33 (0.24) | 0.16 |
| Fasting glucose | 0.08 (0.05) | 0.10 | -0.23 (0.09) | 0.01 | 0.45 (0.32) | 0.15 | -0.11 (0.12) | 0.37 |
| HDL cholesterol | -0.16 (0.05) | 0.0006 | -0.18 (0.08) | 0.02 | 0.31 (0.28) | 0.27 | 0.37 (0.11) | 0.0009 |
| Diabetes mellitus | 0.14 (0.16) | 0.38 | 0.84 (0.28) | 0.003 | -2.91 (1.02) | 0.004 | -0.51 (0.39) | 0.19 |
| Adiponectin | -0.10 (0.05) | 0.06 | 0.17 (0.09) | 0.04 | 0.04 (0.35) | 0.90 | 0.07 (0.14) | 0.64 |
| Leptin | 0.44 (0.08) | <0.0001 | -0.03 (0.12) | 0.81 | 0.66 (0.50) | 0.19 | -1.15 (0.20) | <0.0001 |
| Leptin receptor | -0.08 (0.05) | 0.06 | -0.12 (0.07) | 0.10 | -0.75 (0.29) | 0.01 | 0.32 (0.12) | 0.006 |
| Aldosterone/renin ratio | -0.05 (0.04) | 0.24 | -0.08 (0.07) | 0.25 | 0.09 (0.28) | 0.76 | 0.10 (0.11) | 0.37 |
Beta estimate represents the change in echo variable (strain expressed in %) per 1-standard deviation change in continuous clinical traits, and for the presence versus absence of dichotomous traits. Multivariable model adjusted for age, sex, body mass index, heart rate, systolic blood pressure, hypertension treatment, diabetes mellitus, HDL cholesterol, triglyceride concentrations. Biomarkers are log-transformed.
In order to examine central obesity specifically, we modeled the association of waist circumference with strain measures after adjusting for BMI among other variables (Table 2). We found that each 1-SD increase waist circumference was associated with a 0.52% higher (worse) longitudinal strain (P<0.0001) after adjusting for BMI and other clinical factors. In contrast, there was no association of waist circumference and other strain measures.
Notably, higher triglyceride concentrations and insulin resistance as assessed by HOMA-IR were also associated with mean longitudinal strain (P<0.0001 for both). In addition, we found that participants with diabetes mellitus had worse circumferential and radial strain (short axis). Specifically, individuals with diabetes had a 0.84% higher circumferential and 2.91% lower radial strain (short axis) compared with individuals without diabetes (P≤0.004 for both). After additionally accounting for traditional echocardiographic measures (left atrial diameter, LV mass, fractional shortening or LV ejection fraction), all associations of cardiometabolic traits and strain measures highlighted above remained statistically significant (P≤0.007 for all). Additional adjustment for waist circumference and fasting glucose revealed no substantive changes in previous associations, with the exception of BMI, which was no longer associated with strain measures after adjusting for waist circumference (Supplemental Table 1). In secondary analyses, we accounted for sibling correlations using generalized linear models, and we found minimal differences in effect sizes (Supplemental Table 2).
Cardiometabolic traits are associated with mechanical synchrony
In addition to LV strain measures, BMI was also associated with worse longitudinal segmental synchrony (multivariable-adjusted P=0.003). After accounting for BMI and other clinical variables, waist circumference and diabetes status were also associated with worse longitudinal segmental synchrony (P≤0.006 for both, Table 3). However, after adjusting for traditional echocardiographic measures, the association of BMI and waist circumference with longitudinal segmental synchrony was attenuated (P>0.05 for both). There were no significant associations between cardiometabolic traits and radial (apical) segmental synchrony.
Table 3. Association of cardiometabolic traits and synchrony measures.
| Longitudinal segmental synchrony | Transverse segmental synchrony | |||
|---|---|---|---|---|
| estimate (s.e.) | P | estimate (s.e.) | P | |
| Primary traits | ||||
| Body mass index | 0.02 (0.005) | 0.003 | 0.01 (0.006) | 0.11 |
| Triglycerides | -0.003 (0.005) | 0.59 | -0.003 (0.006) | 0.63 |
| HOMA-IR | 0.02 (0.008) | 0.05 | 0.02 (0.009) | 0.05 |
| C-reactive protein | 0.0003 (0.005) | 0.96 | -0.006 (0.006) | 0.31 |
| Secondary traits | ||||
| Waist circumference | 0.03 (0.01) | 0.006 | 0.01 (0.01) | 0.42 |
| Fasting glucose | -0.008 (0.006) | 0.20 | -0.01 (0.007) | 0.09 |
| HDL cholesterol | -0.007 (0.006) | 0.21 | -0.02 (0.007) | 0.008 |
| Diabetes mellitus | 0.08 (0.02) | <0.0001 | 0.05 (0.02) | 0.02 |
| Adiponectin | 0.0006 (0.005) | 0.90 | 0.004 (0.006) | 0.54 |
| Leptin | 0.01 (0.007) | 0.15 | 0.01 (0.008) | 0.07 |
| Leptin receptor | -0.001 (0.004) | 0.76 | -0.006 (0.005) | 0.20 |
| Aldosterone/renin ratio | 0.006 (0.004) | 0.14 | -0.007 (0.005) | 0.14 |
Beta estimate represents the change in echo variable (synchrony expressed in ms) per 1-standard deviation change in continuous clinical traits, and for the presence versus absence of dichotomous traits. Multivariable model adjusted for age, sex, body mass index, heart rate, systolic blood pressure, hypertension treatment, diabetes mellitus, HDL cholesterol, triglyceride concentrations. Biomarkers are log-transformed.
Adipokines, aldosterone, and LV mechanical function
Higher circulating leptin concentrations were associated with worse longitudinal and radial strain (apical), even after accounting for BMI and other clinical covariates (multivariable-adjusted P<0.0001 for both, Table 3). This finding persisted even after additionally accounting for traditional echocardiographic measures (P<0.0001 for both).
Higher soluble leptin receptor concentrations were associated with higher radial strain (apical), (P=0.006), but not longitudinal strain or other strain measures. Adiponectin concentrations were not associated with strain parameters (P≥0.04 for both). None of the adipokines measured were associated with synchrony measures.
We found no association of aldosterone concentrations or aldosterone-to-renin ratio and measures of LV strain. Further, the association of leptin and longitudinal and radial strain (apical) measures was not attenuated after adjusting for aldosterone concentrations in multivariable analyses (P<0.0001).
Association of metabolic syndrome and strain measures
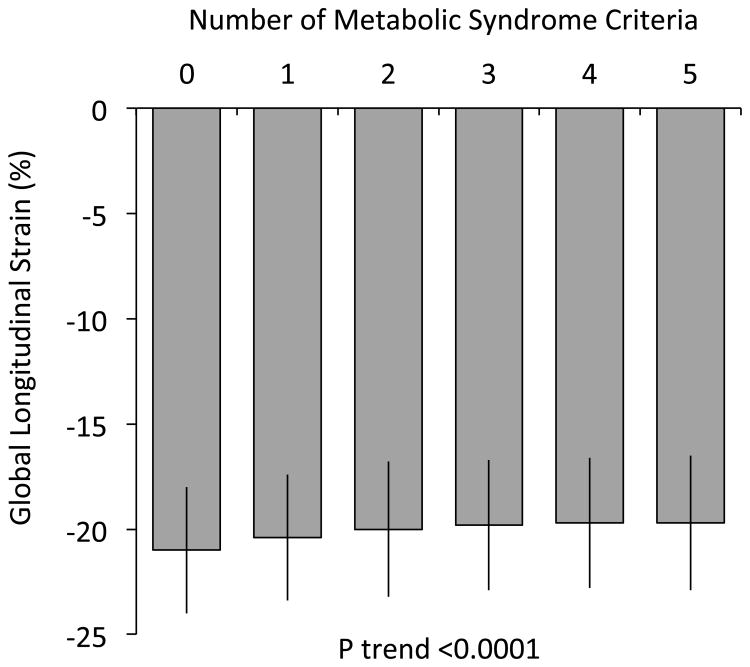
Among the sample studied, 45% of men and 33% of women met criteria for metabolic syndrome. The presence of metabolic syndrome was associated with worse longitudinal and radial (apical) strain in age- and sex-adjusted analyses (P<0.0001 for both). Specifically, individuals with metabolic syndrome had a 1% higher longitudinal strain compared with those without metabolic syndrome (beta estimate 1.06, s.e. 0.09). Figure 1 displays longitudinal strain by number of metabolic syndrome risk factors (between 0 to 5).
Figure 1.
Least squared means of global longitudinal strain by number of metabolic syndrome criteria, adjusted for age and sex. Error bars represent standard errors.
Association of traditional echocardiographic measures with speckle tracking strain
We observed modest correlations between traditional echocardiographic measures and strain measures in age- and sex-adjusted analyses. All four strain measures were correlated with LV ejection fraction (Table 4). Traditional measures of diastolic function included mitral e' velocity, with worse mitral e' correlated with worse longitudinal strain, and the mitral inflow E/e' ratio, with higher E/e' ratio correlated with worse longitudinal and better circumferential strain.
Table 4. Correlation between traditional echocardiographic and strain measures.
| Longitudinal Strain | Circumferential Strain | Radial Strain (short axis) | Radial Strain (apical) | |
|---|---|---|---|---|
|
| ||||
| R* P-value | R* P-value | R* P-value | R* P-value | |
|
| ||||
| LA diameter | 0.03 | -0.11 | 0.04 | 0.003 |
| 0.05 | <0.0001 | 0.002 | 0.85 | |
|
| ||||
| LV mass | 0.12 | 0.007 | -0.001 | -0.03 |
| <0.0001 | 0.58 | 0.93 | 0.06 | |
|
| ||||
| RWT | 0.11 | -0.07 | -0.07 | 0.03 |
| <0.0001 | <0.0001 | <0.0001 | 0.03 | |
|
| ||||
| LVEF | -0.19 | -0.44 | 0.13 | 0.19 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
|
| ||||
| Mitral e' | -0.23 | -0.02 | -0.02 | 0.05 |
| <0.0001 | 0.08 | 0.26 | <0.0001 | |
|
| ||||
| Mitral E/e' ratio | 0.07 | -0.13 | 0.02 | -0.02 |
| <0.0001 | <0.0001 | 0.27 | 0.12 | |
Partial Pearson correlation coefficient adjusted for age and sex
Discussion
Among 6231 participants of the Framingham Heart Study, we found that greater body mass index, and specifically central adiposity as measured by waist circumference, was associated with worse global longitudinal strain, as well as worse longitudinal synchrony. Beyond body mass index itself, other phenotypes accompanying obesity including insulin resistance, dyslipidemia, and diabetes mellitus were associated with distinct aspects of LV mechanical function. Interestingly, we also found that the adipose-derived hormone leptin was associated with global longitudinal and radial strain (apical), whereas no such association was found with adiponectin or CRP. These findings may shed light onto obesity-related cardiac remodeling and heart failure.
The association of obesity and diastolic dysfunction is well-described, whereas the effects of obesity on systolic function are less clear.8 Previous data support normal LV ejection fraction in obesity,25 whereas recent studies examining global longitudinal strain show an association of BMI and central adiposity with LV systolic dysfunction using this more sensitive measure.26-28 Our study extends these findings to a large community-based population of middle-aged and older adults, and demonstrates a robust association of BMI and central adiposity with global longitudinal strain. Further, our results support an association of metabolic phenotypes, including insulin resistance and hypertriglyceridemia with strain measures, above and beyond obesity itself.
Of note, we show a distinct association of BMI, central adiposity, insulin resistance, and hypertriglyceridemia with global longitudinal strain, whereas there were no significant associations with circumferential strain. This mirrors the early decline in longitudinal deformation with relative preservation of radial deformation in models of ischemia and increased afterload states.29,30 In addition, we found that diastolic function as assessed by mitral inflow E/e' ratio correlated with worse longitudinal, and better circumferential strain. Indeed, increasing left atrial to LV gradients in an animal model of pacing-induced heart failure result in greater diastolic lengthening rate of the LV in the anteroposterior and septolateral dimensions, but progressive decline in long-axis lengthening.31
Of note, diabetes mellitus was associated with circumferential but not longitudinal strain after adjusting for BMI. Interestingly, circumferential strain previously was found to be more strongly predictive of incident heart failure compared with longitudinal strain.13 These results are distinct from previous analyses from the Atherosclerosis Risk in the Community (ARIC) study, which showed an association of dysglycemia and diabetes with global longitudinal strain.32 This difference in results may be due to the older mean age of the ARIC participants compared with our study population. Cardiac effects of age-related changes in body composition are often difficult to separate from inherent effects of obesity,33 and it may be that in our younger cohort, we observe more direct associations of cardiometabolic dysfunction on cardiovascular phenotypes, whereas in older samples, age-related effects may be at play. In fact, an earlier study on a subsample of older FHS participants showed a similar association of diabetes with longitudinal strain,23 which we no longer observe with in a younger sample with more than double the number of participants, and after accounting for BMI differences.
The mechanisms underlying obesity-associated cardiac remodeling are likely multifaceted. Prior experimental studies support the potential role of circulating adipose-derived hormones. For example, circulating leptin can have direct myocardial effects on fatty acid metabolism, hypertrophy, and fibrosis, and may also influence cardiac function indirectly via vascular and hypothalamic effects.11 Soluble leptin receptor is the predominant leptin binding protein in the blood, and thus may directly modulate leptin action.34 Circulating adiponectin binds to adiponectin receptor 1 on cardiac myocytes, with downstream cardioprotective effects on cardiac fatty acid and glucose metabolism, hypertrophy, fibrosis, and apoptosis.10 We found that circulating leptin but not adiponectin concentrations were associated with global longitudinal strain. Interestingly, previous studies have suggested that leptin may be linked to increased aldosterone secretion.35 However, leptin remained associated with longitudinal strain even after adjusting for aldosterone concentrations, and there was no association of aldosterone or the aldosterone-to-renin ratio and measures of LV strain. Similarly, we did not find an association of CRP and measures of cardiac strain.
Previous population-based data show that higher leptin concentrations are associated with diastolic dysfunction.36 We now extend prior findings to also highlight a role for leptin in relation to LV systolic mechanics in humans, which corroborates prior findings in leptin-receptor deficient animal models.37 It is important to note that data linking circulating leptin concentrations to clinical outcomes in population-based studies have shown conflicting results, with some studies suggesting higher risk and others a null or even protective effect.38-40
Several limitations of our study should be considered. The observational, cross-sectional nature of our study precludes causal inferences, and the clinical value of strain measures remains unclear. While strain measures have been associated with cardiovascular events among Framingham participants,13 these analyses were limited in power, and further studies are needed to elucidate clinical implications. We acknowledge that effect sizes were modest in our study, and that the clinical significance of observed associations remains unclear. Specifically, although strain measures have been shown to improve with interventions such as bariatric surgery, exercise training, and spironolactone therapy in small trials of select patient groups with cardiometabolic disease,41-43 the clinical impact of improving LV systolic mechanics with regard to disease prevention remain unknown. We measured radial strain along two different imaging planes (short axis and apical views), and note that the mechanistic value or differences between the two imaging planes is not established. Given greater reproducibility for longitudinal and circumferential strain measurements,24 we examined radial strain measures as secondary points of interest. Global longitudinal strain was measured in the apical 4 and 2-chamber views. This may have excluded the anterior septum and inferolateral wall and decreased accuracy of our measurements. However, we expect that this would have biased our results toward the null. Speckle-tracking analyses were performed on previously acquired images that had been obtained without specific focus on optimal endocardial border definition. However, image quality was acceptable for the vast majority of participants, and reproducibility was very good for strain measures across different planes.24 It would have been interesting to examine pericardial fat depots, however this was not assessed contemporaneous with strain measures, given variability in echocardiographic images. Lastly, our study sample was predominantly white, limiting potential generalizability to other populations.
In sum, our findings highlight the association of central obesity and related cardiometabolic phenotypes above and beyond BMI with subclinical measures of LV systolic function and mechanics. We also found that obesity-related traits were associated with distinct aspects of LV mechanics, suggesting potential differential effects along specific LV planes of deformation. Lastly, leptin was associated with global longitudinal strain, suggesting a potential role for circulating adipokines in obesity-related cardiac remodeling. Further work is needed to investigate the possible mechanisms underlying the link between cardiometabolic traits and subclinical alterations in cardiac mechanical dysfunction.
Supplementary Material
Clinical Perspective.
Obesity and cardiometabolic dysfunction are associated with increased risk of heart failure and other cardiovascular diseases. We evaluated the associations of obesity-related phenotypes with echocardiographic measures of left ventricular (LV) mechanical function among 6231 participants of the Framingham Heart Study. Greater body mass index and central adiposity were associated with worse global longitudinal strain and synchrony. Measures of insulin resistance, dyslipidemia, and diabetes mellitus also were associated with distinct aspects of LV mechanical function. Our findings highlight the association of central obesity and related cardiometabolic phenotypes above and beyond body mass index with subclinical measures of LV mechanical function, and may shed light onto obesity-related cardiac remodeling and heart failure.
Acknowledgments
None
Funding sources: This work was partially supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contracts N01-HC-25195 and HHSN268201500001I). Dr. Ho is supported by NIH grant K23-HL116780 and the MGH Hassenfeld Scholar Award. Dr. Cheng is supported in part by R00-HL-107642, R01-HL131532, R01-HL134168, and a grant from the Ellison Foundation. Dr. Tsao is supported by K23-HL118259. Dr. Benjamin was supported by R01-HL128914, R01-HL076784, and R01-AG028321.
Footnotes
Disclosures: None
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Sundström J, Risérus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, Liang CS, Gopal DM, Ayalon N, Donohue C, Santhanakrishnan R, Sandhu H, Perez AJ, Downing J, Gokce N, Colucci WS, Ho JE. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayalon N, Gopal DM, Mooney DM, Simonetti JS, Grossman JR, Dwivedi A, Donohue C, Perez AJ, Downing J, Gokce N, Miller EJ, Liang CS, Apovian CM, Colucci WS, Ho JE. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol. 2014;114:838–842. doi: 10.1016/j.amjcard.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6:142–152. doi: 10.1161/CIRCIMAGING.111.964627. [DOI] [PubMed] [Google Scholar]
- 9.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18:631–644. doi: 10.1007/s10741-012-9337-8. [DOI] [PubMed] [Google Scholar]
- 11.Abel ED, Sweeney G. Modulation of the cardiovascular system by leptin. Biochimie. 2012;94:2097–2103. doi: 10.1016/j.biochi.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Køber L, Pfeffer MA, Solomon SD. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812–1822. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Solomon SD, Benjamin EJ, Vasan RS. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated With Cardiovascular Outcomes and All-Cause Mortality in the Community. J Am Heart Assoc. 2015;4:e002071. doi: 10.1161/JAHA.115.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, Ovize M, Derumeaux G. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr. 2009;10:914–921. doi: 10.1093/ejechocard/jep095. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F, American Heart Association, National Heart Lung and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D'Agostino RB, Wilson PW. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283:221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S, McCabe EL, Larson MG, Chen MH, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Solomon SD, Benjamin EJ, Vasan RS. Left ventricular mechanical function: clinical correlates, heritability, and association with parental heart failure. Eur J Heart Fail. 2015;17:44–50. doi: 10.1002/ejhf.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013;26:1258–1266.e2. doi: 10.1016/j.echo.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JAC, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail. 2016;18:537–544. doi: 10.1002/ejhf.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvaraj S, Martinez EE, Aguilar FG, Kim KYA, Peng J, Sha J, Irvin MR, Lewis CE, Hunt SC, Arnett DK, Shah SJ. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging. 2016;9:e004396. doi: 10.1161/CIRCIMAGING.115.004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR, Carr JJ, Terry JG, Liu K, Goff DC, Lima JAC. Association of Obesity in Early Adulthood and Middle Age With Incipient Left Ventricular Dysfunction and Structural Remodeling: The CARDIA Study. JACC Heart Fail. 2014;2:500–508. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donal E, Roulaud M, Raud-Raynier P, De Bisschop C, Leclercq C, Derumeaux G, Daubert JC, Mabo P, Denjean A. Echocardiographic right ventricular strain analysis in chronic heart failure. Eur J Echocardiogr. 2007;8:449–456. doi: 10.1016/j.euje.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Baltabaeva A, Marciniak M, Bijnens B, Moggridge J, He FJ, Antonios TF, MacGregor GA, Sutherland GR. Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. Eur J Echocardiogr. 2008;9:501–508. doi: 10.1016/j.euje.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa H, Little WC, Ohno M, Brucks S, Morimoto A, Cheng HJ, Cheng CP. Diastolic mitral annular velocity during the development of heart failure. J Am Coll Cardiol. 2003;41:1590–1597. doi: 10.1016/s0735-1097(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 32.Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, Selvin E, Solomon S. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8:448–454. doi: 10.1161/CIRCHEARTFAILURE.114.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 34.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 35.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015;132:2134–2145. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 36.Fontes-Carvalho R, Pimenta J, Bettencourt P, Leite-Moreira A, Azevedo A. Association between plasma leptin and adiponectin levels and diastolic function in the general population. Expert Opin Ther Targets. 2015;19:1283–1291. doi: 10.1517/14728222.2015.1019468. [DOI] [PubMed] [Google Scholar]
- 37.Li RJ, Yang J, Yang Y, Ma N, Jiang B, Sun QW, Li YJ. Speckle tracking echocardiography in the diagnosis of early left ventricular systolic dysfunction in type II diabetic mice. BMC Cardiovasc Disord. 2014;14:141. doi: 10.1186/1471-2261-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 39.Mishra S, Harris TB, Hsueh WC, Hue T, Leak TS, Li R, Mehta M, Vaisse C, Sahyoun NR. The Association of Serum Leptin with Mortality in Older Adults. PLoS ONE. 2015;10:e0140763. doi: 10.1371/journal.pone.0140763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS, Nasir K, Criqui MH, McClelland RL, Hughes-Austin JM, Allison MA. Leptin and incident cardiovascular disease: the Multi-ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239:67–72. doi: 10.1016/j.atherosclerosis.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koshino Y, Villarraga HR, Somers VK, Miranda WR, Garza CA, Hsiao JF, Yu Y, Saleh HK, Lopez-Jimenez F. Changes in myocardial mechanics in patients with obesity following major weight loss after bariatric surgery. Obesity. 2013;21:1111–1118. doi: 10.1002/oby.20168. [DOI] [PubMed] [Google Scholar]
- 42.Schuster I, Vinet A, Karpoff L, Startun A, Jourdan N, Dauzat M, Nottin S, Perez-Martin A. Diastolic dysfunction and intraventricular dyssynchrony are restored by low intensity exercise training in obese men. Obesity. 2012;20:134–140. doi: 10.1038/oby.2011.270. [DOI] [PubMed] [Google Scholar]
- 43.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O'Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging. 2011;4:1239–1249. doi: 10.1016/j.jcmg.2011.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.