Abstract
The transmembrane glycoprotein Mucin 1 (MUC1) is aberrantly glycosylated and overexpressed in a variety of epithelial cancers, and plays a crucial role in progression of the disease. Tumor-associated MUC1 differs from the MUC1 expressed in normal cells with regard to its biochemical features, cellular distribution, and function. In cancer cells, MUC1 participates in intracellular signal transduction pathways and regulates the expression of its target genes at both the transcriptional and post-transcriptional levels. This review highlights the structural and functional differences that exist between normal and tumor-associated MUC1. We also discuss the recent advances made in the use of MUC1 as a biomarker and therapeutic target for cancer.
Keywords: cancer, Mucin 1 (MUC1), VNTR, ABC pumps, cancer stem cell, EMT
Mucin 1: a membrane tethered glycoprotein
Mucin 1 (MUC1; also known as episialin, PEM, H23Ag, EMA, CA15-3, and MCA) is a single pass type I transmembrane protein with a heavily glycosylated extracellular domain that extends up to 200–500 nm from the cell surface [1,2]. MUC1 (see Glossary) is normally expressed in the glandular or luminal epithelial cells of the mammary gland, esophagus, stomach, duodenum, pancreas, uterus, prostate, and lungs, and to a lesser extent, in hematopoietic cells [3,4]. It is absent in the skin epithelium and in mesenchymal cells [5]. In healthy tissues, MUC1 provides protection to the underlying epithelia. The extended negatively charged sugar branches of MUC1 create a physical barrier and impart an anti-adhesive property to MUC1, limiting accessibility and preventing pathogenic colonization [6,7]. The sugar chains oligomerize to form a mucinous gel that lubricates and protects the underlying epithelia from desiccation, changes in pH, pollutants, and microbes [8].
Aberrantly glycosylated MUC1 is overexpressed in most human epithelial cancers and has gained remarkable attention as an oncogenic molecule [9]. The focus of this review is to highlight the recent advances made in determining the structure, the oncogenic role of MUC1 in cancer progression, and the clinical utility of tumor-associated MUC1 (TA-MUC1).
Structure of MUC1
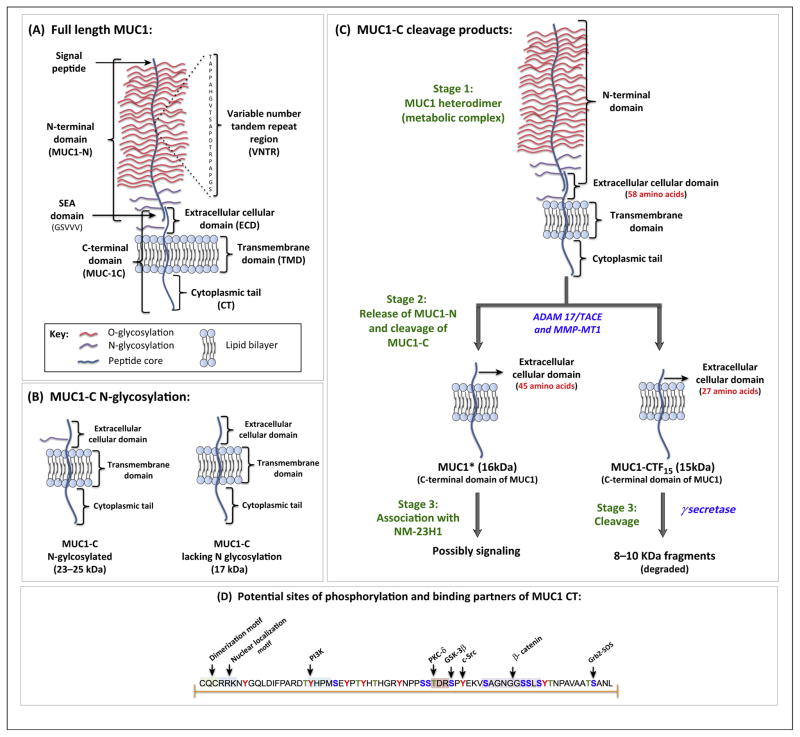
The MUC1 gene encodes a single polypeptide chain which, due to conformational stress, is autoproteolytically cleaved immediately after translation at the GSVVV motif, located within the Sea urchin sperm protein enterokinase and agrin (SEA) domain, into two peptide fragments: the longer N-terminal subunit (MUC1-N) and the shorter C-terminal subunit (MUC1-C) (Figure 1A) [1,10]. Extracellularly, the two subunits remain associated through stable hydrogen bonds.
Figure 1.
Schematic representation of the structure of MUC1, cleavage by proteases, and potential binding motifs on MUC1 cytoplasmic tail (CT). (A) The N-terminal subunit (MUC1-N) and C-terminal subunit (MUC1-C) of MUC1 associate around the SEA domain, forming a stable heterodimeric complex. MUC1-N contains the signal peptide, variable number tandem repeat (VNTR) region, and SEA domain. The VNTR region of MUC1-N is composed of 20 amino acids that are extensively O-glycosylated (red) at the serine and threonine residues. MUC1-N and MUC1-C are sparingly N-glycosylated (violet) at asparagine residues. The C-terminal domain (MUC1-C) consists of the extracellular domain (ECD), transmembrane domain (TMD), and CT. (B) The ECD of MUC1-C contains one N-glycosylation site (asparagine residue). Based on the extent of N-glycosylation, the size of MUC1-C can range between 23 and 25 kDa. MUC1-C lacking N-glycosylation has a molecular weight of 17 kDa. (C) Under normal growth conditions, MUC1 remains in a heterodimeric form (Stage 1). The ECD of MUC1-C is cleaved (Stage 2) by enzymes such as tumor necrosis factor (TNF)-α converting enzyme (TACE), also called disintegrin and metalloprotease domain containing protein-17 (ADAM17) or membrane type 1 matrix metalloprotease (MMP-MT1), to generate peptide fragments: MUC1* (16 kDa) or MUC1-CTF15 (15 kDa) with shorter ECD. Functionally, MUC1* acts as a growth factor receptor for NM23-H1 and MUC1-CTF15 acts as a substrate for γ-secretase. (D) The amino acid sequence of MUC1 CT, highlighting the potential sites of phosphorylation and protein-binding partners. The tyrosine (red), threonine (green), and serine (blue) residues of MUC1 CT are phosphorylated by growth factor receptors and intracellular kinases. The p85 subunit of PI3K, PKC-δ, GSK-3β, and c-Src phosphorylate the tyrosine residue of the YHPM motif, the threonine residue of the TDR motif, the serine residue of the SPY motif, and the tyrosine residue of the YEKV motif, respectively. β-Catenin directly binds to the serine-rich SAGNGGSSLS motif. These phosphorylated residues form potential binding sites for intracellular signaling molecules and thus MUC1 can integrate different signaling cascades or modulate their activation status. For example, the phosphotyrosine residue of the SANL motif acts as a docking site for the Grb-2 protein [109]. MUC1-C monomers dimerize around the CQC motif to form a functional homodimer. The putative nuclear localization motif RRK binds to importin-β, and allows translocation to the nucleus via Nup-62. Adapted from [18]. Abbreviations: MUC1, Mucin 1; SEA, sea urchin sperm protein, enterokinase, and agrin.
MUC1-N is composed of the proline, threonine, and serine-rich (PTS) domain and the SEA domain. The PTS domain, also designated as the variable number tandem repeat (VNTR) region, is encoded by a highly polymorphic exon encoding for multiple 20–21 amino acid sequence repeats [11]. In northern Europeans, the VNTR is composed of 20–120 repeats, with 40–80 repeats being the most common [5]. The amino acid sequence of the VNTR region can vary in different cancer cell lines, consistent with the highly polymorphic nature of this motif [12]. The VNTR region is flanked on both ends by a short degenerate sequence which bears subtle sequence similarity to the VNTR region [5]. Unlike human MUC1, mouse Muc1 contains 16 amino acid long tandem repeats and has only 34% sequence similarity with its human counterpart [13].
MUC1 is extensively O-glycosylated and moderately N-glycosylated to yield mature functional mucin [3]. Glycosylation contributes to 50–90% of the total weight of MUC1. Based on the number of tandem repeats and the degree of glycosylation, MUC1 can weigh between 250 and 500 kDa. Around 40% of the amino acids in the VNTR are serine (Ser) and threonine (Thr) residues, which are abundantly O-glycosylated (Figure 1A). N-Glycosylation occurs at five sites (all asparagine residues), four of which reside in the degenerate sequence of the MUC1-N and one in the extracellular domain (ECD) of the MUC1-C. The glycosylation pattern of MUC1 varies depending upon the tissue-specific expression of the glycosyltransferases [1]. O-Glycosylation correlates with the biological properties of MUC1, whereas N-glycosylation is vital for protein folding, sorting, secretion, and apical expression in polarized cells [14]. In normal cells, MUC1 is heavily glycosylated, with the peptide core masked by the sugar moieties that shield it from undergoing proteolytic cleavage by environmental enzymes. In addition, glycosylation also stabilizes mucins at the cell surface by preventing them from undergoing clathrin-mediated endocytosis [15].
MUC1-C is short, comprising a 58 amino acid ECD, a 28 amino acid transmembrane domain (TMD), and a 72 amino acid cytoplasmic tail (CT) (Figure 1A). Based on the N-glycosylation state of the ECD, the molecular weight of MUC1-C can vary from 17 to 25 kDa (Figure 1B). Under normal conditions, MUC1 exists on the plasma membrane as a heterodimeric complex. However, the complex dissociates following stimulation with the proinflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), and this is catalyzed by the sheddase activities of the enzymes including TNF-α converting enzyme (TACE, also called disintegrin and metalloprotease domain containing protein-17; ADAM17) and matrix metalloproteases (MMPs). These enzymes cause release of MUC1-N from MUC1-C, and also catalyze the cleavage of the ECD of MUC1-C, thereby generating smaller peptide fragments MUC1* and MUC1-CTF15 (Figure 1C) [16–18]. Unlike MUC1-C, the cleavage products of sheddases contain a shorter ECD. MUC1* consists of a 45 amino acid ECD and promotes tumor growth [19]. MUC1* has also been detected in human embryonic stem cells (hESCs), where it functions as a growth factor receptor for a metastasis-associated protein (NM23-H1) (Box 1) [20]. MUC1 CTF15 contains a shorter ECD ‘stub’ of 27 amino acids that is recognized by nicartrin, a substrate receptor for γ-secretase. γ-Secretase further cleaves MUC1-C into shorter peptide fragments with a molecular mass of 8–10 kDa, which are rapidly degraded. In most normal and tumor cells, MUC1 CTF15 is undetectable as it is rapidly degraded [21].
Box 1. MUC1 maintains pluripotency and self-renewal ability in embryonic stem cells.
A recent study showed that MUC1* is expressed on human embryonic pluripotent stem cells and functions as a growth factor receptor for a metastasis-associated protein, NM23-H1. Binding of NM23-H1 to MUC1* boosts proliferation of hESCs and prevents them from differentiating [110]. Further, blocking the interaction of MUC1* with NM23-H1 results in expression of miR-145, which causes the hESCs to exit pluripotency and undergo differentiation [20]. Hyperactivation of the Erk1/2 pathway is frequently observed in MUC1-overexpressing cancer cells and the Erk1/2 pathway inhibits expression of miR-145 in malignant cells [111]. Thus, one can extrapolate that MUC1 may regulate the expression of such miRNAs that favors the CSCs to remain in a dedifferentiated ‘stem cell like’ state. Indeed, a recent report states that MUC1 is highly expressed in pancreatic CSCs in patients with PDA [90]. However, at present, it is not known if and how MUC1 enables the CSCs to maintain their ‘stemness’.
In cancer cells, a significant amount of MUC1-C is detected intracellularly, particularly within the mitochondria and nucleus. MUC1-C monomers dimerize along the CQC motif (Figure 1D) to generate functional homodimers that translocate to the nucleus via importin-β and nucleoprotein 62 (Nup62) [22]. Within the nucleus, MUC1-C is found mostly in the nuclear matrix and nucleoli. Interestingly, MUC1-N has recently been detected in the nuclear speckles suggesting that MUC1 may be involved in pre-mRNA splicing [23]. Finally, it should be noted that the TM domain and the six of the seven tyrosine residues of MUC1 CT are highly conserved among mammalian species, indicating their crucial biological roles [24].
Regulation of MUC1 gene expression
MUC1 is encoded by a gene located on the long arm (q) of chromosome 1 at position 21, a region frequently altered in breast cancer cells [25]. Overexpression of MUC1 in cancer is caused by increases in gene dosage and level of transcription, and by a loss of post-transcriptional regulation. Studies on epigenetic regulation have shown that methylation of histone H3-K9 and the CpG islands in the MUC1 promoter (close to the transcriptional start site; −174 to −182 bp) cause transcriptional repression [26]. By contrast, H3-K9 acetylation is permissive of MUC1 expression. Thus, demethylation of CpG and H3-K9, and the acetylation of H3-K9 in the 5′ flanking region leads to elevated MUC1 expression in cancer cells [26]. The MUC1 promoter contains several putative transcription start sites [27] and several cis-acting elements such as binding sites for Sp1, AP1-4, NF-1, NF- κB, an E-box, GC boxes, peroxisome proliferator-activated receptor (PPAR) responsive region, and estrogen and progesterone receptor sites (reviewed in [3]). Proinflammatory cytokines such as TNF-α and IFN-γ also induce strong MUC1 induction through the independent actions of NF-κB p65 and STAT1α [28]. Furthermore, MUC1 expression is regulated post-transcriptionally. MUC1 mRNA contains the seed sequence for microRNA (miR)-125b in the 3′ untranslated region (UTR) and loss of miR-125b expression in breast cancer cells contributes to MUC1 overexpression [29].
MUC1 isoforms
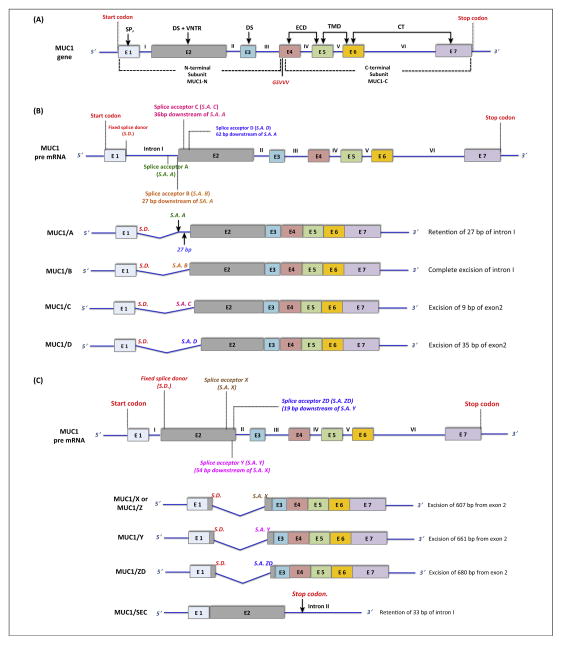
MUC1 contains seven exons, where exons 1–4 encode MUC1-N and exons 4–7 encode MUC1-C (Figure 2A). In humans, there are several isoforms of MUC1 that result from alternative splicing, exon skipping, and intron retention. A recent study identified 78 isoforms of MUC1 [30], with the most common isoforms being MUC1/A, MUC1/B, MUC1/C, MUC1/D, MUC1/X (or MUC1/Z), MUC1/Y, and MUC1/ZD. MUC1/A, MUC1/B, MUC1/C, and MUC1/D, encoding ‘full-length’ MUC1, arise from alternative splicing between sites located in intron I and exon 2 (Figure 2B) and vary only by VNTR length [31,32]. MUC1/B is the so-called ‘normal’ MUC1 mRNA. MUC1/X (or MUC1/Z), MUC1/Y, and MUC1/ZD isoforms are generated from alternative splice acceptor sites located within exon 2, where VNTR encoding exon 2 is skipped (Figure 2C) [33,34]. The MUC1/Y isoform is 54 bp shorter than MUC1/X and is highly expressed in breast, ovarian, and prostate cancer cells [5,35,36]. MUC1/ZD also lacks the VNTR region and the flanking degenerate sequence, but contains a unique C-terminal domain (43 amino acids) that results from a shift in the reading frame [37]. A secreted isoform of MUC1 called MUC1/SEC that lacks both the TMD and CT binds to MUC1/Y causing phosphorylation of the tyrosine residues of MUC1/Y [38]. Presently, there is a lack of clear understanding of the functional significance of each of these spliced MUC1 variants.
Figure 2.
Schematic representation of the MUC1 gene and the different isoforms of MUC1. (A) The MUC1 gene consists of seven exons (E1 to E7, indicated by different colored boxes) and six introns (I to VI, blue lines). Exons 1–3 encode the MUC1 N-terminal and exons 4–7 encode the MUC1 C-terminal subunits. Exons encoding the corresponding domains are indicated by an arrow. Exon 1 (E1) encodes the signal peptide (SP), E2 encodes the N-terminal degenerate sequence (DS) and the VNTR, and E3 encodes the C-terminal DS. E4, E5, E6, and E7 together encode the extracellular domain (ECD), transmembrane domain (TMD), and cytoplasmic tail (CT). MUC1 is encoded as a single polypeptide chain that undergoes spontaneous cleavage at the GSVVV site (red) to generate the MUC1-N and MUC1-C subunits. (B) MUC1 pre-mRNA is spliced into four main variants of mature MUC1 mRNA – MUC1/A, MUC1/B, MUC1/C, and MUC1/D, all encoding ‘full-length’ MUC1. These isoforms are generated by alternative splicing between the fixed splice donor site near the 5′ end of intron I (red) and multiple splice acceptor sites near the 3′ and 5′ end of intron I and exon II, respectively (green, orange, magenta, and blue). In MUC1/A, a portion of the intron I (27 bp) is retained, coding an alternative signal peptide without causing a reading frameshift. In MUC1/B, intron I is completely removed. Portions of exon 2 are spliced out while retaining the reading frame in MUC1/C and MUC1/D, resulting in shorter VNTRs [31]. (C) MUC1 mRNA also contains a cryptic intron in exon 2. Several alternative splice acceptor sites around the 3′ end of exon 2 (brown, magenta, blue) can link with the fixed splice donor site at the 5′ end of exon 2 (red). Splicing between these sites results in the formation of the splice variants MUC1/X (or MUC1/Z), MUC1/Y, and MUC1/ZD, which completely lack the VNTR region. MUC1/SEC, the secreted isoform, is generated by a failure to excise intron 2, leading to premature abortion of transcription caused by the presence of a stop codon within intron 2 [112,113]. Abbreviations: MUC1, Mucin 1; E, exon; VNTR, variable number tandem repeat; MUC1-N, MUC1 N-terminal; MUC1-C, MUC1 C-terminal; S.D., splice donor; S.A., splice acceptor.
Tumor-associated MUC1
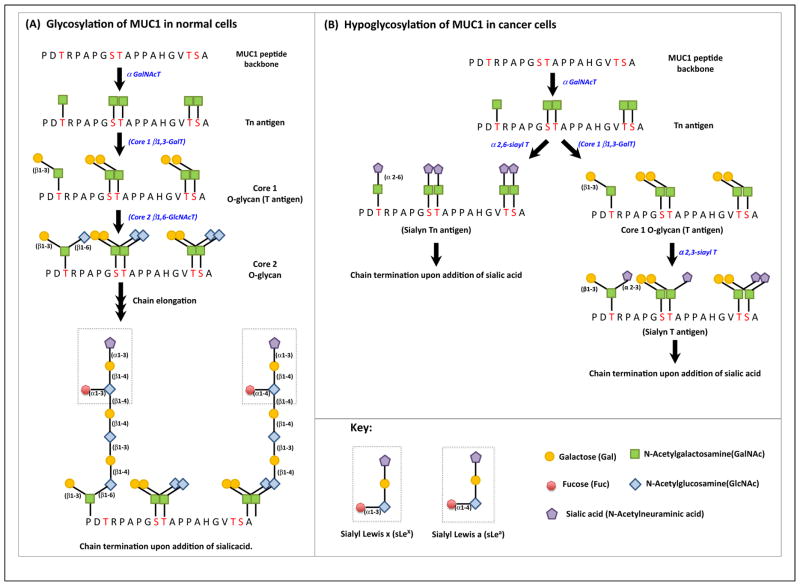
TA-MUC1 differs from that expressed in normal cells, both in its biochemical features and its cellular distribution. Normally expressed MUC1 contains extensively branched Core 2 O-glycans (Figure 3A). By contrast, MUC1 in breast cancer cells mostly exhibits the Core 1 O-glycans [39] as loss of Core 2 β6-GlcNAc-transferase activity results in an absence of Core 2 O-glycans [40]. Additionally, TA-MUC1 is highly sialylated, which causes premature termination of chain elongation and formation of truncated sugar branches (Figure 3B). Increased sialylation may result from an increased expression in the α2,3- and α2,6-sialyl-transferases in cancer cells. In breast cancer cells, α2,3-sialyltransferase (also called ST3 Gal-I) is overexpressed, resulting in the generation of sialylated Core 1 glycans (T antigen) [41]. In other cancer cells, there are alternative mechanisms that dominate. For example, in colon cancer cells, MUC1 overexpress the sialyl Lewisx (sLeX) and sialyl Lewisa (sLea) epitopes and a decrease in O-acetylation appears to contribute to such expression in these cells [42].
Figure 3.
Differences between the glycosylation patterns in normal and tumor-associated MUC1 (TA-MUC1). (A) N-Acetylgalactosamine (GalNAc) is first added to the serine and threonine residues of the MUC1-N VNTR, catalyzed by αGalNAc transferases to generate the Tn antigen. Core 1 β1,3-galactose transferase (Core 1 β1,3-Gal T) catalyzes the addition of galactose to Tn antigen to generate Core 1 O-glycan (also called T antigen). GlcNAc (N-acetyl glucosamine) is then added to the Core 1 O-glycan catalyzed by Core 2 β1,6-N-acetyl glucosamine transferase (Core 2 β1,6-GlcNAc T). The sugar branches of Core 2 glycans undergo further chain elongation followed by termination upon addition of fucose, or sialic acid to the terminal sugar [114]. (B) MUC1 is hypoglycosylated in cancer cells. Tn and T antigens are sialylated to sialyl Tn and sialyl T, respectively, which are catalyzed by the enzyme α2, 6-sialyltransferase and α2, 3-sialyltransferase, respectively. Sialylation causes premature termination of chain elongation. Abbreviations: GalNAc T, N-acetylgalactosamine transferase; GlcNAc T, N-acetyl glucosamine transferase; Gal T, galactose transferase; Sialyl T, sialyl transferase; MUC1, Mucin 1; VNTR, variable number tandem repeat.
Hypoglycosylation impacts the stability and subcellular localization of MUC1 [15]. Compared with fully glycosylated MUC1, hypoglycosylated MUC1 shows increased intracellular uptake by clathrin-mediated endocytosis, without any enhanced degradation. Thus, hypoglycosylation may potentiate MUC1 oncogenic signaling by decreasing its cell surface levels and increasing intracellular accumulation [15]. Moreover, it has been hypothesized that the sugar branches sequester proinflammatory factors such as transforming growth factor α (TGF-α), interleukin 1 (IL-1), IL-4, IL-6, IL-9, and IL-13, which are released upon MUC1-N shedding thereby triggering inflammation [43].
Expression of TA-MUC1
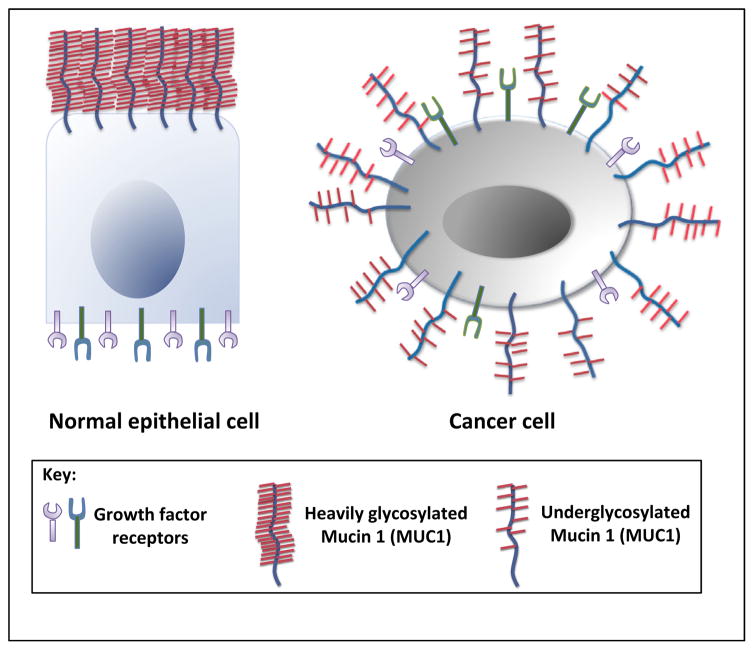
MUC1 is overexpressed in cancer cells and the loss of cell polarity causes TA-MUC1 to be redistributed over the cell surface and within the cytoplasm (Figure 4) [3]. Lack of cell polarity also causes the redistribution of cell surface growth factors that are normally restricted to the basolateral surface of epithelial cells. Growth factors juxtaposed to MUC1 and intracellular kinases such as ZAP-70, PKC-γ, GSK-3β, and c-Src phosphorylate serine, tyrosine, and threonine residues on MUC1 CT (Figure 1C). It is also thought that hypoglycosylation unmasks the peptide core of TA-MUC1 allowing MUC1-N cleavage and release by extracellular proteases. MUC1-N release induces conformational changes in MUC1-C that alter its ligand status and subsequently activates downstream cell signaling pathways such as the mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (P13K/Akt), and wingless type (Wnt) pathways [43]. As a result, MUC1-positive pancreas, breast, lung, and colon cancer cells commonly display hyperactivation of these critical signaling pathways [44–46]. MUC1-C also associates with various transcription factors (STAT3, NF-κB, p53, and β-catenin) and binds the target gene promoter region to drive their expression [46–49]. Several studies have indicated that MUC1 plays a critical role in the transcriptional regulation of genes associated with tumor invasion, metastasis, angiogenesis, proliferation, apoptosis, drug resistance, inflammation, and immune regulation [8,45,48,50–54].
Figure 4.
MUC1 overexpression and loss of polarity in cancer cells. MUC1 and growth factors are confined to the apical surface and basolateral surface of normal epithelial cells, respectively. However, tumor cells lose apicobasal polarity, and hypoglycosylated MUC1 is overexpressed all over the surface of the tumor cells, often in close proximity to the growth factors and their receptors. Abbreviation: MUC1, Mucin 1.
The functional role of MUC1 in malignancy
The importance of MUC1 in disease progression is underscored by a study using mouse models of pancreatic and breast cancer. Muc1−/− mice expressing high levels of polyomavirus middle T antigen in the mammary gland spontaneously develop breast cancer but exhibit a substantial delay in disease progression and metastasis in comparison to Muc1+/+ mice [55]. Similarly, this trend was reported in a mouse model of spontaneous pancreatic ductal adenocarcinoma (PDA) [56,57].
Proliferation
MUC1 mediates production of growth factors such as connective tissue growth factor (CTGF), platelet-derived growth factor A (PDGF-A), and PDGF-B that promote activation of the MAPK and PI3K/Akt pathways, potentiating proliferation and survival of tumor cells [50,53,56,58]. Upon epidermal growth factor (EGF) stimulation, MUC1-C directly associates with epidermal growth factor receptor (EGFR) and translocates to the nucleus. It subsequently binds to cyclin D1 (CCND1) and v-myb myeloblastosis viral oncogene homolog-like 2 (MYBL2) promoters enabling G1/S phase gene expression [59]. PDGF-A stimulation causes MUC1 CT to associate with hypoxia-inducible factor-1α (HIF-1α), and in turn drives the expression of PDGF-A thereby promoting proliferation and invasion of PDA cells [52].
Metabolism
Aberrant glucose metabolism is a cancer hallmark that facilitates cell survival and proliferation. HIF-1α regulates glycolytic pathway enzyme expression and this is the preferred metabolic pathway of proliferating cancer cells. MUC1 acts as a modulator of the hypoxic response by regulating the expression, stability, and activity of HIF-1α [60]. MUC1 physically interacts with HIF-1α to stabilize the protein and then binds to the promoter of multiple glycolytic genes to enhance their expression in a hypoxia-dependent manner. Notably, MUC1 mediates enhanced expression of genes that are involved in glucose uptake and metabolism in orthotopic implantation models of pancreatic cancer [60]. In this manner, this molecule can directly facilitate cancer cell survival and growth by upregulating glucose uptake and metabolism.
Invasion and metastasis
Invasion, an early and prerequisite step to metastasis, is a multistep process which enables cancer cells to detach from the basement membrane, degrade the surrounding matrix, and finally invade the neighboring tissues, and/or enter the bloodstream. Epithelial to mesenchymal transition (EMT) has emerged as an explanation for the biological process by which cancer cells acquire their invasive potential [61].
MUC1 CT translocates to the nucleus in association with β-catenin, represses E-CADHERIN expression, and upregulates expression of the EMT inducers Snail, Slug, Vimentin, and Twist [51]. As a consequence, the adherens junctions are destabilized and profound cytoskeleton rearrangement occurs, reducing contacts between cancer cells and facilitating basement membrane invasion. MUC1 also induces EMT at the post-transcriptional level by modulating the expression of miRNAs that control EMT-related gene expression (Box 2). In addition, PDGF-B stimulation promotes nuclear localization of the MUC1 CT/β catenin transcriptional complex, increasing the invasive potential of PDA cells [62]. Furthermore, MUC1 associates with Cbl-interacting protein of 85 kDa (CIN85) and colocalizes to the invadopodia-like structures aiding breast cancer cell invasion [63]. Such mechanisms could account for clinical findings that MUC1 overexpression leads to metastasis and poor prognosis in pancreas, gall bladder, and colon cancer patients [64–66].
Box 2. MUC1 is a post-transcriptional regulator of genes.
Only recently, it has become apparent that MUC1 impacts the expression of its target genes at the post-transcriptional level. The miR-200c/141 cluster and miRs-192/194/215 are frequently down-regulated in cancer cells and their expression is inversely related to invasion and metastasis. The miR-200c/141 cluster binds to the 3′UTR of Zeb1, a repressor of E-cadherin, thereby initiating EMT. A recent study in pancreatic cancer cells showed that MUC1, in association with ZEB1, binds to the promoter and represses the transcription of the miR-200c/141 cluster [115]. A similar study with breast cancer cells demonstrated that MUC1 in association with NF-κB p65 binds to the promoter of ZEB1 and upregulates its expression. ZEB1 in turn associates with MUC1 and represses miR-200c expression [116]. In addition, the p53 regulated miRs-192/194/215, which are inversely related to invasion and metastasis, are also significantly downregulated in MUC1 high pancreatic cancer cells [115].
It is postulated that altered glycosylation enables TA-MUC1 to function as a ligand for cell adhesion molecules such as selectins and intercellular adhesion molecule-1 (I-CAMs), aiding adherence of MUC1-expressing circulating tumor cells (CTCs) to endothelial cells and seeding at distant sites that establishes secondary tumors [67]. Selectins are known to bind the carbohydrate epitope sLeX [68]. In colon cancer cells, increases in sLeX expression on MUC1 are associated with high metastasis [42], as this antigen interacts with E and P selectins. In melanoma, MUC1 overexpression interferes with integrin-mediated cell adhesion to the extracellular matrix, increasing cancer cell invasiveness [69].
Angiogenesis
Dysregulated cellular proliferation forms a tissue mass that extends beyond the normal vasculature, creating a low nutrient and oxygen microenvironment (hypoxia). However, the proliferating cancer cells adapt to survive in a hypoxic environment by inducing expression of proangiogenic factors and promoting angiogenesis. Under hypoxia, MUC1 induces expression of proangiogenic factors, such as CTGF, PDGF-B, and vascular endothelial growth factor-A (VEGF-A) that, in turn, promote tube formation in endothelial cells and synthesis of new blood vessels within the tumor [70]. Recent findings indicate that MUC1 over-expression in breast and pancreatic cancer cells promote synthesis and secretion of VEGF [51,71]. Furthermore, MUC1 drives the expression of PDGF-A in PDA cells in association with HIF-1α [52]. Interestingly, MUC1 is itself regulated by HIF-1α, which binds to the MUC1 promoter region and drives its expression [72]. MUC1 induces CTGF expression, a potent mediator of metastasis and angiogenesis, by binding to its promoter region through β-catenin and p53 [53]. It is important to note that such MUC1-induced factors not only stimulate angiogenesis but also promote the migratory and invasive properties of cancer cells [52,53,72].
Chemoresistance and resistance to apoptosis
Most anticancer treatments work by inducing apoptosis in cancer cells. However, many cancer cells acquire apoptotic pathway defects and, therefore, do not respond to these treatments. MUC1 assists cancer cells in evading cell death by preventing the activation of the intrinsic apoptotic pathway. In 3Y1 rat fibroblasts, MUC1 overexpression interferes with apoptosis activation by selectively upregulating expression of the antiapoptotic protein, B cell lymphoma extra-large (Bcl-xL), and inactivating the proapoptotic protein, Bcl2-associated agonist of cell death (Bad) [58]. In hypoxic cells, elevated reactive oxygen species (ROS) levels can activate apoptotic pathways. However, MUC1 overexpression decreases intracellular ROS levels by upregulating the expression of superoxide dismutase, catalase, and glutathione peroxidase [73]. Furthermore, it has been reported that MUC1 blocks hypoxia-induced cell death in colon cancer cells by mediating decreases in intracellular ROS concentration and reducing prolyl hydrolase-3 (PHD-3) activity that suppresses HIF-1α stability [74].
Another common mechanism by which cancer cells evade drug-induced cell death is via upregulation of adenosine triphosphate (ATP)-dependent membrane efflux pumps or ATP-binding cassette (ABC) transporters. These transporters and pumps are known to efflux anticancer drugs, thereby reducing drug accumulation within the cancer cells [75]. This form of resistance is mostly seen against amphipathic drugs such as vinka alkaloids and anthracyclines. Recently, it has been shown that MUC1 increases resistance to chemotherapeutic drugs by upregulating multidrug resistance gene and protein expression, in particular, multidrug resistance protein 1 (MRP1) [45].
Inflammation
Epidemiological studies have shown that chronic inflammation caused by microbial infection, tissue injury, or irritation (e.g., from smoke or asbestos) fosters genomic lesions and predisposes individuals to cancer development [76]. Nearly 15% of cancers worldwide are associated with chronic microbial infection [77]. Additionally, oncogene activation can trigger cytokine and chemokine expression, creating an inflammatory milieu in the tumor microenvironment. In cancer cells, constitutively active transcription factors such as NF-κB, STAT3, and HIF-1α can drive the overexpression of proinflammatory mediators, such as colony stimulating factor (CSF), TNF-α, chemokine C–C motif ligand 2 (CCL2), IL-8, IL-1β, IL-6, cyclooxygenase-2 (Cox-2), and MMPs. These inflammatory mediators activate oncogenic pathways in tumor cells, stromal cells, and leukocytes that augment proliferation, resistance to apoptosis, invasion, angiogenesis, and suppress antitumor immune responses [78]. Furthermore, a recent study demonstrated that MUC1 and NF-κB p65 colocalize to the promoter regions of IL-6 and TNF-α, driving their expression in breast cancer cells and thereby creating a proinflammatory milieu in the tumor microenvironment [54].
By contrast, MUC1 has been reported to act as an anti-inflammatory molecule in gastric mucosal cells. MUC1 knockdown in these cells leads to an increase in chemokine secretion when stimulated with TNF-α, pathogens, nucleotide-binding oligomerization domain (NOD), or Toll-like receptor (TLR) ligands [79]. During acute lung infection by Pseudomonas aeruginosa or respiratory syncytial infection (RSV), MUC1 inhibits inflammation [80,81]. Overexpression of MUC1 in HEK293 cells reduces IL-8 production, a key chemokine that promotes neutrophil recruitment to inflammatory sites [82–84]. The anti-inflammatory properties of MUC1 have also been observed in gastric mucosal cell responses to Helicobacter pylori infection [85]. Thus, it may be postulated that MUC1 serves an inflammatory role in cancer cells but has an anti-inflammatory function during infectious disease.
Clinical applications of MUC1
MUC1 as a cancer biomarker
Shed MUC1-N found in the circulation of cancer patients is used as a biomarker for cancer staging and monitoring relapse following therapy. For example, carbohydrate antigen 15.3 (CA 15.3, MUC1) and carbohydrate antigen 19.9 (CA 19.9, sLea antigen, found on several glycoproteins including MUC1) are commonly used for the detection of breast and pancreatic cancers, respectively [86,87]. Because MUC1-N is also released from stressed cells, the clinical utility of MUC1 measurement is confined to monitoring treatment efficacy in cancer patients. A recent clinical study in PDA patients demonstrated that, out of 13 putative biomarkers tested, a significant correlation was observed between elevated MUC1 protein expression and poor patient survival [88]. Recently, new MUC1 specific antibodies, PAM4 and TAB004, have been developed for diagnostic and therapeutic purposes and both of these antibodies exhibit high specificity for TA-MUC1-N. PAM4 shows high specificity and intense reactivity with pancreatic carcinoma tissue and is being developed as an in vitro diagnostic assay, as well as for in vivo targeting of therapeutic agents [89]. TAB004 specifically detects MUC1-N in the carcinoma tissue, cancer stem cells (CSCs), and in the serum of pancreatic cancer patients in a stage-dependent manner [90]. TAB004 is also being developed for use as a diagnostic marker and therapeutic agent for other epithelial cancers, including breast, ovarian, and prostate.
MUC1 as a target for cancer immunotherapy
Recently, a study conducted by the National Cancer Institute (NCI) Translational Research Working Group ranked MUC1 as the second best potential target out of 75 tumor-associated antigens for the development of cancer vaccine [91]. Accordingly, there are currently 30 ongoing MUC1-based clinical trials around the country. Notable examples include ImMucin, which is a 21mer synthetic vaccine composed of the entire signal peptide domain of the MUC1 protein, and L-BLP25 (formally called Stimuvax) which contains 25 amino acids from the immunogenic VNTR region of MUC1 [92,93]. ImMucin successfully completed a PhaseI/II clinical trial inmultiple myeloma patients and is being developed by Vaxil, whereas Stimuvax is being developed by Merck and has recently completed a Phase III trial in patients with unresectable, locally advanced stage IIIA or IIIB non-small cell lung cancer (NSCLC) (http://clinicaltrials.gov/show/NCT01232712) [94].
Several other MUC1-based immunotherapies are in various stages of development, including one for advanced stage colon cancer [95–97]. Some of these strategies have not met expectations, probably due to the conformational disparities between non-glycosylated vaccine sequences and aberrantly glycosylated TA-MUC1. Recently, a fully synthetic aberrantly glycosylated MUC1 peptide vaccine, covalently linked to a TLR agonist, was shown to elicit potent humoral and cellular immune responses and this construct was effective in reversing tolerance and eliciting a therapeutic response in mice [98]. Another major impediment to cancer vaccines is the presence of immunosuppressive cells and factors within the tumor microenvironment that can negate the antitumor immune responses elicited by the vaccine. Therefore, new strategies that combine a MUC1 vaccine with antagonists of tumor-induced immune suppression are currently under development and several preclinical studies utilizing such an approach have been published [99]. However, such combinations have not reached clinical trials.
MUC1 as a target for small molecule inhibitors
In the past, the development of MUC1-based immune therapies was focused on MUC1-N. Only recently has it been recognized that many of the oncogenic properties of MUC1 reside in the CT, making MUC1 CT an attractive druggable target. Currently, a cell penetrating peptide-based inhibitor of MUC1 CT, GO-203, is in Phase I clinical trials for the treatment of breast cancer (http://clinicaltrials.gov/show/NCT01279603). This inhibitor is designed to target the CQC motif of MUC1 CT to prevent MUC1-C dimerization, nuclear translocation, and oncogenic signaling [100]. There is great optimism that a combination therapy of a MUC1 CT inhibitor and chemotherapy will improve anticancer treatment efficacy.
Concluding remarks and future perspectives
Given the multifaceted functions of MUC1 in cancer, it is imperative to determine whether MUC1 plays an initiating role in carcinogenesis. We know that MUC1 is critical for the development of adenocarcinoma from preneoplastic PanIN lesions in the PDA mouse model [56,57]. In the absence of MUC1, PanIN lesions do not progress. Similarly, in the APCmin mouse of spontaneously developing colon polyps, MUC1 drives the polyps to become adenocarcinomas [101]. Thus, one can assume that MUC1 is crucial for the progression of the disease once an initiating oncogenic event has occurred. However, MUC1 overexpression in mouse mammary glands can lead to cancer development, indicating that elevated levels of this molecule can, in some instances, be a tumor-initiating event [102]. Whether this holds true for other epithelial cell cancers is not known, and it must be acknowledged that some epithelial cancers can develop and progress without MUC1 involvement. The incidence rate of such cancers is low and they are usually less aggressive in nature. Based on the available evidence, it can be inferred that most likely, MUC1 does not initiate malignant transformation but plays a critical role in creating the conditions necessary for cancer development. In this review, we discussed the contribution of MUC1 to the seven hallmarks of cancer. Among the MUC1 functions discussed here, it is rather difficult to distinguish which play an initiating role in carcinogenesis, and which foster cancer progression and metastasis.
CSCs have gained remarkable attention in recent years, in part, due to a link with increased recurrence, drug resistance, and metastasis [103]. Thus, gaining a better understanding of the molecular mechanisms underlying drug resistance and metastasis with respect to CSCs is of the utmost importance. Recent reports indicate that MUC1 is expressed in pancreatic and acute myeloid leukemia (AML) CSCs [90,104]. Whether MUC1 contributes to the ‘stemness’ of the CSCs remains unexplored (Box 3). It would be interesting to determine whether there is a link between MUC1 and increased drug resistance in CSCs. Some studies have shown that CSCs overexpress ABC transporters, especially P-glycoprotein (P-gp), MRP1, and ATP-binding cassette subfamily G member 2 (ABCG2) [105]. As such, it is possible that MUC1 promotes CSC aggressiveness via ABC transporter upregulation. Insights gained from such studies will significantly aid in the design of strategies to eradicate highly drug-resistant CSCs.
Box 3. Outstanding questions.
What causes MUC1 gene copy number to increase in malignant cells?
Do the different isoforms of MUC1 play a unique role in the progression of cancer? Is it important to target the MUC1 isoforms individually?
How does MUC1 aid the stem cells to maintain their ‘stemness’? Could an increase in MUC1 expression in cancer cells reprogram the genetic machinery such that it causes the cancer cells to become dedifferentiated and acquire stem cell like properties?
Does MUC1 hyperactivate the Erk1/2 and PI3K/Akt pathways by repressing the expression of the negative regulators of the pathways through miRNA?
MUC1 does not have a DNA-binding domain. Yet, MUC1 occupies the promoter region of its target genes in association with certain transcription factors. What is the exact role of MUC1 in the transcriptional complex?
The available biological and molecular evidence provides a glimpse into the structural and functional complexity of MUC1 as an oncogenic molecule. However, several questions related to the overexpression of MUC1 in cancer, the role of alternatively spliced MUC1 isoforms, its subcellular localization, and its role in stem cell drug resistance remain unanswered. Addressing these questions will be essential to fully understand this molecule and to develop new MUC1-based biomarkers and treatment strategies for cancer.
Acknowledgments
We would like to acknowledge Dr Ian Marriott and Ms Tonya Bates for reviewing the manuscript for clarity.
Glossary
- Epithelial to mesenchymal transition (EMT)
in normal epithelial tissue, intracellular adhesion junctions, such as adherens, tight junctions, and desmosomes, maintain the integrity of the epithelial cell layer, cell polarity, and spatially confine the signaling molecules. During EMT, the junctional complexes are weakened, followed by loss of apicobasal polarity and contact with basement membrane, finally leading to acquisition of invasive properties
- Hypoxia-inducible factor 1α (HIF-1α)
is an oxygen-sensing molecule and the key mediator of cellular responses under oxidative stress (hypoxia). HIF-1α is constitutively expressed and is stable for less than 5 min under normoxic conditions. The stability of HIF-1α is dependent upon the intracellular abundance of prolyl hydroxylase (PHD) and its activity. At normoxic conditions (O2 more than 5%), PHD hydroxylates HIF-1α at Pro-402 and Pro-564, marking it for ubiquitination and subsequent proteosomal degradation. During oxidative stress, the elevated levels of ROS attenuate PHD activity enhancing HIF-1α stability [106]. In hypoxic conditions, stabilized HIF-1α participates in transcriptional regulation of its target genes. Approximately 70 genes are under the direct transcriptional regulation of HIF-1α [107,108]
- microRNA (miRNA)
these are a class of small noncoding RNA (~22 bp long) that engage in post-transcriptional regulation of its target genes. miRNAs bind to partially complementary sequences on the 3′ UTR of multiple target mRNAs, resulting in gene silencing through translational repression, deadenylation, and cleavage of the target mRNA
- Mucins
a class of modular proteins characterized by the presence of a mucin domain (also called the PTS domain) rich in proline/serine/threonine amino acids. Mucins are typically found on the apical surface of the glandular or luminal epithelial cells. They are high molecular weight proteins composed of a long peptide chain called ‘apomucin’, which is extensively modified by O-glycosylation
- MUC1-N, MUC1-C, and MUC1-CT
full-length MUC1 comprises two subunits –N-terminal subunit (MUC1-N) and C-terminal subunit (MUC1-C). MUC1-C again contains a short extracellular region (53 amino acids), transmembrane domain (28 amino acid), and cytoplasmic tail (72 amino acid). The cytoplasmic tail of MUC1 (MUC1-CT) contains highly conserved serine and tyrosine residues that are phosphorylated by growth factor receptors and intracellular kinases. These phosphoserine and phosphotyrosine residues act as binding sites for molecules such as PI3K, c-Src, NF-κB, and β-catenin
- N-Glycosylation
a co-translational event, where mannose-rich oligosaccharide is transferred to an asparagine residue on the acceptor arm consisting of the Asn-Xaa-Ser/Thr consensus motif. The reaction is catalyzed by a number of enzymes belonging to the N-acetylgalactose aminyltransferase (GalNAcT) family. The mannose-rich N-glycans undergo further modification and truncation in the Golgi apparatus
- O-Glycosylation
in the Golgi apparatus, step-by-step O-glycosylation occurs. Galactose, N-acetylgalactosamine (GalNAc), fucose, and/or sialic acid are added to the hydroxyl group of serine and threonine residues of a peptide chain
- VNTR (variable number tandem repeat)
a highly polymorphic sequence motif in the extracellular domain of mucin proteins marked by the presence of a PTS domain rich in serine/proline/threonine residues
References
- 1.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 2.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 3.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 4.Chang JF, et al. The epithelial mucin, MUC1, is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol. 2000;201:83–88. doi: 10.1006/cimm.2000.1643. [DOI] [PubMed] [Google Scholar]
- 5.Hanisch FG, Müller S. MUC1: the polymorphic appearance of a human mucin. Glycobiology. 2000;10:439–449. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- 6.Yolken R, Peterson J. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroten H, et al. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect Immun. 1992;60:2893–2899. doi: 10.1128/iai.60.7.2893-2899.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau SK, et al. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61–69. doi: 10.1309/9R66-73QE-C06D-86Y4. [DOI] [PubMed] [Google Scholar]
- 10.Levitin F, et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–33386. doi: 10.1074/jbc.M506047200. [DOI] [PubMed] [Google Scholar]
- 11.Gendler SJ, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin*. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 12.Muller S. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J Biol Chem. 1999;274:18165–18172. doi: 10.1074/jbc.274.26.18165. [DOI] [PubMed] [Google Scholar]
- 13.Spicer AP, et al. Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem. 1991;266:15099–15109. [PubMed] [Google Scholar]
- 14.Parry S, et al. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology. 2006;16:623–634. doi: 10.1093/glycob/cwj110. [DOI] [PubMed] [Google Scholar]
- 15.Altschuler Y, et al. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell. 2000;11:819–831. doi: 10.1091/mbc.11.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thathiah A, et al. Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 17.Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J. 2004;382:363–373. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson DD. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:pe35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 19.Mahanta S, et al. A minimal fragment of MUC1 mediates growth of cancer cells. PLoS ONE. 2008;3:e2054. doi: 10.1371/journal.pone.0002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smagghe BJ, et al. MUC1* ligand, NM23-H1, is a novel growth factor that maintains human stem cells in a more naïve state. PLoS ONE. 2013;8:e58601. doi: 10.1371/journal.pone.0058601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julian J, et al. MUC1 is a substrate for γ-secretase. J Cell Biochem. 2009;108:802–815. doi: 10.1002/jcb.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raina D, et al. Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. Int J Oncol. 2012;40:1643–1649. doi: 10.3892/ijo.2011.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, et al. The MUC1 extracellular domain subunit is found in nuclear speckles and associates with spliceosomes. PLoS ONE. 2012;7:e42712. doi: 10.1371/journal.pone.0042712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spicer AP, et al. Analysis of mammalian MUC1 genes reveals potential functionally important domains. Mamm Genome. 1995;6:885–888. doi: 10.1007/BF00292441. [DOI] [PubMed] [Google Scholar]
- 25.Merlo G, et al. Frequent alteration of the DF3 tumor-associated antigen gene in primary human breast carcinomas. Cancer Res. 1989;49:6966–6971. [PubMed] [Google Scholar]
- 26.Yamada N, et al. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–2716. doi: 10.1158/0008-5472.CAN-07-6844. [DOI] [PubMed] [Google Scholar]
- 27.Zaretsky JZ, et al. Analysis of the promoter of the MUC1 gene overexpressed in breast cancer. FEBS Lett. 1999;461:189–195. doi: 10.1016/s0014-5793(99)01452-0. [DOI] [PubMed] [Google Scholar]
- 28.Lagow EL, Carson DD. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-γ and tumor necrosis factor-α. J Biol Chem. 2002;86:759–772. doi: 10.1002/jcb.10261. [DOI] [PubMed] [Google Scholar]
- 29.Rajabi H, et al. Mucin 1 oncoprotein expression is suppressed by the miR-125b oncomir. Genes Cancer. 2010;1:62–68. doi: 10.1177/1947601909357933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, et al. Human mucin MUC1 RNA undergoes different types of alternative splicing resulting in multiple isoforms. Cancer Immunol Immunother. 2013;62:423–435. doi: 10.1007/s00262-012-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obermair A, et al. Novel MUC1 splice variants are expressed in cervical carcinoma. Gynecol Oncol. 2001;83:343–347. doi: 10.1006/gyno.2001.6396. [DOI] [PubMed] [Google Scholar]
- 32.Ligtenberg MJ, et al. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990;265:5573–5578. [PubMed] [Google Scholar]
- 33.Zrihan-Licht S, et al. Characterization and molecular cloning of a novel MUC1 protein, devoid of tandem repeats, expressed in human breast cancer tissue. Eur J Biochem. 1994;224:787–795. doi: 10.1111/j.1432-1033.1994.00787.x. [DOI] [PubMed] [Google Scholar]
- 34.Oosterkamp HM, et al. Comparison of MUC-1 mucin expression in epithelial and non-epithelial cancer cell lines and demonstration of a new short variant form (MUC-1/Z) Int J Cancer. 1997;72:87–94. doi: 10.1002/(sici)1097-0215(19970703)72:1<87::aid-ijc13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Schut I, et al. MUC1 expression, splice variant and short form transcription (MUC1/Z, MUC1/Y) in prostate cell lines and tissue. BJU Int. 2003;91:278–283. doi: 10.1046/j.1464-410x.2003.03062.x. [DOI] [PubMed] [Google Scholar]
- 36.Baruch A, et al. Preferential expression of novel MUC1 tumor antigen isoforms in human epithelial tumors and their tumor-potentiating function. Int J Cancer. 1997;71:741–749. doi: 10.1002/(sici)1097-0215(19970529)71:5<741::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Levitin F, et al. A novel protein derived from the MUC1 gene by alternative splicing and frameshifting. J Biol Chem. 2005;280:10655–10663. doi: 10.1074/jbc.M406943200. [DOI] [PubMed] [Google Scholar]
- 38.Baruch A, et al. The breast cancer-associated MUC1 gene generates both a receptor and its cognate binding protein. Cancer Res. 1999;59:1552–1561. [PubMed] [Google Scholar]
- 39.Whitehouse C, Burchell J. A transfected sialyltransferase that is elevated in breast cancer and localizes to the medial/trans-Golgi apparatus inhibits the development of core-2-based O-glycans. J Cell Biol. 1997;137:1229–1241. doi: 10.1083/jcb.137.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brockhausen I, et al. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–617. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- 41.Picco G, et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 2010;20:1241–1250. doi: 10.1093/glycob/cwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann B, et al. Low O-acetylation of sialyl-Lex contributes to its overexpression in colon carcinoma metastases. Int J Cancer. 1997;72:258–264. doi: 10.1002/(sici)1097-0215(19970717)72:2<258::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 43.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 44.Thompson EJ, et al. Tyrosines in the MUC1 cytoplasmic tail modulate transcription via the extracellular signal-regulated kinase 1/2 and nuclear factor-κB pathways. Mol Cancer Res. 2006;4:489–497. doi: 10.1158/1541-7786.MCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 45.Nath S, et al. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. doi: 10.1038/oncsis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, et al. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, et al. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad R, et al. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-κB p65 transcription factor. Cancer Res. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad R, et al. MUC1-C oncoprotein promotes STAT3 activation in an auto-inductive regulatory loop. Sci Signal. 2011;4:ra9. doi: 10.1126/scisignal.2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res. 2006;8:R37. doi: 10.1186/bcr1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy LD, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449–1459. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahraei M, et al. MUC1 regulates PDGFA expression during pancreatic cancer progression. Oncogene. 2012;31:4935–4945. doi: 10.1038/onc.2011.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens ME, et al. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene. 2010;29:5667–5677. doi: 10.1038/onc.2010.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cascio S, et al. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-κB p65 and binding to cytokine promoters: importance of extracellular domain. J Biol Chem. 2011;286:42248–42256. doi: 10.1074/jbc.M111.297630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowse GJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 56.Besmer DM, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432–4442. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tinder TL, et al. MUC1 enhances tumor progression and contributes towards immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181:3116–3125. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raina D, et al. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–20612. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 59.Bitler BG, et al. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaika NV, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1α to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109:13787–13792. doi: 10.1073/pnas.1203339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalluri R, Weinberg RA. Review series. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh PK, et al. Platelet-derived growth factor receptor β-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201–5210. doi: 10.1158/0008-5472.CAN-06-4647. [DOI] [PubMed] [Google Scholar]
- 63.Cascio S, et al. Altered glycosylation of MUC1 influences its association with CIN85: the role of this novel complex in cancer cell invasion and migration. Oncotarget. 2013;4:1686–1697. doi: 10.18632/oncotarget.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lüttges J, et al. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466–471. doi: 10.1097/00000478-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Nakamori S, et al. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 66.Kashiwagi H, et al. DF3 expression in human gallbladder carcinoma: significance for lymphatic invasion. Int J Oncol. 2000;16:455–459. doi: 10.3892/ijo.16.3.455. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi T, et al. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion. 2001;63:87–92. doi: 10.1159/000051917. [DOI] [PubMed] [Google Scholar]
- 68.Rodgers SD, et al. Sialyl Lewisx-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys J. 2000;79:694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wesseling J, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitamoto S, et al. MUC1 enhances hypoxia-driven angiogenesis through the regulation of multiple proangiogenic factors. Oncogene. 2012;32:4614–4621. doi: 10.1038/onc.2012.478. [DOI] [PubMed] [Google Scholar]
- 71.Woo JK, et al. Mucin 1 enhances the tumor angiogenic response by activation of the AKT signaling pathway. Oncogene. 2012;31:2187–2198. doi: 10.1038/onc.2011.410. [DOI] [PubMed] [Google Scholar]
- 72.Aubert S, et al. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res. 2009;69:5707–5715. doi: 10.1158/0008-5472.CAN-08-4905. [DOI] [PubMed] [Google Scholar]
- 73.Yin L, et al. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278:35458–35464. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 74.Yin L, et al. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1α activation in a survival response to hypoxia. J Biol Chem. 2007;282:257–266. doi: 10.1074/jbc.M610156200. [DOI] [PubMed] [Google Scholar]
- 75.Persidis A. Cancer multidrug resistance. Nat Biotechnol. 1999;17:94–95. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 76.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 77.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 78.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 79.Sheng YH, et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2013;6:557–568. doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- 80.Choi S, et al. TNF-α is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011;44:255–260. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Dinwiddie D. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010;298:558–563. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu W, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2013;176:3890–3894. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 83.Ueno K, et al. MUC1 mucin is a negative regulator of Toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38:263–268. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kato K, et al. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J Immunol. 2012;188:2014–2022. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guang W, et al. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J Biol Chem. 2010;285:20547–20557. doi: 10.1074/jbc.M110.121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farouk S, et al. The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. A comparative study with carcinoembryonic antigen. Cancer. 1991;68:574–582. doi: 10.1002/1097-0142(19910801)68:3<574::aid-cncr2820680322>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 87.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 88.Winter JM, et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS ONE. 2012;7:e40157. doi: 10.1371/journal.pone.0040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gold D, et al. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int J Cancer. 1994;57:204–210. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- 90.Curry JM, et al. The use of a novel MUC1 antibody to identify cancer stem cells and circulating MUC1 in mice and patients with pancreatic cancer. J Surg Oncol. 2013;107:713–722. doi: 10.1002/jso.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheever MA, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovjazin R, et al. ImMucin: a novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine. 2011;29:4676–4686. doi: 10.1016/j.vaccine.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 93.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13:s4652–s4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 94.Butts C, et al. Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 95.Kimura T, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takashi K, Finn OJ. MUC1 immunotherapy is here to stay. Expert Opin Biol Ther. 2013;13:35–49. doi: 10.1517/14712598.2012.725719. [DOI] [PubMed] [Google Scholar]
- 97.Beatson R, et al. MUC1 immunotherapy. Immunotherapy. 2010;2:305–327. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 98.Lakshminarayanan V, et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukherjee P, Basu G. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182:216–224. [PMC free article] [PubMed] [Google Scholar]
- 100.Yin L, Kufe D. MUC1-C oncoprotein blocks terminal differentiation of chronic myelogenous leukemia cells by a ROS-mediated mechanism. Genes Cancer. 2011;2:56–64. doi: 10.1177/1947601911405044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akporiaye ET, et al. Characterization of the MUC1. Tg/MIN transgenic mouse as a model for studying antigen-specific immunotherapy of adenomas. Vaccine. 2007;25:6965–6974. doi: 10.1016/j.vaccine.2007.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroeder JA, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 103.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stroopinsky D, et al. MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Res. 2013;73:5569–5579. doi: 10.1158/0008-5472.CAN-13-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dean M, et al. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 106.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 107.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 108.Fruehauf JP, Meyskens FL. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 109.Pandey P, et al. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–4003. [PubMed] [Google Scholar]
- 110.Hikita ST, et al. MUC1* mediates the growth of human pluripotent stem cells. PLoS ONE. 2008;3:e3312. doi: 10.1371/journal.pone.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Y, et al. EGFR-ERK signaling pathway down-regulates miRNA-145 in lung cancer cells. Chin J Oncol. 2013;35:187–192. doi: 10.3760/cma.j.issn.0253-3766.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 112.Smorodinsky N, et al. Detection of a secreted MUC1/SEC protein by MUC1 isoform specific monoclonal antibodies. Biochem Biophys Res Commun. 1996;228:115–121. doi: 10.1006/bbrc.1996.1625. [DOI] [PubMed] [Google Scholar]
- 113.Hey NA, et al. Transmembrane and truncated (SEC) isoforms of MUC1 in the human endometrium and fallopian tube. Reprod Biol Endocrinol. 2003;1:2. doi: 10.1186/1477-7827-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jonckheere N, et al. Membrane-bound mucin modular domains: from structure to function. Biochimie. 2013;95:1077–1086. doi: 10.1016/j.biochi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 115.Mohr AM, et al. MUC1 regulates expression of multiple microRNAs involved in pancreatic tumor progression, including the miR-200c/141 cluster. PLoS ONE. 2013;8:e73306. doi: 10.1371/journal.pone.0073306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajabi H, et al. MUC1-C oncoprotein activates ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene. 2013 doi: 10.1038/onc.2013.114. http://dx.doi.org/10.1038/onc.2013.114. [DOI] [PMC free article] [PubMed]