Abstract
Introduction
The aim of this study was to evaluate efficacy of maintenance sunitinib after first-line chemotherapy for stage IIIB/IV NSCLC.
Methods
Cancer and Leukemia Group B 30607 trial was a randomized, double-blind, placebo-controlled, phase III study that enrolled patients without progression after four cycles of first-line platinum-based doublet chemotherapy with or without bevacizumab. Bevacizumab was allowed only during the four cycles of chemotherapy. Patients were randomized to receive sunitinib, 37.5 mg/d, or placebo and were treated until unacceptable adverse event(s), progression, or death. The primary end point was progression-free survival (PFS).
Results
A total of 210 patients were enrolled, randomized, and included in the intent-to-treat analysis. Ten patients did not receive maintenance therapy (four who received placebo and six who received sunitinib). Grade 3/4 adverse events affecting more than 5% of the patients were fatigue (25%), thrombocytopenia (12%), hypertension (12%), rash (11%), mucositis (11%), neutropenia (7%), and anemia (6%) for sunitinib and none for placebo. There were three grade 5 events in patients receiving sunitinib (one pulmonary hemorrhage, one other pulmonary event, and one death not associated with a Common Terminology Criteria for Adverse Events term) and two grade 5 events in patients receiving placebo (one other pulmonary event and one thromboembolism). Median PFS was 4.3 months for sunitinib and 2.6 months for placebo (hazard ratio = 0.62, 95% confidence interval: 0.47–0.82, p = 0.0006). Median overall survival was 11.7 months for sunitinib versus 12.1 months for placebo (hazard ratio = 0.98, 95% confidence interval: 0.73–1.31, p = 0.89).
Conclusions
Maintenance sunitinib was safe and improved PFS as maintenance therapy in stage IIIB/IV NSCLC but had no impact on overall survival. There is no room for future investigations of sunitinib in this setting.
Keywords: Sunitinib, Maintenance, NSCLC, Tyrosine kinase inhibitor
Introduction
Lung cancer affects more than 220,000 people in the United States annually and is the leading cause of cancer death.1 Platinum-based two-agent chemotherapy as initial therapy is the standard of care in patients with advanced-stage disease without actionable mutations. At the time of this trial’s initiation in 2008, the role of maintenance cytotoxic therapy in stage IIIB/IV NSCLC had not yet been established, and it remained controversial2 during the course of the trial’s accrual. Multiple trials investigated the use of maintenance therapy in an attempt to prolong progression-free survival (PFS) and improve overall survival (OS).
Sunitinib, a tyrosine kinase inhibitor that targets platelet derived growth factor receptors, vascular endothelial growth factor receptors, KIT proto-oncogene receptor tyrosine kinase receptors, and fms related tyrosine kinase 3 receptors,3 was explored as an oral antiangiogenic agent. It demonstrated single-agent activity in the setting of second- or third-line treatment of recurrent NSCLC,4 with overall response rates (ORRs) of 11.1% and a median PFS of 12.0 weeks. A small phase II study reported that there may be some value with the use of sunitinib as maintenance therapy after first-line therapy, but the primary end point of improved 1-year survival was not met.5 However, the trial design allowed some patients to proceed to maintenance therapy without completing four cycles of first-line chemotherapy and/or with progressive disease after first-line therapy. Because of the promising nature of the data, Cancer and Leukemia Group B (CALGB) 30607, a large double-blind, placebo-controlled phase III study, was undertaken. CALGB is now part of the Alliance for Clinical Trials in Oncology, a part of the National Cancer Institute National Clinical Trials Network.
During the conduct of CALGB 30607 (2008–2013), pemetrexed, erlotinib, and bevacizumab were approved as maintenance therapy, although controversy continued regarding their impact on survival.2 Because of limitations of the other agents in certain patient populations (those with the squamous histologic type, those who required use of nonsteroidal anti-inflammatory medication, those with decreased renal function, and those with uncontrolled third-space fluid collections), it was important to continue to investigate other agents in the maintenance setting.
Patients and Methods
Patients
Eligible patients had histologic or cytological documentation of stage IIIB/IV NSCLC. Patients must have received one chemotherapy regimen including four cycles of platinum-based doublet chemotherapy with or without bevacizumab and no other primary or prior treatment for NSCLC. Bevacizumab was allowed only during the four cycles of chemotherapy. Patients must have achieved a complete response (CR), partial response (PR), or stable disease to first-line chemotherapy with no evidence of disease progression. They may have had measurable or nonmeasurable disease. Eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 to 1 and standard initial laboratory test results showing adequate hematologic status (granulocyte count ≥1500/μL and platelet count ≥100,000/μL), liver function (bilirubin level ≤1.5 of the upper limit of normal and aspartate transaminase and alanine transaminase levels ≤2.5 of the upper limits of normal), and renal function (creatinine level ≤1.5 mg/dL). Patients were not eligible if they had any of the following: cavitary lesions; symptomatic or untreated brain metastases, spinal cord compression, or carcinomatous meningitis; abdominal fistula, gastrointestinal perforation, intra-abdominal abscess, serious or nonhealing wounds, or ulcer or bone fracture; and uncontrolled hypertension. Patients with bleeding disorders or significant hemoptysis and those requiring therapeutic anticoagulation were also excluded. Patients had to be able to take oral medication and could not require any cytochrome P450 family 3 subfamily A member 4 inhibitors or inducers.
Enrollment
Enrollment and registration were managed by Alliance Statistics and Data Center (Durham, NC). Each participant signed an institutional review board– approved, protocol-specific informed consent form in accordance with federal and institutional guidelines. All enrolled patients were also offered participation in two substudies: CALGB 70701 (Quality of Life [QOL] Studies in CALGB 30607) and CALGB 60702 (Pharmacogenetic Studies in CALGB 30607). Patients were enrolled and treated on study at Alliance member institutions and other National Clinical Trials Network member sites.
Treatment Plan
Patients were registered 3 to 5 weeks after day 1 of cycle 4 of prior therapy and then randomly assigned 1:1 in a double-blind fashion to receive maintenance sunitinib, 37.5 mg/d continuously, or placebo until disease progression or intolerable toxicity. Patients were stratified by performance status (0 versus 1), stage (IIIB versus IV), prior use of bevacizumab (yes or no), and sex (male versus female). Patients experiencing reversible grade 3 or 4 toxicity had sunitinib held until toxicity had decreased to grade 2 or lower, and sunitinib was restarted or dose-reduced to 25 mg/d continuously or 25 mg/d for 4 of 6 weeks. Sunitinib was permanently discontinued in patients who experienced recurrent or irreversible grade 3 or higher toxicity, grade 4 hypertension, grade 3 or higher hemorrhage, a grade 3 or higher cardiac event, or other severe toxicity.
A comprehensive history and physical examination (including assessment of performance status) were performed before enrollment and before each treatment cycle. Complete blood counts, serum chemistry tests, and liver function tests were also performed at each visit. Patients were reassessed by Response Evaluation Criteria in Solid Tumors, version 1.0, after every two cycles (6 weeks) and at the completion of therapy.
For patients who consented to the pharmacogenetic substudy, additional blood samples were drawn at three time points (before treatment and at the start of cycles 2 and 3) to evaluate for vascular endothelial growth factor genotyping and plasma vascular endothelial growth factor concentrations. For patients who consented to the QOL substudy, patients were asked to complete three questionnaires (European Organization for Research and Treatment of Cancer [EORTC] Quality of Life Questionnaire [QLQ]–Core 30, EORTC QLQ–Lung Cancer 13, and European Quality of Life Five Dimensions) at the start of each 3-week cycle.
Trial Design and Statistical Considerations
The trial was designed as a randomized, double-blind, placebo-controlled phase III trial of sunitinib as maintenance therapy with a primary objective to evaluate the effect of sunitinib (S) on PFS compared with that of placebo (P) in patients with stage IIIB/IV NSCLC. Secondary objectives assessed included toxicity, ORR, OS, and time to QOL or symptom deterioration. With a total of 232 eligible patients (116 patients per arm) to be accrued and use of a log-rank test at a two-sided significance level of 0.05, the study had approximately 90% power to reject the null hypothesis of λS/λP equal to 1 and accept the alternative hypothesis of λS/λP less than 1 when the true λS/λP equaled 0.647. A stratified permuted block randomization was used to restrict any significant imbalance of the stratification factors (performance status, stage, use of bevacizumab, and sex) between the two study arms. The trial was designed with a two-stage stopping rule for grade 3 or higher dose-limiting toxicity among the first 42 patients (10 of 42) treated in the sunitinib arm. After 65 events had been observed, formal interim analyses were to be conducted semiannually for early stopping for superiority and for futility on the basis of PFS.
PFS was defined as the time from the date of randomization to the date of disease progression or death from any cause, whichever came first. Progression was defined by Response Evaluation Criteria in Solid Tumors, version 1.0. OS was defined as the time from the date of randomization to death from any cause; living patients were censored at the date of last follow-up. For efficacy end points, all randomized patients were included in the intention-to-treat analysis. Kaplan-Meier curves6 were used to characterize PFS and OS. Median survival times and their 95% confidence intervals (CIs) were computed. A Cox proportional hazards model7 was used to estimate the hazard ratios (HRs) and their 95% CIs. The reported p values were two sided. Only the p value testing PFS between the two treatment arms in the intention-to-treat analysis was regarded as confirmatory.
Data collection was managed by the Alliance Statistics and Data Center. Data quality was ensured by careful review by Alliance Statistics and Data Center staff and the study chairperson following Alliance policies. Analysis of data was performed by Alliance statisticians using SAS 9.4 software (SAS Institute, Inc., Cary, NC). For tabulation of safety data, all patients for whom data on adverse events had been submitted during treatment were included. Adverse events were recorded by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. This phase III therapeutic trial was monitored at least twice annually by the Data and Safety Monitoring Board (DSMB), a standing committee composed of individuals from within and outside of the Alliance. As part of the quality assurance program, Alliance audit committee members visit all participating institutions at least once every 3 years to review source documents and verify compliance with federal regulations and protocol requirements.
Results
CALGB 30607 was activated in June 2008 and closed in November 2013; it accrued 210 patients. The adverse events reported in the first 42 patients receiving sunitinib were proved safe and did not cross the stopping rule. Beginning in July 2010 (after 65 events had been observed), formal interim analyses based on PFS were conducted semiannually for possible early stopping because of superiority and futility. At the interim analysis performed on October 7, 2013, the superiority test based on PFS was crossed (one-sided p = 0.0004) for the HR. With clear evidence that patients receiving sunitinib had prolonged PFS, the Alliance DSMB decided on November 11, 2013, to terminate its new patient accrual, and patients were unblinded and informed of their treatment assignment. For patients randomized to the sunitinib arm, continuation of sunitinib was recommended; however, the Alliance DSMB did not recommend crossover to sunitinib for patients randomized to receive placebo because of the magnitude of benefit. Trial accrual was terminated early and data based on the interim analysis of 210 patients were released. Data for the final analysis were locked on January 30, 2015. The median time of follow-up of the surviving patients was 20.6 months, with a range of 6.3 to 60.9 months. Of 210 enrolled patients, 200 received maintenance therapy, with 100 receiving placebo and 100 receiving sunitinib. Ten randomly assigned patients (four in the placebo arm and six in the sunitinib arm) did not receive maintenance therapy as a result of early disease progression, adverse event, or refusal. Baseline characteristics for the patients are listed in Table 1. Most of the patients were male (56%) and white (82%), with stage IV disease (88%) and no previous treatment with bevacizumab (78%). Both the adenocarcinoma (46%) and squamous (33%) histologic types were common. The median age of the patients was 66 years (range 25–89). There were no significant differences in baseline characteristics between the two arms (p > 0.05 for those baseline factors).
Table 1.
Patient Characteristics
| Characteristic | Placebo (n=104) |
Sunitinib (n=106) |
Total (n = 210) |
|---|---|---|---|
| Age, y | |||
| Mean ± SD | 66.3 ± 9.3 | 63.6 ± 10.0 | 64.9 ± 9.7 |
| Median | 67.0 | 65.0 | 66.0 |
| Q1, Q3 | 58.5, 73.0 | 57.0, 70.0 | 58.0, 72.0 |
| Range | 44.0–89.0 | 25.0–84.0 | 25.0–89.0 |
| Race, n (%) | |||
|
| |||
| White | 85 (81.7%) | 87 (82.1%) | 172 (81.9%) |
| Black | 13 (12.5%) | 18 (17.0%) | 31 (14.8%) |
| Asian | 3 (2.9%) | 0 (0.0%) | 3 (1.4%) |
| Other | 3 (2.9%) | 1 (0.9%) | 4 (1.9%) |
|
| |||
| Sex, n (%) | |||
| Female | 44 (42.3%) | 49 (46.2%) | 93 (44.3%) |
| Male | 60 (57.7%) | 57 (53.8%) | 117 (55.7%) |
|
| |||
| Performance status, n (%) | |||
| 0 | 42 (40.4%) | 40 (37.7%) | 82 (39.0%) |
| 1 | 62 (59.6%) | 66 (62.3%) | 128 (61.0%) |
|
| |||
| Stage, n (%) | |||
| IIIB | 12 (11.5%) | 14 (13.2%) | 26 (12.4%) |
| IV | 92 (88.5%) | 92 (86.8%) | 184 (87.6%) |
|
| |||
| Prior use of bevacizumab, n (%) | |||
| No | 80 (76.9%) | 83 (78.3%) | 163 (77.6%) |
| Yes | 24 (23.1%) | 23 (21.7%) | 47 (22.4%) |
|
| |||
| Histologic type, n (%) | |||
| Adenocarcinoma (including BAC) | 43 (41.3%) | 53 (50.0%) | 96 (45.7%) |
| Squamous cell | 39 (37.5%) | 31 (29.2%) | 70 (33.3%) |
| Undifferentiated NSCLC | 13 (12.5%) | 15 (14.2%) | 28 (13.3%) |
| Large cell | 5 (4.8%) | 4 (3.8%) | 9 (4.3%) |
| Other | 4 (3.8%) | 3 (2.8%) | 7 (3.3%) |
|
| |||
| Smoking history, n (%) | |||
| Nonsmoker | 10 (9.6%) | 5 (4.7%) | 15 (7.1%) |
| Past smoker | 67 (64.4%) | 76 (71.7%) | 143 (68.1%) |
| Current smoker | 27 (26.0%) | 25 (23.6%) | 52 (24.8%) |
Q, quartile.
Maintenance Efficacy
The median PFS (Fig. 1A) was 2.6 months for placebo (95% CI: 1.8–3.0) versus 4.3 months for sunitinib (95% CI: 3.2–4.9). The two-sided log-rank test p value for PFS was 0.0006 (HR = 0.62, 95% CI: 0.47–0.82). Therefore, the primary end point of improved PFS was met. OS (Fig. 1B) was 12.1 months for placebo (95% CI: 9.8–15.3) versus 11.7 months for sunitinib (95% CI: 9.9–14.0). The two-sided log-rank test p value for OS was 0.89 (HR = 0.98, 95% CI: 0.73–1.31). The effect on PFS and OS did not differ by histologic type (Supplementary Figs. 1–4). Multivariate analysis of PFS showed two significant variables (Fig. 2A): sunitinib versus placebo (HR = 0.60, 95% CI: 0.45–0.80) and age (HR = 0.98, 95% CI: 0.96–1.00). There was no significant association of PFS with sex, race, performance status, stage, histologic type, smoking status, or previous bevacizumab treatment. ORR, defined as CR or PR, was 11.0% in the sunitinib arm and 5.0% in the placebo arm, but this difference was not statistically significant (p = 0.19). All of the patients in the placebo arm who responded (five patients) had previously responded to initial chemotherapy (four with a CR or PR and one with stable disease). Disease progression was the most frequent reason for patients discontinuing treatment, with 90.1% of the patients receiving placebo progressing versus 48.1% of the patients receiving sunitinib.
Figure 1.
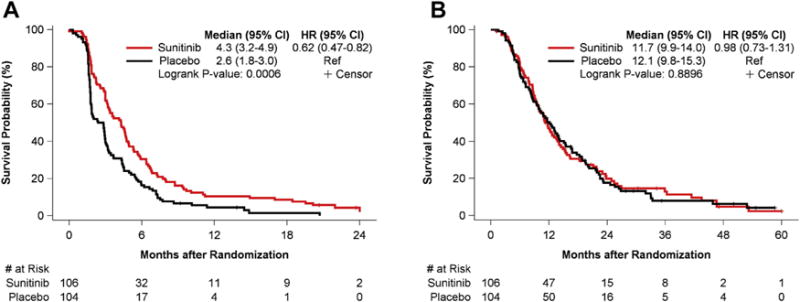
Kaplan-Meier curves of progression-free survival (A) and overall survival (B) for all patients. Abbreviations: HR, hazard ratio; CI, confidence interval; Ref, reference.
Figure 2.
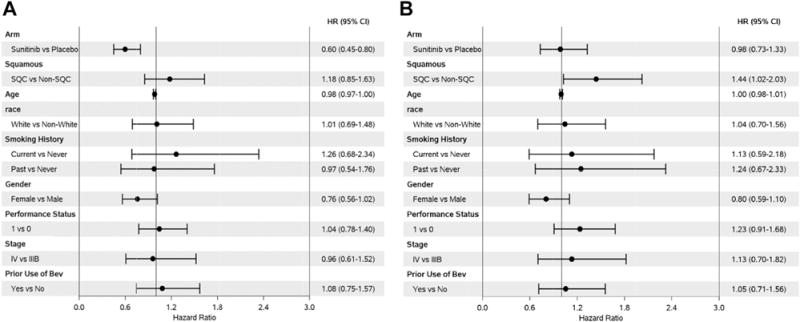
Cox proportional hazard multivariate analysis based on progression-free survival (A) and overall survival (B). Abbreviations: HR, hazard ratio; CI, confidence interval; SQC, squamous cell carcinoma.
Toxicity
One patient in each arm received maintenance therapy and failed to have adverse events evaluated. The remaining 198 patients (99 in each arm) were included in the adverse event analysis. Table 2 summarizes hematologic and nonhematologic adverse events. Overall, sunitinib was well tolerated. Grade 3/4 adverse events affecting more than 5% of the patients were fatigue (25%), thrombocytopenia (12%), hypertension (12%), rash (11%), mucositis (11%), neutropenia (7%), and anemia (6%) for the sunitinib arm and none for the placebo arm. There were three grade 5 events in the sunitinib arm (one pulmonary hemorrhage, one other pulmonary event, and one death not associated with a Common Terminology Criteria for Adverse Events term) and two grade 5 events in the placebo arm (one other pulmonary event and one thromboembolism). The most frequent and statistically significant toxicities of any grade for sunitinib compared with for placebo were fatigue (71%), diarrhea (44%), and nausea (41%) (p < 0.05). There was also a statistically significant increase in the prevalence of all grades of anorexia, mucositis, rash, hypertension, thrombocytopenia, anemia, neutropenia, vomiting, and pulmonary hemorrhage in the sunitinib arm (p < 0.05). These toxicities were generally easily managed medically or with dose interruption or modification; 83 patients in the sunitinib arm underwent sunitinib dose modifications, either planned (i.e., 56 patients on account of an adverse event), unplanned (17 patients), or planned in some cycles and unplanned in other cycles (10 patients). These dose modifications were determined to be planned if the patient had a toxicity in a previous cycle of therapy that required a modification in a subsequent cycle. These dose modifications were determined to be unplanned if a patient had a toxicity in a current cycle that required modification in the same cycle of therapy.
Table 2.
Hematologic and Nonhematologic Toxicity
| Toxicity | Placebo, % (n = 99) |
Sunitinib, % (n = 99) |
||
|---|---|---|---|---|
| All Grade | Grade III/V | All Grade | Grade III/V | |
| Hematologic | ||||
| Neutropenia | 1 | 1/0 | 12a | 7/0a |
| Thrombocytopenia | 1 | 0/0 | 18a | 11/1a |
| Anemia | 1 | 0/0 | 14a | 6/0a |
|
| ||||
| Nonhematologic | ||||
| Nausea | 18 | 1/0 | 41a | 2/1 |
| Vomiting | 1 | 1/0 | 7a | 0/1 |
| Mucositis | 5 | 0/0 | 35a | 11/0a |
| Diarrhea | 12 | 0/0 | 44a | 2/0 |
| Anorexia | 15 | 0/0 | 36a | 3/0 |
| Rash | 8 | 0/0 | 28a | 11/0a |
| Neuropathy | 2 | 0/0 | 5 | 2/0 |
| Hypertension | 9 | 0/0 | 27a | 12/0a |
| Fatigue | 56 | 4/0 | 71a | 23/2a |
| Hemorrhage, GI | 0 | 0/0 | 3 | 3/0 |
| Hemorrhage, pulmonary | 1 | 0/0 | 8a | 0/0 |
| Hypothyroidism | 3 | 0/0 | 6 | 0/0 |
Note: There were three grade 5 events in patients receiving sunitinib (one pulmonary hemorrhage, one other pulmonary event, and one death not associated with a Common Terminology Criteria for Adverse Events term). There were two grade 5 events in patients receiving placebo (one other pulmonary event and one thromboembolism).
p < 0.05.
GI, gastrointestinal.
QOL Assessment
For the QOL substudy (Supplementary Table 1), patients were asked to complete three questionnaires (EORTC QLQ–Core 30, EORTC QLQ–Lung Cancer 13, and European Quality of Life Five Dimensions) at the start of each cycle. A total of 179 patients (85%) reported QOL data for at least one time point, with 163 (78%) at baseline, 121 (58%) at 3 months, and 43 (20%) at 6 months. The demographics of the 179 patients with QOL measures were similar between arms except that those in the sunitinib arm had a median age 3 years younger (p = 0.02) and retired patients were more likely to report QOL components (p = 0.005). Patients in the sunitinib arm reported significantly worse problems with appetite, diarrhea, nausea/vomiting, dyspnea, and sore mouth or tongue at 3 months (p < 0.05). They also had significantly worse cognition and overall QOL (p < 0.05), but this QOL difference was not large enough to indicate a difference in quality-adjusted survival (p > 0.33).
Discussion
This trial of sunitinib as an antiangiogenic agent in maintenance therapy met its primary objective. It showed statistically significant improvement in PFS versus that with placebo after four cycles of standard platinum-based doublet chemotherapy for untreated advanced NSCLC. Subgroup analysis revealed that the benefit of sunitinib existed in patients with non-squamous and squamous disease. It is unclear whether there was any effect on PFS or other secondary objectives by molecular profiling because biomarker analysis of the tumors was not performed given the time of the trial’s activation in 2008.
Secondary objectives included evaluation of OS, ORR, toxicity, and QOL. There was no significant difference in OS. Single-agent activity of sunitinib was noted, with an ORR of 11.0% and CR in three patients. This was not statistically significant compared with the placebo arm.
Patients receiving sunitinib did relatively well, with no new toxicity signals. Problems with appetite, diarrhea, nausea/vomiting, dyspnea, fatigue, and sore mouth or tongue were significantly increased compared with in the placebo arm, leading 27.9% of the patients to discontinue sunitinib treatment. There was no increased incidence of hemorrhage or fistula formation. In addition to this side effect profile, patients treated with sunitinib had significantly worse cognition and overall QOL, but this did not result in a difference in quality-adjusted survival.
Most of the patients were discontinued from treatment on account of disease progression. There was a statistically significant difference between those who went on to receive another line of therapy after progression (placebo 82% versus sunitinib 64% [p = 0.002]). This difference in subsequent therapy may reflect the toxicity differences between the two arms and may also account for the lack of difference in OS found in this trial. Another concern is the discontinuation of bevacizumab upon study entrance and its possible effect on response in the placebo arm. However, as both arms were well matched for previous use of bevacizumab with initial chemotherapy (21.7% versus 23.1%), this is likely not a significant factor.
This trial was activated in June 2008 and closed in November 2013. At the time of activation, the role of maintenance chemotherapy had not yet been established. During the course of the study, two agents were approved by the U.S. Food and Drug Administration for maintenance therapy. Pemetrexed was approved in July 2009 for switch maintenance therapy after a randomized phase III study8 showed statistically significant improvements in PFS and OS in patients with nonsquamous NSCLC. This approval was expanded in October 2012 for continuation maintenance with the PARAMOUNT study,9 which demonstrated improved median PFS and reduction in the risk for disease progression. Erlotinib also improved PFS and OS in the maintenance setting in unselected patients with squamous and nonsquamous histologic types10 and was approved for maintenance therapy in April 2010. Bevacizumab had been previously approved in 2006 as part of first-line combination therapy that was then continued as single-agent therapy, but it was not specifically studied for continuation maintenance therapy.11
Despite these approvals, there was some controversy raised regarding the role of maintenance therapy. Although PFS improvement was clearly seen, some concerns2 were raised regarding the impact on OS, QOL, and financial toxicity. One concern was whether the patients in the control groups in those trials served as an adequate comparison because many (up to 60%) did not receive any therapy upon progression. This would be an unusually high percentage because the patients in these trials would have had either stable or responding disease, good performance status, and normal organ function to meet eligibility criteria. With regard to those who did receive second-line therapy, it was difficult to quantify which specific agents they received to ensure adequacy of treatment. Additionally, there were some patient populations that could not receive these approved agents. For these reasons, we believed that it was important to continue our study of sunitinib. Ultimately, the trial was closed in November 2013, terminating accrual early after interim analysis.
With three agents already approved in the maintenance setting, there could still be a role for sunitinib in maintenance therapy in particular patient populations that may not be able to undergo treatment with the aforementioned three agents owing to restrictions on pemetrexed use (squamous histologic type, decreased creatinine clearance, need for nonsteroidal anti-inflammatory agents, or uncontrolled effusions), erlotinib use (pneumonitis, liver function, diarrhea, or rash), or bevacizumab use (hypertension, proteinuria, hemorrhage risk, or fistula risk). However, because of the absence of a survival signal in the current trial and the current focus on newer agents such as the programmed cell death 1 inhibitors, there are no plans to further investigate the use of sunitinib in the maintenance setting.
Supplementary Material
Figure 3. PFS Curve for Squamous Patients
Figure 4. OS Curve for Squamous Patients
Figure 5. PFS Curve for Non-Squamous Patients
Figure 6. OS Curve for Non-Squamous Patients
Table 3. Mean Change in QOL Subscales from Baseline to 3 and 6 Months by Arm
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA031946, U10CA180821, U10CA033601, and U10CA180882 (to the Alliance for Clinical Trials in Oncology) and U10CA180790, U10CA180833, U10CA180836, U10CA180838, U10CA180844, and U10CA180857. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: Dr. Baggstrom has received research funding from the Academic and Community Center Research United, AstraZeneca, CtyRx, and MedImmune outside the submitted work. Dr. Socinski has received research funding from the University of North Carolina, University of Pittsburgh, and Pfizer and has received honoraria from and functioned in a consulting/advisory role for Genentech outside the submitted work. Dr. Stinchcombe had functioned in a consulting/advisory role for Bristol-Myers Squibb, Celgene, Boehringer Ingelheim, ARIAD, and Helsinn Healthcare and received research funding from Genentech, Bristol-Myers Squibb, and EMD Serono outside the submitted work. Dr. Edelman has received research funding from Eli Lilly, Bristol-Myers Squibb, Merck, Endocyte, Genentech, and ARIAD and functioned in a consulting/advisory role for Eli Lilly, Bristol-Myers Squibb, Merck, Endocyte, Genentech, and ARIAD outside the submitted work. Dr. Baker Jr. has functioned in a consulting/advisory role for Boehringer Ingelheim outside the submitted work. Dr. Feliciano has functioned in a consulting/advisory role for AstraZeneca and received research funding from Merck and Genentech outside the submitted work. Dr. Hahn has received honoraria from Cardinal Health and compensation for travel, accommodations, and expenses from Cardinal Health outside the submitted work. Mr. Crawford has functioned in a consulting/advisory role for Merck, Novartis, and Pfizer; received research funding from Amgen, AstraZeneca, Bayer, Clovis, and GTx; and served as a data and safety monitoring board member of Celgene, G1Therapeutics, Janssen, Merrimack, and Roche outside the submitted work. The remaining authors declare no conflict of interest.
Trial Registration: Sunitinib Malate as Maintenance Therapy in Treating Patients with Stage III or Stage IV Non–Small Cell Lung Cancer Previously Treated with Combination Chemotherapy. NCT00693992
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology. May 30–June 3, 2014; Chicago, IL.
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at http://dx.doi.org/10.1016/j.jtho.2017.01.022.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Edelman MJ, Le Chevalier T, Soria JC. Maintenance therapy and advanced non-small-cell lung cancer: a skeptic’s view. J Thorac Oncol. 2012;7:1331–1336. doi: 10.1097/JTO.0b013e3182629e37. [DOI] [PubMed] [Google Scholar]
- 3.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor B in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2013;2:471–478. [PubMed] [Google Scholar]
- 4.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gervais R, Hainsworth JD, Blais N, et al. Phase II study of sunitinib as maintenance therapy in patients with locally advanced or metastatic non-small cell lung cancer. Lung Cancer. 2011;74:474–480. doi: 10.1016/j.lungcan.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Cox DR. Regression models and life tables. J Royal Stat Soc Ser B. 1971;20:187–220. [Google Scholar]
- 8.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomized, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomized controlled trial. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 10.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomized, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 3. PFS Curve for Squamous Patients
Figure 4. OS Curve for Squamous Patients
Figure 5. PFS Curve for Non-Squamous Patients
Figure 6. OS Curve for Non-Squamous Patients
Table 3. Mean Change in QOL Subscales from Baseline to 3 and 6 Months by Arm


