Abstract
Objective
We sought to describe the relationship between age, sex, and race-ethnicity with transcranial Doppler (TCD) hemodynamic characteristics from major intracerebral arterial segments in a large elderly population with varying demographics.
Methods
We analyzed 369 stroke-free subjects aged ≥70 years from the Einstein Aging Study. Single-gate, non-imaging TCD, a noninvasive ultrasonic technique that assesses real-time cerebrovascular hemodynamics, was used to interrogate nine cerebral arterial segments. Individual Doppler spectra and cerebral blood flow velocities were acquired and Pulsatility index (PI) and resistance index (RI) were calculated using the device’s automated waveform tracking function. Multiple linear regression models were used to examine the independent associations of age, sex, and race-ethnicity with TCD measures, adjusting for hypertension, history of MI or revascularization, and history of diabetes.
Results
Among enrolled subjects, 303 individuals had at least one vessel insonated (mean age, 80±6 years; 63% women; 58% white, 32% black). With age, TCD measures of mean blood flow velocity (MBFV) were significantly decreased in the BA (p=0.001) and PCA (right: p=0.003, left: p=0.02). PIs were increased in the left MCA (p=0.01) and left ACA (p=0.03), and RI was increased in the left MCA (p=0.007) with age. Women had higher PI and RI when compared to men in several vessels.
Conclusions
We report decreased MBFV and weakly increased arterial pulsatility and resistance with aging in a large, elderly stroke-free population. These referential trends in cerebrovascular hemodynamics may carry important implications in vascular pathology associated with advanced age, increased risk of cerebrovascular disease, cognitive decline and dementia.
Keywords: cerebral hemodynamics, transcranial Doppler, blood flow velocities, Pulsatiliy Index, Resistance Index, aging
Introduction
Transcranial Doppler (TCD) ultrasonography is a rapid, inexpensive, and noninvasive technique to measure real-time cerebrovascular hemodynamics of major cerebral arteries [1]. A low frequency (≤2MHz) transducer emits ultrasound waves through skull windows of adequate penetration and receives reflections off the surfaces of red blood cells within intracerebral vessels. Computer assistance analyzes the information to produce a visual and numerical output, which has predictive and diagnostic value in a clinical setting. TCD has proven to be a useful technology for identifying stroke risk in children with sickle cell disease [2], adults at risk of vasospasm after subarachnoid hemorrhage [3–5], intra- and extracranial steno-occlusive disease [6,7], angiographic occlusion in acute ischemic stroke [8], brain stem death [9], raised intracranial pressure from head injury [10], right-to-left cardiopulmonary shunt [11], and impaired vasomotor function in a wide range of neurological and metabolic conditions [12]. TCD is increasingly becoming an essential tool in peri- and post-operative monitoring of coronary artery bypass graft and carotid endarterectomy [13,14], in acute emergency settings [15], vascular clinics [16], and in athletics and traumatic brain injury patients [17]. Several professional national organizations have published practice guidelines and recommendations for the use of TCD [18].
Normal aging is associated with a wide range of anatomical and functional changes and with an increasing risk of age-related cerebrovascular diseases. Epidemiological, neuroimaging and clinical studies have suggested vascular contributions to age-related memory loss, cognitive decline and dementia. [19,20]. Therefore, understanding changes in cerebral hemodynamics in older adults free of stroke and dementia is the initial step in characterization of normal from abnormal changes in brain hemodynamics during aging. Cerebral hypoperfusion has been reported in conditions with structural and neuronal brain injury [21] but the role of normal aging in these processes is not clear. While numerous studies have documented the validity of TCD in cerebrovascular disease (CVD), less has been reported on the hemodynamic characteristics as measured by TCD in a community-based cohort of older adults. Previous groups have established normative values in healthy populations [22–24], but these cohorts have been either smaller in size or limited to younger individuals.
Hemodynamic abnormalities may be a useful early clinical marker in the development of age-related neurodegenerative disorders with vascular etiology [20]. Describing the variability of TCD values across age, sex and race-ethnicity in a large elderly population may better guide further studies. Therefore, this study sought to describe the relationship between age, sex and race-ethnicity with TCD hemodynamic characteristics from major intracerebral arterial segments in a large elderly population with varying demographics.
Methods
Study Subjects
The Einstein Aging Study (EAS) is a longitudinal study of cognitive aging and dementia. Study methods have been described in detail elsewhere [25]. Briefly, the cohort includes systematically recruited community-residing older adults from Bronx County, NY. Between 1993 and 2004, participants were recruited using Health Care Financing Administration/Centers for Medicaid and Medicare Services (HCFA/CMS) rosters of Medicare eligible persons aged 70 and above. Since 2004, New York City Board of Elections registered voter lists for the Bronx have been used due to changes in policies for release of HCFA/CMS rosters. Eligible participants are at least 70 years of age, Bronx residents, non-institutionalized, and English speaking. Exclusion criteria included visual or auditory impairments that preclude neuropsychological testing, active psychiatric symptomatology that interfered with the ability to complete assessments, and non-ambulatory status. This study was approved by the Albert Einstein College of Medicine and University of Miami Institutional Review Boards. Written informed consent was obtained at the initial clinic visit according to protocols approved by the local institutional review board. Annual clinic visits include measures of blood pressure, weight and height, neurological examinations, neuropsychological assessments, as well as demographic and medical history assessments. Hypertension was defined as self-reported history of hypertension defined as systolic ≥ 140 mmHg and/or diastolic ≥ 90 mmHg or use of antihypertensive medication. Participants were asked whether a doctor had ever told them they had stroke, myocardial infarction, or diabetes, whether they had ever had coronary artery bypass surgery or other revascularization procedures for a coronary artery. Beginning in 2012, TCD assessments were included in the annual clinic visits. This report includes data for the first 408 individuals who completed TCD. Of the 408 who had a TCD assessment, 39 had a history of stroke and were excluded from the present analyses. Among the remaining 369 individuals, 66 (18%) did not have an adequate bone window to insonate any of the anterior (ACA), posterior (PCA) and middle (MCA) cerebral arteries. This report is based on the remaining 303 individuals.
TCD Examination
A complete TCD examination was performed by a single experienced sonographer in NY using a single gate, non-imaging TCD (TOCM Neurovision system with 2MHz probes) and a fixation device. Complete, bilateral TCD examination was performed in supine position using standardized TCD protocol [26]. Temporal bone windows were used to interrogate nine arterial segments including bilateral middle cerebral arteries (MCA; proximal and distal M1 segment at a 50–65 mm depth); bilateral anterior cerebral arteries (ACA segment; A1 at a 60–70 mm depth); and bilateral posterior cerebral artery (PCA; P1 segment at a 60–65 mm depth). The foraminal window was used to interrogate the bilateral vertebral arteries (VA at a 60 mm depth) and the basilar artery (BA a 80–100 mm depth). We used a 5-second sweep per screen that created in average 5 waveforms per page. A single technologist identified screens with clear waveforms and saved them for the off-line analyses. Individual Doppler spectra and cerebral blood flow velocities were saved for each arterial segment. Dimensionless parameters, Pulsatility Index (PI); defined as [(Peak-systolic velocity, PSV) – (End-diastolic velocity, EDV)/Mean flow velocity, MFV] and resistance index (RI); defined as [(PSV – EDV)/PSV], were calculated using the device’s automated waveform tracking function. The images were stored in a CD and sent to the University of Miami Department of Neurology, Ultrasound Reading Center for processing and analyses. The velocity spectra were reviewed by the study lead sonologist in Miami to assure the adherence to the protocol and waveform quality and to perform the analyses.
Statistical Analyses
Not all arterial segments were able to be insonated in each individual, thus analysis is based on available TCD measures for each intracerebral artery. Bivariate associations of TCD measures with age (in years) were examined using Pearson’s correlation coefficients. For ease of presentation, the mean values for each TCD measure are presented in 5-year age categories. Unadjusted associations of TCD measures with sex and race-ethnic group were examined using and Student’s t-tests (men vs. women; whites vs. blacks). Analyses by race-ethnicity were restricted to whites and blacks given that the number of participants in all other race-ethnic groups was small (Total N all groups=30). Additional analyses examined the independent associations of age, sex and race-ethnicity with TCD measures using linear regression models in which with TCD measures modeled as a function of age (years), sex (male/female), race-ethnicity (black/white), hypertension, self-reported history of MI or revascularization and self-reported history of diabetes. All analyses were completed using SAS version 9.3.
Results
Among the 303 subjects who had at least one vessel insonated, the mean age was 80±6 years; 63% were female, and 58% were white and 32% black. There were 30 individuals (10%) who identified as Hispanic, Asian, or other race-ethnicity. The distribution of demographics and vascular risk factors are shown in Table 1.
Table 1.
Demographics and clinical characteristics of the study population (N = 303)
| Characteristic | Men (N=111) N (%) or Mean (SD) |
Women (N=192) N (%) or Mean (SD) |
Total (N=303) N (%) or Mean (SD) |
|---|---|---|---|
| Age, years | 79 (6) | 81 (6) | 80 (6) |
| Education, years | 15 (3) | 15 (3) | 15 (3) |
| Race: White Black Other |
75 (68) 25 (22) 11 (10) |
102 (53) 71 (37) 19 (10) |
177 (58) 96 (32) 30 (10) |
| Hypertension | 82 (75) | 146 (76) | 228 (75) |
| Self-reported history of diabetes | 30 (27) | 35 (18) | 65 (22) |
| Self-reported history of MI or revascularization | 20 (18) | 7 (4) | 27 (9) |
| BMI | 29 (5) | 29 (6) | 29 (5) |
| Smoking: Never Prior Current |
35 (32) 70 (64) 4 (4) |
99 (52) 89 (47) 1 (1) |
134 (45) 159 (53) 5 (2) |
TCD measures of mean blood flow velocity (MBFV) (Table 2), pulsatility index (PI) (Table 3), and resistance index (RI) (Table 4) in the basilar artery (BA), left and right segments of vertebral artery (VA), middle cerebral artery (MCA), anterior cerebral artery (ACA), and posterior cerebral artery (PCA) were examined by age, sex, and race-ethnicity groups.
Table 2.
Mean Blood Flow Velocities by age, sex, race, in key vessel segments
| Mean Blood Flow Velocity (cm/second) (Mean and Standard Error) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BA | VA | MCA | ACA | PCA | |||||
| (N=292) | Left (N=301) |
Right (N=298) |
Left (N=268) |
Right (N=270) |
Left (N=255) |
Right (N=257) |
Left (N=256) |
Right (N=254) |
|
| Age Group | |||||||||
| 70–74 | 24.6 (0.8) | 20.7 (0.6) | 20.9 (0.6) | 33.8 (0.9) | 34.8 (0.9) | 32.2 (1.0) | 32.9 (1.1) | 29.8 (0.6) | 29.0 (0.6) |
| 75–79 | 22.7 (0.8) | 19.4 (0.6) | 21.0 (0.6) | 33.9 (1.0) | 33.6 (0.7) | 29.4 (1.0) | 32.3 (1.0) | 28.3 (0.6) | 27.7 (0.6) |
| 80–84 | 22.8 (0.8) | 20.9 (0.7) | 20.2 (0.7) | 32.9 (1.0) | 32.0 (1.0) | 30.1 (1.1) | 29.9 (1.1) | 26.9 (0.6) | 27.4 (0.8) |
| 85+ | 21.0 (0.8) | 19.0 (0.6) | 19.9 (0.7) | 32.2 (1.1) | 32.8 (1.1) | 31.0 (1.1) | 31.4 (1.0) | 27.6 (0.6) | 26.7 (0.6) |
| Pearson Correlation (p-value) * Adjusted p-value† |
−0.21 (0.0003) 0.001 |
−0.079 (0.17) 0.26 |
−0.10 (0.08) 0.07 |
−0.076 (0.20) 0.26 |
−0.11 (0.07) 0.40 |
−0.059 (.35) 0.45 |
−0.12 (0.06) 0.05 |
−0.19 (0.003) 0.02 |
−0.18 (0.004) 0.003 |
| Sex | |||||||||
| Men | 22.0 (0.7) | 20.3 (0.5) | 20.5 (0.6) | 33.9 (0.9) | 34.6 (0.7) | 30.9 (0.9) | 32.9 (0.9) | 28.7 (0.5) | 28.6 (0.6) |
| Women | 23.3 (0.5) | 19.8 (0.4) | 20.5 (0.4) | 32.8 (0.6) | 32.5 (0.6) | 30.6 (0.7) | 30.9 (0.6) | 27.9 (0.4) | 27.2 (0.4) |
| t-test p-value | 0.14 | 0.46 | 0.96 | 0.31 | 0.02 | 0.76 | 0.06 | 0.28 | 0.05 |
| Adjusted p-value† | 0.04 | 0.59 | 0.78 | 0.67 | 0.10 | 0.76 | 0.11 | 0.91 | 0.18 |
| Race | |||||||||
| White | 22.1 (0.5) | 19.3 (0.4) | 20.2 (0.4) | 33.5 (0.6) | 33.3 (0.6) | 31.5 (0.7) | 32.0 (0.7) | 28.5 (0.4) | 28.0 (0.4) |
| Black | 23.8 (0.7) | 21.1 (0.6) | 21.1 (0.5) | 32.2 (0.9) | 32.6 (0.9) | 29.6 (1.0) | 31.8 (1.0) | 27.2 (0.6) | 26.7 (0.6) |
| t-test p-value | 0.05 | 0.02 | 0.18 | 0.22 | 0.48 | 0.10 | 0.91 | 0.07 | 0.09 |
| Adjusted p-value† | 0.24 | 0.02 | 0.31 | 0.19 | 0.59 | 0.09 | 0.92 | 0.07 | 0.09 |
Pearson correlation coefficients for age as a continuous value (in years) with mean blood flow velocity
P-values from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes.
Table 3.
Pulsatility indices by age, sex, race, in key vessel segments
| Pulsatility Index (Mean and Standard Error) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BA | VA | MCA | ACA | PCA | |||||
| (N= 292) | Left (N=301) |
Right (N=298) |
Left (N=268) |
Right (N=270) |
Left (N=255) |
Right (N=257) |
Left (N=256) |
Right (N=254) |
|
| Age Group | |||||||||
| 70–74 | 1.42 (0.04) | 1.29 (0.03) | 1.35 (0.03) | 1.55 (0.04) | 1.59 (0.04) | 1.61 (0.05) | 1.71 (0.05) | 1.60 (0.04) | 1.60 (0.04) |
| 75–79 | 1.40 (0.04) | 1.32 (0.03) | 1.39 (0.03) | 1.56 (0.03) | 1.62 (0.04) | 1.71 (0.04) | 1.72 (0.04) | 1.58 (0.04) | 1.61 (0.03) |
| 80–84 | 1.46 (0.04) | 1.37 (0.03) | 1.38 (0.04) | 1.61 (0.04) | 1.62 (0.04) | 1.71 (0.04) | 1.70 (0.04) | 1.63 (0.04) | 1.65 (0.04) |
| 85+ | 1.42 (0.05) | 1.33 (0.04) | 1.42 (0.05) | 1.66 (0.04) | 1.64 (0.04) | 1.73 (0.05) | 1.77 (0.04) | 1.64 (0.03) | 1.65 (0.03) |
| Pearson Correlation (p-value)* |
−0.002 (0.98) |
0.090 (0.12) | 0.033 (0.57) | 0.15 (.01) | 0.063 (0.31) | 0.15 (0.02) | 0.071 (0.26) | 0.074 (.24) | 0.084 (0.18) |
| Adjusted p-value† | 0.63 | 0.17 | 0.85 | 0.01 | 0.50 | 0.03 | 0.39 | 0.28 | 0.28 |
| Sex | |||||||||
| Men | 1.45 (0.04) | 1.33 (0.03) | 1.34 (0.03) | 1.51 (0.03) | 1.55 (0.03) | 1.59 (0.03) | 1.68 (0.04) | 1.55 (0.03) | 1.60 (0.03) |
| Women | 1.41 (0.03) | 1.33 (0.02) | 1.39 (0.02) | 1.64 (0.03) | 1.66 (0.03) | 1.75 (0.03) | 1.75 (0.03) | 1.65 (0.02) | 1.65 (0.02) |
| t-test p-value | 0.47 | 0.97 | 0.14 | 0.0008 | 0.008 | 0.0006 | 0.11 | 0.008 | 0.16 |
| Adjusted p-value† | 0.21 | 0.64 | 0.08 | 0.004 | 0.06 | 0.002 | 0.17 | 0.02 | 0.28 |
| Race | |||||||||
| White | 1.41 (0.03) | 1.32 (0.02) | 1.34 (0.02) | 1.56 (0.03) | 1.59 (0.03) | 1.68 (0.03) | 1.70 (0.03) | 1.59 (0.02) | 1.61 (0.02) |
| Black | 1.45 (0.04) | 1.33 (0.03) | 1.42 (0.02) | 1.61 (0.04) | 1.69 (0.04) | 1.71 (0.04) | 1.76 (0.04) | 1.65 (0.03) | 1.65 (0.03) |
| t-test p-value | 0.41 | 0.81 | 0.01 | 0.25 | 0.03 | 0.57 | 0.23 | 0.15 | 0.24 |
| Adjusted p-value† | 0.60 | 0.98 | 0.06 | 0.71 | 0.12 | 0.96 | 0.32 | 0.37 | 0.41 |
Pearson correlation coefficients for age as a continuous value (in years) with mean blood flow velocity
P-values from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes.
Table 4.
Resistance indices by age, sex, race, in key vessel segments
| Resistance Index (Mean and Standard Error) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BA | VA | MCA | ACA | PCA | |||||
| (N=292) | Left (N=301) |
Right (N=298) |
Left (N=268) |
Right (N=270) |
Left (N=255) |
Right (N=257) |
Left (N=256) |
Right (N=254) |
|
| Age Group | |||||||||
| 70–74 | 0.72(0.01) | 0.69 (0.01) | 0.70 (0.01) | 0.75 (0.01) | 0.76 (0.01) | 0.77 (0.01) | 0.79 (0.01) | 0.76 (0.01) | 0.77 (0.01) |
| 75–79 | 0.71(0.01) | 0.70 (0.01) | 0.72 (0.01) | 0.76 (0.01) | 0.77 (0.01) | 0.79 (0.01) | 0.79 (0.01) | 0.76 (0.01) | 0.78 (0.01) |
| 80–84 | 0.73(0.01) | 0.71 (0.01) | 0.71 (0.11) | 0.76 (0.01) | 0.77 (0.01) | 0.78 (0.01) | 0.79 (0.01) | 0.77 (0.01) | 0.78 (0.01) |
| 85+ | 0.72(0.01) | 0.69 (0.01) | 0.71 (0.01) | 0.78 (0.01) | 0.78 (0.01) | 0.80 (0.01) | 0.80 (0.01) | 0.78 (0.01) | 0.78 (0.01) |
| Pearson Correlation (p-value) * |
0.02 (0.65) | 0.070 (0.22) | 0.007 (0.91) | 0.17 (0.01) | 0.070 (0.25) | 0.10 (0.10) | 0.078 (0.21) | 0.10 (0.10) | 0.096 (0.13) |
| Adjusted p-value† | 0.50 | 0.32 | 0.80 | 0.007 | 0.42 | 0.18 | 0.31 | 0.10 | 0.19 |
| Sex | |||||||||
| Men | 0.72 (0.01) | 0.69 (0.01) | 0.70 (0.01) | 0.74 (0.01) | 0.75 (0.01) | 0.76 (0.01) | 0.78 (0.01) | 0.76 (0.01) | 0.77 (0.01) |
| Women | 0.72 (0.01) | 0.70 (0.01) | 0.72 (0.01) | 0.78 (0.01) | 0.78 (0.01) | 0.80 (0.01) | 0.80 (0.01) | 0.78 (0.01) | 0.78 (0.01) |
| t-test p-value | 0.57 | 0.64 | 0.06 | 0.001 | 0.01 | 0.001 | 0.10 | 0.06 | 0.07 |
| Adjusted p-value† | 0.27 | 0.37 | 0.03 | 0.006 | 0.06 | 0.0004 | 0.16 | 0.15 | 0.08 |
| Race | |||||||||
| White | 0.72 (0.01) | 0.69 (0.01) | 0.70 (0.01) | 0.76 (0.01) | 0.76 (0.01) | 0.78 (0.01) | 0.79 (0.01) | 0.76 (0.01) | 0.77 (0.01) |
| Black | 0.73 (0.01) | 0.70 (0.01) | 0.73 (0.01) | 0.77 (0.01) | 0.79 (0.01) | 0.79 (0.01) | 0.80 (0.01) | 0.78 (0.01) | 0.78 (0.01) |
| t-test p-value | 0.25 | 0.68 | 0.002 | 0.33 | 0.04 | 0.45 | 0.14 | 0.12 | 0.35 |
| Adjusted p-value† | 0.41 | 0.94 | 0.03 | 0.81 | 0.16 | 0.93 | 0.27 | 0.23 | 0.71 |
Pearson correlation coefficients for age as a continuous value (in years) with mean blood flow velocity
P-values from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes.
MBFV showed tendency of being lower with advanced age in all arteries. After adjusting for sex, race-ethnicity, hypertension, diabetes and history of MI or revascularization, a significant inverse relationship between MBFV and age was observed in the BA (r=−0.21, p=0.001), bilateral segments of the PCA (left: r=−0.19, p=0.02; right: r=−0.18, p=0.003), and in the right ACA (r=−0.12, p=0.05). Women had lower MBFV than men in all arteries overall, except the BA where women had higher velocities. However, with the exception of the BA (adjusted p=0.04), the sex difference was not significant after full adjustments. Only the left segment of the VA showed significant differences in MBFV between black and white participants, for which black participants had higher velocities (adjusted p=0.02).
PI showed weak positive correlations with advancing age in most arteries, with a significant trend only in the left MCA (r=0.15, adjusted p=0.01) and in the left ACA (r=0.15, adjusted p=0.03). Among women, PI was higher in bilateral segments of the MCA, and in the left segments of the ACA and PCA, compared with men. After full adjustments, the differences remained significant for only the left segments of all three vessels. In unadjusted analyses, black participants had increased PI as compared to whites in the right VA and in the right MCA, however the associations were attenuated after controlling for demographics and cardiovascular history.
Similar to PI, RI showed weak positive correlations with age. After full adjustments, a significant positive correlation was found between RI and age only in the left MCA (p=0.007). With the exception of the BA, women had higher RI than men in all vessels. The sex difference was statistically significant in the right VA (adjusted p=0.03), left MCA (adjusted p=0.006), and left ACA (adjusted p=0.0004). RI did not differ for black and white participants after adjustment, with the exception of higher RI in the right VA for blacks (adjusted p=0.03).
Figures 1–3 display MBFV, PI, and RI for major arterial segments by age groups, respectively. For all figures, age groups are represented by color bars and the asterisk denotes statistically significant associations between increasing age and TCD parameter after full adjustments.
Figure 1. Mean blood flow velocities (cm/second) by age group in key arterial segments.
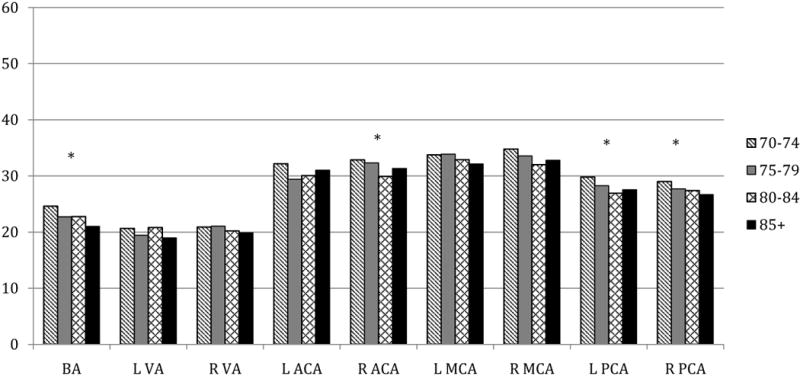
* p <0.05 for association of age with MFV from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes.
Figure 3. Resistance indices by age groups in key arterial segments.
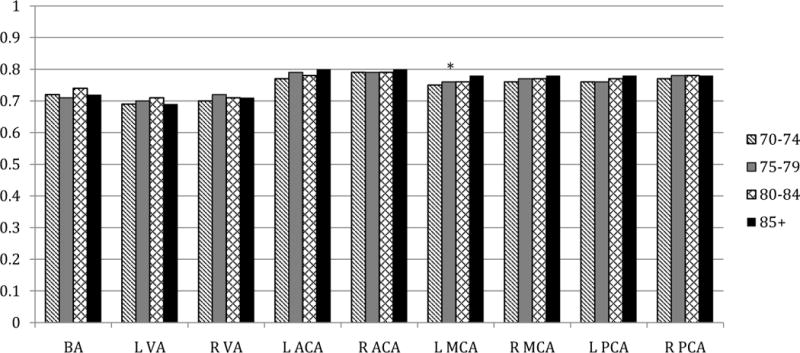
* p < 0.05 for association of age with RI from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes
Discussion
In a population of older adults free of clinical stroke or dementia, our TCD study finds that advancing age is correlated with decreasing cerebral blood flow velocities (MBFV), in particular in the posterior circulation (the PCA and BA). In addition, we observed increased arterial stiffness as indicated by higher PI in the left segments of the MCA and ACA, and higher RI in the left MCA with increasing age. We also found that women had higher PI and RI than men in key arterial segments. Differences in TCD parameters between race-ethnicity were seen in the VA and PCA, where black participants were found to have in increased then decreased MBFV, respectively, when compared to white individuals. Our results are in general consistent with other studies of TCD variables [24,27–29] and add further support to cerebral hemodynamic changes associated with normal aging. Our findings suggest less favorable indicators of vascular stiffness among women, which in part may explain greater risk of stroke in older women compared with men [30] and potentially greater rate of age-related cognitive decline and dementia among women in comparison to men as previously reported [31].
Age-related decreases in cerebral blood flow have been identified through several neuroimaging methods including positron emission tomography (PET), single-photon emission computed tomography (SPECT), functional magnetic resonance imaging (fMRI), and TCD [27,32,33]. The inverse association between cerebral blood flow and increasing age may be related to decreased metabolic demands, higher hematocrit [34], vessel-size changes [35], lower cardiac output [36], and changes in cerebral autoregulation associated with vascular risk factor burden and cardiac autonomic abnormalities [28]. Conversely, increases in arterial stiffness with age may lead to greater cerebral hemodynamic pulsatility [29]. Population-based epidemiologic studies have shown that age-related increase in central arterial stiffness is an important risk factor for white-mater damage in the brain and cognitive decline in older adults [37].
Together, post-mortem studies suggest vascular pathologies can cause cerebral ischemia through impaired blood flow, leading to neurocognitive decline and dementia [38,39]. Examinations of cerebral blood flow with normal aging using PET note specific decreases in blood flow to frontal [40] and limbic cortices believed to play a role in higher-order functions [41], which have been correlated with decreases in glucose consumption of neurons in these regions [30]. Our results therefore contribute to the “vascular” hypothesis of age-related cognitive changes, which is a subject of our further investigations.
Sex has also been shown to affect TCD values in some studies [24,28], but not others [22,27]. Decreased MBFV in women may be mediated by differences in hematocrit, which are lower in women. Further, hormonal fluctuations, especially estrogen levels, appear to contribute to reactivity and state of tone in the cerebral microcirculation of women [42]. Additionally, other studies report that female sex may be associated with elevations in cerebrovascular pulsatility through extension of central pulse pressure [29]. Given the differences in risk of CVD between men and women [30], the influence of sex on cerebral hemodynamic characteristics may have important implications for stroke and cognitive decline disparities between men and women and deserve further investigations.
Reports on race-ethnic differences in TCD parameters are lacking due to small sample sizes or populations composed of predominantly white participants [24,27]. In large multi-ethnic cohorts, diabetes and hypertension were main drivers of brain atrophy and ischemic changes reflected in brain MRI in the Atherosclerosis in Communities study [43] and correlated with poorer cognitive performance in the Northern Manhattan Study [44]. Specifically with posterior circulation, vertebral artery origin atherosclerosis leading to artery stenosis or occlusion was found to be less prevalent in a stroke registry comprised of mostly Hispanics and blacks than in separate studies with predominantly white populations [45]. This difference may be mediated by a high prevalence of intracranial atherosclerosis, which was previously shown to be increased in black populations due to higher prevalence of hyperglycemia [46]. While many associations between black and white participants were attenuated after adjusting for vascular risk factors, our results suggest possible race-ethnic disparities in TCD measures in the VA and PCA, which warrant further studies in diverse populations.
Limitations of this study include the cross-sectional design that limits conclusive inferences on changes with age. Further, our TCD parameters are measured only at rest and vasomotor reactivity was not included. Functional TCD testing with cognitive and memory tasks have been shown to affect cerebral hemodynamics [47]. Additionally, we while we adjusted for hypertension using a definition that includes antihypertensive medications, we did not adjust specifically for medications and did not assess cardiac output status, thus limiting our ability to analyze further potential influences on intracerebral hemodynamics. However, our results remained similar after accounting for presence of hypertension and history of heart disease or diabetes, which increased our confidence that the age-related hemodynamic differences are mainly due to the intracranial vascular changes. Lastly, we observed some side differences for MBFVs and PIs in several arterial segments, such as the ACA, that most likely resulted from variable angles of insonation [48] or variable courses of these vessels. Similarly, the same reasons could have accounted for absence of the relationships between MBFVs and several arterial segments at the same side (e.g. for left ACA). However, we did not specifically investigate the differences in TCD parameter across left-to-right arterial segments and additional research is needed to assess the clinical relevance of these inter-hemispherical asymmetries. Further work is needed to determine if changes in cerebral blood flow, pulsatility, and vessel resistance with age are linked to specific cerebral vascular pathologies during aging.
In conclusion, we report decreasing cerebral blood flow velocities and increasing vessel pulsatility and resistance significant in a few arterial segments with aging in a large, healthy, elderly population. Women had higher PI and RI when compared to men in several vessels. There may also be race-ethnic differences in cerebral hemodynamics with aging. These referential trends in cerebrovascular hemodynamics may carry important implications in vascular pathology associated with advanced age, increased risk of stroke, cognitive decline and dementia.
Figure 2. Pulsatility indices by age groups in key arterial segments.
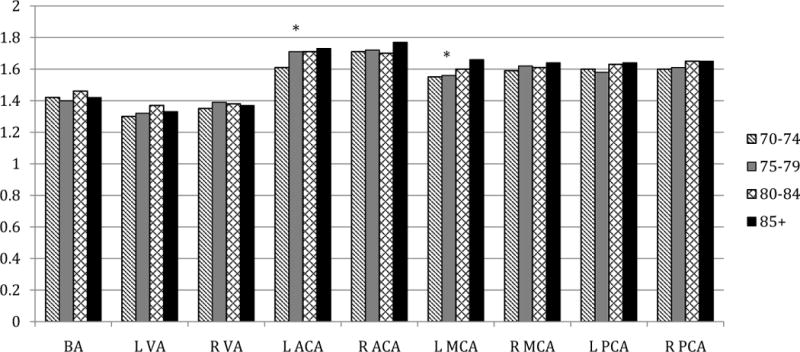
* p < 0.05 for association of age with PI from linear regression models that included age (years), sex, race-ethnicity (black/white), history of MI or revascularization, hypertension, and history of diabetes.
Acknowledgments
This study was supported by NIH: NIA P01-AG03949.
Footnotes
No other financial disclosures.
Contributor Information
Dixon Yang, Department of Neurology, Miller School of Medicine, University of Miami, FL.
Digna Cabral, Department of Neurology, Miller School of Medicine, University of Miami, FL.
Emmanuel N Gaspard, Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY.
Richard B. Lipton, Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY.
Tatjana Rundek, Department of Neurology, Miller School of Medicine, University of Miami, FL.
Carol A. Derby, Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY.
References
- 1.Aaslid R, Markwalder TM, Nornes H. Non invasive transcranial Dopplar ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 2.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 3.Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55:112–123. doi: 10.1007/BF03016323. [DOI] [PubMed] [Google Scholar]
- 4.Sviri GE, Ghodke B, Britz GW, et al. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59:360–366. doi: 10.1227/01.NEU.0000223502.93013.6E. [DOI] [PubMed] [Google Scholar]
- 5.Kincaid MS, Souter MJ, Treggiari MM, Yanez ND, Moore A, Lam AM. Accuracy of transcranial Doppler ultrasonography and single-photon emission computed tomography in the diagnosis of angiographically demonstrated cerebral vasospasm. J Neurosurg. 2009;110:67–72. doi: 10.3171/2008.4.17520. [DOI] [PubMed] [Google Scholar]
- 6.Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Alvarez-Sabín J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898–2904. doi: 10.1161/hs1201.099652. [DOI] [PubMed] [Google Scholar]
- 7.Christou I, Felberg RA, Demchuk AM, et al. A broad diagnostic battery for bedside transcranial Doppler to detect flow changes with internal carotid artery stenosis or occlusion. J Neuroimaging. 2001;11:236–242. doi: 10.1111/j.1552-6569.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrov AV, Bladin CF, Norris JW. Intracranial blood flow velocities in acute cerebral ischemia. Stroke. 1994;25:1378–1383. doi: 10.1161/01.str.25.7.1378. [DOI] [PubMed] [Google Scholar]
- 9.Ducrocq X, Braun M, Debouverie M, Junges C, Hummer M, Vespignani H. Brain death and transcranial Doppler: experience in 130 cases of brain dead patients. J Neurol Sci. 1998;160:41–46. doi: 10.1016/s0022-510x(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 10.Moreno JA, Mesalles E, Gener J, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8:1–7. doi: 10.3171/foc.2000.8.1.1702. [DOI] [PubMed] [Google Scholar]
- 11.Klingelhofer J, Conrad B, Benecke R, Frank B. Transcranial Doppler ultrasonography of carotidobasilar collateral circulation in subclavian steal. Stroke. 1985;14:721–728. doi: 10.1161/01.str.19.8.1036. [DOI] [PubMed] [Google Scholar]
- 12.Muller M, Voges M, Piepgras U, Schimrigk K. Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding: a comparison with acetazolamide as vasodilatory stimulus. Stroke. 1995;26:96–100. doi: 10.1161/01.str.26.1.96. [DOI] [PubMed] [Google Scholar]
- 13.Gerriets T, Schwarz N, Sammer G, et al. Protecting the brain from gaseous and solid micro-emboli during coronary artery bypass grafting: a randomized controlled trial. Eur Heart J. 2010;31:360–368. doi: 10.1093/eurheartj/ehp178. [DOI] [PubMed] [Google Scholar]
- 14.Halsey JH, McDowell HA, Gelmon S, Morawetz RB. Blood velocity in the middle cerebral artery and regional cerebral blood flow during carotid endarterectomy. Stroke. 1989;20:53–58. doi: 10.1161/01.str.20.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Jaffres P, Brun J, Declety P, et al. Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med. 2005;31:785–790. doi: 10.1007/s00134-005-2630-4. [DOI] [PubMed] [Google Scholar]
- 16.Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol. 2012;32:411–420. doi: 10.1055/s-0032-1331812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner AJ, Tan CO, Ainslie PN, et al. Cerebrovascular reactivity assessed by transcranial Doppler ultrasound in sport-related concussion: a systematic review. Br J Sports Med. 2015;49:1050–1055. doi: 10.1136/bjsports-2014-093901. [DOI] [PubMed] [Google Scholar]
- 18.American College of Radiology (ACR), Society for Pediatric Radiology (SPR), Society of Radiologists in Ultrasound (SRU) AIUM practice guideline for the performance of a transcranial Doppler ultrasound examination for adults and children. J Ultrasound Med. 2012;31:1489–1500. doi: 10.7863/jum.2012.31.9.1489. [DOI] [PubMed] [Google Scholar]
- 19.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68:S74–87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keage HA, Churches OF, Kohler M, et al. Cerebrovascular function in aging and dementia: a systematic review of transcranial Doppler studies. Dement Geriatr Cogn Dis Extra. 2012;2:258–270. doi: 10.1159/000339234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 22.Ringelstein EB, Kahlscheuer B, Niggemeyer E, Otis SM. Transcranial doppler sonography: anatomical landmarks and normal velocity values. Ultrasound Med Biol. 1990;16:745–761. doi: 10.1016/0301-5629(90)90039-f. [DOI] [PubMed] [Google Scholar]
- 23.Liboni W, Allais G, Mana O, et al. Transcranial Doppler for monitoring the cerebral blood flow dynamics: normal ranges in the Italian female population. Panminerva Med. 2006;48:187–191. [PubMed] [Google Scholar]
- 24.Tegeler CH, Crutchfield K, Katsnelson M, et al. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging. 2013;23:466–472. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 25.Katz MJ, Lipton RB, Hall CB, et al. Age and sex-specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer dementia in blacks and whites: a report From the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandrov AV, Sloan MA, Wong LK, et al. Practice standards for transcranial Doppler ultrasound: Part I- Test performance. J Neuroimaging. 2007;17:11–18. doi: 10.1111/j.1552-6569.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Demirkaya S, Uluc K, Bek S, Vural O. Normal blood flow velocities of basal cerebral arteries decrease with advancing age: a transcranial Doppler sonography study. Tohoku J Exp Med. 2008;214:145–149. doi: 10.1620/tjem.214.145. [DOI] [PubMed] [Google Scholar]
- 28.Fu CH, Yang CC, Kuo TB. Age-related changes in cerebral hemodynamics and their correlations with cardiac autonomic functions. Neurol Res. 2006;28:871–876. doi: 10.1179/016164106X110463. [DOI] [PubMed] [Google Scholar]
- 29.Tarumi T, Ayaz Khan M, Liu J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34:971–978. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves MJ, Bushnell C, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease: the impact of mortality on risk estimates in the Framingham Study. Neurology. 2007;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 32.Bentourkia M, Bol A, Ivanoiu A, et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci. 2000;181:19–28. doi: 10.1016/s0022-510x(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, Ito H, Goto R, et al. Apparent CBF decrease with normal aging due to partial volume effects: MR-based partial volume correction on CBF SPECT. Ann Nucl Med. 2005;19:283–290. doi: 10.1007/BF02984620. [DOI] [PubMed] [Google Scholar]
- 34.Melamed E, Lacy S, Bentin S, Cooper G, Rinot Y. Reduction in regional cerebral blood flow during normal aging in man. Stroke. 1980;11:31–35. doi: 10.1161/01.str.11.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Kusunoki K, Oka Y, Saito M, et al. Changes in visibility of intracranial arteries on MRA with normal ageing. Neuroradiology. 1999;41:813–819. doi: 10.1007/s002340050847. [DOI] [PubMed] [Google Scholar]
- 36.Safar M. Ageing and its effects on the cardiovascular system. Drugs. 1990;39(Suppl 1):1–8. doi: 10.2165/00003495-199000391-00003. [DOI] [PubMed] [Google Scholar]
- 37.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernando MS, Ince PG, MRC Cognitive Function and Ageing Neuropathology Study Group Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Keage HA, Carare RO, Friedland RP, et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3. doi: 10.1186/1471-2377-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar B. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- 41.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- 42.Shamma FN, Fayad P, Brass L, Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertil Steril. 1992;57:1022–1025. [PubMed] [Google Scholar]
- 43.Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin BE, Llabre MM, Dong C, et al. Modeling metabolic syndrome and its association with cognition: the Northern Manhattan study. J Int Neuropsychol Soc. 2014;20:951–960. doi: 10.1017/S1355617714000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon Perue GL, Narayan R, Zangiabadi AH, et al. Prevalence of vertebral artery origin stenosis in a multirace-ethnic posterior circulation stroke cohort: Miami Stroke Registry (MIAMISR) Int J Stroke. 2015;10:185–187. doi: 10.1111/ijs.12321. [DOI] [PubMed] [Google Scholar]
- 46.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 47.Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt EA, Piechnik SK, Smielewski P, Raabe A, Matta BF, Czosnyka M. Symmetry of cerebral hemodynamic indices derived from bilateral transcranial Doppler. J Neuroimaging. 2003;13:248–254. [PubMed] [Google Scholar]


