Abstract
The human homologue of mouse double minute 2 (MDM2) is overexpressed in tumors and contributes to tumorigenesis through inhibition of p53 activity. We investigated the effect of the anti-estrogen fulvestrant on MDM2 expression and sensitivity of estrogen receptor positive human breast cancer cell lines to chemotherapeutics. Fulvestrant down-regulated MDM2 through increased protein turnover. Fulvestrant blocked estrogen-dependent up-regulation of MDM2 and decreased basal expression of MDM2 in the absence of estradiol. As combinations of fulvestrant with doxorubicin, etoposide or paclitaxel were synergistic, altering cell cycle distribution and increasing cell death, this provides rationale for testing combinatorial chemotherapy with fulvestrant as a novel therapeutic strategy for patients with advanced breast cancer.
Keywords: Fulvestrant, MDM2, Chemotherapy, Estrogen receptor, Breast cancer
1. Introduction
Fulvestrant, a newer type of estrogen receptor (ER) antagonist that lacks estrogen agonist effect and cross-resistance with other hormonal agents, is currently used in clinic to treat patients with ER positive (ER+), advanced and metastatic breast cancers. Fulvestrant downregulates intracellular ER levels and results in abrogation of estrogen-sensitive gene transcription. One of the genes whose expression is upregulated in response to estrogen is mouse double minute 2 (MDM2), first identified as an oncoprotein encoded by double minute chromosomes in murine sarcoma cells, and later found to be overexpressed in a variety of human cancers. Hdm2 represents the human ortholog (hereafter in this report, the human ortholog is denoted as MDM2). Transcriptional activation of MDM2 by estrogen is mediated via an estrogen response element in breast cancer cells homozygous or heterozygous for the single nucleotide polymorphism in the MDM2 promoter (SNP 309) [1].
MDM2 mainly acts as a negative regulator of p53 activity, thus prohibiting cells from entering into cell cycle arrest, senescence, or apoptosis. In addition, MDM2 also plays a regulatory role in cell-cycle progression independent of p53. For instance, MDM2 promotes the degradation of the phosphorylated retinoblastoma protein (pRB) [2] and p21 [3], thereby modulating their activities. MDM2 also interacts with the S-phase promoting factor E2F1 and increases its function [4]. Due to its ability to determine the fate of critical regulators of cell cycle, the activity and expression of MDM2 have been shown to affect the sensitivity of cancer cells to chemotherapeutic agents [5,6].
In the current study, we sought to determine whether the anti-estrogenic agent, fulvestrant, could suppress MDM2 expression and enhance the response of breast cancer cells to treatment with standard chemotherapeutic drugs. Our study shows that treatment of ER+ breast cancer cells with fulvestrant resulted in increased turnover and down-regulation of MDM2 protein, and sensitized tumor cells to chemotherapeutic drugs doxorubicin, etoposide, and paclitaxel. These results suggest that combined use of fulvestrant with these cytotoxic drugs may enhance effectiveness of chemotherapy in patients with ER+ breast cancers.
2. Materials and methods
2.1. Cell lines and culture
T47D and MCF7 breast cancer cell lines (ATCC, Manassas, VA) were maintained in RPMI 1640 (Invitrogen Life Technologies, Grand Island, NY) supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere containing 5% CO2/95% air. For estrogen and anti-estrogen treatments, cells were cultured in phenol red-free RPMI supplemented with charcoal-stripped 10% fetal bovine serum for 48 h prior to drug treatment.
2.2. Reagents and antibodies
Doxorubicin hydrochloride (DOX), etoposide (VP-16), paclitaxel (TAX), and fulvestrant (Fulv) (Sigma Aldrich, St. Louis, MO) were dissolved in DMSO. β-Estradiol–water soluble (Sigma Aldrich, St. Louis, MO) was dissolved in water. Primary antibodies used for Western blotting and immunoprecipitation were as follows: MDM2 (SMP14), ERα (HC-20), p53 (DO-1), (Santa Cruz Biotechnology, Dallas, TX); p21 (Ab-1), (EMD Millipore, Billerica, MA); monoclonal anti-β-actin clone AC-15 (Sigma Aldrich, St. Louis, MO); PARP, caspase-8 (1C12) (Cell Signaling, Danvers, MA). Proteins were visualized using enhanced chemiluminescence (ECL) detection (Pierce Biotechnology Inc., Rockford, IL). Senescence was detected using senescence β-Galactosidase Staining Kit (Cell Signaling, Danvers, MA).
2.3. Real-time RT–PCR
Cells plated in 6-well plates were treated with fulvestrant as indicated. Total RNA was extracted from treated cells with RNeasy Mini Kit (QIAGEN, Germantown, MD) and quantified by UV absorbance spectroscopy. Two-step quantitative RT–PCR was performed as follows: (1) cDNA synthesis was performed using TaqMan Reverse Transcription Kit (Life Technologies, Grand Island, NY). The thermal profile for cDNA synthesis was 25 °C for 10 min, 48 °C for 30 min, and 95 °C for 5 min. (2) Quantitative PCR was performed using TaqMan gene expression assays for MDM2 (Hs00242813_m1) and GAPDH (endogenous control) (Hs99999905_m1). Quantitative PCR amplifications were performed on the Applied Biosystems 7900 HT Fast Real Time PCR system. Reactions were carried out in 20 μl volume containing 10 μl of 2× TaqMan Universal PCR Master Mix (Life Technologies, Grand Island, NY). The thermal profile for real-time PCR was 50 °C for 2 min, then 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The threshold cycle (CT) values were determined.
2.4. Western blot analysis
Cell lysates were prepared using the CelLytic™ MT Cell Lysis Reagent (Sigma Aldrich, St. Louis, MO) with Protease Inhibitor Cocktail. Lysates were clarified by centrifugation at 12,000g for 30 min at 4 °C. Protein concentration was determined by the Bradford method using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). Lysates (10–50 μg proteins) were separated onto 8% SDS–PAGE gels followed by transfer to nitrocellulose membranes. Membranes were incubated in blocking solution consisting of 5% powered milk in PBST (PBS plus 0.1% Tween 20) at room temperature for 1 h, then immunoblotted with the indicated primary antibody overnight at 4 °C. Detection by enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol (ECL; Pierce Biotechnology Inc., Rockford, IL). Quantification of protein bands was performed using ImageJ software (http://rsb.info.nih.gov/ij).
2.5. Co-Immunoprecipitation
T47D cells were plated in 100-mm dishes. After the respective treatment (fulvestrant 1 μM for 16 h or vehicle), cells were washed twice with ice-cold PBS, scraped off the dishes and pelleted at 1500g for 5 min. Cell pellets were then lysed in NETN buffer [50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 0.1% IGEPAL CA-630 (Sigma Aldrich, St. Louis, MO), 1 mM EDTA, and Protease Inhibitor Cocktail (Sigma Aldrich, St. Louis, MO)] for 30 min at 4 °C in a rotating wheel. Lysates were clarified by centrifugation at 16,000g for 20 min at 4 °C. Protein concentration was determined by Bradford assay and equal protein amounts were pre-cleared with Protein A/G Plus-Agarose beads (Santa Cruz Biotechnology, Dallas, TX) for 1 h at 4 °C. Pre-cleared lysates were then incubated with MDM2 (SMP14) antibody or mouse IgG for 6 h at 4 °C. Protein A/G Plus-Agarose beads were added and incubated overnight at 4 °C. Beads were then washed five times with lysis buffer containing 0.5% of IGE-PAL and one time with PBS and boiled with 2× Laemmli sample buffer. Pre-cleared lysates prior to immunoprecipitation served as input controls. Extracted proteins were loaded onto a 4–15% SDS–PAGE gradient gel followed by transfer to PVDF membrane. Blots were assayed for the expression of MDM2, ERα, and β-actin (loading control).
2.6. Cycloheximide treatment
Cells were treated with vehicle or 1 μM of fulvestrant for 16 h, and then pulse-chased for MDM2 protein in the presence of 20 μg/ml of cycloheximide (CHX). Cell extracts from the treated cells collected at the indicated times were analyzed by Western blotting.
2.7. Drug sensitivity assay
Cells were plated in 96-well tissue culture plates, allowed to attach for 5–6 h, and then treated with different drug combinations for 66 h. Fifty microliters of 2.5 mg/ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma Aldrich, St. Louis, MO) in PBS were then added to each well, and cells were incubated at 37 °C for 4 h. Formazan crystals were dissolved in DMSO. Absorbance was determined at 570 nm using a Wallac 1420 Victor3 plate reader (Perkin Elmer, Waltham, MA). Viability was expressed as a percentage of control by dividing the absorbance of each treated sample by the average of the untreated controls. Combination index (CI) for drug interaction (e.g., synergy) was calculated using the CompuSyn software (ComboSyn, Inc.). CI values at different effect and dose levels and isobolograms were generated using this software.
2.8. Cell cycle analysis
Cells were plated in 6 well tissue culture plates and treated with different drug combinations for 72 h. After treatment, all cells were harvested, and cell number was determined using the Vi-CELL Cell Viability Analyzer (Beckman Coulter, Indianapolis, IN). A single cell suspension with equal cell number was prepared for each sample. Cells were fixed with absolute ethanol dropwise while vortexing and incubated overnight. Fixed cells were washed twice with 1X PBS and stained with staining solution (10 μg/ml propidium iodide and 100 μg/ml RNase A in PBS (Sigma Aldrich, St. Louis, MO). Cell cycle distribution was analyzed by flow cytometry (Cytomics FC 500 Series; Beckman Coulter, Indianapolis, IN). The data were analyzed using CXP software (Beckman Coulter, Indianapolis, IN).
2.9. Senescence assay
Cells were plated in 12 well tissue culture plates and treated with different drug combinations for 72 h. Cells were washed with 1X PBS, and fixed for 15 min per manufacturers instructions. Cells were then washed twice with 1X PBS. β-Galactosidase staining solution (pH 6.0) was added to the cells for overnight incubation. Stained and unstained cells from three representative fields were counted.
2.10. Statistical analysis
For estrogen and anti-estrogen treatments, Student’s t-test was used to calculate the P value of the difference between control and treated cells from three independent experiments where a P value of less than 0.05 was considered statistically significant. For drug combination effects on cell cycle, apoptosis protein markers, and senescence, Student’s t-test and one way ANOVA were used to calculate the P value between treatments from three independent experiments where a P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Treatment with fulvestrant down-regulates MDM2 protein in human breast cancer cells
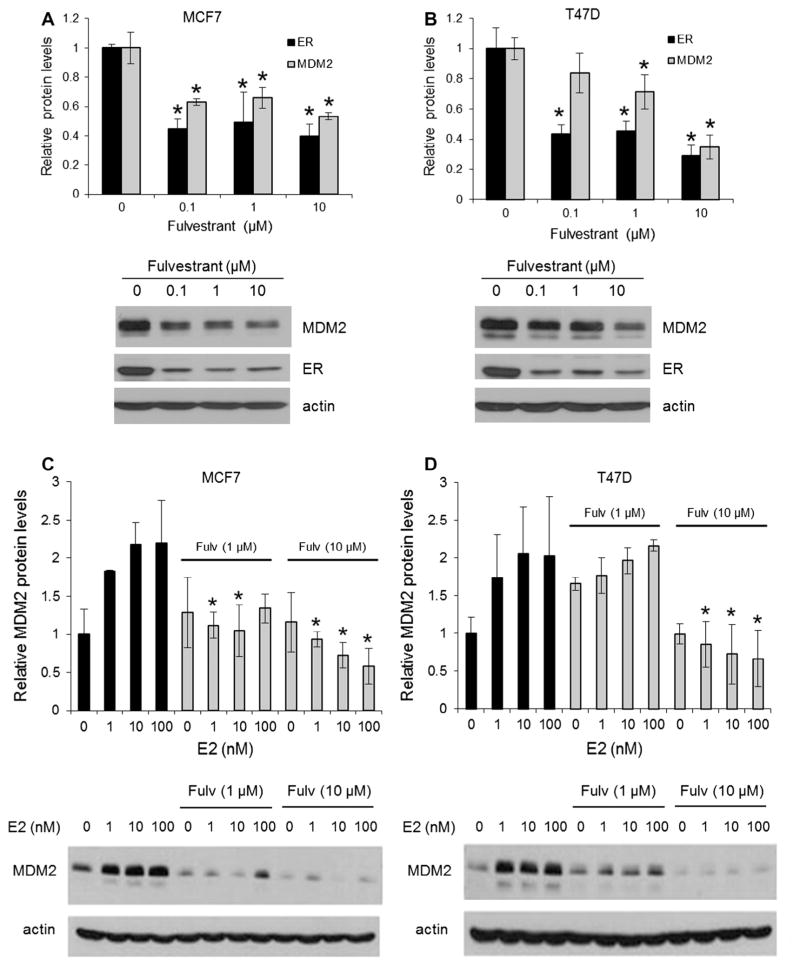
To test the effect of anti-estrogen on the expression of MDM2, the ER+ human breast cancer cell lines, MCF7 and T47D, were treated with different concentrations of fulvestrant and then MDM2 protein expression measured. Fig. 1A and B show that fulvestrant treatment caused a significant decrease in MDM2 protein expression in both cell lines and that the reduction of MDM2 correlated with the decrease in ER expression. Treatment of MCF7 and T47D cells with estradiol increased MDM2 expression. However, fulvestrant not only reduced basal MDM2 expression (in the absence of estradiol), but also blocked the up-regulation of MDM2 induced by estradiol (Fig. 1C and D).
Fig. 1.
Fulvestrant decreases MDM2 protein expression and abolishes the effect of estradiol on MDM2 expression. MCF7 (A) and T47D (B) cells were cultured in the presence of different concentrations of fulvestrant (Fulv) for 66 h. ER and MDM2 were detected by Western blot and normalized to β-actin. The decrease in protein expression (shown relative to vehicle control treatment) after fulvestrant treatment was calculated for each drug concentration. *P < 0.05 compared to vehicle control treatment. MCF7 (C) and T47D (D) cells were cultured in the presence of different concentrations of estradiol (E2) for 72 h, with or without fulvestrant. MDM2 protein level was measured by Western blot and normalized to β-actin. *P < 0.05 compared to corresponding E2 treatment without fulvestrant. Representative Western blots of MCF7 and T47D lysates from three independent experiments are shown below the corresponding graphs.
3.2. p53 Activity is not affected by fulvestrant
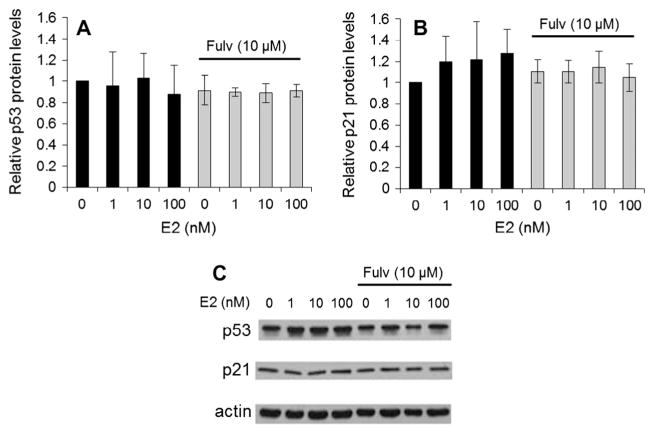
Because MDM2 is a p53-regulated gene and there are known interactions between ER and p53, the potential role of p53 in MDM2 down-regulation with fulvestrant was investigated. The ER+ human breast cancer cell lines, MCF7 and T47D, were treated with different concentrations of fulvestrant and p53 expression measured (Fig. 2). MDM2 depletion by fulvestrant did not correlate with an increase in p53, as might have been expected according to the regulatory role of MDM2 on p53. Instead a slight though not significant decrease in p53 was observed. In addition, activation of p53 was not affected by fulvestrant as measured by expression of p21, a gene that is tightly controlled by p53. Fulvestrant did not alter levels of p21.
Fig. 2.
MDM2 depletion by fulvestrant does not correlate with p53 expression or activation. MCF7 cells (wild-type for p53) were cultured in the presence of different concentrations of estradiol (E2) for 72 h, with or without fulvestrant (Fulv). The p53 (A) and p21 (B) protein levels were measured by Western blot and normalized to the levels of β-actin. No significant changes were observed. (C) Representative Western blots from three independent experiments are shown.
3.3. Fulvestrant treatment does not alter MDM2 mRNA level
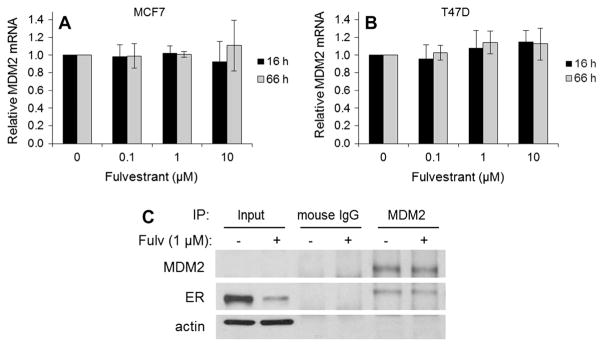
To determine whether the down-regulation of MDM2 caused by fulvestrant resulted from altered transcription of MDM2 gene, MDM2 mRNA in MCF7 and T47D cells treated with vehicle or fulvestrant was measured using quantitative PCR. This was performed at both 16 and 66 h for several concentrations of fulvestrant in both MCF7 and T47D cells. The shorter time period was chosen as fulvestrant treatment can affect multiple transcriptional systems. While MDM2 protein levels decrease with all doses of fulvestrant at 66 h (Fig. 1A and B), mRNA levels are unchanged or slightly increased for both cells lines (Fig. 3A and B). Similar patterns were noted at 16 h treatment with fulvestrant in both cell lines (Fig. 3A and B). These results suggest that fulvestrant does not suppress transcription of MDM2 gene.
Fig. 3.
Fulvestrant does not reduce MDM2 mRNA abundance or disrupt the ERα–MDM2 complex. MCF7 (A) and T47D (B) cells were cultured in the presence of different concentrations of fulvestrant (Fulv) for 66 h (gray bars) or 16 h (black bars). MDM2 mRNA levels were evaluated at two different fulvestrant treatment times. mRNA levels were determined by quantitative PCR (qPCR), and the quantification data were analyzed following the delta delta Ct method after normalization to GAPDH (endogenous control) levels. No significant changes were observed. (C) T47D cells were cultured with or without 1 μM of fulvestrant for 16 h after which immunoprecipitation (IP) for MDM2 was performed. IP for mouse IgG served as an isotype-matched negative control for non-specific interactions. Western blot was done to assess expression of MDM2 and ERα. Input lanes confirm the decrease in ERα expression as a result of fulvestrant treatment (decrease of ~80%). ERα is detected in MDM2 IP lanes with and without fulvestrant treatment. As expected, no protein is detectable in mouse IgG IP lanes.
3.4. Fulvestrant treatment does not disrupt the ERα–MDM2 complex
ERα is known to interact with other proteins. As fulvestrant results in decreased ERα as well as reduced MDM2 levels, coimmunoprecipitation was performed to identify ERα–MDM2 protein interactions. T47D cells were cultured with or without 1 μM of fulvestrant for 16 h after which immunoprecipitation was performed (Fig. 3C). Western blot of input protein (pre-cleared lysates) confirmed reduced expression of ERα as a function of fulvestrant treatment, i.e. there was an 80% decrease in ERα expression. As expected, MDM2 was present in both MDM2 immunoprecipitation lanes with and without fulvestrant treatment (Fig. 3C). MDM2 was also present in the input lanes when a longer exposure was done (data not shown). In both samples with and without drug treatment, ERα was immunoprecipitated with MDM2. This suggests that an ERα–MDM2 complex remains intact with fulvestrant treatment.
3.5. Fulvestrant increases the turnover rate of MDM2 protein
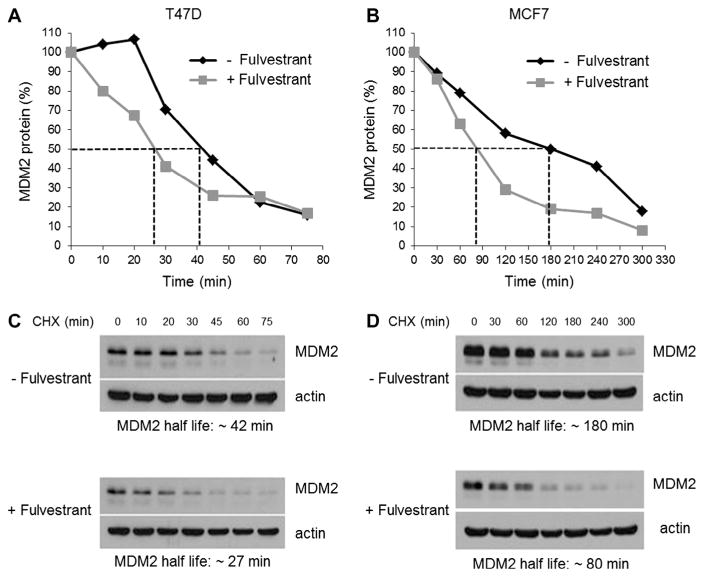
As fulvestrant seemed to directly affect the protein levels of MDM2 without down-regulating the mRNA levels of this gene, the effect of fulvestrant on MDM2 protein half-life was evaluated. MDM2 protein turnover rate was evaluated in T47D and MCF-7 cells treated with fulvestrant or vehicle, in order to determine the effect of this anti-estrogen on stability of MDM2 protein. The pulse-chase experiments demonstrated that fulvestrant facilitated degradation of MDM2 protein, as reflected in the shortened half-life of this protein in the presence of fulvestrant (27 min vs. 42 min in T47D cells; 80 min vs. 180 min in MCF7 cells) (Fig. 4). Thus, down-regulation of MDM2 expression by fulvestrant appeared to be attributable to enhanced MDM2 turnover that was unrelated to ERα–MDM2 protein interaction.
Fig. 4.
Fulvestrant reduces MDM2 protein half-life. T47D (A) and MCF7 (B) cells were cultured with or without 1 μM of fulvestrant for 16 h. After drug treatment, cells were exposed to cycloheximide (CHX) for different incubation times. MDM2 protein was measured by Western blot and normalized to β-actin. Representative Western blots of T47D (C) and MCF7 (D) lysates are shown.
3.6. Fulvestrant enhances the sensitivity of human breast cancer cells to chemotherapeutic drugs
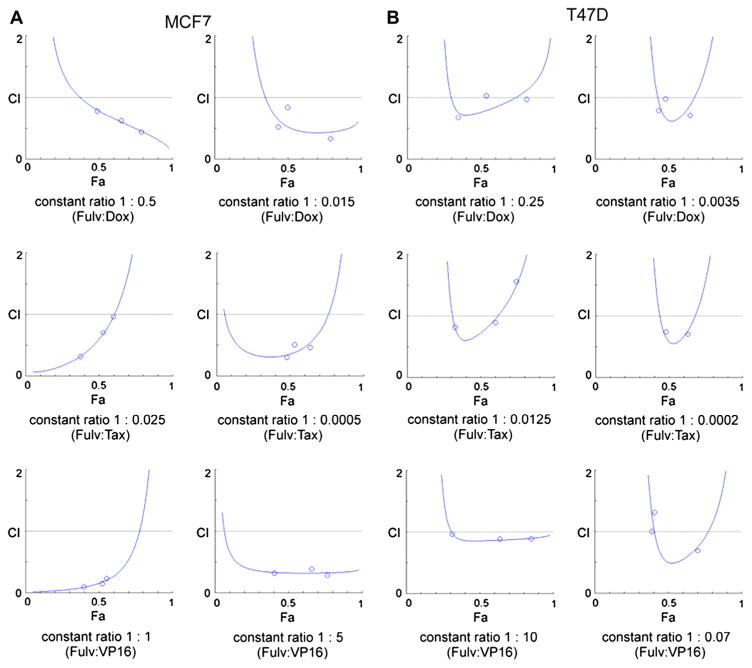
Inhibition of MDM2 has been reported to potentiate cytotoxic effects of chemotherapeutic drugs such as paclitaxel [5]. Therefore, using MCF7 and T47D breast cancer cell lines, it was evaluated whether down-regulation of MDM2 by fulvestrant could enhance the effectiveness of cytotoxic drugs that are commonly used for treatment of breast cancer. Dose-response studies of doxorubicin, paclitaxel or etoposide in combination with fulvestrant were performed, and the data from MTT assays were analyzed using the CompuSyn software. CompuSyn analyses showed that combined use of doxorubicin, paclitaxel or etoposide with fulvestrant resulted in different degrees of synergism in both of the breast cancer cell lines tested (Table 1, Fig. 5).
Table 1.
Combination of doxorubicin, paclitaxel or etoposide with fulvestrant results in different degrees of synergism.
| Cell line | Treatment | Constant ratio | CIa | Effectb |
|---|---|---|---|---|
| MCF7c | Fulvestrant:Doxorubicin | 1:0.5 | 0.45; 0.63; 0.79 | Syn*, Syn** |
| 1:0.015 | 0.34; 0.53; 0.84 | Syn*, Syn** | ||
| Fulvestrant:Paclitaxel | 1:0.025 | 0.33; 0.71; 0.97 | Syn**/Add | |
| 1:0.0005 | 0.31; 0.47; 0.51 | Syn**, Syn*** | ||
| Fulvestrant:Etoposide | 1:1 | 0.10; 0.15; 0.23 | Syn***, Syn**** | |
| 1:5 | 0.29; 0.32; 0.39 | Syn**, Syn*** | ||
| T47Dc | Fulvestrant:Doxorubicin | 1:0.25 | 0.68; 0.97; 1.04 | Syn**/Add |
| 1:0.0035 | 0.71; 0.79; 0.98 | Syn*/Add | ||
| Fulvestrant:Paclitaxel | 1:0.0125 | 0.82; 0.89; 1.56 | Syn*, Syn-/Ant** | |
| 1:0.0002 | 0.71; 0.74; 3.02 | Syn*/Ant** | ||
| Fulvestrant:Etoposide | 1:10 | 0.89; 0.89; 0.96 | Syn-/Add | |
| 1:0.07 | 0.69; 1.00; 1.32 | Syn**/Add/Ant* | ||
| MCF7d | Fulvestrant:Doxorubicin | 1:0.5 | 0.76; 0.76; 0.95; 1.02 | Syn/Add |
| Fulvestrant:Paclitaxel | 1:0.025 | 0.15; 0.20; 0.76; 1.46 | Syn/Ant | |
| Fulvestrant:Etoposide | 1:1 | 0.25; 0.36; 0.37; 1.22 | Syn/Ant | |
| T47Dd | Fulvestrant:Doxorubicin | 1:0.5 | 0.68; 0.96; 1.17; 1.37 | Syn/Add/Ant |
| Fulvestrant:Paclitaxel | 1:0.025 | 0.80; 1.49; 1.58; 2.57 | Syn/Ant | |
| Fulvestrant:Etoposide | 1:1 | 1.33; 1.50; 7.57; 64.76 | Ant |
CI: combination index.
CI < 1 Synergism: Syn (slight: Syn-, moderate: Syn*, synergism: Syn**, strong: Syn***, very strong: Syn****). CI = 1 Additive effect: Add. CI > 1 Antagonism: Ant (slight: Ant-, moderate: Ant*, antagonism: Ant**, strong: Ant***, very strong: Ant****).
Analysis of combination effect at optimal doses for each cell line.
Comparison of MCF7 and T47D response at the same constant ratios and doses.
Fig. 5.
Isobolograms for combination treatment with fulvestrant and chemotherapy. Three different drug combinations at constant ratios were assessed for each cell line: MCF7 (A), T47D (B). MTT assays were carried out and the results of the drug combination analysis using CompuSyn software are shown. Isobolograms from the three different drug combinations for doxorubicin (Dox), paclitaxel (Tax), and etoposide (VP16) at two different constant ratios were assessed for each cell line and were used to calculate the CI values listed in Table 1. CI: combination index. CI 〈1: synergism (Syn); CI = 1: additive effect (Add); CI〉 1: antagonism (Ant).
3.7. Combination of fulvestrant and chemotherapeutic drugs induces altered cell cycle distribution, apoptosis, and senescence
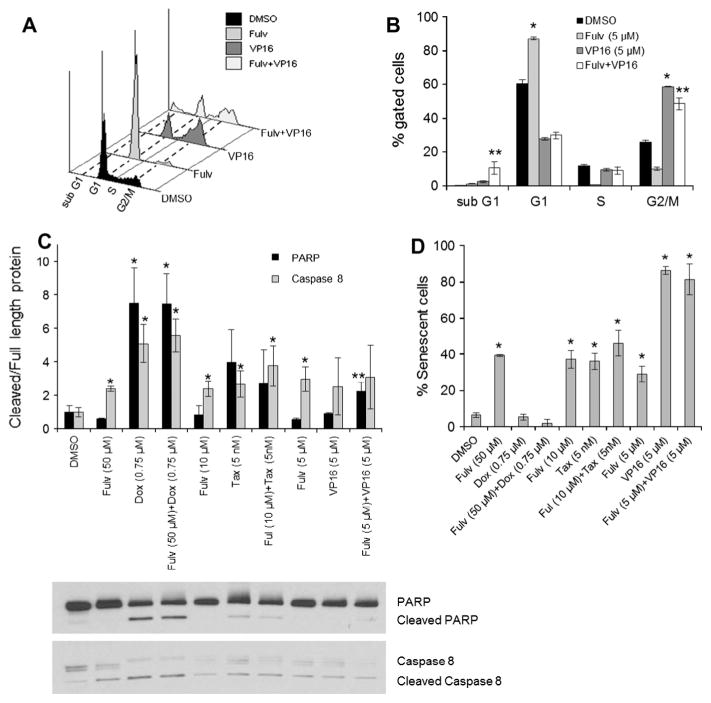
Since the combination of fulvestrant with cytotoxic drugs was synergistic, the mechanism of cell death was evaluated. MCF7 and T47D cells were treated for 72 h with each drug or combined at synergistic concentrations to assess cell cycle distribution, apoptosis, and senescence. A representative cell cycle histogram for MCF7 treated with fulvestrant, etoposide, or the combination demonstrates treatment-related alterations in cell cycle distribution (Fig. 6A). Quantitative analysis of cell cycle alterations is shown in Fig. 6B. A collective summary of cell cycle distribution for all treatments is depicted in Table 2. Cell cycle analysis revealed that fulvestrant increased the G1 population in both cell lines consistent with induction of G1 arrest. In MCF7 cells, doxorubicin or etoposide alone induced G2/M arrest. While the combination of fulvestrant and doxorubicin had a similar effect to doxorubicin alone, the combination of fulvestrant and etoposide significantly increased the sub G1 population of cells compared to either agent alone (Table 2, Fig. 6A and B). This suggests that the combination of fulvestrant and etoposide more effectively induced cell cycle arrest and apoptosis. Paclitaxel treatment of MCF7 and T47D cells increased the sub G1 population. However, the combination of fulvestrant and paclitaxel increased both G2/M and sub G1 cell populations (Table 2).
Fig. 6.
Combination of fulvestrant and cytotoxic drugs induces altered cell cycle distribution, apoptosis, and senescence. MCF7 and T47D cells were treated for 72 h with fulvestrant (Fulv), doxorubicin (Dox), paclitaxel (Tax), and etoposide (VP16) either alone or in combination. A representative histogram (A) and bar graph depiction (B) of MCF7 cell cycle distribution are shown for fulvestrant, etoposide, or the combination of fulvestrant and etoposide. Quantitation of cell cycle distribution for all treatments is shown in Table 2. (C) Levels of cleaved PARP and cleaved caspase 8 in MCF7 cells were analyzed by Western blot and normalized to full length PARP and caspase 8, respectively. Representative Western blots of MCF7 lysates from three independent experiments are shown below the corresponding graph. (D) Senescence in MCF7 treated cells was evaluated based on β-galactosidase staining. *P < 0.05 compared to vehicle control treatment. **P < 0.05 for combination treatments compared to each agent alone or to control.
Table 2.
Cell cycle distribution of MCF7 and T47D cells treated with cytotoxic drugs and fulvestrant alone or in combination.
| Cell line | Treatment | % Sub G1 (s.d.)a | % G1 (s.d.) | % S (s.d.) | % G2/M (s.d.) |
|---|---|---|---|---|---|
| MCF7 | DMSO | 0.1 (0.1) | 60.4 (2.4) | 11.5 (0.9) | 26.0 (1.0) |
| Fulvb (50 μM) | 0.5 (0.1) | 88.5 (0.2) | 1.0 (0.3) | 9.1 (0.2) | |
| Doxb (0.75 μM) | 3.7 (0.5) | 37.2 (0.61) | 7.6 (0.4) | 50.4 (0.1) | |
| Fulv (50 μM) + Dox (0.75 μM) | 2.4 (0.1) | 40.4 (1.0) | 12.1 (1.4) | 44.2 (1.3) | |
| Fulv (10 μM) | 1.1 (0.4) | 88.3 (0.9) | 0.9 (0.2) | 8.8 (0.3) | |
| Taxb (5 nM) | 39.5 (1.0) | 21.9 (0.4) | 15.0 (0.4) | 18.5 (2.0) | |
| Ful (10 μM) + Tax (5 nM) | 26.8 (4.0) | 18.3 (1.2) | 15.0 (1.3) | 37.2 (4.3) | |
| Fulv (5 μM) | 1.1 (0.2) | 87.3 (1.0) | 0.7 (0.1) | 9.8 (0.9) | |
| VP16b (5 μM) | 2.4 (0.5) | 27.7 (0.6) | 9.4 (0.8) | 58.8 (0.1) | |
| Fulv (5 μM) + VP16 (5 μM) | 10.5 (3.9) | 29.9 (2.0) | 8.9 (2.1) | 48.6 (3.7) | |
| T47D | DMSO | 0.7 (0.3) | 66.7 (0.9) | 8.4 (0.7) | 23.0 (0.6) |
| Fulv (50 μM) | 0.9 (0.1) | 82.4 (1.4) | 2.2 (0.5) | 13.7 (0.9) | |
| Dox (0.175 μM) | 6.1 (0.6) | 3.8 (0.4) | 47.9 (4.2) | 37.4 (3.5) | |
| Fulv (50 μM) + Dox (0.175 μM) | 1.7 (0.6) | 37.9 (0.4) | 32.4 (2.5) | 26.1 (2.0) | |
| Tax (10 nM) | 42.7 (5.3) | 16.9 (1.3) | 13.2 (1.7) | 23.7 (4.2) | |
| Ful (50 μM) + Tax (10 nM) | 15.8 (0.8) | 30.0 (1.9) | 8.5 (1.0) | 43.0 (1.8) | |
| VP16 (3.5 μM) | 11.3 (1.4) | 6.6 (0.5) | 54.9 (0.7) | 22.3 (1.4) | |
| Fulv (50 μM) + VP16 (3.5 μM) | 3.1 (0.9) | 39.2 (1.0) | 33.7 (1.8) | 21.7 (1.3) |
s.d.: standard deviation.
Fulv: fulvestrant; Dox: doxorubicin; Tax: paclitaxel; VP16: etoposide.
As measures of apoptosis, PARP and caspase 8 cleavage were measured in MCF7 protein lysates by Western blotting. Doxorubicin alone or in combination with fulvestrant significantly increased PARP cleavage (Fig. 6C). While fulvestrant or etoposide alone had no effect on PARP cleavage, the combination induced significant cleavage as compared to control or to either agent alone (Fig. 6C). Consistent with the increased sub G1 and G2/M populations, this suggests that the fulvestrant and etoposide combination synergistically induces cell cycle arrest and apoptosis. As MCF7 cells are caspase 3 deficient, caspase 8 cleavage was alternatively assessed. Caspase 8 cleavage significantly increased with fulvestrant, doxorubicin, and paclitaxel without further enhanced effect from the combination (Fig. 6C). Etoposide and the combination with fulvestrant showed a non-significant trend to increased cleaved caspase 8.
Senescence, detected by β-galactosidase staining after 72 h drug treatment, significantly increased with fulvestrant, paclitaxel, etoposide, and their combination with fulvestrant as compared to control (Fig. 6D). Combination treatment was not significantly different from either agent alone.
4. Discussion
It was hypothesized that fulvestrant mediates its anti-tumor effect through reduced estrogen-regulated MDM2 transcription. This study demonstrates that fulvestrant reduces MDM2 expression through decreased MDM2 protein half-life and this is not related to protein destabilization from altered ERα–MDM2 protein interactions.
Resistance to endocrine therapy and chemotherapy has been a critical issue in the treatment of ER+ breast cancers. Clinical and preclinical studies evaluating the combination of fulvestrant with other agents to elicit improved therapeutic response have shown conflicting results for drug interaction studies [7–15], though fulvestrant appears to act as a radiosensitizer [16]. It was hypothesized that blocking expression of MDM2 with anti-estrogen would restore sensitivity to chemotherapeutic drugs in ER+ breast cancer cell lines. These data demonstrate that fulvestrant exerts synergistic effects in combination with several chemotherapeutic drugs, consistent with a recent report showing in vivo synergism between fulvestrant and doxorubicin or docetaxel [17]. In support of these synergistic effects are the enhanced cell death mechanisms of apoptosis and sensecence, as well as altered cell cycle distribution that were observed. Interestingly, though low dose paclitaxel alone produces prolonged mitotic arrest followed by tripolar mitosis [18] as evidenced by the appearance of a wide sub G1 peak, addition of fulvestrant accelerates the movement of the cells into G2/M arrest with a population of cells in sub G1.
The therapeutic mechanism of fulvestrant is thought to be due to classic reduction in ERα and its resultant reduction in estrogen-regulated gene expression. Numerous studies have identified associations between MDM2 and ERα expression in breast tissue and breast cancer cell lines as the first intron of MDM2 contains an estrogen response element [19–25]. As many estrogen regulated genes are pro-survival, reduced gene expression could contribute to the effectiveness of fulvestrant. However, alternative mechanisms of regulation of ER-associated genes have clinical relevance including the novel role for MDM2 in regulating cell adhesion and cell motility through endosomal targeting of proteins [26]. The mechanism supports observations correlating MDM2 expression with breast cancer stage and outcomes [27–30].
This study describes a novel effect of fulvestrant on altered protein stability. In contrast to the lack of ERα-mediated effect on MDM2 protein half-life, MDM2 has been demonstrated to regulate ERα turnover through their direct interaction, and MDM2 ubiquitin-ligase activity with targeted ERα degradation and transactivation [31,32]. This occurs through direct interaction with ERα and p53 in a ternary complex both in the absence or presence of estrogens. MDM2 exerts its effects both dependent on and independent of p53. In this study, fulvestrant-induced reduction in both ERα and MDM2 is independent of p53 expression and is consistent with that of Brekman et al. [24] showing that estrogen-induced breast cancer cell proliferation required a p53-independent role of MDM2.
In summary, this study demonstrates that fulvestrant possesses a suppressive effect on MDM2 expression and that this may contribute mechanistically to the observed synergistic effect with chemotherapeutic drugs and fulvestrant in ER+ human breast cancer cells. Cytotoxic drug-fulvestrant combinations demonstrating additive or synergistic interactions should be further evaluated in in vivo models for breast cancer to determine their effectiveness. These results provide a rationale and support for testing the combination of fulvestrant with chemotherapy as a novel therapeutic strategy for patients with advanced breast cancers.
Acknowledgments
We thank J. Harris at Rutgers Cancer Institute of New Jersey. This research was supported by the Department of Defense [Breast Cancer Research Program under award number (W81XH-07-1-0403)]. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
5. Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Bond GL, Bond EE, Robins H, Yip L, Hwang S, Strong LC, Lozano G, Levine AJ. A polymorphism in the human MDM2 promoter associates with attenuation of the p53 pathway and increased incidence of cancer in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Uchida C, Miwa S, Kitagawa K, Hattori T, Isobe T, Otani S, Oda T, Sugimura H, Kamijo T, Ookawa K, Yasuda H, Kitagawa M. Enhanced MDM2 activity inhibits pRB function via ubiquitin-dependent degradation. EMBO J. 2005;24:160–169. doi: 10.1038/sj.emboj.7600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279:16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 4.Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Oliver P, Zhang Z, Agrawal S, Zhang R. Chemosensitization and radiosensitization of human cancer by antisense anti-MDM2 oligonucleotides: in vitro and in vivo activities and mechanisms. Ann NY Acad Sci. 2003;1002:217–235. doi: 10.1196/annals.1281.025. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci USA. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson RW, O’Neill A, Vidaurre T, Gomez HL, Badve SS, Sledge GW. A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2012;133(3):1049–1056. doi: 10.1007/s10549-012-1997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Diaz MR, Yee D. Fulvestrant regulates epidermal growth factor (EGF) family ligands to activate EGF receptor (EGFR) signaling in breast cancer cells. Breast Cancer Res Treat. 2013;139(2):351–360. doi: 10.1007/s10549-013-2541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thrane S, Lykkesfeldt AE, Larsen MS, Sorensen BS, Yde CW. Estrogen receptor α is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signaling. Breast Cancer Res Treat. 2013;139(1):71–80. doi: 10.1007/s10549-013-2485-2. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JF, Dixon JM, Sibbering DM, Jahan A, Ellis IO, Channon E, Hyman-Taylor P, Nicholson RI, Gee JM. A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res. 2013;15(2):R18. doi: 10.1186/bcr3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, Lew DL, Hayes DF, Gralow JR, Livingston RB, Hortobagyi GN. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367(5):435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyams DM, Chan A, de Oliveira C, Snyder R, Vinholes J, Audeh MW, Alencar VM, Lombard J, Mookerjee B, Xu J, Brown K, Klein P. Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a randomized phase II study. Invest New Drugs. 2013;31(5):1345–1354. doi: 10.1007/s10637-013-9991-2. [DOI] [PubMed] [Google Scholar]
- 13.Tan WW, Dueck AC, Flynn P, Steen P, Anderson D, Rowland K, Northfelt D, Perez EA. N0539 phase II trial of fulvestrant and bevacizumab in patients with metastatic breast cancer previously treated with an aromatase inhibitor: a North Central Cancer Treatment Group (now Alliance) trial. Ann Oncol. 2013;24(10):2548–2554. doi: 10.1093/annonc/mdt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson JF, Ferrero JM, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I, Loh E, Gansert JL, Kaufman PA. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14(3):228–235. doi: 10.1016/S1470-2045(13)70026-3. [DOI] [PubMed] [Google Scholar]
- 15.De Vincenzo R, Scambia G, Benedetti Panici P, Fattorossi A, Bonanno G, Ferlini C, Isola G, Pernisco S, Mancuso S. Modulatory effect of tamoxifen and ICI 182,780 on adriamycin resistance in MCF-7 human breast-cancer cells. Int J Cancer. 1996;68(3):340–348. doi: 10.1002/(SICI)1097-0215(19961104)68:3<340::AID-IJC12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Yang Q, Haffty BG, Li X, Moran MS. Fulvestrant radiosensitizes human estrogen receptor-positive breast cancer cells. Biochem Biophys Res Commun. 2013;431(2):146–151. doi: 10.1016/j.bbrc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda H, Taira N, Nogami T, Shien K, Okada M, Shien T, Doihara H, Miyoshi S. Combination treatment with fulvestrant and various cytotoxic agents (doxorubicin, paclitaxel, docetaxel, vinorelbine, and 5-fluorouracil) has a synergistic effect in estrogen receptor-positive breast cancer. Cancer Sci. 2011;102(11):2038–2042. doi: 10.1111/j.1349-7006.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 18.Demidenko ZN, Kalurupalle S, Hanko C, Lim CU, Broude E, Blagosklonny MV. Mechanism of G1-like arrest by low concentrations of paclitaxel: next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene. 2008;27(32):4402–4410. doi: 10.1038/onc.2008.82. [DOI] [PubMed] [Google Scholar]
- 19.Hori M, Shimazaki J, Inagawa S, Itabashi M. Overexpression of MDM2 oncoprotein correlates with possession of estrogen receptor alpha and lack of MDM2 mRNA splice variants in human breast cancer. Breast Cancer Res Treat. 2002;71(1):77–83. doi: 10.1023/a:1013350419426. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh MS, Shao ZM, Hussain A, Fontana JA. The p53-binding protein MDM2 gene is differentially expressed in human breast carcinoma. Cancer Res. 1993;53(14):3226–3228. [PubMed] [Google Scholar]
- 21.Marchetti A, Buttitta F, Girlando S, Dalla Palma P, Pellegrini S, Fina P, Doglioni C, Bevilacqua G, Barbareschi M. MDM2 gene alterations and MDM2 protein expression in breast carcinomas. J Pathol. 1995;175(1):31–38. doi: 10.1002/path.1711750106. [DOI] [PubMed] [Google Scholar]
- 22.Hu W, Feng Z, Ma L, Wagner J, Rice JJ, Stolovitzky G, Levine AJ. A single nucleotide polymorphism in the MDM2 gene disrupts the oscillation of p53 and MDM2 levels in cells. Cancer Res. 2007;67(6):2757–2765. doi: 10.1158/0008-5472.CAN-06-2656. [DOI] [PubMed] [Google Scholar]
- 23.Okumura N, Saji S, Eguchi H, Nakashima S, Saji S, Hayashi SI. Distinct promoter usage of MDM2 gene in human breast cancer. Oncol Repub. 2002;9(3):557–563. [PubMed] [Google Scholar]
- 24.Brekman A, Singh KE, Polotskaia A, Kundu N, Bargonetti J. A p53-independent role of MDM2 in estrogen-mediated activation of breast cancer cell proliferation. Breast Cancer Res. 2011;13(1):R3. doi: 10.1186/bcr2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps M, Darley M, Primrose JM, Blaydes JP. P53-independent activation of the Hdm2-P2 promoter through multiple transcription factor response elements results in elevated Hdm2 expression in estrogen receptor alpha-positive breast cancer cells. Cancer Res. 2003;63(10):2616–2623. [PubMed] [Google Scholar]
- 26.Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, Broglio K, Berry DA, Hung MC. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26(19):7269–7282. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann AH, Kirley A, Carney DN, Corbally N, Magee HM, Keating G, Dervan PA. Amplification of the MDM2 gene in human breast cancer and its association with MDM2 and p53 protein status. Br J Cancer. 1995;71(5):981–985. doi: 10.1038/bjc.1995.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turbin DA, Cheang MC, Bajdik CD, Gelmon KA, Yorida E, De Luca A, Nielsen TO, Huntsman DG, Gilks CB. MDM2 protein expression is a negative prognostic marker in breast carcinoma. Mod Pathol. 2006;19(1):69–74. doi: 10.1038/modpathol.3800484. [DOI] [PubMed] [Google Scholar]
- 29.Bueso-Ramos CE, Manshouri T, Haidar MA, Yang Y, McCown P, Ordonez N, Glassman A, Sneige N, Albitar M. Abnormal expression of MDM-2 in breast carcinomas. Breast Cancer Res Treat. 1996;37(2):179–188. doi: 10.1007/BF01806499. [DOI] [PubMed] [Google Scholar]
- 30.Bankfalvi A, Tory K, Kemper M, Breukelmann D, Cubick C, Poremba C, Fuzesi L, Lelle RJ, Bocker W. Clinical relevance of immunohistochemical expression of p53-targeted gene products mdm-2, p21 and bcl-2 in breast carcinoma. Pathol Res Pract. 2000;196(7):489–501. doi: 10.1016/S0344-0338(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 31.Duong V, Boulle N, Daujat S, Chauvet J, Bonnet S, Neel H, Cavaille V. Differential regulation of estrogen receptor alpha turnover and transactivation by MDM2 and stress-inducing agents. Cancer Res. 2007;67:5513–5521. doi: 10.1158/0008-5472.CAN-07-0967. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Burghardt R, Barhoumi R, Lee S, Liu X, Safe S. MDM2 regulates estrogen receptor α and estrogen responsiveness in breast cancer cells. Mol Endocrinol. 2011;46(2):67–79. doi: 10.1677/JME-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]