Summary
Five million individuals with chronic heart failure (CHF) in the United States have poor clinical outcomes including high death rates. Observational studies have indicated a reverse epidemiology of traditional cardiovascular risk factors in CHF; in contrast to trends seen in the general population, obesity and hypercholesterolemia are associated with improved survival. The temporal discordance between the overnutrition (long-term killer) and undernutrition (short-term killer) not only can explain some of the observed paradoxes but also may indicate a role for malnutrition, inflammation and oxidative stress that result in cachexia contributing to poor survival in CHF. Diminished appetite or anorexia may be both a cause and a consequence of this so-called malnutrition-inflammation-cachexia (MIC) or wasting syndrome in CHF. Neurohumoral activation, insulin resistance, cytokine activation and survival selection resultant genetic polymorphisms may also contribute to the prominent inflammatory and oxidative characteristics of this population. In CHF patients with wasting, nutritional strategies may be a promising therapeutic approach in CHF, especially if the provision of additional protein and energy also includes nutrients with anti-inflammatory and anti-oxidant properties. Regardless of the etiology of anorexia, appetite stimulating agents especially with anti-inflammatory properties such as megesterol acetate or pentoxyphylline may be appropriate adjuncts to dietary supplementation. Understanding the factors that modulate the MIC and wasting and their associations with clinical outcomes in CHF may lead to the development of nutritional strategies that alter the pathophysiology of CHF and improve outcomes
Keywords: Malnutrition-inflammation-cachexia (MIC), chronic heart failure, protein-energy malnutrition, wasting, reverse epidemiology
CHF Patients and Poor Outcome
In the United States, there are currently approximatelty five million individuals with chronic heart failure (CHF).1,2 CHF is the only major cardiovascular (CV) disease with rising incidence and prevalence in past decades. The life-time attributable risk for CHF for men and women is approximately 1 in 5. Increasing age of the population, greater awareness, and improved diagnostic techniques for the detection of CHF may have contributed to the steady rise in incidence.3–5 Another reason for the rising incidence of CHF is improved treatment and survival of patients with ischemic heart disease, the most common etiology of CHF.3–6 CHF is characterized by a high rate of hospital readmission and death, significant functional compromise, reduced health related quality of life (QoL), and increased caregiver burden.7–9. Data from the National Health and Nutrition Examination Survey (NHANES) indicated that 72% of men and 60% of women aged 65 to 74 years died within 10 yrs of their self-reported onset of CHF.7,10 Increased severity of CHF, as measured by the New York Heart Association (NYHA), is associated with higher mortality risk.11,12 The increased CHF prevalence represents an enormous burden to our health care system when coupled with extended and frequent hospital stays and poor HRQOL and survival.1–5
Improving Clinical Outcomes in CHF Patients
Because most CHF patients die prematurely and because their death is attributed to CV disease states, efforts to improve traditional CV risk factors might appear promising. Indeed, the known CV risk factors, i.e. hypercholesterolemia, hypertension and obesity, are associated with independently increased risks for developing CHF and mortality in the general population.13,14 Hence, it would be reasonable to infer that once CHF develops, these CV risk factors would continue to be associated with increased mortality. However, there has been increasing data indicating that in CHF patients, conventional CV risk factors are not positively associated with mortality risk (see below).
Therapy with angiotension converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), and ß-blockers are well studied and widely available treatment modalities that ameliorate symptoms, reduces morbidity, and improve survival in CHF patients.6,15 Aldosterone antagonists improve survival in patients with moderately-severe to severe symptoms as well.15,16 However even among CHF patients who receive evidence based medical therapy CHF mortality has remained unacceptably high. Hence, additional and/or novel therapeutic alternatives that are safe and practical should be sought and examined to ascertain whether they can improve poor CHF survival.
The Concept of Reverse Epidemiology
Many reports indicate that there is a high prevalence of body wasting and cachexia in CHF.17–21 CHF patients with cachexia have an extremely high death rate, i.e., up to 50% over 18 months,22 a mortality worse than many cancers. In industrialized and affluent countries, undernutrition and cachexia are uncommon cause of poor outcome in the general population, whereas “over-nutrition” is associated with a greater risk of CV and other diseases and leads to shortened survival.23 In contrast, in CHF patients “undernutrition” and wasting are common and strong risk factors for death.21 Consistent with this notion, most epidemiologic studies have shown survival advantages of obesity in CHF patients (Figure 1).24 The terms “reverse epidemiology” or “obesity paradox” underscore such paradoxical observations.25–27 These terms indicate that certain markers which predict a low likelihood of CV events and indeed an improved survival in the general population, such as low body mass index (BMI), low blood pressure, and low serum cholesterol, become paradoxically strong risk factors for increased morbidity and mortality in CHF patients. Moreover, some indicators of over-nutrition such, as obesity and hypercholesterolemia, actually predict improved outcome in CHF patients.25,28 In fact, observational studies of CHF patients have shown that weight gain is associated with improved survival while weight loss is associated with markedly increased mortality risk (unpublished data of S.D. Anker).
Figure 1.

Obesity paradox in CHF patients: Association of body mass index (BMI) as a continuous variable and unadjusted all-cause mortality using polynomial logistic regression. Data from 7767 patients with stable CHF enrolled in the Digitalis Investigation Group trial (adapted with permission from Curtis et al. Arch Intern Med 2005;165:55–6124)
The reverse epidemiology phenomenon is not unique to CHF population. Patients with renal failure undergoing maintenance hemodialysis (MHD)25, geriatric populations,29,30 patients with rheumatoid arthritis,31 AIDS,32 chronic pulmonary disease,33 or malignancy,34 and possibly several other vulnerable populations with chronic disease states may have similar risk factor reversals.25,35,36 Hence, the key to improved survival in CHF patients and other similar populations may lie in interventions that modulate conditions related to the reverse epidemiology phenomenon. And although counterintuitive, the obesity paradox is supported by a growing body of medical literature.
Anorexia and Malnutrition in CHF
Diminished appetite and inadequate food intake, together known as anorexia, may be engendered in CHF as a consequence of clinical symptoms such as fatigue and dyspnea, or via intestinal edema causing nausea, diminished absorption, and protein-losing enteropathy.37–43 Anorexia might also be an iatrogenic consequence of drug therapy, e.g., digoxin.37 Decreased food intake may also be a result of imposed dietary restrictions.44 CHF patients with cachexia appear to suffer from gastrointestinal fat malabsorption but not from gastro-intestinal protein loss.45 Another important aspect is a significantly higher resting metabolic rate in CHF patients,46–48 which increases with the CHF severity48,49. Nutritional intake that may have been adequate prior to developing CHF may be inadequate after CHF is established. Finally, the robust, systemic inflammatory activation characteristic of CHF may lead to anorexia, similar to what has recently shown in dialysis patients.50–55
The term malnutrition, even though may be used for both inadequate and excessive nutrition, generally indicate conditions related to under-nutrition. Malnutrition may be related to macronutrients, i.e., the protein and energy malnutrition, or micronutrients such as vitamins and minerals including trace elements. The protein-energy malnutrition can be defined as the state of decreased body pools of protein with or without fat depletion or a state of diminished functional capacity, which is caused at least partly by inadequate nutrient intake relative to nutrient demand and/or which can potentially be improved by nutritional repletion.56 Hence, the protein-energy malnutrition is engendered when the body’s need for protein or energy fuels or both cannot be satisfied by the current dietary intake. Although various studies using different criteria have been used to establish the presence of protein-energy malnutrition in the CHF population, its exact prevalence is not known, but it is estimated between 20% and 70% depending on assessment criteria.
Per definition the protein-energy malnutrition should not involve micronutrients; however, many protein-energy malnourished CHF patients may also have a relative deficiency in vitamins and minerals. Moreover, it is important to appreciate that, even though cachexia traditionally relates to weight loss, there is no clear-cut separation between malnutrition and cachexia in CHF patients, especially since inflammation, oxidative stress or other conditions may lead to both in CHF patients (see below). Furthermore, even among CHF patients who fall into an overweight or obese category by body mass index, evidence of malnutrition, such as hypoalbuminemia, may still be present.57–59 Hence, a cross-sectional body size measure may be misleading.
Chronic Inflammation
An important and inadequately appreciated feature of malnutrition and wasting in CHF is the presence of chronic inflammation.60–63 Tumor necrosis factor alpha (TNF-α) is significantly increased in CHF associated with cachexia64 as in the cases of cachectic patients with a variety of cancers, infections, renal failure or vascular diseases.52,65,66 The effect of TNF-α on wasting might be mediated by effects on endothelium or on the control of apoptosis.67 The chronic inflammatory state may result from bacterial or endotoxin translocation enhanced by bowel-wall edema.68–71. Inflammation may be responsible for the wasting syndrome and hypoalbuminemia in CHF.72–75 It has also been postulated that lower body weight is associated with a heightened catabolic state, which is associated with higher levels of TNF-α and other cytokines, as well as increased cortisol/dehydroepiandrosterone balance.76,77 Hence, nutritional interventions with anti-inflammatory and anti-oxidant properties may be more effective in CHF patients than the mere provision of protein and energy.
Cardiac Cachexia and Malnutrition-Inflammation Complex
According to the literature, 20% to 30% of CHF patients have hypoalbuminemia <3.5 g/dL and up to 70% have muscle atrophy.57–59 We recently showed that hypoalbuminemia is associated with a 2-fold increase in death risk in a group of CHF outpatients.59 Although “cardiac cachexia” (CC) traditionally implies the presence of extreme wasting in the setting of CHF, more inclusive definitions of CC have recently been evolving, according to which any significant weight loss or signs of protein-energy malnutrition and inflammation such as hypoalbuminemia are included. Anker et al defined CC as 6% loss in edema-free weight over at least 6 months20. Some investigators have proposed other terms such as malnutrition-inflammation-cachexia (MIC) or wasting syndrome21 which has also been used for a chronic renal failure associated cachexia, also known as Kidney Disease Wasting.56 Another analogous condition is the “cancer anorexia-cachexia syndrome” (CACS), which is described for malnutrition and wasting in malignancies.78,79 No matter what nomenclature is used, malnutrition and inflammation are both strong predictors of increased mortality in CHF patients and other similar populations ; furthermore, it is likely that similar pathophysiologic mechanisms of wasting are at play in all these chronic disease states (Figure 2).21,22,56
Figure 2.
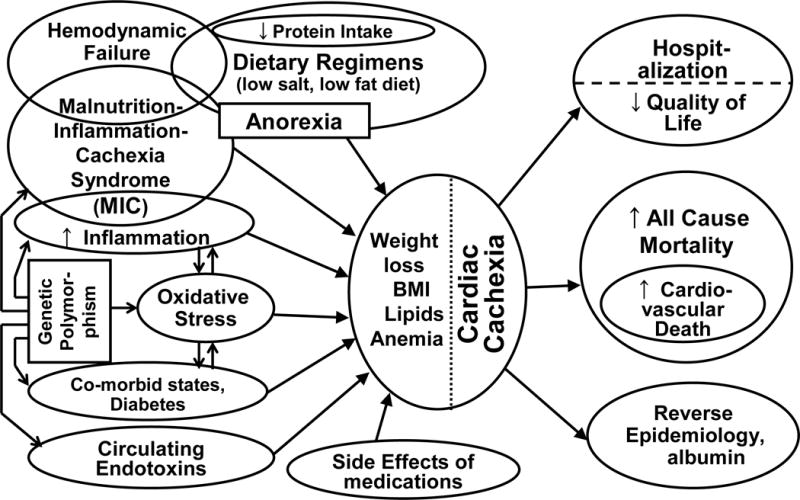
Schematic representation of the contributors and consequences of cardiac cachexia in CHF patients.
Oxidative Stress (SOX) and MIC in CHF
Oxidative stress (SOX) implies the potential of tissue damage from an imbalance between an excessive generation of oxidant compounds and insufficient anti-oxidant defense mechanisms.80,81 The generation of oxidative compounds represents part of the defense mechanisms against invading microorganisms and malignant cells, as well as of tissue healing and remodeling.81 However, an improper or maladaptive activation of oxidative processes may be chronically present in pathological situations including in CHF patients.82–86 Several deficiencies in different components of the anti-oxidant defense mechanisms may be responsible for the CHF associated SOX.86–91 Reduced intake and reduced levels of vitamin C and some other anti-oxidant vitamins are probably due to anorexia and malnutrition.88,92,93 At the same time, pro-oxidant activity is increased in CHF patients due to advanced age, higher prevalence of diabetes, circulating endotoxin, and chronic inflammation.86 Blood levels of several lipid and protein oxidation products such as F2-istoprostanes are increased in CHF patients.94,95 SOX may also promote formation of advanced glycation end-products (AGEs) independently of glucose levels.96–98 Hence, the SOX resultant “carbonyl stress” products may also be associated with further damage in CHF.99,100
Anti-Oxidants Interventions in CHF
Several clinical observations indicate that SOX play a role in human heart failure, although to date the clinical data have been conflicting.82,101,102 Treatment with vitamin C inhibits endothelial cell apoptosis in CHF.103 Anti-inflammatory interventions may mitigate SOX in CHF.104 The superiority of carvedilol to other ß-blockers in CHF may be in part due to its antioxidant effects.105–107 However, large clinical trials of the antioxidant vitamins have not shown benefit in preventing morbidity or mortality in the general population or in those at risk of cardiovascular disease.108–110 The mixed and often negative results of clinical trials using antioxidant therapy may also reflect the lack of effectiveness of a single anti-oxidant agent;111 an integrated approach using more than one anti-oxidant component, especially in combination with anti-inflammatory and nutritional interventions, focusing on malnourished CHF patients, may hold more promise.104
The Endotoxin-Lipoprotein Hypothesis
A low serum cholesterol is not only a predictor of poor outcome in CHF patients21,112,113 but also in other disease states characterized by inflammation and SOX, such as malignancy, AIDS and renal failure,25,35,60,114–117. Serum cholesterol is a surrogate of the totality of lipoproteins. Freely circulating lipoplysaccharides (LPS) activate pro-inflammatory cytokine cascade leading to the MIC/SOX complex69,118,119. Hence, intact (not oxidized) lipoproteins may be a defense mechanism against inflammation by binding to and neutralizing the circulating LPS.70,120 Higher levels of endotoxins have been observed in both CHF and dialysis patients.118,119,121,122 Hypothetically, in malnourished or inflamed CHF patients with a low cholesterol level or dysfunctional lipoproteins, the LPS bioactivity can practically increase even without more absolute LPS on board, leading to pro-inflammatory cytokine activation cascade21,123, esp. in the setting of pro-inflammatory genetic polymorphism (see below).
The Temporal Discordance Hypothesis
In contrast to the conventional CV risk factors and over-nutrition that require several years to decades to exert their deleterious effect, the impact of wasting and MIC appears rapid, resulting in decreased short-term survival.5 This “time discrepancy” or temporal discordance between the two sets of competing risk factors, i.e., over-nutrition as the long-term killer vs. under-nutrition as the short-term killer, may explain the clinical relevance of the reverse epidemiology phenomenon observed in CHF patients, in whom the under-nutrition overwhelms the presence of over-nutrition, leading to poor short-term survival.25,36 (Figure 3) Hence, no matter how strongly such CV risk factors as hypercholesterolemia or obesity are present, CHF patients will continue to die excessively and fast as long as the short-term impact of cachexia and MICS prevails. In other words, underweight CHF patients will not live long enough to die of obesity, hypercholesterolemia or hypertension, because they die much faster of cachexia.36 Hence, if the main issue is indeed the high rate of short-term mortality, it is also expected that short-term interventions to correct the underlying condition, i.e., the MIC/SOX complex, can improve survival. Based on this plausible hypothesis, nutritional interventions may be promising.
Figure 3.
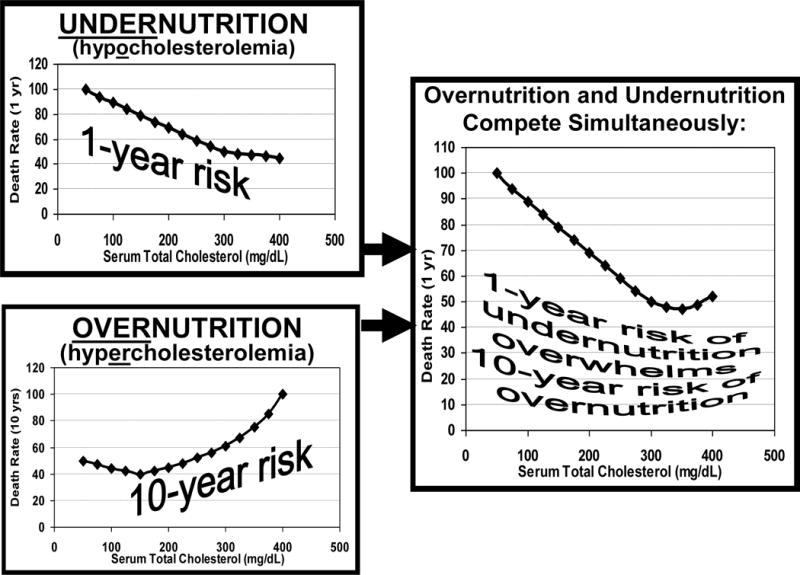
The Temporal Discordance Hypothesis: Competition between the short-term killer (undernutrition) and long-term killer (overnutrition) in CHF patients. (adapted with permission from Kalantar-Zadeh et al Seminars in Nephrology 2006;26:18–33235)
Gene Polymorphism and MIC in CHF Patients
The great inter- and intra-individual variability in the prevalence of cachexia and MIC/SOX complex in CHF patients, which cannot be explained by inflammatory or oxidative factors alone, indicates that genetic differences may be involved. Many individuals with atherosclerotic heart disease die before they reach CHF. Thus, we hypothesize that a significant “survival selection process” leads to a higher prevalence of certain genotypes with higher prevalence of MIC/SOX amongst the survivors, i.e., the full-blown and yet surviving CHF patients. This hypothesis may also explain why the traditional cardiovascular risk factors are less relevant or even paradoxical in CHF patients, whereas such non-traditional factors as the MICS/SOX complex emerge as the strongest survival predictors.
A substantial heritability (35–40%) for CRP and albumin levels and leukocytes have been reported in non-CHF populations with heart disease.124 Similarly, individual factors may significantly influence the levels of inflammatory markers in CHF and dialysis patients125. The example of Asian dialysis patients may be illuminating: Because dialysis patients of Asian origin have a lower CRP levels and better survival,126 it could be argued that either the Asian diet or genetic factors or both may account for the observed differences. Indeed, Szalai et al127 reported that the prevalence of a polymorphic GT-repeat in the intron of the CRP gene, which contributes to variations in baseline CRP, was 2-fold higher in Caucasians than in African Americans. Thus, single nucleotide polymorphisms (SNPs) in cytokine genes may have a significant influence on inflammation and its attendant morbidity in CHF patients as well. Although genetic factors such as SNPs may have a modest effect at an individual level, because of their presumably high frequency in the CHF population (see above our “survival selection” hypothesis above), these genetic variants can be associated with a high attributable risk of cardiovascular disease and death.
Can Nutritional Interventions Correct Wasting, Inflammation and SOX?
To the best of our knowledge, nutritional interventions to prevent or ameliorate the MIC/SOX complex in CHF have been rarely, if ever, studied. Two recent studies based on nutritional interventions using unconventional vegetarian128 or Mediterranean-style129 diets showed that diet might be effective in correcting inflammation and associated CV risk in non-CHF populations. Many foods contain factors that can modulate the synthesis or activity of pro-inflammatory mediators51,130. The efficacy of dietary fish oil has been demonstrated in several studies.91,131–135 Fish oil is an abundant source of eicosapentaenoic acid (EPA), a precursor of certain prostaglandins and leukotrienes with anti-inflammatory properties.136–138 In addition, borage oil, a plant seed with a high concentration of gamma linolenic acid (GLA) has anti-inflammatory, anti-oxidant and vaso-protective properties.139–144 GLA is elongated to dihomo-gamma-linolenic acid (DGLA), the fatty acid precursor to prostaglandin E1, known to have vasodilator and anti-aggregator properties.143,145 Carnitine is another nutraceutical that mitigates pro-inflammatory cytokine levels in patients with liver disease146 and heart failure147 and in dialysis patients,147–149 although it may not be effective in CJF. Recently, a commercially available nutritional supplement that contains relatively large proportions of fish oil, borage oil, carnitine and other anti-oxidants and is designed for critically ill patients with inflammation and SOX137,138,150,151 corrected hypoalbuminemia in a small group of malnourished dialysis patients in a non-randomized pilot study.151 However, this or similar nutritional supplements have not yet been tested in CHF patients.
Nutritional Interventions for CHF
A number of different modalities can be employed to improve nutritional, inflammatory and oxidative status in CHF patients, as listed in Table 1. Among more intensive modalities, tube feeding and parenteral interventions are cumbersome, invasive, and cannot be imposed to the average CHF outpatient. Hormonal interventions including growth hormones (such as IGF-I)152,153, ghrelin154–159, and melano-cortin antagonists55,160,161 have been under investigation, but some of their related studies in CHF have yielded mixed results.37,90,154,162 With Regard to ghrelin and melanocortin antagonists, further research will be necessary to identify the exact pathways involved, to examine their safety profile in human subjects with CHF, and to find the best therapeutic strategies of using these agents. Some appetite stimulants and anti-inflammatory/anti-oxidant agents appear promising for use in CHF patients. Nevertheless, as we have recently discussed for dialysis patients, who have a similar conundrum,5,163,164 it is less likely to find one single agent (e.g., fish oil alone or vitamin E alone) to correct MIC. Integrated oral supplements, especially if they contain a combination of several nutritional, anti-inflammatory and anti-oxidant agents and provide supplemental protein and energy, are more promising modalities.
Table 1.
Nutritional/anti-inflammatory interventions in CHF patients
|
Can Oral Interventions Correct MIC and Improve Survival in CHF?
To date there are no large-scale, randomized prospective interventional studies that have examined this question. According to some reports, aggressive attempts to increase nutritional intake may improve nutritional or clinical status in CHF patients.38,39,87,165–168 However, achieving a moderate increase in food intake without concurrent provision of anti-inflammatory or anti-oxidant nutrients may not be successful. The clinical potential of improving nutritional status in CHF patients with MIC/SOX by means of oral nutritional therapies should be demonstrated in well-designed clinical trials. However, before large-scale and expensive trials are launched, smaller pilot/feasibility studies are needed.
Anti-Inflammatory and Anti-Oxidant Agents
Although epidemiologic evidence links wasting to inflammation and SOX and to poor outcome in CHF patients, there are no randomized trials to indicate improvement of nutritional status or clinical outcome by simple oral nutritional supplements with anti-inflammatory and antioxidant properties. A number of treatment modalities have been implicated to target inflammation and/or SOX in CHF patients. (Table 2) These include existing and emerging CHF therapies ACE inhibitors,169 ARB, carvedilol, and statins.170–172 Antioxidant vitamins including vitamin E173–176 and vitamin C177–183 have also been suggested as potential treatments for CHF, even though some recent randomized trials including HOPE fail to show any beneficial effect.109 There are however discrepancies, for instance, pertaining to the role of vitamin E in survival in both CHF patients173, coronary heart disease, and in the general population110,184. More directly targeting the myocardium and vasculature with potent antioxidant therapies may be necessary in order to derive therapeutic benefit.
Table 2.
Anti-inflammatory & anti-oxidant agents for use in CHF patients
|
Orexigenics
Among appetite stimulants that are studied clinically (Table 3), megestrol acetate (MA) is by far the most utilized and best-studied agent, although not among CHF patients. MA, 800 mg/day, increases appetite and food intake in cancer or AIDS patients.185–189 MA reduces the in vitro production of cytokines and serotonin in peripheral blood mononuclear cells of cancer patients, down-regulates the synthesis and release of pro-inflammatory cytokines, and mitigates SOX.34,190–195 However, there are some serious concerns about adverse effect of MA. There are virtually no studies concerning MA in CHF patients, although our group196 and others197–200 have used MA in dialysis patients. An increase in serum albumin and weight gain can be observed, but increase in fat mass appears to be the dominant feature.196,198 MA is associated with many side effects including thrombotic events, diarrhea, confusion/hallucinations, hyperglycemia, headache and dizziness, elevated LDH, Cushing syndrome, and cerebrovascular accident and anti-testosterone effects in men197,201–205. Hence, it appears doubtful whether MA will be used routinely in low risk and stable CHF patients without extreme wasting given its unfavorable side effect profile.
Table 3.
Appetite stimulants for potential use in CHF patients.
|
Pentoxifylline
Pentoxifylline (PTX) is a tri-substituted xanthine derivative. Due to its hemorrheologic and anti-platelet properties, PTX has been safely used for 3 decades to treat peripheral vascular disease.206–210 PTX down-regulates local pro-inflammatory cytokine-mediated NO synthase pathway,211 inhibits TNF-α and nuclear factor kappa B (NF-κB) production,212–215 and decreases body weight loss and muscle protein wasting in acutely ill patients.216 The anti-TNF-α and orexigenic properties have rendered PTX a promising agent for treating rheumatoid arthritis and cancer anorexia/cachexia,213,217–222 as well as to treat the MIC complex and anemia in dialysis patients.223,224 PTX attenuates cardiac dysfunction and reduces TNF-α and NF-κB levels in ischemic-reperfused heart.225
The anti-inflammatory and cardioprotective effect of PTX may improve clinical outcomes in CHF patients with cachexia. To our knowledge, only 2 groups have conducted RCTs to compare PTX with placebo in CHF patients. Sliwa and colleagues from South Africa have reported 4 clinical trials (PTX 400 mg tid for up to 6 mo vs. placebo) in 18 to 49 black African patients (total of 144 subjects) and reported improved ejection fraction and reduced plasma cytokine levels226–233. Bahrmann et al234 conducted a similar RCT in 47 subjects in Germany (PTX 600 mg bid) and did not find any difference in two groups after 6 months. Both South African and German studies had serious methodological and analytic limitations and did not target patients with MIC or wasting, nor did they measure nutritional parameters and appetite in studied subjects. Moreover, these studies did not examine the effect of PTX combined with nutritional supplements. It is possible that PTX alone cannot correct the catabolic state in CHF, whereas its combination with nutritional supplements with anti-inflammatory and anti-oxidant ingredients may have synergistic effects.
Conclusions and Future Steps
The poor clinical outcomes in patients with established CHF may not be addressed by targeting traditional cardiovascular risk factors of obesity, hypercholesterolemia, and hypertension. Malnutrition and inflammation are both strong predictors of increased mortality in CHF patients. Addressing these factors has the potential to improve outcomes. Nutritional intervention may be an effective means to that end. The National Heart Lung and Blood Institute (NHLBI) of the National institutes of Health has recently issued a new program announcement (PA 05-089) to encourage investigators to examine nutritional avenues in CHF patients.
A complex set of conditions that are related to the cachexia, inflammation and oxidative stress may be the etiology of the risk factor reversal or reverse epidemiology and high death rate in CHF patients. The short-term death risk due to undernutrition overwhelms the long-term effects of overnutrition leading to poor survival in malnourished and/or cachectic CHF patients. If our foregoing hypotheses are true, then the key to improving survival in heart failure patients as well as in other 20 to 30 million Americans with other disease processes exhibiting a reverse epidemiology may be nutritional interventions that can correct MIC. If a drop in weight over time is associated with poor outcome in CHF patients and if weight gain confers improved survival, nutritional interventions esp. with anti-inflammatory and anti-oxidant properties may be the most promising alternatives. However, since the wasting process in CHF is multi-factorial, narrowly-targeted therapeutic strategies are not likely to be successful. Integrated nutritional interventions that target several aspects of the MIC and wasting in form of combined nutritional treatment strategies with novel micronutrient components that have anti-oxidant and anti-inflammatory properties may be a solution and need to be tested. Appetite stimulating agents especially with anti-inflammatory properties such as MA or anti-inflammatory agents such as PTX may be appropriate adjuncts to dietary supplementation in CHF patients.
The ongoing focus with treating such conventional risk factors as hypertension, hypercholesterolemia, and obesity utilizing treatment targets derived from community cohorts are not likely to lead to an immediate improvement of high mortality rate in CHF patients, as long as the short-term survival is the issue at hand. Such practices as imposing “ideal” BMI ranges based on the general population norms or mandatory weight loss programs for heart transplant wait-listed patients may need to be reevaluated. Dismissing the theory of Reverse Epidemiology as counterintuitive and potentially harmful may not be the most scientifically rigorous approach in dealing with this conundrum. The characteristics of a surviving CHF patient stand in a clear contradiction to those predicted by traditional cardiovascular risk factors. For CHF patients it may be time to go beyond the traditional framingham risk factors and try to explore new paradigms and novel modalities such as nutritional interventions that can correct specific risk factors in them.
Acknowledgments
KKZ is supported by a grant from the National Institute of Diabetes Digestive and Kidney Diseases of the National Institutes of Health (R01DK078106), the American Heart Association Grant-in-Aid 0655776Y, and research grants from Harold Simmons. SDA and Division of Applied Cachexia Research are supported with a grant from Charité Medical School. TBH was funded by NIH training grant 401357JI30608. GCF holds the Eliot Corday Chair in Cardiovascular Medicine and Science.
Footnotes
Relevant Conflicts of Interests: Gregg C. Fonarow, MD: Research grants, consultant and speaker for GlaxoSmithKline. None declared by the other authors.
References
- 1.Redfield MM. Heart failure–an epidemic of uncertain proportions. N Engl J Med. 2002;347:1442–4. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 4.van Jaarsveld CH, Ranchor AV, Kempen GI, Coyne JC, van Veldhuisen DJ, Sanderman R. Epidemiology of heart failure in a community-based study of subjects aged >/=57 years: Incidence and long-term survival. Eur J Heart Fail. 2005 doi: 10.1016/j.ejheart.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients; special review article for the 25th anniversary of the Seminars in Nephrology. Semin Nephrol. 2006;26:118–133. doi: 10.1016/j.semnephrol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 7.Basinski ASH. Hospitalization for cardiovascular medical diagnoses. In: Naylor CD, Slaughter PM, editors. Cardiovascular Health and Services in Ontario: An ICES Atlas Toronto. Ontario: Institute for Clinical Evaluative Sciences; 1999. pp. 15–49. [Google Scholar]
- 8.Gwadry-Sridhar FH, Flintoft V, Lee DS, Lee H, Guyatt GH. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–20. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- 9.Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, Sutton GC. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–10. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 11.Madsen BK, Videbaek R, Stokholm H, Mortensen LS, Hansen JF. Prognostic value of echocardiography in 190 patients with chronic congestive heart failure. A comparison with New York Heart Association functional classes and radionuclide ventriculography. Cardiology. 1996;87:250–6. doi: 10.1159/000177096. [DOI] [PubMed] [Google Scholar]
- 12.van den Broek SA, van Veldhuisen DJ, de Graeff PA, Landsman ML, Hillege H, Lie KI. Comparison between New York Heart Association classification and peak oxygen consumption in the assessment of functional status and prognosis in patients with mild to moderate chronic congestive heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70:359–63. doi: 10.1016/0002-9149(92)90619-a. [DOI] [PubMed] [Google Scholar]
- 13.Contaldo F, Pasanisi F, Finelli C, de Simone G. Obesity, heart failure and sudden death. Nutr Metab Cardiovasc Dis. 2002;12:190–7. [PubMed] [Google Scholar]
- 14.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 16.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. Jama. 2002;287:883–9. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 17.Strassburg S, Springer J, Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol. 2005 doi: 10.1016/j.biocel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Florea VG, Moon J, Pennell DJ, Doehner W, Coats AJ, Anker SD. Wasting of the left ventricle in patients with cardiac cachexia: a cardiovascular magnetic resonance study. Int J Cardiol. 2004;97:15–20. doi: 10.1016/j.ijcard.2003.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–29. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 20.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–83. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 23.Chopra M, Galbraith S, Darnton-Hill I. A global response to a global problem: the epidemic of overnutrition. Bull World Health Organ. 2002;80:952–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa Y, Shoji T, Ishimura E, Inaba M, Morii H. Paradox of risk factors for cardiovascular mortality in uremia: is a higher cholesterol level better for atherosclerosis in uremia? Am J Kidney Dis. 2001;38:S4–7. doi: 10.1053/ajkd.2001.27380. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann EH, Bower JD, Salahudeen AK. Risk factor paradox in hemodialysis: better nutrition as a partial explanation. Asaio J. 2001;47:74–81. doi: 10.1097/00002480-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Salahudeen AK. Obesity and survival on dialysis. Am J Kidney Dis. 2003;41:925–32. doi: 10.1016/s0272-6386(03)00189-6. [DOI] [PubMed] [Google Scholar]
- 29.Morley JE. Anorexia and weight loss in older persons. J Gerontol A Biol Sci Med Sci. 2003;58:131–7. doi: 10.1093/gerona/58.2.m131. [DOI] [PubMed] [Google Scholar]
- 30.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–23. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 31.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Grosvenor M, Lillington L, Sayre J, Beall G. Dietary intake and counseling, weight maintenance, and the course of HIV infection. J Am Diet Assoc. 1995;95:428–32. doi: 10.1016/S0002-8223(95)00115-8. quiz 433–5. [DOI] [PubMed] [Google Scholar]
- 33.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog. 1998;9:99–106. doi: 10.1615/critrevoncog.v9.i2.10. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh KFG, Coresh J, Block G, Kopple JD. Identifying populations with a reverse epidemiology of cardiovascular disease. Annual conference of the American College of Epidemiology, Annals of Epidemiology. 2003 Nov; abstract. [Google Scholar]
- 36.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, Wu DY. Reverse epidemiology: a spurious hypothesis or a hardcore reality? Blood Purif. 2005;23:57–63. doi: 10.1159/000082012. [DOI] [PubMed] [Google Scholar]
- 37.Akashi YJ, Springer J, Anker SD. Cachexia in chronic heart failure: prognostic implications and novel therapeutic approaches. Curr Heart Fail Rep. 2005;2:198–203. doi: 10.1007/BF02696650. [DOI] [PubMed] [Google Scholar]
- 38.Nicol SM, Carroll DL, Homeyer CM, Zamagni CM. The identification of malnutrition in heart failure patients. Eur J Cardiovasc Nurs. 2002;1:139–47. doi: 10.1016/s1474-5151(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsson A, Pihl-Lindgren E, Fridlund B. Malnutrition in patients suffering from chronic heart failure; the nurse’s care. Eur J Heart Fail. 2001;3:449–56. doi: 10.1016/s1388-9842(01)00139-8. [DOI] [PubMed] [Google Scholar]
- 40.Schwengel RH, Gottlieb SS, Fisher ML. Protein-energy malnutrition in patients with ischemic and nonischemic dilated cardiomyopathy and congestive heart failure. Am J Cardiol. 1994;73:908–10. doi: 10.1016/0002-9149(94)90825-7. [DOI] [PubMed] [Google Scholar]
- 41.Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1989;63:709–13. doi: 10.1016/0002-9149(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 42.Helies-Toussaint C, Moinard C, Rasmusen C, Tabbi-Anneni I, Cynober L, Grynberg A. Aortic banding in rat as a model to investigate malnutrition associated with heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1325–31. doi: 10.1152/ajpregu.00320.2004. [DOI] [PubMed] [Google Scholar]
- 43.Leiter L. Heart failure and malnutrition. Arch Intern Med. 1960;105:825–9. doi: 10.1001/archinte.1960.00270180003001. [DOI] [PubMed] [Google Scholar]
- 44.Don’t fade away with heart failure. Changes wrought by heart failure can attack your appetite and whittle away your weight. Some simple steps can help. Harv Heart Lett. 2003;14:6–7. [PubMed] [Google Scholar]
- 45.King D, Smith ML, Chapman TJ, Stockdale HR, Lye M. Fat malabsorption in elderly patients with cardiac cachexia. Age Ageing. 1996;25:144–9. doi: 10.1093/ageing/25.2.144. [DOI] [PubMed] [Google Scholar]
- 46.Poehlman ET, Scheffers J, Gottlieb SS, Fisher ML, Vaitekevicius P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med. 1994;121:860–2. doi: 10.7326/0003-4819-121-11-199412010-00006. [DOI] [PubMed] [Google Scholar]
- 47.Vaisman N, Silverberg DS, Wexler D, Niv E, Blum M, Keren G, Soroka N, Iaina A. Correction of anemia in patients with congestive heart failure increases resting energy expenditure. Clin Nutr. 2004;23:355–61. doi: 10.1016/j.clnu.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Obisesan TO, Toth MJ, Donaldson K, Gottlieb SS, Fisher ML, Vaitekevicius P, Poehlman ET. Energy expenditure and symptom severity in men with heart failure. Am J Cardiol. 1996;77:1250–2. doi: 10.1016/s0002-9149(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 49.Toth MJ, Gottlieb SS, Goran MI, Fisher ML, Poehlman ET. Daily energy expenditure in free-living heart failure patients. Am J Physiol. 1997;272:E469–75. doi: 10.1152/ajpendo.1997.272.3.E469. [DOI] [PubMed] [Google Scholar]
- 50.Lennie TA, Steward DK. Energy regulation in inflammation-induced anorexia: implications for treatment. Nutrition. 2001;17:740–1. doi: 10.1016/s0899-9007(01)00621-9. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy DO. Rethinking nutritional support for persons with cancer cachexia. Biol Res Nurs. 2003;5:3–17. doi: 10.1177/1099800403005001001. [DOI] [PubMed] [Google Scholar]
- 52.Yeh SS, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr. 1999;70:183–97. doi: 10.1093/ajcn.70.2.183. [DOI] [PubMed] [Google Scholar]
- 53.Mak RH, Cheung W, Cone RD, Marks DL. Orexigenic and anorexigenic mechanisms in the control of nutrition in chronic kidney disease. Pediatr Nephrol. 2005;20:427–31. doi: 10.1007/s00467-004-1789-1. [DOI] [PubMed] [Google Scholar]
- 54.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 55.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115:1659–65. doi: 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis. 2003;42:864–81. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Pasini E, Aquilani R, Gheorghiade M, Dioguardi FS. Malnutrition, muscle wasting and cachexia in chronic heart failure: the nutritional approach. Ital Heart J. 2003;4:232–5. [PubMed] [Google Scholar]
- 58.Filippatos GS, Anker SD, Kremastinos DT. Pathophysiology of peripheral muscle wasting in cardiac cachexia. Curr Opin Clin Nutr Metab Care. 2005;8:249–54. doi: 10.1097/01.mco.0000165002.08955.5b. [DOI] [PubMed] [Google Scholar]
- 59.Horwich TB, Kalantar-Zadeh K, Fonarow GC. Hypoalbuminemia is a frequent finding in lean as well as obese heart failure patients and independently predicts mortality. Circulation. 2004;110:III–555. abstract. [Google Scholar]
- 60.Conraads VM, Bosmans JM, Vrints CJ. Chronic heart failure: an example of a systemic chronic inflammatory disease resulting in cachexia. Int J Cardiol. 2002;85:33–49. doi: 10.1016/s0167-5273(02)00232-2. [DOI] [PubMed] [Google Scholar]
- 61.Milani RV, Mehra MR, Endres S, Eigler A, Cooper ES, Lavie CJ, Jr, Ventura HO. The clinical relevance of circulating tumor necrosis factor-alpha in acute decompensated chronic heart failure without cachexia. Chest. 1996;110:992–5. doi: 10.1378/chest.110.4.992. [DOI] [PubMed] [Google Scholar]
- 62.Steele L. Invited commentary: unexplained health problems after Gulf War Service–finding answers to complex questions. Am J Epidemiol. 2001;154:406–9. doi: 10.1093/aje/154.5.406. [DOI] [PubMed] [Google Scholar]
- 63.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–70. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 65.Furukawa S, Imai K, Matsubara T, Yone K, Yachi A, Okumura K, Yabuta K. Increased levels of circulating intercellular adhesion molecule 1 in Kawasaki disease. Arthritis Rheum. 1992;35:672–7. doi: 10.1002/art.1780350611. [DOI] [PubMed] [Google Scholar]
- 66.Girardin E, Grau GE, Dayer JM, Roux-Lombard P, Lambert PH. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 67.Ceconi C, Curello S, Bachetti T, Corti A, Ferrari R. Tumor necrosis factor in congestive heart failure: a mechanism of disease for the new millennium? Prog Cardiovasc Dis. 1998;41:25–30. doi: 10.1016/s0033-0620(98)80028-5. [DOI] [PubMed] [Google Scholar]
- 68.Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–47. doi: 10.1378/chest.115.3.836. [DOI] [PubMed] [Google Scholar]
- 69.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–42. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 70.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–3. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 71.Sharma R, von Haehling S, Rauchhaus M, Bolger AP, Genth-Zotz S, Doehner W, Oliver B, Poole-Wilson PA, Hans-Dieter V, Coats AJ, Adcock IM, Anker SD. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur J Heart Fail. 2005;7:479–84. doi: 10.1016/j.ejheart.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J Parenter Enteral Nutr. 1998;22:156–66. doi: 10.1177/0148607198022003156. [DOI] [PubMed] [Google Scholar]
- 73.Kalantar-Zadeh K, Anker SD. Inflammation, cholesterol levels, and risk of mortality among patients receiving dialysis. Jama. 2004;291:1834. doi: 10.1001/jama.291.15.1834-a. author reply 1834–5. [DOI] [PubMed] [Google Scholar]
- 74.Rauchhaus M, Anker SD. Plasma concentrations of bacterial lipopolysaccharide: a marker of infection or inflammation? J Am Coll Cardiol. 2000;36:656–7. doi: 10.1016/s0735-1097(00)00750-6. [DOI] [PubMed] [Google Scholar]
- 75.Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–22. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- 76.Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, Coats AJ. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18:259–69. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- 77.Anker SD, Clark AL, Kemp M, Salsbury C, Teixeira MM, Hellewell PG, Coats AJ. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30:997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 78.Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427–34. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 79.Mantovani G, Madeddu C, Maccio A, Gramignano G, Lusso MR, Massa E, Astara G, Serpe R. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol Biomarkers Prev. 2004;13:1651–9. [PubMed] [Google Scholar]
- 80.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 81.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–80. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 82.Mak S, Newton GE. The oxidative stress hypothesis of congestive heart failure: radical thoughts. Chest. 2001;120:2035–46. doi: 10.1378/chest.120.6.2035. [DOI] [PubMed] [Google Scholar]
- 83.Handelman GJ. Current studies on oxidant stress in dialysis. Blood Purif. 2003;21:46–50. doi: 10.1159/000067868. [DOI] [PubMed] [Google Scholar]
- 84.Handelman GJ. Evaluation of oxidant stress in dialysis patients. Blood Purif. 2000;18:343–9. doi: 10.1159/000014460. [DOI] [PubMed] [Google Scholar]
- 85.Freeman LM, Rush JE, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med. 2005;19:537–41. doi: 10.1892/0891-6640(2005)19[537:asaboo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37:1765–74. doi: 10.1016/s0735-1097(01)01227-x. [DOI] [PubMed] [Google Scholar]
- 88.Witte KK, Clark AL. Nutritional abnormalities contributing to cachexia in chronic illness. Int J Cardiol. 2002;85:23–31. doi: 10.1016/s0167-5273(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 89.Witte KK, Clark AL. Chronic heart failure and multiple micronutrient supplementation: realistic hope or idealistic conjecture? Heart Fail Monit. 2005;4:123–9. [PubMed] [Google Scholar]
- 90.Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, Clark AL, Cleland JG. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–44. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 91.Witte KK, Clark AL. Fish oils–adjuvant therapy in chronic heart failure? Eur J Cardiovasc Prev Rehabil. 2004;11:267–74. doi: 10.1097/01.hjr.0000136728.27524.f5. [DOI] [PubMed] [Google Scholar]
- 92.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 93.Dagenais GR, Marchioli R, Yusuf S, Tognoni G. Beta-carotene, vitamin C, and vitamin E and cardiovascular diseases. Curr Cardiol Rep. 2000;2:293–9. doi: 10.1007/s11886-000-0084-4. [DOI] [PubMed] [Google Scholar]
- 94.Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis. 2003;42:286–94. doi: 10.1016/s0272-6386(03)00653-x. [DOI] [PubMed] [Google Scholar]
- 95.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 96.Inagi R, Miyata T. Oxidative protein damage with carbohydrates and lipids in uremia: ‘Carbonyl stress’. Blood Purif. 1999;17:95–8. doi: 10.1159/000014380. [DOI] [PubMed] [Google Scholar]
- 97.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 98.Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–8. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 99.Heidland A, Sebekova K, Frangiosa A, De Santo LS, Cirillo M, Rossi F, Cotrufo M, Perna A, Klassen A, Schinzel R, De Santo NG. Paradox of circulating advanced glycation end product concentrations in patients with congestive heart failure and after heart transplantation. Heart. 2004;90:1269–74. doi: 10.1136/hrt.2003.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith HM, Hamblin M, Hill MF. Greater propensity of diabetic myocardium for oxidative stress after myocardial infarction is associated with the development of heart failure. J Mol Cell Cardiol. 2005;39:657–65. doi: 10.1016/j.yjmcc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–74. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 102.Jaatinen P, Saukko P, Hervonen A. Chronic ethanol exposure increases lipopigment accumulation in human heart. Alcohol Alcohol. 1993;28:559–69. [PubMed] [Google Scholar]
- 103.Rossig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, Dimmeler S. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation. 2001;104:2182–7. doi: 10.1161/hc4301.098284. [DOI] [PubMed] [Google Scholar]
- 104.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–20. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 105.Castro P, Vukasovic JL, Chiong M, Diaz-Araya G, Alcaino H, Copaja M, Valenzuela R, Greig D, Perez O, Corbalan R, Lavandero S. Effects of carvedilol on oxidative stress and chronotropic response to exercise in patients with chronic heart failure. Eur J Heart Fail. 2005;7:1033–9. doi: 10.1016/j.ejheart.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 106.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 107.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann-Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–6. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 108.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 109.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 110.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–27. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 111.Mak S, Newton GE. Redox modulation of the inotropic response to dobutamine is impaired in patients with heart failure. Am J Physiol Heart Circ Physiol. 2004;286:H789–95. doi: 10.1152/ajpheart.00633.2003. [DOI] [PubMed] [Google Scholar]
- 112.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–24. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 113.Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, Coats AJ, Anker SD. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–40. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 114.Kalantar-Zadeh K, Block G, Fonarow GC. Reverse epidemiology of cardiovascular risks in chronic heart failure. (SUBMITTED to: J Am Coll Cardiol) doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 115.Horwich TB, Fonarow GC. The impact of obesity on survival in patients with heart failure. Heart Fail Monit. 2002;3:8–14. [PubMed] [Google Scholar]
- 116.Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J. 1998;19:761–5. doi: 10.1053/euhj.1997.0858. [DOI] [PubMed] [Google Scholar]
- 117.Rauchhaus M, Koloczek V, Volk H, Kemp M, Niebauer J, Francis DP, Coats AJ, Anker SD. Inflammatory cytokines and the possible immunological role for lipoproteins in chronic heart failure. Int J Cardiol. 2000;76:125–33. doi: 10.1016/s0167-5273(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 118.Hosoya N, Sakai K. Backdiffusion rather than backfiltration enhances endotoxin transport through highly permeable dialysis membranes. ASAIO Trans. 1990;36:M311–3. [PubMed] [Google Scholar]
- 119.Laude-Sharp M, Caroff M, Simard L, Pusineri C, Kazatchkine M, Haeffner-Cavaillon N. Induction of IL-1 during hemodialysis: transmembrane passage of intact endotoxin (LPS) Kidney Int. 1990;38:1089–1094. doi: 10.1038/ki.1990.317. [DOI] [PubMed] [Google Scholar]
- 120.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19:203–14. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- 121.Tielemans C, Husson C, Schurmans T, Gastaldello K, Madhoun P, Delville JP, Marchant A, Goldman M, Vanherweghem JL. Effects of ultrapure and non-sterile dialysate on the inflammatory response during in vitro hemodialysis. Kidney Int. 1996;49:236–43. doi: 10.1038/ki.1996.33. [DOI] [PubMed] [Google Scholar]
- 122.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79:1426–30. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 123.Kalantar-Zadeh K, Fouque D, Kopple JD. Outcome research, nutrition, and reverse epidemiology in maintenance dialysis patients. J Ren Nutr. 2004;14:64–71. doi: 10.1053/j.jrn.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–9. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 125.Tsirpanlis G, Bagos P, Ioannou D, Bleta A, Marinou I, Lagouranis A, Chatzipanagiotou S, Nicolaou C. The variability and accurate assessment of microinflammation in haemodialysis patients. Nephrol Dial Transplant. 2004;19:150–7. doi: 10.1093/ndt/gfg486. [DOI] [PubMed] [Google Scholar]
- 126.Yao Q, Lindholm B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodial Int. 2004;8:118–129. doi: 10.1111/j.1492-7535.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 127.Szalai AJ, McCrory MA, Cooper GS, Wu J, Kimberly RP. Association between baseline levels of C-reactive protein (CRP) and a dinucleotide repeat polymorphism in the intron of the CRP gene. Genes Immun. 2002;3:14–9. doi: 10.1038/sj.gene.6363820. [DOI] [PubMed] [Google Scholar]
- 128.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, Emam A, Parker TL, Vidgen E, Lapsley KG, Trautwein EA, Josse RG, Leiter LA, Connelly PW. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. Jama. 2003;290:502–10. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 129.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 130.Watson J, Byars ML, McGill P, Kelman AW. Cytokine and prostaglandin production by monocytes of volunteers and rheumatoid arthritis patients treated with dietary supplements of blackcurrant seed oil. Br J Rheumatol. 1993;32:1055–8. doi: 10.1093/rheumatology/32.12.1055. [DOI] [PubMed] [Google Scholar]
- 131.Curtis CL, Harwood JL, Dent CM, Caterson B. Biological basis for the benefit of nutraceutical supplementation in arthritis. Drug Discov Today. 2004;9:165–72. doi: 10.1016/S1359-6446(03)02980-5. [DOI] [PubMed] [Google Scholar]
- 132.Vergili-Nelsen JM. Benefits of fish oil supplementation for hemodialysis patients. J Am Diet Assoc. 2003;103:1174–7. doi: 10.1016/s0002-8223(03)00984-2. [DOI] [PubMed] [Google Scholar]
- 133.Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W. Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol. 2003;171:4837–43. doi: 10.4049/jimmunol.171.9.4837. [DOI] [PubMed] [Google Scholar]
- 134.Pakala R, Sheng WL, Benedict CR. Vascular smooth muscle cells preloaded with eicosapentaenoic acid and docosahexaenoic acid fail to respond to serotonin stimulation. Atherosclerosis. 2000;153:47–57. doi: 10.1016/s0021-9150(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 135.McCarty MF. A central role for protein kinase C overactivity in diabetic glomerulosclerosis: implications for prevention with antioxidants, fish oil, and ACE inhibitors. Med Hypotheses. 1998;50:155–65. doi: 10.1016/s0306-9877(98)90202-x. [DOI] [PubMed] [Google Scholar]
- 136.Hirai A, Terano T, Tamura Y, Yoshida S. Eicosapentaenoic acid and adult diseases in Japan: epidemiological and clinical aspects. J Intern Med Suppl. 1989;225:69–75. doi: 10.1111/j.1365-2796.1989.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 137.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–20. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 138.Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Claycombe KJ, Smith GT, Gregory TJ, Karlstad MD. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med. 1997;25:523–32. doi: 10.1097/00003246-199703000-00024. [DOI] [PubMed] [Google Scholar]
- 139.Dirks J, van Aswegen CH, du Plessis DJ. Cytokine levels affected by gamma-linolenic acid. Prostaglandins Leukot Essent Fatty Acids. 1998;59:273–7. doi: 10.1016/s0952-3278(98)90141-7. [DOI] [PubMed] [Google Scholar]
- 140.Purasiri P, McKechnie A, Heys SD, Eremin O. Modulation in vitro of human natural cytotoxicity, lymphocyte proliferative response to mitogens and cytokine production by essential fatty acids. Immunology. 1997;92:166–72. doi: 10.1046/j.1365-2567.1997.d01-2308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Utsunomiya T. Inhibition of neutrophil respiratory burst and cytokine priming by gamma-linolenic acid. Br J Surg. 1997;84:426. doi: 10.1002/bjs.1800840353. [DOI] [PubMed] [Google Scholar]
- 142.Jiang WG, Puntis MC, Horrobin DF, Scott C, Hallett MB. Inhibition of neutrophil respiratory burst and cytokine priming by gamma-linolenic acid. Br J Surg. 1996;83:659–64. doi: 10.1002/bjs.1800830523. [DOI] [PubMed] [Google Scholar]
- 143.Rotondo D, Earl CR, Laing KJ, Kaimakamis D. Inhibition of cytokine-stimulated thymic lymphocyte proliferation by fatty acids: the role of eicosanoids. Biochim Biophys Acta. 1994;1223:185–94. doi: 10.1016/0167-4889(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 144.Fan YY, Ramos KS, Chapkin RS. Dietary gamma-linolenic acid suppresses aortic smooth muscle cell proliferation and modifies atherosclerotic lesions in apolipoprotein E knockout mice. J Nutr. 2001;131:1675–81. doi: 10.1093/jn/131.6.1675. [DOI] [PubMed] [Google Scholar]
- 145.Palombo JD, DeMichele SJ, Boyce PJ, Lydon EE, Liu JW, Huang YS, Forse RA, Mizgerd JP, Bistrian BR. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med. 1999;27:1908–15. doi: 10.1097/00003246-199909000-00032. [DOI] [PubMed] [Google Scholar]
- 146.Bykov I, Jarvelainen H, Lindros K. L-carnitine alleviates alcohol-induced liver damage in rats: role of tumour necrosis factor-alpha. Alcohol Alcohol. 2003;38:400–6. doi: 10.1093/alcalc/agg109. [DOI] [PubMed] [Google Scholar]
- 147.Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. 2003;41:S4–12. doi: 10.1016/s0272-6386(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 148.Yllmaz Selcuk N, San A, Tonbul HZ, Aksoy H, Ika I, Bakan E. Effects of nutritional status and oral essential amino acid replacement on serum L-carnitine levels of chronically hemodialyzed patients. Nephron. 1996;72:341–2. doi: 10.1159/000188877. [DOI] [PubMed] [Google Scholar]
- 149.Borum PR, Bennett SG. Carnitine as an essential nutrient. J Am Coll Nutr. 1986;5:177–82. doi: 10.1080/07315724.1986.10720124. [DOI] [PubMed] [Google Scholar]
- 150.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 151.Kalantar-Zadeh K, Braglia A, Chow J, Kwon O, Kuwae N, Colman S, Cockram DB, Kopple JD. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr. 2005;15:318–31. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 152.Dalla Libera L, Ravara B, Volterrani M, Gobbo V, Della Barbera M, Angelini A, Danieli Betto D, Germinario E, Vescovo G. Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am J Physiol Cell Physiol. 2004;286:C138–44. doi: 10.1152/ajpcell.00114.2003. [DOI] [PubMed] [Google Scholar]
- 153.Dreifuss P. Congestive heart failure and the growth hormone/insulin-like growth factor-1 (GH/IGF-1) system. Am J Cardiol. 2003;92:245–6. doi: 10.1016/s0002-9149(03)00418-1. [DOI] [PubMed] [Google Scholar]
- 154.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–9. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 155.Nagaya N, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of chronic heart failure. Regul Pept. 2003;114:71–7. doi: 10.1016/s0167-0115(03)00117-4. [DOI] [PubMed] [Google Scholar]
- 156.Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol. 2003;3:146–51. doi: 10.1016/s1471-4892(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 157.Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854–9. doi: 10.1210/jcem.86.12.8115. [DOI] [PubMed] [Google Scholar]
- 158.Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–8. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 159.Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–5. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 160.Scarlett JM, Marks DL. The use of melanocortin antagonists in cachexia of chronic disease. Expert Opin Investig Drugs. 2005;14:1233–9. doi: 10.1517/13543784.14.10.1233. [DOI] [PubMed] [Google Scholar]
- 161.Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, Fleck BA, Brown BT, Marks DL. The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology. 2005;146:2766–73. doi: 10.1210/en.2005-0142. [DOI] [PubMed] [Google Scholar]
- 162.von Haehling S, Anker SD. Future prospects of anticytokine therapy in chronic heart failure. Expert Opin Investig Drugs. 2005;14:163–76. doi: 10.1517/13543784.14.2.163. [DOI] [PubMed] [Google Scholar]
- 163.Kalantar-Zadeh K, Stenvinkel P, Bross R, Khawar OS, Rammohan M, Colman S, Benner D. Kidney insufficiency and nutrient-based modulation of inflammation. Curr Opin Clin Nutr Metab Care. 2005;8:388–396. doi: 10.1097/01.mco.0000172578.56396.9e. [DOI] [PubMed] [Google Scholar]
- 164.Kalantar-Zadeh K. Recent Advances in Understanding the Malnutrition-Inflammation-Cachexia Syndrome in Chronic Kidney Disease Patients: What is Next? Semin Dial. 2005;18:365–9. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 165.Bourdel-Marchasson I, Emeriau JP. Nutritional strategy in the management of heart failure in adults. Am J Cardiovasc Drugs. 2001;1:363–73. doi: 10.2165/00129784-200101050-00006. [DOI] [PubMed] [Google Scholar]
- 166.Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19:617–22. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- 167.Chin-Dusting JP, Kaye DM, Lefkovits J, Wong J, Bergin P, Jennings GL. Dietary supplementation with L-arginine fails to restore endothelial function in forearm resistance arteries of patients with severe heart failure. J Am Coll Cardiol. 1996;27:1207–13. doi: 10.1016/0735-1097(95)00611-7. [DOI] [PubMed] [Google Scholar]
- 168.Broqvist M, Arnqvist H, Dahlstrom U, Larsson J, Nylander E, Permert J. Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure–effects of long-term dietary supplementation. Eur Heart J. 1994;15:1641–50. doi: 10.1093/oxfordjournals.eurheartj.a060447. [DOI] [PubMed] [Google Scholar]
- 169.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. Jama. 1995;273:1450–6. [PubMed] [Google Scholar]
- 170.Haehling S, Okonko DO, Anker SD. Statins: a treatment option for chronic heart failure? Heart Fail Monit. 2004;4:90–7. [PubMed] [Google Scholar]
- 171.von Haehling S, Anker SD. Statins for heart failure: at the crossroads between cholesterol reduction and pleiotropism? Heart. 2005;91:1–2. doi: 10.1136/hrt.2004.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–8. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 173.Vitamin E gets an F. Research linking high doses of vitamin E to heart failure is causing new worries about the AREDS vitamins for macular degeneration. Harv Health Lett. 2005;30:6. [PubMed] [Google Scholar]
- 174.Bauersachs J, Fleming I, Fraccarollo D, Busse R, Ertl G. Prevention of endothelial dysfunction in heart failure by vitamin E: attenuation of vascular superoxide anion formation and increase in soluble guanylyl cyclase expression. Cardiovasc Res. 2001;51:344–50. doi: 10.1016/s0008-6363(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 175.Keith ME, Jeejeebhoy KN, Langer A, Kurian R, Barr A, O’Kelly B, Sole MJ. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am J Clin Nutr. 2001;73:219–24. doi: 10.1093/ajcn/73.2.219. [DOI] [PubMed] [Google Scholar]
- 176.Klein HH, Pich S, Lindert-Heimberg S, Nebendahl K, Niedmann P. Failure of chronic, high-dose, oral vitamin E treatment to protect the ischemic, reperfused porcine heart. J Mol Cell Cardiol. 1993;25:103–12. doi: 10.1006/jmcc.1993.1011. [DOI] [PubMed] [Google Scholar]
- 177.Mak S, Overgaard CB, Newton GE. Effect of vitamin C and L-NMMA on the inotropic response to dobutamine in patients with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2424–8. doi: 10.1152/ajpheart.00453.2005. [DOI] [PubMed] [Google Scholar]
- 178.Piccirillo G, Raffaele Q, Fimognari F, Moise A, Mario M, Lionetti M, Naso C, Di Carlo S, Nocco M, Magri D. Influence of L-arginine and vitamin C on the autonomic nervous system in chronic heart failure secondary to ischemic cardiomyopathy. Am J Cardiol. 2004;93:650–4. doi: 10.1016/j.amjcard.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 179.Nightingale AK, Schmitt M, Frenneaux MP. Vitamin C in heart failure: hype or hope? Hypertension. 2004;43:e5–6. doi: 10.1161/01.hyp.0000112025.25724.8a. author reply e5–6. [DOI] [PubMed] [Google Scholar]
- 180.Piccirillo G, Nocco M, Moise A, Lionetti M, Naso C, Carlo SD, Marigliano V. Response: Vitamin C in Heart Failure: Hope! Hypertension. 2004 doi: 10.1161/01.HYP.0000073581.74107.22. [DOI] [PubMed] [Google Scholar]
- 181.Piccirillo G, Nocco M, Moise A, Lionetti M, Naso C, di Carlo S, Marigliano V. Influence of vitamin C on baroreflex sensitivity in chronic heart failure. Hypertension. 2003;41:1240–5. doi: 10.1161/01.HYP.0000073581.74107.22. [DOI] [PubMed] [Google Scholar]
- 182.Zittermann A. German physicians discover a possible correlation. Heart failure caused by vitamin D deficiency? (interview by Dr. Kirsten Westphal) MMW Fortschr Med. 2003;145:18. [PubMed] [Google Scholar]
- 183.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 184.Hoogwerf BJ, Young JB, The HOPE study Ramipril lowered cardiovascular risk, but vitamin E did not. Cleve Clin J Med. 2000;67:287–93. doi: 10.3949/ccjm.67.4.287. [DOI] [PubMed] [Google Scholar]
- 185.Karcic E, Philpot C, Morley JE. Treating malnutrition with megestrol acetate: literature review and review of our experience. J Nutr Health Aging. 2002;6:191–200. [PubMed] [Google Scholar]
- 186.Chang AY. Megestrol acetate as a biomodulator. Semin Oncol. 1998;25:58–61. [PubMed] [Google Scholar]
- 187.Strang P. The effect of megestrol acetate on anorexia, weight loss and cachexia in cancer and AIDS patients (review) Anticancer Res. 1997;17:657–62. [PubMed] [Google Scholar]
- 188.Loprinzi CL, Bernath AM, Schaid DJ, Malliard JA, Athmann LM, Michalak JC, Tschetter LK, Hatfield AK, Morton RF. Phase III evaluation of 4 doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. Oncology. 1994;51(Suppl 1):2–7. doi: 10.1159/000227407. [DOI] [PubMed] [Google Scholar]
- 189.Aisner J, Tchekmedyian NS, Tait N, Parnes H, Novak M. Studies of high-dose megestrol acetate: potential applications in cachexia. Semin Oncol. 1988;15:68–75. [PubMed] [Google Scholar]
- 190.Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine activity in cancer-related anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate. Semin Oncol. 1998;25:45–52. [PubMed] [Google Scholar]
- 191.Mantovani G. Does megestrol acetate down-regulate interleukin-6 in patients? Support Care Cancer. 2002;10:566–7. doi: 10.1007/s00520-002-0351-6. author reply 568. [DOI] [PubMed] [Google Scholar]
- 192.Lambert CP, Sullivan DH, Evans WJ. Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2003;58:165–70. doi: 10.1093/gerona/58.2.m165. [DOI] [PubMed] [Google Scholar]
- 193.Yeh SS, Wu SY, Levine DM, Parker TS, Olson JS, Stevens MR, Schuster MW. The correlation of cytokine levels with body weight after megestrol acetate treatment in geriatric patients. J Gerontol A Biol Sci Med Sci. 2001;56:M48–54. doi: 10.1093/gerona/56.1.m48. [DOI] [PubMed] [Google Scholar]
- 194.Yeh S, Wu SY, Levine DM, Parker TS, Olson JS, Stevens MR, Schuster MW. Quality of life and stimulation of weight gain after treatment with megestrol acetate: correlation between cytokine levels and nutritional status, appetite in geriatric patients with wasting syndrome. J Nutr Health Aging. 2000;4:246–51. [PubMed] [Google Scholar]
- 195.Mantovani G, Maccio A, Bianchi A, Curreli L, Ghiani M, Santona MC, Del Giacco GS. Megestrol acetate in neoplastic anorexia/cachexia: clinical evaluation and comparison with cytokine levels in patients with head and neck carcinoma treated with neoadjuvant chemotherapy. Int J Clin Lab Res. 1995;25:135–41. doi: 10.1007/BF02592554. [DOI] [PubMed] [Google Scholar]
- 196.Rammohan M, Kalantar-Zadeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15:345–55. doi: 10.1016/j.jrn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 197.Boccanfuso JA, Hutton M, McAllister B. The effects of megestrol acetate on nutritional parameters in a dialysis population. J Ren Nutr. 2000;10:36–43. doi: 10.1016/s1051-2276(00)90021-9. [DOI] [PubMed] [Google Scholar]
- 198.Burrowes JD, Bluestone PA, Wang J, Pierson RN., Jr The effects of moderate doses of megestrol acetate on nutritional status and body composition in a hemodialysis patient. J Ren Nutr. 1999;9:89–94. doi: 10.1016/s1051-2276(99)90006-7. [DOI] [PubMed] [Google Scholar]
- 199.Lien YH, Ruffenach SJ. Low dose megestrol increases serum albumin in malnourished dialysis patients. Int J Artif Organs. 1996;19:147–50. [PubMed] [Google Scholar]
- 200.Costero O, Bajo MA, del Peso G, Gil F, Aguilera A, Ros S, Hevia C, Selgas R. Treatment of anorexia and malnutrition in peritoneal dialysis patients with megestrol acetate. Adv Perit Dial. 2004;20:209–12. [PubMed] [Google Scholar]
- 201.Thomas DR. Incidence of venous thromboembolism in megestrol acetate users. J Am Med Dir Assoc. 2004;5:65–6. doi: 10.1097/01.JAM.0000105070.61741.9D. author reply 66–7. [DOI] [PubMed] [Google Scholar]
- 202.Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4:255–6. doi: 10.1097/01.JAM.0000083384.84558.75. [DOI] [PubMed] [Google Scholar]
- 203.Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1:248–52. [PubMed] [Google Scholar]
- 204.Koller E, Gibert C, Green L, Mann M, Bernstein B. Thrombotic events associated with megestrol acetate in patients with AIDS cachexia. Nutrition. 1999;15:294–8. doi: 10.1016/s0899-9007(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 205.Force L, Barrufet P, Herreras Z, Bolibar I. Deep venous thrombosis and megestrol in patients with HIV infection. Aids. 1999;13:1425–6. doi: 10.1097/00002030-199907300-00031. [DOI] [PubMed] [Google Scholar]
- 206.Moriau M, Lavenne-Pardonge E, Crasborn L, von Frenckell R, Col-Debeys C. The treatment of severe or recurrent deep venous thrombosis. Beneficial effect of the co-administration of antiplatelet agents with or without rheological effects, and anticoagulants. Thromb Res. 1995;78:469–82. doi: 10.1016/0049-3848(95)00081-2. [DOI] [PubMed] [Google Scholar]
- 207.Lucas MA. Prevention of post-operative thrombosis in peripheral arteriopathies. Pentoxifylline vs. conventional antiaggregants: a six-month randomized follow-up study. Angiology. 1984;35:443–50. doi: 10.1177/000331978403500707. [DOI] [PubMed] [Google Scholar]
- 208.Radmilovic A, Boric Z, Naumovic T, Stamenkovic M, Musikic P. Shunt thrombosis prevention in hemodialysis patients--a double-blind, randomized study: pentoxifylline vs placebo. Angiology. 1987;38:499–506. doi: 10.1177/000331978703800701. [DOI] [PubMed] [Google Scholar]
- 209.Colen LB, Stevenson A, Sidorov V, Potparic Z, Pacelli E, Searles J, Lee S, Li L. Microvascular anastomotic thrombosis in experimental diabetes mellitus. Plast Reconstr Surg. 1997;99:156–62. doi: 10.1097/00006534-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 210.De Sanctis MT, Cesarone MR, Belcaro G, Incandela L, Steigerwalt R, Nicolaides AN, Griffin M, Geroulakos G. Treatment of retinal vein thrombosis with pentoxifylline: a controlled, randomized trial. Angiology. 2002;53(Suppl 1):S35–8. [PubMed] [Google Scholar]
- 211.Stosic-Grujicic SD, Maksimovic DD, Stojkovic MB, Lukic ML. Pentoxifylline prevents autoimmune mediated inflammation in low dose streptozotocin induced diabetes. Dev Immunol. 2001;8:213–21. doi: 10.1155/2001/37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Whitehouse MW. Anti-TNF-alpha therapy for chronic inflammation: reconsidering pentoxifylline as an alternative to therapeutic protein drugs. Inflammopharmacology. 2004;12:223–7. doi: 10.1163/1568560042342293. [DOI] [PubMed] [Google Scholar]
- 213.Wallis RS, Johnson JL, Okwera A, Nsubuga P, Whalen CC, Mugerwa RD, Ellner JJ. Pentoxifylline in human immunodeficiency virus- positive tuberculosis: safety at 4 years. J Infect Dis. 1998;178:1861. doi: 10.1086/314528. [DOI] [PubMed] [Google Scholar]
- 214.Wallis RS, Nsubuga P, Whalen C, Mugerwa RD, Okwera A, Oette D, Jackson JB, Johnson JL, Ellner JJ. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J Infect Dis. 1996;174:727–33. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]
- 215.Dezube BJ, Sherman ML, Fridovich-Keil JL, Allen-Ryan J, Pardee AB. Down-regulation of tumor necrosis factor expression by pentoxifylline in cancer patients: a pilot study. Cancer Immunol Immunother. 1993;36:57–60. doi: 10.1007/BF01789132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Breuille D, Farge MC, Rose F, Arnal M, Attaix D, Obled C. Pentoxifylline decreases body weight loss and muscle protein wasting characteristics of sepsis. Am J Physiol. 1993;265:E660–6. doi: 10.1152/ajpendo.1993.265.4.E660. [DOI] [PubMed] [Google Scholar]
- 217.Mora C, Macia M, Navarro JF. Renal failure, anaemia, cytokines and inflammation. Nephrol Dial Transplant. 2001;16:1524–5. doi: 10.1093/ndt/16.7.1524. [DOI] [PubMed] [Google Scholar]
- 218.Porter MH, Hrupka BJ, Altreuther G, Arnold M, Langhans W. Inhibition of TNF-alpha production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2113–20. doi: 10.1152/ajpregu.2000.279.6.R2113. [DOI] [PubMed] [Google Scholar]
- 219.Thorne PS, McCray PB, Howe TS, O’Neill MA. Early-onset inflammatory responses in vivo to adenoviral vectors in the presence or absence of lipopolysaccharide-induced inflammation. Am J Respir Cell Mol Biol. 1999;20:1155–64. doi: 10.1165/ajrcmb.20.6.3632. [DOI] [PubMed] [Google Scholar]
- 220.McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999;52:465–77. doi: 10.1054/mehy.1997.0684. [DOI] [PubMed] [Google Scholar]
- 221.Oberyszyn TM, Tober KL, Ross MS, Robertson FM. Inhibitory effects of pentoxifylline on ultraviolet B light-induced cutaneous inflammation. Mol Carcinog. 1998;22:16–25. [PubMed] [Google Scholar]
- 222.Swain MG, Appleyard CB, Wallace JL, Maric M. TNF-alpha facilitates inflammation-induced glucocorticoid secretion in rats with biliary obstruction. J Hepatol. 1997;26:361–8. doi: 10.1016/s0168-8278(97)80053-0. [DOI] [PubMed] [Google Scholar]
- 223.Guarnieri G, Toigo G, Situlin R, Ciocchi B, Biolo G. Modulation of protein kinetics in chronic renal failure. Mineral and Electrolyte Metabolism. 1997;23:214–217. [PubMed] [Google Scholar]
- 224.Guarnieri G, Toigo G, Fiotti N, Ciocchi B, Situlin R, Giansante C, Vasile A, Carraro M, Faccini L, Biolo G. Mechanisms of malnutrition in uremia. Kidney International. 1997;52:S41–S44. [PubMed] [Google Scholar]
- 225.Zhang M, Xu YJ, Saini HK, Turan B, Liu PP, Dhalla NS. Pentoxifylline attenuates cardiac dysfunction and reduces TNF-alpha level in ischemic-reperfused heart. Am J Physiol Heart Circ Physiol. 2005;289:H832–9. doi: 10.1152/ajpheart.00178.2005. [DOI] [PubMed] [Google Scholar]
- 226.Sliwa K, Woodiwiss A, Libhaber E, Zhanje F, Libhaber C, Motara R, Essop R. C-reactive protein predicts response to pentoxifylline in patients with idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2004;6:731–4. doi: 10.1016/j.ejheart.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 227.Sliwa K, Woodiwiss A, Kone VN, Candy G, Badenhorst D, Norton G, Zambakides C, Peters F, Essop R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation. 2004;109:750–5. doi: 10.1161/01.CIR.0000112568.48837.60. [DOI] [PubMed] [Google Scholar]
- 228.Sliwa K, Woodiwiss A, Candy G, Badenhorst D, Libhaber C, Norton G, Skudicky D, Sareli P. Effects of pentoxifylline on cytokine profiles and left ventricular performance in patients with decompensated congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;90:1118–22. doi: 10.1016/s0002-9149(02)02779-0. [DOI] [PubMed] [Google Scholar]
- 229.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2002;4:305–9. doi: 10.1016/s1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 230.Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation. 2001;103:1083–8. doi: 10.1161/01.cir.103.8.1083. [DOI] [PubMed] [Google Scholar]
- 231.Skudicky D, Sliwa K, Bergemann A, Candy G, Sareli P. Reduction in Fas/APO-1 plasma concentrations correlates with improvement in left ventricular function in patients with idiopathic dilated cardiomyopathy treated with pentoxifylline. Heart. 2000;84:438–9. doi: 10.1136/heart.84.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P. Randomised investigation of effects of pentoxifylline on left-ventricular performance in idiopathic dilated cardiomyopathy. Lancet. 1998;351:1091–3. doi: 10.1016/S0140-6736(97)09338-0. [DOI] [PubMed] [Google Scholar]
- 233.Kremsner PG, Grundmann H, Neifer S, Sliwa K, Sahlmuller G, Hegenscheid B, Bienzle U. Pentoxifylline prevents murine cerebral malaria. J Infect Dis. 1991;164:605–8. doi: 10.1093/infdis/164.3.605. [DOI] [PubMed] [Google Scholar]
- 234.Bahrmann P, Hengst UM, Richartz BM, Figulla HR. Pentoxifylline in ischemic, hypertensive and idiopathic-dilated cardiomyopathy: effects on left-ventricular function, inflammatory cytokines and symptoms. Eur J Heart Fail. 2004;6:195–201. doi: 10.1016/j.ejheart.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 235.Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol. 2006;26:118–33. doi: 10.1016/j.semnephrol.2005.09.005. [DOI] [PubMed] [Google Scholar]


