Abstract
Objective
Modifications of lipid constituents within atherosclerotic lesions generates neoepitopes that activate innate and adaptive immune responses. We aimed to define the prevalence, distribution, and relationship of autoantibody titers of oxidized lipoproteins to subclinical atherosclerosis and major adverse cardiovascular events (MACE) in different ethnic groups.
Approach and Results
IgG and IgM autoantibodies to malondialdehyde(MDA)-LDL and apolipoprotein B-100-immune complexes (ApoB-IC) were measured in 3509 individuals (1814 Blacks, 1031 Whites, 589 Hispanics, 85 no race identifier) from the Dallas Heart Study with median 10.5-year follow-up. Coronary artery calcium score (CAC), abdominal aortic plaque by MRI and MACE were quantified. IgG MDA-LDL and IgG and IgM apoB-IC were significantly different between groups, with Blacks having the highest levels of IgG MDA-LDL and IgG ApoB-IC and Hispanics the highest levels of IgM ApoB-IC (p<0.001 for all). IgGs tended to be higher and IgMs lower with age for all markers. In multivariable-adjusted binary logistic regression analysis, a doubling of IgG MDA-LDL levels was associated with prevalent CAC > 10 Agatson Units (OR (95%CI) 1.21 (1.07–1.36, p=0.002). Multivariable-adjusted Cox regression analysis revealed that IgG MDA-LDL was independently associated with time to incident MACE in the entire group (HR (95% CI) 1.76 (1.16–2.72, p=0.009) for 4th vs. 1st quartile). This effect was particularly prominent in Black subjects (HR 2.52 (1.39–4.57), p=0.002).
Conclusion
Autoantibodies to oxidized lipoproteins and immune complexes with apoB-100 lipoproteins vary significantly by sex, age and ethnicity. Higher baseline IgG MDA-LDL titers independently associate with new MACE. These findings may contribute to the understanding of differences in ethnic-specific MACE events.
Keywords: Oxidation, biomarkers, lipids, ethnicity, cardiovascular disease, autoantibodies, innate immunity
INTRODUCTION
Cardiovascular disease (CVD) continues to be the major cause of morbidity and mortality in the developed world1 and is rapidly becoming a key determinant of premature death in developing nations. Known risk factors, such as lipid and inflammatory genes and biomarkers, predict a relatively small fraction of risk and additional insights are needed to identify all determinants of CVD risk.2 This is particularly true among sexes as well as certain ethnic groups, where similarly potent risk factors do not seem to have the same impact.1,3–6
It has been established that atherosclerosis, the main etiological driver of CVD, is primarily a disease of lipid abnormalities with superimposed chronic inflammation. Modification of the lipid constituents within atherosclerotic lesions generates neoepitopes, called danger-associated molecular patterns (DAMPs),7 that activate innate and adaptive immune responses. These responses initiate a pro-inflammatory response to inactivate and clear such antigens.8,9 The generation of pro-inflammatory oxidation-specific epitopes (OSE) in vivo is well described.10
At the clinical level, one can measure plasma IgG and IgM autoantibodies that target chemically and pathophysiologically well-defined OSE such as malondialdehyde(MDA)-lysine adducts on proteins. IgG and IgM present on apolipoprotein B-100 lipoproteins as part of immune complexes can also be measured.11,12 In general, elevated plasma levels of IgG autoantibodies to OSE represent responses to antigen exposure and tend to correlate with worse cardiovascular risk. In contrast, IgM autoantibodies to OSE often represent “natural” antibodies present at birth that are likely evolutionarily conserved to protect against such DAMPs present in sites of inflammation, such as apoptotic cells and cell walls of infectious pathogens. These so-called natural antibodies also bind to OSE on modified lipoproteins as the target DAMPs are either identical or act as molecular mimics of those present on apoptotic cells and infectious agents.9,13 In contrast to IgGs to OSE, IgMs tend to be associated with lower CVD events.11,12,14,15
The prevalence and impact on sub-clinical and clinical CVD of such biomarkers of oxidized lipoproteins in different sexes and ethnicities has not been well studied. Because the titers of OSE-autoantibodies are strongly influenced by hereditary,16 it might be anticipated that sex/ethnic differences might have an important impact on their titers. Therefore, the purpose of the present study is to examine the relationship between IgG and IgM autoantibodies to MDA-LDL and apoB-IC and subclinical atherosclerosis and major adverse cardiovascular events (MACE) over a 10.5-year prospective follow-up in a large, multi-ethnic, population-based epidemiological cohort from the Dallas Heart Study.
Materials and Methods
Materials and Methods are shown in the Supplementary Appendix.
RESULTS
Subject characteristics
The participant characteristics in the entire group combined and by ethnicity are summarized in Table 1. The study consisted of 3509 participants, with a mean age of 43.7±10.1 years and 44.1% of the subjects were male, 1814 (51.7%) were African American, 1021 (29.1%) Caucasian, and 589 (16.8%) Hispanic. Eighty-five subjects did not self-report ethnicity. Significant differences were observed in baseline characteristics between ethnic groups. There was a high prevalence of traditional cardiovascular risk factors in the population; 34.4% hypertension, 11.6% diabetes, and 29.3% current smokers. Notably, Blacks had the lowest total cholesterol, triglycerides, VLDL-C levels, and highest HDL-C levels. LDL-C levels were comparable among the three ethnic groups. Hispanics had a lower prevalence of current smoking, while Blacks had the highest prevalence of hypertension.
Table 1.
Baseline characteristics of study subjects in the entire group and by ethnicity
| Entire Group (N=3509) | Black (N=1814) | White (N=1031) | Hispanic (N=589) | P-Value for ethnicity | |
|---|---|---|---|---|---|
| Age, years (SD) | 43.7 (10.1) | 44.5 (10.2) | 44.7 (10.0) | 40.1 (9.1) | <0.001 |
| Male, N (%) | 1546 (44.1) | 765 (49.5) | 493 (47.8) | 244 (41.4) | 0.001 |
| BMI (SD) | 30.5 (7.6) | 31.6 (8.2) | 29.0 (6.7) | 30.4 (6.6) | <0.001 |
| HTN, N (%) | 1177 (34.4)* | 679 (38.2) | 287 (29.0) | 180 (32.6) | <0.001 |
| Diabetes, N (%) | 408 (11.6) | 258 (14.2) | 70 (6.8) | 72 (12.2) | <0.001 |
| Current smoking, N (%) | 1026 (29.3) | 605 (33.4) | 288 (28.0) | 120 (20.4) | <0.001 |
| Laboratory variables | |||||
| Total Cholesterol, mg/dl | 180.3 (39.6) | 177.7 (40.1) | 183.6 (38.4) | 181.9 (40.1) | 0.001 |
| LDL-C, mg/dl | 106.3 (35.4) | 104.8 (36.6) | 108.3 (34.4) | 107.0 (33.4) | 0.071 |
| HDL-C, mg/dl | 59.8 (14.8) | 52.2 (15.3) | 48.2 (15.0) | 45.7 (11.2) | <0.001 |
| VLDL-C, mg/dl | 24.2 (18.8) | 20.8 (17.7) | 27.1 (18.0) | 29.3 (21.5) | <0.001 |
| Triglycerides, mg/dl | 96 (67–147) | 85 (62–123) | 110 (75–167) | 118 (81–175) | <0.001 |
| IgG MDA-LDL, RLU | 5280 (3522–7969) | 6010 (4204–8857) | 4179 (2952–6435) | 4689 (3094–7247) | <0.001 |
| IgM MDA-LDL, RLU | 15355 (10943–21243) | 15427 (10738–21119) | 15349 (11051–21418) | 15357 (11422–21328) | 0.64 |
| IgG ApoB-IC, RLU | 4179 (3243–5304) | 4272 (3281–5382) | 4023 3158–5073) | 4064 (3246–5252) | <0.001 |
| IgM ApoB-IC, RLU | 3391 (2233–4944) | 3285 (2172–4971) | 3227 (2108–4534( | 4056 (2768–5700) | <0.001 |
75 Individuals either did not have ethnicity identified or identified as “other” and were not included in the ethnicity analysis.
In the database, of the 3509 individuals, 3419 had an entry for prevalent hypertension.
Significant differences were also noted among autoantibodies and ApoB-IC, with Blacks having highest levels of IgG MDA-LDL and IgG ApoB-IC and Hispanics having the highest levels of IgM ApoB-IC, measured as relative light units (RLU) (Table 1).
Relationship between sex, age and autoantibody titers to oxidized lipoproteins
In the entire group, median (IQR) IgM MDA-LDL (13525(9546–18954) vs. 16678(12344–22724), p<0.001) and IgM ApoB-IC (3065(2044–4494) vs. 3653(2410–5330), p<0.001) were lower and IgG ApoB-IC (4240(3302–5353) vs. 4113(3208–5232), p=0.018) were higher in males versus females. However, IgG MDA-LDL levels were not different between males and females 5055(3401–7771) vs. 5485(3593–8150), p=0.98). Within ethnic groups, in Blacks, IgM MDA-LDL (p<0.001) were lower and IgG ApoB-IC (p<0.001) were higher in males. In Whites and Hispanics, IgM MDA-LDL (p<0.001 for both) and IgM ApoB-IC (p<0.001 for both) were lower in males.
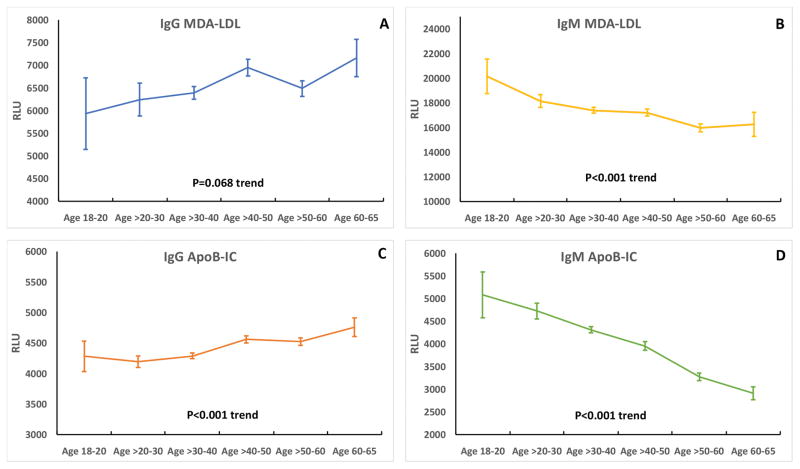
In the entire group, IgG MDA-LDL and IgG apoB-IC levels tended to be higher with advancing age and IgM MDA-LDL and IgM ApoB-IC tended to be lower with age (Figure 1). Comparing biomarkers within ethnic groups, in Blacks IgG MDA-LDL (p=0.086) and IgG ApoB-IC (p= 0.002) tended to be higher with age, while in contrast IgM MDA-LDL (p = 0.03) and IgM ApoB-IC (p<0.001) were significantly lower with age. In Whites, only IgM ApoB-IC (p<0.001) was lower with age, but in Hispanics both IgG MDA-LDL (p = 0.007) and IgM ApoB-IC (p<0.001) were significantly lower with age (Table 2).
Figure 1.
Relationship of age per decade to autoantibodies to MDA-LDL and apoB-immune complexes in the entire Dallas Heart Study group. A= IgG MDA-LDL, B= IgM MDA-LDL, C= IgG apoB-IC, D= IgM apoB-IC.
Table 2.
Relationship of Age to Autoantibodies to MDA-LDL and ApoB-Immune Complexes in Different Ethnic Groups
| Variable | Age 18–20 (N=33) | Age >20–30 (N=246) | Age >30–40 (N=1157) | Age >40–50 (N=1091) | Age >50–60 (N=816) | Age 60–65 (N=166) | P-Value for age |
|---|---|---|---|---|---|---|---|
| Black | |||||||
|
|
|||||||
| IgG MDA-LDL, RLU* | 5.2 (3.1–7.8) | 6.1 (4.6–8.5) | 5.8 (4.0–8.4) | 6.5 (4.4–9.5) | 5.8 (4.1–8.9) | 7.0 (4.6–9.1) | 0.086 |
| IgM MDA-LDL, RLU* | 19.2 (13.4–26.4) | 16.7 (13.1–22.9) | 16.3 (11.4–21.3) | 15.2 (10.8–21.5) | 14.4 (9.5–19.8) | 13.9 (8.7–19.8) | 0.03 |
| IgG ApoB-IC, RLU* | 4.4 (2.9–5.2) | 4.1 (3.2–5.3) | 4.0 (3.2–5.1) | 4.4 (3.3–5.5) | 4.4 (3.2–5.6) | 4.5 (3.4–5.8) | 0.002 |
| IgM ApoB-IC, RLU* | 4.4 (2.7–7.2) | 3.7 (2.9–5.7) | 3.8 (2.5–5.6) | 3.2 (2.2–5.1) | 2.8 (1.6–3.4) | 2.2 (1.6–3.4) | <0.001 |
| White | |||||||
|
|
|||||||
| IgG MDA-LDL, RLU* | 3.7 (3.3–6.4) | 3.8 (2.8–5.8) | 4.3 (3.0–6.3) | 4.0 (2.9–7.1) | 4.1 (2.9–5.8) | 5.5 (3.2–8.2) | 0.11 |
| IgM MDA-LDL, RLU* | 16.9 (14.4–25.1) | 16.9 (10.7–23.3) | 16.4 (11.5–22.1) | 15.6 (11.1–22.0) | 14.4 (10.4–20.2) | 12.3 (9.2–18.3) | 0.16 |
| IgG ApoB-IC, RLU* | 3.9 (2.8–4.5) | 3.8 (3.1–4.5) | 3.9 (3.0–4.8) | 4.1 (3.2–5.3) | 4.2 (3.3–5.1) | 4.5 (3.7–5.4) | 0.068 |
| IgM ApoB-IC, RLU* | 4.5 (3.6–5.0) | 3.8 (2.9–5.7) | 3.7 (2.6–4.9) | 3.2 (2.2–4.6) | 2.7 (1.6–4.0) | 2.3 (1.6–3.6) | <0.001 |
| Hispanic | |||||||
|
|
|||||||
| IgG MDA-LDL, RLU* | 2.9 (−) | 4.6 (3.3–6.8) | 4.8 (3.2–7.4) | 4.5 (2.8–6.6) | 5.2 (3.3–8.4) | 5.8 (3.4–7.8) | 0.28 |
| IgM MDA-LDL, RLU* | 17.2 (−) | 16.3 (12.0–22.0) | 16.3 (12.8–22.4) | 15.5 (10.2–20.8) | 12.7 (9.7–17.3) | 10.7 (7.2–16.8) | 0.007 |
| IgG ApoB-IC, RLU* | 6.1 (−) | 3.9 (3.2–4.9) | 4.0 (3.2–5.2) | 4.2 (43.2–5.3) | 4.1 (3.3–5.7) | 3.9 (3.0–6.0) | 0.17 |
| IgM ApoB-IC, RLU* | 4.5 (−) | 4.4 (2.9–6.5) | 4.5 (3.1–6.2) | 3.9 (2.4–5.4) | 3.0 (2.2–4.2) | 3.9 (1.7–4.3) | <0.001 |
Values represent RLU X 1000. The Hispanic age group 18–20 years old had only 2 subjects.
Supplemental Table I shows the median (IQR) levels of the autoantibody titers and apoB-immune complexes in patients according to sex. Women of all ethnicities tended to have higher IgM MDA-LDL and apoB-IC levels. Supplemental Table II shows the median (IQR) levels of autoantibody titers and apoB-immune complexes in patients according to demographic variables. Diabetic subjects had lower levels of IgM MDA-LDL and IgM ApoB-IC, and subjects with hypertension had higher levels of IgG MDA-LDL but lower levels of IgG ApoB-IC.
Supplemental Table III shows the Spearman correlations between continuous variables. IgM MDA-LDL and IgM apoB-IC were modestly correlated (r=0.61, p<0.001), while weak to modest correlations were present for other variables.
Relationship between biomarkers of oxidized lipoproteins to coronary calcium score and abdominal aortic plaque area
Of the 2740 subjects with a CAC scan, 582 (21.2%) had a CAC score >10. When evaluated as a continuous variable, CAC showed weak but significant positive Spearman correlations with IgG MDA-LDL (r=0.073, p<0.001) and IgG ApoB-IC (r=0.040, p=0.036). In contrast, inverse correlations were noted with CAC score and IgM MDA-LDL (r= −0.107, p<0.001) and IgM ApoB-IC (r= −0.167, p<0.001).
When using CAC >10 as a positive score and CAC <10 as a negative score to remove artifact bias of very low CAC scores, and evaluating the data with binary logistic analysis with multivariable adjustment with the variables in Table 1, (age per decile, male sex, ethnicity, diabetes, current smoking, BMI, LDL-C per SD, HDL per SD, log triglycerides per SD, HTN, and log of the 4 oxidative biomarker variables per SD), the log IgG MDA-LDL per 1 SD OR (5% CI) was 1.21 (1.07–1.36), p=0.002) and log IgG ApoB-IC per 1 SD was 0.88 (0.79–0.99, p=0.026) were independently associated with CAC >10 (Table 3). The p-value for the interaction test of ethnicity (included all 3 groups)*log IgG MDA-LDL per 1 SD was p=0.27. Evaluating these data by ethnicity, similar trends were noted (Supplemental Table IV),
Table 3.
Multiple logistic regression analysis for the presence or absence of coronary artery calcium and the presence or absence of abdominal aortic plaque in the entire group
| Odds ratio | 95% CI | |||
|---|---|---|---|---|
|
|
||||
| CAC | Lower | Upper | P-Value | |
|
|
||||
| Age per decile | 4.29 | 3.68 | 5.01 | <0.001 |
| Male sex | 3.33 | 2.58 | 4.30 | <0.001 |
| Black | 1.08 | 0.83 | 1.40 | 0.56 |
| White | 0.79 | 0.55 | 1.13 | 0.20 |
| Hispanic | 0.58 | 0.96 | 1.52 | 0.25 |
| Hypertension | 1.21 | 0.96 | 1.52 | 0.11 |
| Diabetes | 2.05 | 1.49 | 2.81 | <0.001 |
| Current smoking | 2.48 | 1.94 | 3.18 | <0.001 |
| BMI | 1.03 | 1.01 | 1.05 | 0.006 |
| LDL-C per SD | 1.08 | 0.97 | 1.21 | 0.14 |
| HDL-C per SD | 1.02 | 0.90 | 1.15 | 0.81 |
| Triglycerides per SD | 1.10 | 0.97 | 1.24 | 0.14 |
| IgG MDA-LDL per SD | 1.21 | 1.07 | 1.36 | 0.002 |
| IgM MDA-LDL per SD | 1.00 | 0.87 | 1.15 | 0.99 |
| IgG ApoB-IC per SD | 0.88 | 0.79 | 0.99 | 0.026 |
| IgM ApoB-IC per SD | 1.05 | 0.91 | 1.21 | 0.48 |
|
| ||||
| Odds ratio | 95% CI | |||
|
|
||||
| Abdominal Plaque | Lower | Upper | P-Value | |
|
|
||||
| Age per decile | 2.27 | 2.04 | 2.52 | <0.001 |
| Male sex | 1.07 | 0.88 | 1.30 | 0.52 |
| Black | 0.84 | 0.67 | 1.05 | 0.12 |
| White | 0.91 | 0.69 | 1.20 | 0.52 |
| Hispanic | 1.36 | 0.72 | 1.56 | 0.34 |
| Hypertension | 0.94 | 0.79 | 1.16 | 0.55 |
| Diabetes | 1.41 | 1.08 | 1.95 | 0.025 |
| Current smoking | 2.12 | 1.73 | 2.61 | <0.001 |
| BMI | 0.97 | 0.96 | 0.99 | <0.001 |
| LDL-C per SD | 1.09 | 0.99 | 1.19 | 0.076 |
| HDL-C per SD | 0.86 | 0.78 | 0.96 | 0.006 |
| Triglycerides per SD | 1.19 | 1.08 | 1.32 | 0.001 |
| IgG MDA-LDL per SD | 1.08 | 0.98 | 1.19 | 0.13 |
| IgM MDA-LDL per SD | 1.10 | 0.97 | 1.24 | 0.13 |
| IgG ApoB-IC per SD | 0.89 | 0.81 | 0.98 | 0.019 |
| IgM ApoB-IC per SD | 0.93 | 0.80 | 1.05 | 0.23 |
For abdominal aortic atherosclerosis measured as aortic plaque burden by magnetic resonance imaging, 977 (39.5%) out of 2475 had a positive scan. Weak but significant positive correlations were noted with IgG MDA-LDL (r=0.053, p=0.009) and inverse correlations were noted with IgG ApoB-IC (r=−0.048, p=0.017) and IgM ApoB-IC (r=−0.094, p<0.001).
When using plaque area =0 or >0 mm2 as a binary variable in a multivariable, logistic regression analysis including variables in Table 1, age per decile, diabetes, current smoking, BMI, HDL-C per 10 mg/dL, LDL-C per 25 mg/dL, log2 triglycerides (1.21 (1.08–1.36, p = 0.001) and log2 IgG ApoB-IC (0.81 (0.68–0.97, p=0.022), were independent predictors of plaque area >0 mm2 (Table 3). The p-value for the interaction test of ethnicity (included all 3 groups)*log IgG ApoB-IC per 1 SD was p=0.43. Evaluating these data by ethnicity, generally similar findings were noted (Supplemental Table V).
Relationship of biomarkers of oxidized lipoproteins to cardiovascular outcomes
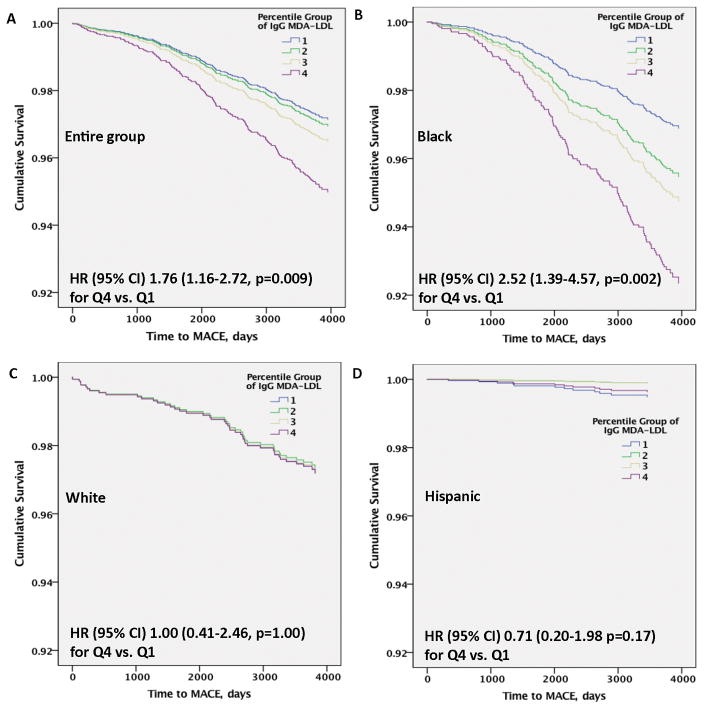
There were a total of 190 MACE events out of total of 2914 subjects with adjudicated MACE until Dec 31 2011, with 132 (out of 1498 subjects or 8.81%) in Blacks, 44 (out of 911 subjects, or 4.83%) in White and 12 (out of 447 subjects, or 2.68%) in Hispanics. There were 2 events in subjects whose ethnicity was not identified and who were not included in the analyses. Cox regression analysis with time to MACE was performed in the entire group in 3 models; Model 1: oxidation biomarkers alone; Model 2: plus age in deciles, sex, hypertension, diabetes, current smoking, LDL-C per 1 SD, HDL-C per 1 SD and log triglycerides per 1 SD; and Model 3: plus ethnicity. IgG MDA-LDL were independently associated with time to MACE with an HR (95% CI) of 2.10 (1.40–3.15, p=0.001) for 4th quartile vs. first (Table 4). Further adjustment in model 2 and model 3 attenuated the results slightly but significance was maintained. The ethnicity*quartile IgG MDA-LDL interaction was significant for Q3 (p=0.006) and Q4 (p<0.001) vs Q1. Removing all revascularization events did not appreciably change the HR (95% CI) 1.75 (1.12–2.75, p=0.014) for Q4 vs.Q1 in the fully adjusted model. Figure 2A demonstrates the temporal relationship of time to MACE in the entire group for IgG MDA-LDL autoantibodies. The IgM MDA-LDL, IgG ApoB-IC and IgM ApoB-IC were not significantly different after full adjustment (Table 4). In the model of IgG MDA-LDL including all covariates including ethnicity, addition of IgM MDA-LDL per 1 SD, IgG ApoB-IC per 1 SD, IgM ApoB-IC per 1 SD did not appreciably affect the findings: Q4 HR 1.88 (1.19–2.95), p=0.006.
Table 4.
Cox Regression Hazard Ratios for MACE According to Quartiles of IgG and IgM Autoantibodies to MDA-LDL and ApoB-IC.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value Q1 vsQ4 | |
|---|---|---|---|---|---|
|
|
|||||
| Model 1: IgG MDA-LDL | 1 | 1.25 (0.82–1.95) | 1.38 (0.89–2.12) | 2.10 (1.40–3.15) | <0.001 |
| Model 2: Plus age in deciles, sex, HTN diabetes, smoking, BMI, LDL-C per SD, HDL-C per SD, log TG per SD | 1 | 1.13 (0.72–1.78) | 1.29 (0.83–2.01) | 1.97 (1.30–2.99) | 0.001 |
| Model 3: Plus Ethnicity (Black vs others) | 1 | 1.07 (0.67–1.70) | 1.23 (0.79–1.94) | 1.76 (1.16–2.72) | 0.009 |
| Model 1: IgM MDA-LDL | 1 | 0.70 (0.48–1.01) | 0.62 (0.42–0.92) | 0.58 (0.39–0.86) | 0.007 |
| Model 2: Plus age in deciles, sex, HTN diabetes, smoking, BMI, LDL-C per SD, HDL-C per SD, log TG per SD | 1 | 0.91 (0.62–1.33) | 0.92 (0.63–1.36) | 0.96 (0.63–1.45) | 0.84 |
| Model 3: Plus Ethnicity (Black vs others) | 1 | 0.92 (0.62–1.36) | 0.90 (0.61–1.34) | 0.95 (0.63–1.44) | 0.81 |
| Model 1: IgG ApoB-IC | 1 | 0.74 (0.49–1.12) | 1.02 (0.69–1.50) | 1.04 (0.71–1.53) | 0.83 |
| Model 2: Plus age in deciles, sex, HTN diabetes, smoking, BMI, LDL-C per SD, HDL-C per SD, log TG per SD | 1 | 0.92 (0.60–1.41) | 1.22 (0.82–1.80) | 1.08 (0.73–1.60) | 0.71 |
| Model 3: Plus Ethnicity (Black vs others) | 1 | 0.97 (0.63–1.49) | 1.20 (0.81–1.78) | 1.07 (0.72–1.61) | 0.730 |
| Model 1: IgM ApoB-IC | 1 | 0.66 (0.46–0.95) | 0.60 (0.41–0.88) | 0.47 (0.31–0.71) | <0.001 |
| Model 2: Plus age in deciles, sex, HTN diabetes, smoking, BMI, LDL-C per SD, HDL-C per SD, log TG per SD | 1 | 0.87 (0.60–1.27) | 0.96 (0.65–1.42) | 1.04 (0.67–1.60) | 0.88 |
| Model 3: Plus Ethnicity (Black vs others) | 1 | 0.83 (0.561.21) | 0.95 (0.64–1.41) | 1.00 (0.64–1.55) | 1.00 |
Figure 2.
Multivariable adjusted Cox regression analysis of IgG MDA-LDL with time to MACE over a 10.5-year follow-up in the entire group and by ethnic cohort. A= entire group, B= Black, C= White, D= Hispanic.
Similar analyses were performed in individual ethnic groups. In fully adjusted analyses, the results in the entire group were primarily driven by the Black group, which had an HR (95% CI) of 2.52 (1.39–4.57, p=0.002) comparing 4th vs 1st quartile. No significant differences were noted in Whites HR (95% CI) of 1.00 (0.41–2.46, p=1.00) or Hispanics HR (95% CI) of 0.71 (0.20–1.98, p=0.17) (Figure 2B–D).
DISCUSSION
This study demonstrates several novel observations regarding circulating autoantibodies and immune complexes reflecting OSE over a 10.5 year prospective follow-up in the Dallas Heart Study: First, significant differences were present between ethnic groups in several measures, with Blacks having highest levels of IgG MDA-LDL and ApoB-IC, while whites had the lowest levels and Hispanics had the highest levels of IgM ApoB-IC; Second, a significant age effect was noted, with IgGs trending higher and IgMs lower with advancing age; Third, IgG MDA-LDL levels were associated with prevalent CAC; Fourth, elevated IgG MDA-LDL levels were predictive of time to MACE in the entire group, an effect particularly prominent in Black subjects. Remarkably, these findings were present even though individuals were relatively young (mean age ~44 years old) upon entry into the study.
Since the initial description of autoantibodies to oxidized LDL in atherosclerotic lesions and plasma of both animals and humans,17–19 a large database has been generated on experimental13,14,20,21 and clinical aspects of such autoantibodies.11,12,22,23 Furthermore, the research realm has expanded beyond CVD to other inflammatory conditions, such as lupus, rheumatoid arthritis,24 infections,25 renal failure26 and Alzheimer’s disease.27 However, most of these studies have been performed in subjects of European descent and little data is present in other ethnic groups. This is the first dedicated study evaluating autoantibody titers to oxidized lipoproteins and immune complexes with apoB-100 lipoproteins in the context of race and ethnicity. Significant differences in racial/ethnic groups were present, and in turn, higher IgG MDA-LDL titers were associated with higher CAC score and likelihood for MACE. Although the underlying etiology of these differences cannot be determined from this study, the data suggest that appropriate interpretation of these measurements in epidemiological studies should include race/ethnicity as a variable.
The association of age with oxidative biomarkers is also of interest, particularly since age is universally one of the strongest risk factors for CVD. The DHS has the advantage of evaluating the relationship of age to these biomarkers since it includes a broad range of 18–65 year old subjects. Although age was analyzed in a cross-sectional rather than a prospective analysis in DHS, one can speculate that continued exposure to the various OSE led to increasing IgG titers as subjects age. Conversely, as IgMs are thought to be derived from B-1 cells present at birth or shortly thereafter, a decline in titers to OSE over time may suggest loss of certain B-1 cell subsets, resulting in decreased innate immune responses to chronic diseases caused by OSE and certain infections.13,21,28–30 Consistent with a genetic influence of circulating autoantibodies to OSE, a recent genome-wide linkage study and genetic association in twins, the heritability (h2) of IgM MDA-LDL and IgM apoB-IC were 0.69 and 0.80, respectively, and for IgG MDA-LDL and apoB-IC was 0.62 and 0.53, respectively, which was similar or higher than physiological, inflammatory, or lipid traits.16
In the current study, IgG MDA-LDL titers were predictive of subclinical atherosclerosis as measured by CAC, which to our knowledge is the first study to evaluate this phenotype with autoantibodies to OSE. This is consistent with some,12,18,31–33 but not all studies,34–36 that show similar associations with carotid or peripheral arterial disease. Analyses by ethnicity showed that this association was only significant in Blacks, although the interaction test for ethnicity was not significant but trends across ethnicity were consistent. It is also interesting that Blacks in the United States, as reflected in the Dallas area in this study, have up to 25% European or Native American genes, which further complicates analysis based on race/ethnicity.37
This study also showed that elevated levels of IgG MDA-LDL were independently predictive of time to MACE over a median of 10.5 years, which seemed to be primarily driven by the Black cohort. These observations are consistent with the Bruneck study in Whites, where IgG titers to Cu-OxLDL were associated with higher risk of MACE (HR: 1.18; 95% CI: 1.02 to 1.37, p=0.028 for 1-SD unit increase),12 as well as in other studies.11,12,38–40 However, these data are not entirely consistent, as other studies have shown no association.11,36,39,41,42 Additionally, a recent study has shown that low levels of IgG to selected apoB-100 peptides and MDA-modified apoB-100 peptides (which may not reflect IgG titers to MDA epitopes) are associated with higher risk of CVD.43 In a recent report from the Dallas Heart Study, we reported that lipoprotein(a) [Lp(a)], oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB), apolipoprotein(a) isoforms and LPA snps varied significantly by ethnicity. In turn, elevated Lp(a) and/or OxPL-apoB were independent predictors of MACE, even after adjusting for apo(a) isoforms and LPA snps with consistent results in the 3 ethnic groups.44
IgM biomarkers in this study were not predictive of CVD events. In other studies, low levels of IgM autoantibodies to a variety of OSE predict worse outcomes,12,45 consistent with a potentially atheroprotective effect, which has been consistently observed in experimental studies (reviewed in 9,22) In DHS, with a decline in IgM levels in more advanced ages, power may not have been optimal to assess these relationships, compared to prior studies that recruited more elderly individuals.
The underlying reasons for the divergent results in these studies is not clear, but our current observations suggest that the failure to consider ethnic/race of the populations studied may be one such factor. Aside from other biases in the populations studied, study design, methodological assessments of subclinical atherosclerosis endpoints and different endpoints confound these studies. In addition, there are likely multiple technical issues in the way these antibody titers were assessed including differences in assay antigen preparation. It is well known that variations in the extent of oxidation result in different preparations of MDA-LDL, or other MDA modified proteins, which can result in differences in measured autoantibody titers46,47 The generation of standardized antigens has been suggested to overcome this limitation and standardize assays across laboratories. In that regard, we have described the development of small amino acid mimotopes apparently reflecting the 3-dimensional structure of MDA and validated that they have similar immunological properties of MDA and may be useful in future studies.48 Ultimately, whether such autoantibody titers to OSE are causally related to CVD or are epiphenomena await genome wide association and other studies evaluating causality. For example, a recent genome-wide association study using the PROMIS database showed that novel variants on chromosome 6 at the HLA-B locus and on chromosome 12 at the CIT locus were associated with circulating IgM titers to MDA-LDL, IgM to phosphocholine-modified bovine serum albumin and ApoB-IC, and these variants were subsequently linked to CVD events in PROMIS, as well as in the CARDIOGRAmplusC4D consortium database.49 These clinical observations in conjunction with experimental data strongly suggest genetic influences on circulating levels of autoantibody titers to OSE, which may then be reflected as differences in circulating levels among different ethnic groups, and finally to cardiovascular events.
Limitations of this study included that the Hispanic group was small with a relatively small number of events and therefore the study may have been underpowered to evaluate relationships to CVD.
In conclusion, significant age and ethnic-specific difference are present in IgG and IgM autoantibody titers to OSE and ApoB-IC. Elevated IgG MDA-LDL is associated with time to MACE, particularly in Black individuals and may provide some explanations for the disparity in outcomes among different ethnic groups.
Supplementary Material
Highlights.
IgG and IgM autoantibodies to malondialdehyde(MDA)-LDL and apolipoprotein B-100-immune complexes (ApoB-IC) were measured in 3509 individuals from the Dallas Heart Study with median 10.5-year follow-up.
IgG MDA-LDL and IgG and IgM apoB-IC were significantly different between groups, with Blacks having the highest levels of IgG MDA-LDL and IgG ApoB-IC and Hispanics the highest levels of IgM ApoB-IC (p<0.001 for all).
A doubling of IgG MDA-LDL levels was associated with prevalent CAC > 10 Agatson Units (OR (95%CI) 1.21 (1.07–1.36, p=0.002).
IgG MDA-LDL was independently associated with time to incident MACE in the entire group (HR (95% CI) 1.76 (1.16–2.72, p=0.009) for 4th vs. 1st quartile). This effect was particularly prominent in Black subjects (HR 2.52 (1.39–4.57), p=0.002).
These findings may contribute to the understanding of differences in ethnic-specific MACE events.
Acknowledgments
A) Acknowledgements: None
B) B) Sources of Funding: NIH R01-HL119828, P01-HL088093, P01-HL055798, R01-HL093767 R01-HL086599 (ST, JLW).
C) C) Disclosure: ST and JLW are co-inventors and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies. ST has a dual appointment at UCSD and Ionis Pharmaceuticals. JLW is a consultant for Ionis Pharmaceuticals, Intercept, CymaBay and Prometheus. The other co-authors have no conflicts of interest.
Abbreviations
- CAD
Coronary Calcium Score
- CVD
Cardiovascular Disease
- DAMPs
Danger associated molecular patterns
- HR
Hazard Ratio
- IgM
Immunoglobulin M
- IgG
Immunoglobulin G
- MDA-LDL
Malondialdehyde-modified low density lipoprotein
- OSE
Oxidation-specific epitopes
- ApoB-IC
Apolipoprotein B-100-immune complexes
- MACE
Major adverse cardiovascular events
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res. 2016;118:579–585. doi: 10.1161/CIRCRESAHA.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, de Lemos JA, Witztum JL. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: Results from the Dallas Heart Study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duru OK, Li S, Jurkovitz C, Bakris G, Brown W, Chen SC, Collins A, Klag M, McCullough PA, McGill J, Narva A, Pergola P, Singh A, Norris K. Race and sex differences in hypertension control in CKD: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;51:192–198. doi: 10.1053/j.ajkd.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brilakis ES, Khera A, McGuire DK, See R, Banerjee S, Murphy SA, de Lemos JA. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: Observations from the Dallas Heart Study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P, Lichtman Andrew H, Hansson Göran K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16:485–497. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 11.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 13.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmon DB, Srikakulapu P, Kaplan JL, Oldham SN, McSkimming C, Garmey JC, Perry HM, Kirby JL, Prohaska TA, Gonen A, Hallowell P, Schirmer B, Tsimikas S, Taylor AM, Witztum JL, et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arterioscler Thromb Vasc Biol. 2016;36:682–691. doi: 10.1161/ATVBAHA.116.307166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao F, Schork AJ, Maihofer AX, Nievergelt CM, Marcovina SM, Miller ER, Witztum JL, O’Connor DT, Tsimikas S. Heritability of biomarkers of oxidized lipoproteins: Twin pair study. Arterioscler Thromb Vasc Biol. 2015;35:1704–1711. doi: 10.1161/ATVBAHA.115.305306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. PNAS. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 19.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 21.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–U834. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 22.Hulthe J. Antibodies to oxidized LDL in atherosclerosis development--clinical and animal studies. Clinica Chimica Acta. 2004;348:1–8. doi: 10.1016/j.cccn.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson J, Kovanen PT. Will autoantibodies help to determine severity and progression of atherosclerosis? Curr Opin Lipidol. 2004;15:499–503. doi: 10.1097/00041433-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Peters MJ, van Halm VP, Nurmohamed MT, Damoiseaux J, Tervaert JW, Twisk JW, Dijkmans BA, Voskuyl AE. Relations between autoantibodies against oxidized low-density lipoprotein, inflammation, subclinical atherosclerosis, and cardiovascular disease in rheumatoid arthritis. J Rheumatol. 2008;35:1495–1499. [PubMed] [Google Scholar]
- 25.Orellana RV, Fonseca HA, Monteiro AM, Ortega KL, Gallottini MH, Gidlund M, Pobocik AM. Association of autoantibodies anti-OxLDL and markers of inflammation with stage of HIV infection. Int J Cardiol. 2013;168:1610–1612. doi: 10.1016/j.ijcard.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Bossola M, Tazza L, Merki E, Giungi S, Luciani G, Miller ER, Lin EB, Tortorelli A, Tsimikas S. Oxidized low-density lipoprotein biomarkers in patients with end-stage renal failure: Acute effects of hemodialysis. Blood Purif. 2007;25:457–465. doi: 10.1159/000112465. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson UK, Sjoberg BG, Bennet AM, de Faire U, Pedersen NL, Frostegard J. Low levels of antibodies against phosphorylcholine in Alzheimer’s disease. J Alzheimers Dis. 2010;21:577–584. doi: 10.3233/JAD-2010-091705. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein TL. Natural antibodies as rheostats for susceptibility to chronic diseases in the aged. Frontiers in immunology. 2016;7:127. doi: 10.3389/fimmu.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holodick NE, Rothstein TL. B cells in the aging immune system: time to consider B-1 cells. Ann N Y Acad Sci. 2015;1362:176–187. doi: 10.1111/nyas.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holodick NE, Vizconde T, Hopkins TJ, Rothstein TL. Age-related decline in natural IgM function: Diversification and selection of the B-1a cell pool with age. J Immunol. 2016;196:4348–4357. doi: 10.4049/jimmunol.1600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meraviglia MV, Maggi E, Bellomo G, Cursi M, Fanelli G, Minicucci F. Autoantibodies against oxidatively modified lipoproteins and progression of carotid restenosis after carotid endarterectomy. Stroke. 2002;33:1139–1141. doi: 10.1161/01.str.0000014420.15948.2e. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves I, Gronholdt ML, Soderberg I, Ares MP, Nordestgaard BG, Bentzon JF, Fredrikson GN, Nilsson J. Humoral immune response against defined oxidized low-density lipoprotein antigens reflects structure and disease activity of carotid plaques. Arterioscler Thromb Vasc Biol. 2005;25:1250–1255. doi: 10.1161/01.ATV.0000166518.96137.38. [DOI] [PubMed] [Google Scholar]
- 33.Chen HW, Kuo CL, Huang CS, Kuo SJ, Liu CS. Oxidized low-density lipoproteins, autoantibodies against oxidized low-density lipoproteins and carotid intima media thickness in a clinically healthy population. Cardiology. 2008;110:252–259. doi: 10.1159/000112409. [DOI] [PubMed] [Google Scholar]
- 34.Uusitupa MI, Niskanen L, Luoma J, Vilja P, Mercuri M, Rauramaa R, Yla-Herttuala S. Autoantibodies against oxidized LDL do not predict atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:1236–1242. doi: 10.1161/01.atv.16.10.1236. [DOI] [PubMed] [Google Scholar]
- 35.Mayr M, Kiechl S, Tsimikas S, Miller E, Sheldon J, Willeit J, Witztum JL, Xu Q. Oxidized low-density lipoprotein autoantibodies, chronic infections, and carotid atherosclerosis in a population-based study. J Am Coll Cardiol. 2006;47:2436–2443. doi: 10.1016/j.jacc.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Bertoia ML, Pai JK, Lee JH, Taleb A, Joosten MM, Mittleman MA, Yang X, Witztum JL, Rimm EB, Tsimikas S, Mukamal KJ. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61:2169–2179. doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96:37–53. doi: 10.1016/j.ajhg.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erkkila AT, Narvanen O, Lehto S, Uusitupa MI, Yla-Herttuala S. Autoantibodies against oxidized low-density lipoprotein and cardiolipin in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:204–209. doi: 10.1161/01.atv.20.1.204. [DOI] [PubMed] [Google Scholar]
- 39.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, Schwartz GG. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 41.Rossi GP, Cesari M, De Toni R, Zanchetta M, Maiolino G, Pedon L, Ganzaroli C, Maiolino P, Pessina AC. Antibodies to oxidized low-density lipoproteins and angiographically assessed coronary artery disease in white patients. Circulation. 2003;108:2467–2472. doi: 10.1161/01.CIR.0000097122.19430.48. [DOI] [PubMed] [Google Scholar]
- 42.Geller BJ, Mega JL, Morrow DA, Guo J, Hoffman EB, Gibson CM, Ruff CT. Autoantibodies to phosphorylcholine and cardiovascular outcomes in patients with acute coronary syndromes in the ATLAS ACS-TIMI 46 trial. J Thromb Thrombolysis. 2014;37:310–316. doi: 10.1007/s11239-013-0968-y. [DOI] [PubMed] [Google Scholar]
- 43.Bjorkbacka H, Alm R, Persson M, Hedblad B, Nilsson J, Fredrikson GN. Low levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler Thromb Vasc Biol. 2016;36:765–771. doi: 10.1161/ATVBAHA.115.306938. [DOI] [PubMed] [Google Scholar]
- 44.Lee SR, Prasad A, Choi YS, Xing C, Clopton P, Witztum JL, Tsimikas S. The LPA Gene, Ethnicity, and Cardiovascular Events. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.024611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 46.Karabina SA, Elisaf MC, Goudevenos J, Siamopoulos KC, Sideris D, Tselepis AD. PAF-acetylhydrolase activity of Lp(a) before and during Cu(2+)-induced oxidative modification in vitro. Atherosclerosis. 1996;125:121–134. doi: 10.1016/0021-9150(96)05872-8. [DOI] [PubMed] [Google Scholar]
- 47.Papathanasiou AI, Lourida ES, Tsironis LD, Goudevenos JA, Tselepis AD. Short- and long-term elevation of autoantibody titers against oxidized LDL in patients with acute coronary syndromes. Role of the lipoprotein-associated phospholipase A2 and the effect of atorvastatin treatment. Atherosclerosis. 2008;196:289–297. doi: 10.1016/j.atherosclerosis.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 48.Amir S, Hartvigsen K, Gonen A, Leibundgut G, Que X, Jensen-Jarolim E, Wagner O, Tsimikas S, Witztum JL, Binder CJ. Peptide mimotopes of malondialdehyde epitopes for clinical applications in cardiovascular disease. J Lipid Res. 2012;53:1316–1326. doi: 10.1194/jlr.M025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taleb A, Zhao W, Shahzada A, Rasheed A, Binder CJ, Witztum JL, Danesh J, Rader DJ, Tsimikas S, Saleheen D. Increased Levels of IgM Autoantibodies to Oxidation-Specific Epitopes are Inversely Associated With Coronary Heart Disease: Analyses of Data From 204,257 Participants. Circulation. 2015;132:A14351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.