Abstract
Indeterminate cystic kidney lesions found incidentally on abdominal imaging are an increasingly prevalent diagnostic challenge. The standard workup includes Bosniak classification with contrast-enhanced CT or MRI. However, these tests are costly and not without risks. Contrast-enhanced ultrasound (CEUS) is a relatively new imaging technique with lower risk of adverse events than iodine-containing contrast or gadolinium. In our review of the evidence for characterization of cystic kidney lesions with CEUS, CEUS displayed sensitivity (89–100%) and negative predictive value (86–100%) comparable to contrast-enhanced CT or MRI with no decrease in specificity compared to CT and only a slight decrease compared to MRI.
Keywords: Contrast, ultrasound, kidney, cyst
INTRODUCTION
Due to a rise in abdominal imaging, particularly among chronic kidney disease patients and the elderly, increasingly physicians detect kidney cysts and other indeterminate lesions in the kidney. The best approach to these cystic lesions, sometimes referred to as “incidentalomas,” is not clear. The Bosniak classification system of kidney cysts, developed in 1986,1 classifies patients into categories (I, II, IIF, III and IV) based on lesion size and density, number, thickness and enhancement of septa, calcifications, and nodularity. Categories I and II are generally considered benign, IIF requires follow-up and III and IV are generally surgical lesions.
The recommendation for Class IIF and III lesions, which make up the greatest proportion of complex cystic lesions, is to perform repeat or follow-up contrasted imaging studies which can often invoke patient anxiety and add to the burden of healthcare costs. Biopsy is an alternative, but poses risks to the patient and can be often be non-diagnostic. The tests used to follow indeterminate lesions suffer from serious limitations in certain patient populations. For example, these studies are contraindicated in patients with allergies to contrast agents, claustrophobia, or inability to lie flat or hold their breath. Most significantly, patients with moderate to severe renal insufficiency are both the highest risk group for developing complex lesions,2 and have the highest risk of morbidity and mortality from CT or MRI contrast agents. An ideal diagnostic imaging modality would have fewer of these adverse features but remain highly sensitive and specific in differentiating malignancy from benign disease.
More access to sensitive alternative imaging tools could improve management of these lesions and aid in the special at risk populations. Current alternative imaging approaches include several highly non-sensitive modalities: B-mode ultrasound, Doppler ultrasound, and unenhanced CT and MRI. Contrast enhanced ultrasound (CEUS) is also an emerging strategy that is being applied to these lesions. CEUS of the kidneys, initially introduced in 1994,3 utilizes a microbubble contrast agent which, unlike CT and MRI agents, is not filtered through the glomeruli but remains intravascular, making CEUS ideal for visualizing tissue vascularity without opacifying the urinary tract or surrounding parenchyma. The contrast agents Sonovue, Definity and Optison are currently FDA approved only for cardiac use in the United States but approved for use in abdominal imaging outside the United States in Europe, India, China, South Korea and Brazil.
While a recent review of new imaging modalities for indeterminate cystic kidney lesions included CEUS,4 among other modalities, our review looks in depth at the evidence specifically related to CEUS, summarizing the current guidelines and diagnostic tools for characterizing cystic kidney lesions, reporting the existing evidence for application of CEUS to patients with indeterminate cystic kidney lesions, and comparing CEUS to conventional imaging modalities for cystic lesions to explore potential future clinical applications of CEUS in the kidney. To achieve this, we conducted a formal literature search with criteria shown in Supplemental Table 1. Our selection criteria were full-text, English language experimental studies in humans investigating CEUS for kidney lesions.
CURRENT CLINICAL GUIDELINES
While international urologic and radiologic associations provide guidelines for management of indeterminate cystic kidney lesions, numerous differences across societies exist. The major urologic associations advise use of contrast-enhanced CT as the primary test to characterize indeterminate cystic kidney lesions with contrast-enhanced MRI rated either equivalently or as a second option. The American College of Radiology rates contrast-enhanced CT highest for those without kidney impairment while ratings for patients with kidney impairment are significantly different due to the nephrotoxic potential of contrast agents; subsequently, non-contrasted studies, though suboptimal, are relied upon (Supplemental Table 2).
The current role of ultrasound in clinical guidelines is variable. The American Urologic Association does not specifically address indeterminate cystic lesions; guidelines are therefore extrapolated from sections on asymptomatic hematuria and stage T1 incidentally detected renal masses. They indicate the need for alternative, low-risk imaging methods, including screening ultrasound. Similarly, the British Association of Urologic Surgeons/British Uro-oncology Group guidelines do not specifically address indeterminate cystic lesions, but in the guidelines for renal cancer, ultrasound is described as an initial screening modality. CEUS is mentioned only by the European Association of Urology, where SonoVue is approved for non-cardiac applications and more widely utilized than in the United States.
BOSNIAK CLASSIFICATION OF CYSTIC KIDNEY LESIONS
The rate of malignancy of cystic kidney lesions correlates with Bosniak classification. The classification scheme initially sorted cystic kidney lesions into 4 categories based on lesion complexity and thus likelihood of malignancy. Category I and II lesions are generally considered benign with very minimal chance of malignancy. Radiographically, Bosniak I lesions have non-enhancing thin walls with no septa or solid components. Bosniak II lesions have minimally enhancing hairline thin septa with fine to slightly thickened calcifications. Class II lesions also include completely intrarenal, marginated, non-enhancing cystic masses less than 3 cm. Category IV lesions are nearly 100% malignant warranting surgical removal in appropriate candidates. These lesions are cystic masses with enhancing soft tissue within the lesion and thickened, irregular and enhancing walls or septa. Many category III lesions were initially removed surgically but found to be benign, prompting the development of category IIF - lesions worrisome enough to warrant follow-up but not worrisome enough to warrant surgery.5 Management of these two categories, IIF and III, is the most challenging.
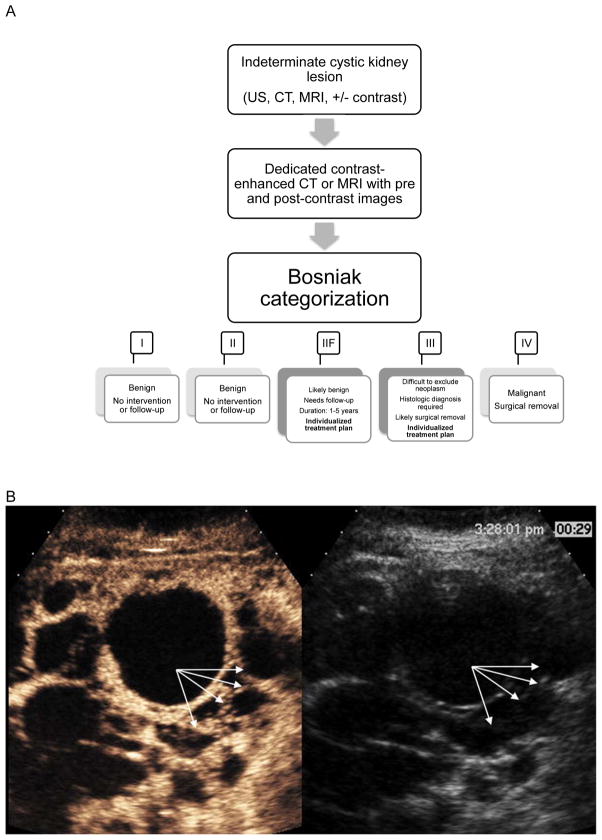
Category III lesions are generally accepted to be malignant 40–60% of the time,6, 7 warranting surgical removal in the majority.7 These lesions have thickened, enhancing walls or septa with or without calcifications. Category IIF lesions have malignancy rates between 5–25%6, 8, 9 and are generally followed with repeat imaging. These lesions are more complex than category II lesions with more septa and/or calcifications with minimal thickening and enhancement. This category also includes intrarenal, marginated, non-enhancing cystic masses larger than 3 cm. Recommended duration and frequency of follow-up is not clear and ranges from 1–5 years, depending on lesion complexity.9, 10 Repeated imaging with contrast-enhanced CT or MRI leads to high levels of radiation and/or contrast agent exposure, particularly an issue in younger patients. Therefore ultrasound is sometimes used, although CT or MRI remain the most accurate test. A summary of the current management strategy, based on Bosniak classification, is provided in Figure 1A.
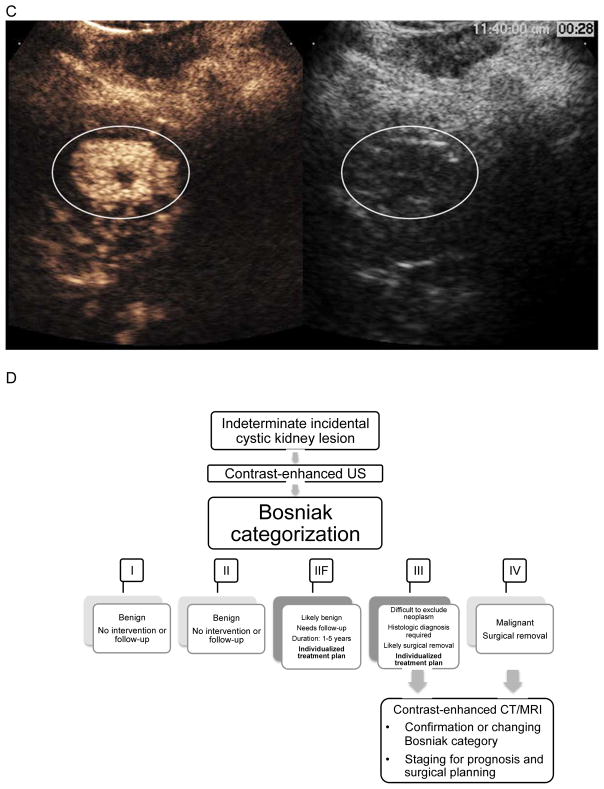
Figure 1.
A. Current management strategy for indeterminate cystic kidney lesions
B. CEUS image from a patient with polycystic kidney disease. Conventional gray-scale ultrasound images are on the right, single time point contrast-enhanced images are on the left. Arrows point to the lesion wall and septa that clearly enhance with CEUS but are difficult to detect with gray-scale ultrasound. This was classified as a Bosniak III lesion by CEUS.
C. CEUS image from a patient with renal cell carcinoma. Conventional gray-scale ultrasound images are on the right, single time point contrast-enhanced images are on the left. The circle encloses the enhancing solid portion of this lesion that is difficult to differentiate from surrounding parenchyma on gray-scale ultrasound. This was classified as a solid renal lesion by CEUS.
D. Proposed management strategy for indeterminate cystic kidney lesions seen on conventional ultrasound
IMAGING/DIAGNOSTIC APPROACHES TO CYSTIC KIDNEY LESIONS
A summary of the standard diagnostic approaches to indeterminate cystic kidney lesions is provided in Supplemental Table 3. A brief description of the current role for each modality is provided below.
Conventional ultrasound with Doppler
As many as 83%11 of renal cell carcinomas (RCC) are revealed incidentally on conventional B-mode ultrasound. Conventional ultrasound is also an excellent test for simple cyst (Bosniak I and II) identification, but lacks diagnostic accuracy for complex cysts (Bosniak IIF, III and IV) as it provides no information on enhancement and small lesions < 2cm which are difficult to visualize. Currently, unenhanced ultrasound is most commonly used in patients with chronic kidney disease when contrast-enhanced CT or MRI is contraindicated and as a follow-up imaging modality.
Contrast-enhanced CT
Contrast-enhanced CT is the most commonly used imaging test for characterization of indeterminate kidney lesions and is the test on which the Bosniak criteria were initially based. Although there has been some variability reported regarding the reliability of the CT-based Bosniak classification scheme,12 CT remains the first study recommended by both radiologic and urologic associations (Supplemental Table 2).
Contrast-enhanced MRI
Contrast-enhanced MRI is widely accepted as an alternative first-line study to characterize cystic kidney lesions. In direct comparisons, the two modalities showed similar findings in 81% of cases. The differences primarily led to upgraded Bosniak classification with MRI based on findings of additional septa, wall thickness and enhancement.13
Biopsy
The utility of biopsy/aspiration in the diagnosis of complex cystic kidney lesions is not clear but is becoming more widely accepted as a way to spare patients unnecessary surgery.7, 14 However, because there is less tissue to sample in a cystic lesion, there is a high chance of sampling error. Therefore a negative test will not necessarily exclude the need for surgery.15, 16 Moreover, instrumentation during biopsy can change the natural course of a lesion and its radiographic appearance, making follow-up studies challenging. Despite these limitations, the kidney biopsy literature reflects reasonable accuracy. For example, a study of 312 lesions showed misclassification rate of only 2.25% based on CT-guided biopsy.17
CEUS TECHNOLOGY
Contrast-enhanced ultrasound consists of two components: an FDA approved intravenous microbubble contrast agent and ultrasound software capable of detecting contrast agents. The use of agitated saline as a contrast agent has been around for over 40 years.18 The basic principle is that gas microbubbles in the blood increase ultrasound scattering intensity, providing enhanced contrast between blood and surrounding tissue. However, gas bubbles from agitated saline dissolve quickly and do not pass the lungs, making agitated saline contrast useful for detecting right-to-left cardiac shunts (bubbles will not be visible in the left heart unless a shunt is present) but not useful as a contrast agent elsewhere in the circulation.
The first generation of dedicated microbubble contrast agents was introduced in the 1990’s (Echovist by Schering, Levovist by Schering, Albunex by Molecular Biosystem).19 Lipid or albumin shells provide circulation persistence beyond agitated saline, but the high solubility of the air core in blood still led to rapid degradation. Newer generation agents utilize high molecular weight gas (Sulfur hexafluoride, Perfluoropropane, or Perfluorobutane) with lower solubility and greater stability in blood (Sonovue by Bracco Imaging, Definity by Lantheus Medical Imaging, Sonazoid by Daiichi Pharmaceutical/GE Healthcare, Optison by Mallinckrodt/GE Healthcare), increasing imaging time from seconds to minutes.19 Once dissolved, the gas is then exhaled through the lungs with no excretion through the liver or kidneys, making these agents safe for use in renal or hepatic insufficiency.
Although microbubble contrast agents can be visualized using traditional B-mode imaging, the difference between the microbubbles and surrounding tissue is minimal. Hence, modern ultrasound systems now utilize non-linear imaging techniques to differentiate the signal from tissue and microbubbles. Biological tissue produces a linear acoustic response. In contrast, microbubbles respond non-linearly, exhibiting differences in the way they respond to phase and amplitude of exciting pulses, as well as producing energy which contains subharmonics20 and harmonics21, 22 of the transmitted pulse that are not observed from tissue. This unique acoustic response allows for high sensitivity imaging of blood flow in small vessels.
In standard CEUS, a bolus of contrast is administered, typically 0.2–2 mL (depending on the manufacturer and concentration of bubbles in solution, patient mass, and imaging target) followed by 5–10 mL saline. A low mechanical index (MI) setting is typically used to avoid bubble destruction with continuous imaging.23 Real-time images are then obtained as the microbubbles flow through the region of interest. Qualitatively, features such as net enhancement, wash-in, and wash-out may be observed to provide an indication of vascularity and flow properties of the target tissue. Using cine clips and specialized software, time-intensity curves may be calculated from the change in image intensity to provide numerical values for wash-in rate, wash-out rate, or other quantitative parameters.
One of the advantages of CEUS is the low rate of serious adverse events with microbubble contrast agents, ranging from 0.009% to 0.014%,24, 25 significantly lower than gadolinium contrast. They are however contraindicated in the presence of right-to-left shunts, with caution recommended in certain cardiopulmonary conditions including recent acute myocardial infarction, severe heart failure, severe pulmonary hypertension, pregnancy and breastfeeding. Current ultrasound guidelines advise MI settings lower than 1.9, regardless of contrast use. Animal studies in which CEUS of the kidneys was performed show glomerular capillary hemorrhage and gross petechiae on the kidney surface with transient microhematuria.26 However, these studies used MIs higher than those used clinically for standard CEUS imaging.
One challenge to clinical application of CEUS is inter-operator and inter-observer variability. For example, ultrasonographers may choose different imaging windows, look at different angles, and choose different angles or imaging depths. The technique inherently requires more expertise and training to obtain high quality images than CT or MR. Interpretation will also vary as radiologists are not used to reading this type of study. As with the implementation of any new technology, a significant amount of training and experience will be required.
COMPARISON OF CEUS vs. OTHER IMAGING MODALITIES
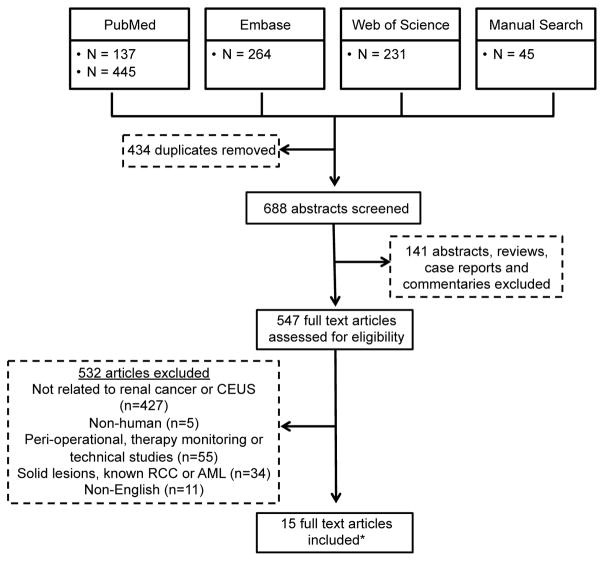
Our search strategy, displayed in Figure 2, identified 547 articles for full-text review after removal of duplicates, abstracts, reviews, case reports and commentaries. 532 full text articles were excluded based on selection criteria. A total of 15 full text articles were included in the final analysis. Nine of the 15 studies were not excluded by the search strategy but were not analyzed statistically because lesion inclusion criteria differed,27–30 they used an ultrasound detection technique not optimized for contrast detection,31 or they focused on a specific subgroup or aspect of CEUS.32–35 These 9 studies provide valuable information and are summarized in supplemental table 4.
Figure 2.
Flow chart for search strategy
* Only 6 of the articles are included in table 3. The remaining 9 articles are reviewed and included in supplemental table 2.
Of the remaining 6 studies, all included lesions were based on indeterminate findings on an imaging study and are presented in Table 1. While we attempted to limit the studies to those that included only cystic lesions, two studies did not specifically exclude solid lesions, although the percentage of solid lesions is low. Five of the six studies were conducted outside the US, due in large part to the approval for microbubble use in other countries.36–40 Follow-up times varied, ranging from 12 months to 10 years, although most fell in the 2-year time frame.
Table 1.
Characteristics of studies evaluating CEUS for characterization of indeterminate cystic kidney lesions
| Author | Country (Year) | Study type (N) | Inclusion criteria | CEUS contrast agent, volume and needle gauge (US technique) | Gold Standard (N) | Modalities compared | Method of analysis | Resultsa |
|---|---|---|---|---|---|---|---|---|
| Ascentib | Italy (2007) | Prospective (44) | Complex cystic kidney lesion identified on conv US | SonoVue 2.4 mL (harmonic, low MI) |
Histology (9) Imaging fu (31) |
CECT | 3 blinded readers (Bosniak and enhancement) |
CECT: sens 100%, spec 87%, PPV 50%, NPV 100%, Acc 87% CEUS: sens 100%, spec 87%, PPV 50%, NPV 100%, Acc 87% |
| Clevertb | Germany (2008) | Prospective (37) | Complex kidney cysts referred to radiology | SonoVue 1.6–2.4 mL 18g (CPS, low MI) |
Histology (14) Imaging fu (23) |
CECT | Blinded readers (Bosniak) |
CECT: sens 83%, spec 80%, PPV 67%, NPV 91%, Acc 81% CEUS: sens 100%, spec 80%, PPV 71%, NPV 100%, Acc 86% |
| Quaia | Italy (2008) | Observational (40) | Complex cystic kidney lesions identified on conv US or CT | SonoVue 2.4 mL 10 or 22g (CPS or contrast tuned imaging, low MI) |
Histology (32) Imaging fu (10) |
CECT Conv US+PDUS/CDUS |
Three different blinded readers (enhancement, 5-grade scale of diagnostic confidence) |
Conv US+PDUS/CDUS: sens 46%, spec 13%, PPV 37%, NPV 17%, Acc 30% CECT: sens 86%, spec 51%, PPV 67%, NPV 77%, Acc 69% CEUS: sens 89%, spec 74%, PPV 79%, NPV 86%, Acc 82% |
| Nicolaub | Spain (2014) | Prospective (83, primarily cystic but includes an undesignated number of solid lesions) | Indeterminate CT studies | SonoVue 2.4 mL 21g (CPS, low MI) |
Histology (32) Imaging fu (51) |
Conv US +CDUS | Two blinded readers (Bosniak) |
Conv US+CDUS: sens 52%, spec 36%, PPV 35%, NPV 53%, Acc 42% Conv US+CEUS: sens 94%, spec 96%, PPV 94%, NPV 96%, Acc 95% |
| Barrb | US (2014) | Observational (1018, 950 cystic) | Clinically indeterminate solid or cystic kidney lesions | Definity/Optison (low MI) 0.3–3 mL |
Histology (306) Imaging fu (712) |
Modality chosen by clinician | Single reader (enhancement) | CEUS: sens 100%, spec 99%, PPV 97%, NPV 100% |
| Chen | China (2014) | Prospective (71) | Complex cystic kidney lesions (includes Bosniak II) on conv US | SonoVue (PIHI, low MI) 2.4 mL 20g |
Histology (43) Imaging fu (28) |
MRI +/− contrast | Two blinded readers (Bosniak) |
MRI: sens 81%, spec 77%, PPV 78%, NPV 79%, Acc 79% CEUS: sens 97%, spec 71%, PPV 78 %, NPV 96%, Acc 85% |
Calculated based on classification of Bosniak I,II and IIF as negative and Bosniak III and IV as positive.
Not all statistics provided by reference. All or some of the statistics were calculated by authors of this review. When Bosniak classification scheme used, Bosniak I, II and IIF counted as a negative test and Bosniak III and IV counted as positive test.
Abbreviations: fu (follow-up), conv (conventional), Acc (accuracy), CPS (cadence pulse sequencing), PIHI (pulse inversion harmonic imaging), CECT (contrast-enhanced CT), US (ultrasound), PDUS (power Doppler US), CDUS (color Doppler US)
From a technical standpoint, all studies used either SonoVue or Definity. Technique was similar, including a contrast agent volume of 1.2–2.4 mL, use of low MI, and contrast specific imaging techniques, including cadence pulse sequencing, harmonic imaging and pulse inverted harmonic imaging. Examples of a single image from cine clips obtained with CEUS are shown in figures 1B and C. For most studies, the contrast-enhanced CT based Bosniak criteria were used for lesion characterization. Septa and wall enhancement were the main lesion characteristics detected on CEUS that contributed to Bosniak classification. All studies except one41 used multiple readers to address inter-observer variability.
Due to challenges such as variable follow-up time, different ways of controlling for variability between readers, and the fine gradation of the Bosniak classification system, uniform interpretation of the studies as presented was not possible. In calculations of sensitivity, specificity, positive and negative predictive values, we thus placed Bosniak I, II and IIF into the negative category and Bosniak III and IV into positive category as this is the delineation between primarily non-surgical and surgical lesions. One drawback to this strategy is that a change in classification from Bosniak I to IIF would not be detected based on this strategy. This was deemed acceptable, as it would not greatly affect clinical management. Any follow-up of at least a year was accepted.
The most notable and consistent finding across the six studies was the high sensitivity of CEUS for detection of malignant cystic kidney lesions. Sensitivity ranged from 89–100% and reached 100% in three studies,36, 37, 41 including the large, US-based study with over 900 cystic lesions.41 This is attributed to the ability of CEUS to detect subtle enhancement in thin septa that are more difficult to detect with either CT or MRI. Although not reflected in the statistics, upgrade of Bosniak classification from I/II to IIF also occurred with CEUS, due to increased sensitivity in detection of enhancement.
Specificity was moderate with CEUS, ranging from 71–99%. This was due in large part to false positives. As reported by Ascenti, of five false positives, two were inflammatory cysts, one was xanthogranulomatous pyelonephritis, one was multilocular cystic RCC (an indolent malignancy) and one refused intervention and was stable at 18-month follow-up. The false positive findings reported by Clevert were all Bosniak III and also reported as false positives by contrast-enhanced CT. Compared to MRI, CEUS had a higher false positive rate with 10 out of 35 benign lesions being misdiagnosed by CEUS and only 8 out of 35 being misdiagnosed by MRI. These consisted primarily of benign cystic kidney lesions and angiomyolipomas.
Negative predictive value was also consistently high between the studies, ranging from 86–100%. Because both per study cost and risks associated with CEUS are lower than either CT or MRI, with a high negative predictive value, use of CEUS prior to CT or MRI could limit the number of costly, potentially harmful studies. However, positive findings would still likely require further evaluation by either CT, MRI or biopsy. But routine use of CEUS has the potential to significantly alter management both for individual patients and for the field as a whole.
SPECIALIZED TOPICS
The remaining 9 studies were not excluded based on any of the named exclusion criteria but did not specifically compare the overall diagnostic accuracy of CEUS compared to other modalities. Thus they were not included in the formal analysis. However, they do provide useful information that can inform development of the technology for clinical use. A summary is provided in Supplemental Table 4.
In studies that included patients based on histologic diagnosis,27–30 sensitivity was comparable to the 6 main studies (80–100%). However, specificity was decreased, likely due to inclusion criteria. The vast majority of cases were malignant lesions, as these were most likely to be resected or biopsied, resulting in omission of numerous true negative cases and leading to a wide range in specificity (0–80%). Although false negative cases might also be absent, skewing sensitivity, all studies had at least one other imaging study, usually contrast-enhanced CT. Therefore, decision to resect was based on these studies and the lesion would not have been detected, even with the current standard of care.
The ability of CEUS to predict histologic subtype has also been investigated with minimal success thus far. There is certainly ability to differentiate a solid from a cystic lesion32 and define necrotic areas from solid carcinoma or cystic regions.33 However, correlation of enhancement patterns with histologic subtype, i.e. clear cell, papillary cell or multilocular cystic RCC, has not yet been clearly defined.35 Whether CEUS can predict histologic subtype and thus help with prognostication, is yet to be determined.
Inclusion of the specific population that might benefit most from CEUS, chronic kidney disease patients, has not yet been conducted. However, a subgroup of this population, renal transplant patients with acquired cystic kidney disease, has been studied.42 This patient population is clinically relevant, both because of their increased risk for cancer but also because contrasted CT or MRI is often avoided in these patients. In a cohort of 43 renal transplant patients with acquired cystic kidney disease, 35 lesions were detected in the native kidneys of 15 patients on conventional ultrasound.34 Each lesion was subsequently evaluated with CEUS. Twenty-seven lesions were classified as Bosniak I or II based on CEUS; four lesions were classified as Bosniak III or IV. Those four lesions underwent contrast-enhanced CT followed by surgical resection with confirmed cancer in 100%.
FUTURE DIRECTIONS
Numerous other applications for CEUS in the kidneys have been investigated, including trauma, pyelonephritis, ischemia and renal artery stenosis.43 Additionally, even more applications are under current investigation. Post-surgical surveillance after nephron sparing surgery, peri-operational use to aid surgical location and complete resection, and peri-procedural monitoring during radiofrequency ablation44 are three such applications. The sensitivity of CEUS can also be very useful to detect recurrence of disease and spare patients repeated exposure to radiation and costly tests. In addition, in experimental models of anti-angiogenic drug therapies, the use of CEUS may track response to therapy.45
In other applications, molecular ultrasound imaging with targeted microbubbles is being explored as a way to further increase diagnostic accuracy and ability to detect inflammation, thrombus, microvascular changes associated with early diabetic nephropathy46 and other vascular phenomena with sensitivity beyond CT or MRI. Microbubbles are also being investigated as a way to deliver drug or gene therapy to a specific organ, reducing potential toxic effects to other organs.22, 46
In addition to characterizing renal lesions, characterization of enhancement of renal parenchyma is also an area with potential clinical impact. Loss of vascularization of the failing kidney occurs throughout the course of chronic kidney disease. The ability to detect changes in perfusion in kidneys with a minimally invasive imaging technique may help clinicians detect patients with early stages of chronic kidney disease and target them for early interventions.
CONCLUSIONS
CEUS has potential to become an option as an initial diagnostic test for indeterminate cystic kidney lesions. The high sensitivity and negative predictive value, along with its excellent adverse effect profile and low cost, make it a candidate for a first line test. One challenge to widespread CEUS adoption is the current lack of experience by technicians and radiologists. Standardized CEUS administration and interpretation protocols and specialized training in this modality are needed. The potential payoff, in terms of patient safety and potential healthcare dollars saved, though, may be well worth the effort.
A proposed management strategy is shown in Figure 1D. However, more large, multi-center trials comparing CEUS to the current standard along with analysis of cost benefits are needed. CEUS also has potential to be utilized as the follow-up imaging study of choice for Bosniak IIF and III lesions for which patients opt to manage with surveillance as there would be significantly lower exposure to radiation from repeated imaging. Efforts made by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) and The International Contrast Ultrasound Society (ICUS) to encourage appropriate utilization of CEUS are supported by the authors.
Supplementary Material
Acknowledgments
Jennifer E. Flythe, Ronald J. Falk, Susan L. Hogan
References
- 1.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Ordonez J, Udaltsova N, Russo P, Go AS. CKD and the Risk of Incident Cancer. Journal of the American Society of Nephrology : JASN. 2014;25:2327–2334. doi: 10.1681/ASN.2013060604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippone A, Muzi M, Basilico R, Di Giandomenico V, Trapani AR, Bonomo L. Color Doppler flow imaging of renal disease. Value of a new intravenous contrast agent: SH U 508 A (Levovist) La Radiologia medica. 1994;87:50–58. [PubMed] [Google Scholar]
- 4.Ellimoottil C, Greco KA, Hart S, et al. New Modalities for Evaluation and Surveillance of Complex Renal Cysts. The Journal of urology. 2014;192:1604–1611. doi: 10.1016/j.juro.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 5.Israel GM, Bosniak MA. Follow-up CT of moderately complex cystic lesions of the kidney (Bosniak category IIF) AJR. American journal of roentgenology. 2003;181:627–633. doi: 10.2214/ajr.181.3.1810627. [DOI] [PubMed] [Google Scholar]
- 6.Smith AD, Remer EM, Cox KL, et al. Bosniak category IIF and III cystic renal lesions: outcomes and associations. Radiology. 2012;262:152–160. doi: 10.1148/radiol.11110888. [DOI] [PubMed] [Google Scholar]
- 7.Harisinghani MG, Maher MM, Gervais DA, et al. Incidence of malignancy in complex cystic renal masses (Bosniak category III): should imaging-guided biopsy precede surgery? AJR. American journal of roentgenology. 2003;180:755–758. doi: 10.2214/ajr.180.3.1800755. [DOI] [PubMed] [Google Scholar]
- 8.O’Malley RL, Godoy G, Hecht EM, Stifelman MD, Taneja SS. Bosniak category IIF designation and surgery for complex renal cysts. The Journal of urology. 2009;182:1091–1095. doi: 10.1016/j.juro.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Hindman NM, Hecht EM, Bosniak MA. Follow-up for Bosniak category 2F cystic renal lesions. Radiology. 2014;272:757–766. doi: 10.1148/radiol.14122908. [DOI] [PubMed] [Google Scholar]
- 10.Bosniak MA. The Bosniak renal cyst classification: 25 years later. Radiology. 2012;262:781–785. doi: 10.1148/radiol.11111595. [DOI] [PubMed] [Google Scholar]
- 11.Siemer S, Uder M, Humke U, et al. Value of ultrasound in early diagnosis of renal cell carcinoma. Der Urologe Ausg A. 2000;39:149–153. doi: 10.1007/s001200050023. [DOI] [PubMed] [Google Scholar]
- 12.Wilson TE, Doelle EA, Cohan RH, Wojno K, Korobkin M. Cystic renal masses: a reevaluation of the usefulness of the Bosniak classification system. Academic radiology. 1996;3:564–570. doi: 10.1016/s1076-6332(96)80221-2. [DOI] [PubMed] [Google Scholar]
- 13.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231:365–371. doi: 10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 14.Lang EK, Macchia RJ, Gayle B, et al. CT-guided biopsy of indeterminate renal cystic masses (Bosniak 3 and 2F): accuracy and impact on clinical management. European radiology. 2002;12:2518–2524. doi: 10.1007/s00330-001-1292-z. [DOI] [PubMed] [Google Scholar]
- 15.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology. 2005;236:441–450. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 16.Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR. American journal of roentgenology. 2003;180:1281–1287. doi: 10.2214/ajr.180.5.1801281. [DOI] [PubMed] [Google Scholar]
- 17.Richter F, Kasabian NG, Irwin RJ, Jr, Watson RA, Lang EK. Accuracy of diagnosis by guided biopsy of renal mass lesions classified indeterminate by imaging studies. Urology. 2000;55:348–352. doi: 10.1016/s0090-4295(99)00468-9. [DOI] [PubMed] [Google Scholar]
- 18.Gramiak R, Shah PM. Echocardiography of the aortic root. Investigative radiology. 1968;3:356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg B, Raichlen J, Forsberg Flemming. Ultrasound Contrast Agents: Basic Princples and Clinical Applications. London: Martin Duntz Ltd; 2001. [Google Scholar]
- 20.Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonics. 2000;38:93–98. doi: 10.1016/s0041-624x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 21.Frinking PJ, Bouakaz A, Kirkhorn J, Ten Cate FJ, de Jong N. Ultrasound contrast imaging: current and new potential methods. Ultrasound in medicine & biology. 2000;26:965–975. doi: 10.1016/s0301-5629(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 22.Martin KH, Dayton PA. Current status and prospects for microbubbles in ultrasound theranostics. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2013;5:329–345. doi: 10.1002/wnan.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosgrove D. Ultrasound contrast agents: an overview. European journal of radiology. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Kitzman DW, Goldman ME, Gillam LD, Cohen JL, Aurigemma GP, Gottdiener JS. Efficacy and safety of the novel ultrasound contrast agent perflutren (definity) in patients with suboptimal baseline left ventricular echocardiographic images. The American journal of cardiology. 2000;86:669–674. doi: 10.1016/s0002-9149(00)01050-x. [DOI] [PubMed] [Google Scholar]
- 25.Piscaglia F, Bolondi L Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound in medicine & biology. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Miller DL, Dou C, Wiggins RC. Contrast-enhanced diagnostic ultrasound causes renal tissue damage in a porcine model. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2010;29:1391–1401. doi: 10.7863/jum.2010.29.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park BK, Kim B, Kim SH, Ko K, Lee HM, Choi HY. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. European journal of radiology. 2007;61:310–314. doi: 10.1016/j.ejrad.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Peng Y, Jia L, Sun N, et al. Assessment of cystic renal masses in children: comparison of multislice computed tomography and ultrasound imaging using the Bosniak classification system. European journal of radiology. 2010;75:287–292. doi: 10.1016/j.ejrad.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Ignee A, Straub B, Brix D, Schuessler G, Ott M, Dietrich CF. The value of contrast enhanced ultrasound (CEUS) in the characterisation of patients with renal masses. Clinical hemorheology and microcirculation. 2010;46:275–290. doi: 10.3233/CH-2010-1352. [DOI] [PubMed] [Google Scholar]
- 30.Xue LY, Lu Q, Huang BJ, et al. Contrast-enhanced ultrasonography for evaluation of cystic renal mass: in comparison to contrast-enhanced CT and conventional ultrasound. Abdominal imaging. 2014;39:1274–1283. doi: 10.1007/s00261-014-0171-4. [DOI] [PubMed] [Google Scholar]
- 31.Kim AY, Kim SH, Kim YJ, Lee IH. Contrast-enhanced power Doppler sonography for the differentiation of cystic renal lesions: preliminary study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1999;18:581–588. doi: 10.7863/jum.1999.18.9.581. [DOI] [PubMed] [Google Scholar]
- 32.Siracusano S, Quaia E, Bertolotto M, Ciciliato S, Tiberio A, Belgrano E. The application of ultrasound contrast agents in the characterization of renal tumors. World journal of urology. 2004;22:316–322. doi: 10.1007/s00345-004-0410-3. [DOI] [PubMed] [Google Scholar]
- 33.Wink MH, de la Rosette JJ, Laguna P, Lagerveld BW, Wijkstra H. Ultrasonography of renal masses using contrast pulse sequence imaging: a pilot study. Journal of endourology / Endourological Society. 2007;21:466–472. doi: 10.1089/end.2006.0255. [DOI] [PubMed] [Google Scholar]
- 34.Paudice N, Zanazzi M, Agostini S, et al. Contrast-enhanced ultrasound assessment of complex cystic lesions in renal transplant recipients with acquired cystic kidney disease: preliminary experience. Transplantation proceedings. 2012;44:1928–1929. doi: 10.1016/j.transproceed.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Zhang S, Wei X, Pan Y, Hao J. Contrast enhanced ultrasonography prediction of cystic renal mass in comparison to histopathology. Clinical hemorheology and microcirculation. 2014;58:429–438. doi: 10.3233/CH-131799. [DOI] [PubMed] [Google Scholar]
- 36.Ascenti G, Mazziotti S, Zimbaro G, et al. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology. 2007;243:158–165. doi: 10.1148/radiol.2431051924. [DOI] [PubMed] [Google Scholar]
- 37.Clevert DA, Minaifar N, Weckbach S, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clinical hemorheology and microcirculation. 2008;39:171–178. [PubMed] [Google Scholar]
- 38.Quaia E, Bertolotto M, Cioffi V, et al. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR. American journal of roentgenology. 2008;191:1239–1249. doi: 10.2214/AJR.07.3546. [DOI] [PubMed] [Google Scholar]
- 39.Nicolau C, Bunesch L, Pano B, et al. Prospective evaluation of CT indeterminate renal masses using US and contrast-enhanced ultrasound. Abdominal imaging. 2015;40:542–551. doi: 10.1007/s00261-014-0237-3. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Wu N, Xue T, Hao Y, Dai J. Comparison of contrast-enhanced sonography with MRI in the diagnosis of complex cystic renal masses. Journal of clinical ultrasound : JCU. 2015;43:203–209. doi: 10.1002/jcu.22232. [DOI] [PubMed] [Google Scholar]
- 41.Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology. 2014;271:133–142. doi: 10.1148/radiol.13130161. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz A, Vatandaslar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2007;2:750–756. doi: 10.2215/CJN.03661106. [DOI] [PubMed] [Google Scholar]
- 43.Granata A, Zanoli L, Insalaco M, et al. Contrast-enhanced ultrasound (CEUS) in nephrology: Has the time come for its widespread use? Clinical and experimental nephrology. 2014;19:606–615. doi: 10.1007/s10157-014-1040-8. [DOI] [PubMed] [Google Scholar]
- 44.Yang R, Lian H, Zhang G, et al. Laparoscopic radiofrequency ablation with intraoperative contrast-enhanced ultrasonography for T1bN0M0 renal tumors: Initial functional and oncologic outcomes. Journal of Endourology. 2014;28:4–9. doi: 10.1089/end.2013.0397. [DOI] [PubMed] [Google Scholar]
- 45.Lassau N, Koscielny S, Albiges L, et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1216–1225. doi: 10.1158/1078-0432.CCR-09-2175. [DOI] [PubMed] [Google Scholar]
- 46.Leong-Poi H. Contrast ultrasound and targeted microbubbles: diagnostic and therapeutic applications in progressive diabetic nephropathy. Seminars in nephrology. 2012;32:494–504. doi: 10.1016/j.semnephrol.2012.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.