Abstract
Vertebrates are endowed with a closed circulatory system, the evolution of which required novel structural and regulatory changes. Furthermore, immune cell trafficking paradigms adapted to the barriers imposed by the closed circulatory system. How did such changes occur mechanistically? We propose that spatial compartmentalization of the lipid mediator sphingosine 1-phosphate (S1P) may be one such mechanism. In vertebrates, S1P is spatially compartmentalized in the blood and lymphatic circulation, thus comprising a sharp S1P gradient across the endothelial barrier. Circulatory S1P has critical roles in maturation and homeostasis of the vascular system as well as in immune cell trafficking. Physiological functions of S1P are tightly linked to shear stress, the key biophysical stimulus from blood flow. Thus, circulatory S1P confinement could be a primordial strategy of vertebrates in the development of a closed circulatory system. This review discusses the cellular and molecular basis of the S1P gradients and aims to interpret its physiological significance as a key feature of the closed circulatory system.
Keywords: sphingolipid, lipid mediators, G protein-coupled receptor, vascular biology, angiogenesis, immune cell trafficking, HDL, lipid chaperones
INTRODUCTION
In contrast to the open circulatory system of invertebrates, that of vertebrates warranted the efficient transport of nutrients and gases to various organs as well as precise control of such processes. This required drastic changes in vascular structure, control mechanisms, and the development of novel hematopoietic cell trafficking paradigms. Vertebrates developed a true endothelium, a continuous layer of endothelial cells (ECs) connected by specialized junctional complexes. ECs are ensheathed with mural cells, namely pericytes and vascular smooth muscle cells (VSMCs), which allow specialized properties of organ-specific ECs (1). The closed system also necessitated that the vessels withstand the high pressure, whereas the permeability of solutes and fluids is finely controlled to enable rapid and selective exchange between blood and tissues. In addition, precise organ-specific regulation of blood flow is needed. Furthermore, new immune cell trafficking systems are developed in a site- and context-specific manner.
Sphingosine 1-phosphate (S1P) is synthesized intracellularly by the sphingosine kinase (SphK) enzyme(s)-dependent phosphorylation of sphingosine. Many species of yeast, plants, invertebrates such as Caenorhabditis elegans, and vertebrates produce S1P as a key metabolic intermediate linking sphingolipids to glycerophospholipids (2). However, vertebrates (and perhaps chordates) started to use S1P as an extracellular bioactive lipid mediator by activating G protein-coupled receptors (GPCRs) localized in the plasma membrane of target cells (3). In mammals, S1P is spatially compartmentalized in the blood (~1 µM) and lymph (~0.1 µM) circulation. Meanwhile, it is estimated to be much lower in interstitial fluids of tissues (<1 nM), creating a sharp S1P gradient across the endothelial barrier (4, 5).
Why and how do vertebrates develop this circulatory S1P gradient? Recent studies have begun to unravel the complex mechanisms underlying the establishment and maintenance of the circulatory S1P gradient. In addition, critical functions of circulatory S1P in the maturation and homeostasis of the vascular system are now appreciated. Notably, the regulation and function of S1P are tightly linked to blood flow, a major biophysical stimulus of the vascular system (6, 7). An S1P gradient is also critical for the immune cell trafficking between peripheral tissues, lymph, and blood circulation (4, 8). Thus, circulatory S1P confinement seems to be a primordial strategy of vertebrates to adapt to the closed circulatory system. In this review, we summarize the current state of knowledge of the cellular and molecular basis for a circulatory S1P gradient. We also aim to interpret its physiological significance in vascular development and maintenance as well as immune cell trafficking. We discuss recent studies that reveal the spatial S1P distribution patterns in vascular and extravascular microenvironments.
CIRCULATORY COMPARTMENTALIZATION OF SPHINGOSINE 1-PHOSPHATE
S1P is present in a variety of species and cell types. How do vertebrates achieve the compartmentalized enrichment of S1P in the circulatory system? Recent advances in conditional knockout technology together with identification of S1P transporters and chaperones have begun to unravel the mechanisms involved.
Cellular Sources of Blood S1P
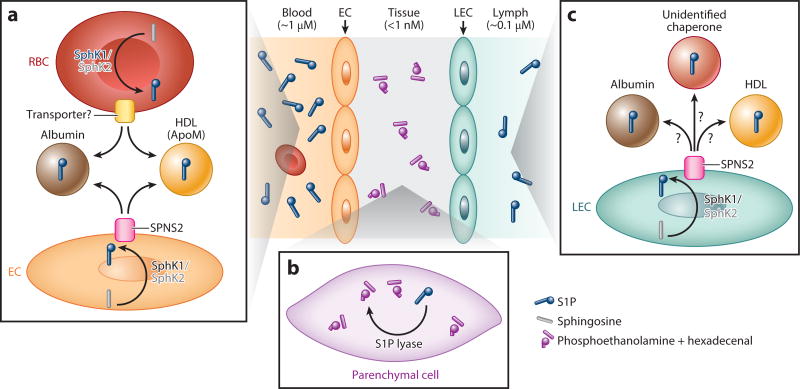
Red blood cells (RBCs) and ECs are the major sources of plasma S1P (Figure 1a). Significant progress toward understanding the cellular basis of circulatory S1P was made by the establishment of a mouse strain lacking S1P in plasma by the conditional gene deletion of two SphK enzymes, Sphk1 and Sphk2 (pS1Pless mice) (9). Adoptive transfer of wild-type RBCs to pS1Pless mice restored plasma S1P to the normal levels (9).In addition, a 30% reduction of plasma S1P levels was seen in irradiated wild-type mice reconstituted with RBC-specific Sphk1/Sphk2 double knockout fetal liver (10). The contribution of nonhematopoietic cells, especially of ECs, to plasma S1P was implicated by hematopoietic cell depletion experiments and cell culture experiments (11, 12). Accordingly, EC-specific Sphk1/Sphk2 double knockout mice displayed a 30% reduction of plasma S1P levels (10).
Figure 1.
Circulatory compartmentalization of S1P. (a) RBCs and ECs are the major sources of plasma S1P. In RBCs, S1P is synthesized from sphingosine mainly by SphK1. Intracellular S1P is secreted by S1P transporter(s). In ECs, S1P is produced mainly by SphK1 and secreted by SPNS2. In plasma, more than half of S1P is found in the ApoM-positive HDL fraction, and 30–40% is found in the albumin fraction. (b) In tissues, high S1P lyase activity and irreversible degradation of S1P facilitate their low S1P levels. (c) LECs are major sources of lymphatic S1P. S1P is synthesized mainly by SphK1 and secreted through SPNS2. S1P carriers in lymphatic circulation are not yet elucidated. Abbreviations: ApoM, apolipoprotein M; EC, endothelial cell; HDL, high-density lipoprotein; LEC, lymphatic endothelial cell; RBC, red blood cell; S1P, sphingosine 1-phosphate; SphK1, sphingosine kinase; SPNS2, spinster homolog 2.
Platelets may also participate in the circulatory enrichment of plasma S1P, especially under nonhomeostatic conditions (13). Platelets have high SphK activity (14) and lack S1P lyase that irreversibly degrades S1P (15, 16). In addition, S1P release was enhanced by platelet activation (15–17). Serum S1P levels are consistently higher than those in plasma (13). However, platelets are unlikely to contribute to plasma S1P levels under homeostasis because mice that lack platelets display normal plasma S1P levels (9). Acute platelet depletion by antibodies against platelet glycoprotein GPIba also failed to change plasma S1P levels (12). Consistently, Sphk2 knockout mice lacking platelet S1P did not show plasma S1P reduction (18). Instead, local exaggerated generation of S1P by platelets may regulate platelet activation or thrombus formation (18). Importantly, local S1P production by platelets is also essential for the barrier function of high endothelial venules (HEVs), a specialized vasculature through which lymphocytes home to lymph nodes (LNs) and secondary lymphoid organs (SLOs), as discussed below. Additional cellular sources of circulatory S1P may exist and await further conclusive experiments using tissue-specific SphK enzyme knockouts.
Two SphK enzymes, SphK1 and SphK2, show unique contributions to blood S1P levels. Although these enzymes show redundant functions, they display differential tissue distribution and subcellular localization. Pharmacological or genetic targeting of SphK1 caused a >50% decrease in circulatory S1P (18–23). Surprisingly, elevated circulatory S1P levels (1.5- to 4-fold) were observed in Sphk2 knockout mice (20, 21, 23–27). Acute treatment with SphK2 inhibitors increased blood S1P levels (1.5- to 3-fold) in wild-type mice (23, 27). However, ablation of both Sphk1 and Sphk2 resulted in the complete loss of plasma S1P, as mentioned above (9). SphK2 inhibitor also substantially reduced blood S1P levels in Sphk1 knockout mice (23, 27). Therefore, SphK1 is necessary for the SphK2 inhibition-dependent increase of plasma S1P.
How can these observations be explained? It is conceivable that SphK2 blockage may lead to compensatory upregulation of Sphk1 expression in select cell types. Indeed, some cell types or tissues from Sphk2 knockout mice displayed substantially increased Sphk1 expression (10, 26). However, this might not be the sole mechanism for the SphK2 blockage effects. For example, Sphk2 deletion did not change Sphk1 mRNA expression or activity in various tissues and cell types, including RBCs (18, 20, 24). In addition, an increase of SphK1 activity is unlikely to be the entire reason for elevated plasma S1P levels, as the transgenic overexpression of Sphk1 failed to change plasma S1P levels (28). Furthermore, SphK2 inhibitors rapidly (<3 min) increased blood S1P levels (23, 27), which is too rapid for transcriptional induction of Sphk1. Alternatively, it is conceivable that SphK2 blockage increases its substrate sphingosine, which might be redirected to other cells for SphK1-dependent conversion to S1P. Sphk1 knockout RBCs have virtually no S1P (18), so RBCs produce S1P predominantly by SphK1. Significantly, Sphk2 knockout mice displayed increased levels of sphingosine and S1P in RBCs (24). This observation supports the idea of sphingosine redistribution to SphK1 when SphK2 is inhibited. Contrasting phenomena were observed in platelets. Sphk2 deficient platelets have virtually no S1P, indicating the dominant role of SphK2 in platelet S1P production (18). Conversely, Sphk1 knockout mice showed a 50% increase of platelet S1P levels (18). Together, these observations suggest that blockage of a single isoform of SphK enzyme results in the redistribution of its substrate to the other isoform. Substrate redistribution might occur not only intracellularly but also in a transcellular manner. When wild-type mice were injected with labeled S1P–loaded RBCs, S1P redistributed from RBCs to lymphoid tissues (24). This process seems to require S1P dephosphorylation and rephosphorylation (24). On the other hand, Sphk2 knockout failed to redistribute RBC-S1P to lymphoid tissues (24). Notably, S1P is more susceptible to degradation in lymphoid tissues than in RBCs (discussed below). Therefore, SphK2 blockage might facilitate the escape of S1P from degradation-prone tissues (e.g., lymphoid tissues) to S1P secretion-oriented cells (e.g., RBCs). This hypothesis is further supported by the observation that pharmacological or genetic targeting of SphK2 substantially lengthens the S1P half-life in circulation (27).
Taken together, these studies provide us with various insights into the SphK subtype- or cell type-specific regulation of the circulatory S1P. However, the precise molecular mechanisms are not fully understood. For example, it is hypothesized that S1P synthesized by SphK1 or SphK2 appears to be destined for secretion or intracellular metabolism, respectively (24). If so, how are these distinct fates determined? Furthermore, how does the differential subcellular localization of SphK isoenzymes affect this phenomenon? Better understanding of how plasma S1P is regulated is important to fully appreciate its biological, pathological, and pharmacological impact.
S1P Export Into Plasma
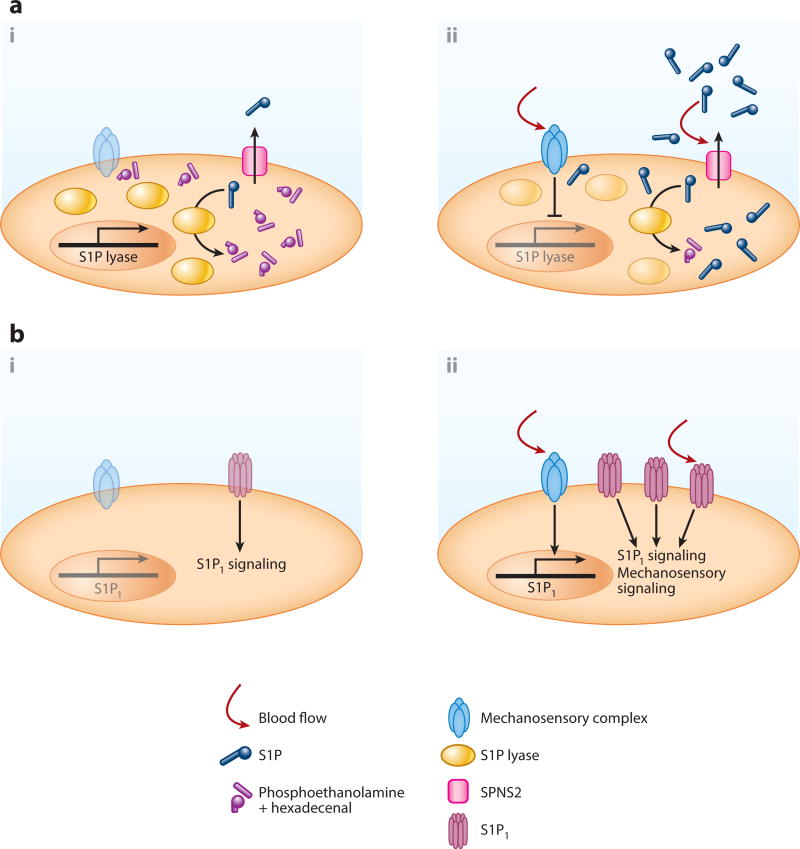
S1P produced in the intracellular space is exported via specific transporters (Figure 1a). To date, only spinster homolog 2 (SPNS2) is proven as a bona fide S1P transporter (29, 30). SPNS2 is highly expressed in ECs (31, 32). Spns2 global or EC-specific knockout mice showed substantial reduction (20–60%) in plasma S1P (31–34). S1P release from ECs is constitutive and fully dependent on SPNS2 (31, 32). Notably, laminar shear stress induced S1P release within 5 min in ECs (12). Although the underlying mechanism is still unknown, shear stress may be critical for the constitutive supply of circulatory S1P (Figure 2a). Nonetheless, S1P release from RBCs was unchanged in Spns2 global knockout mice (32). Several members of the ATP-binding cassette (ABC) superfamily were reported as other candidate S1P transporters in some cell types (35–39). However, their identity as S1P transporters is tentative. First, mice that lack these ABC-type transporters showed normal plasma S1P levels (11). Moreover, heterologous overexpression of these transporters failed to promote S1P secretion (40). In RBCs, S1P seems to be present at the outer surface of plasma membrane (41), and S1P can be extracted into a soluble form with the help of S1P chaperones (discussed below). Thus, unidentified transporters/phospholipid scramblases might flip S1P to the outer leaflet to facilitate continuous enrichment of circulatory S1P.
Figure 2.
Blood flow-dependent regulation of endothelial sphingosine 1-phosphate (S1P) degradation/secretion and the receptor S1P1 expression/signaling. (a) Blood flow enhances endothelial S1P secretion. At the static state, S1P lyase transcription is highly active, which would limit endothelial S1P production and secretion (i). Shear stress substantially downregulates S1P lyase expression, which results in an increase of endothelial S1P levels (ii). In addition, blood flow rapidly enhances S1P secretion via spinster homolog 2 (SPNS2); however, the precise molecular mechanisms are still unknown (ii). (b) Flow-dependent regulation of S1P1 transcription and signaling. Without blood flow, S1P1 transcription is less active, which leads to less S1P1 activity (i). Shear stress induces S1P1 expression presumably by flow-regulated transcription factors (ii). Furthermore, shear stress enhances S1P–dependent S1P1 signaling or even directly activates S1P1 in an S1P–independent manner (ii).
S1P Chaperones in Blood Circulation
Circulating S1P is bound to plasma protein chaperones, which function not only as S1P carriers but also activate its receptors in a ligand-dependent manner to evoke specific biological responses (42–46) (Figure 1a). Recent studies suggest that chaperones in part determine blood S1P levels. More than half (50–60%) of plasma S1P is found in the high-density lipoprotein (HDL) fraction, approximately 30–40% in the albumin fraction, and much smaller amounts in other lipoprotein fractions (47). In the HDL particles, S1P is exclusively bound to apolipoprotein M (ApoM) (48), which is found only in 5%of plasma HDL particles (49). S1P is absent in the HDL fraction of Apom knockout mice, which show an approximately 45% reduction in plasma S1P levels (48). Moreover, transgenic mice overexpressing Apom show increased plasma S1P levels in a transgene dosage-dependent manner (48). These data suggest that S1P chaperones determine the S1P concentration in blood. How do they determine the blood S1P levels? First, plasma S1P chaperones may stabilize S1P by limiting access to degradative enzymes. Indeed, overexpression of ApoM in HepG2 cells increased S1P levels in a conditioned medium via the specific blockage of S1P degradation (50). The complete docking of S1P into ApoM, as shown in crystal structure (48, 51), likely protects S1P from degradation. Another possibility is that chaperones accelerate S1P release from cells. This hypothesis is supported by the observations that S1P was not released from platelets (52) or RBCs (41) in the absence of carrier proteins. Furthermore, a monoclonal S1P antibody also extracted S1P from cells (41). Remarkably, HDL showed faster and more efficient extraction of S1P from RBCs compared to albumin (41). This property of HDL (presumably facilitated by ApoM) may explain why S1P is more abundant in ApoM, a minor protein in plasma, than in albumin, the most abundant plasma protein. However, the precise mechanisms by which S1P is transferred to chaperones are not yet determined.
Limiting S1P Levels
S1P enzymatic degradation in tissues is a key component in the formation of the circulatory S1P gradient. In tissues, intracellular S1P levels are limited mainly by the irreversible degradation to phosphoethanolamine and hexadecenal via S1P lyase located at the endoplasmic reticulum (16) (Figure 1b). Either pharmacological or genetic targeting of S1P lyase induced a 10–1000-fold elevation of S1P levels in various tissues (4, 53–55). S1P lyase is expressed in a wide range of tissues (55), whereas RBCs lack S1P lyase activity (13, 56). This distribution of S1P lyase expression can partly explain the formation of the circulatory S1P gradient. In addition, laminar flow markedly downregulates endothelial S1P lyase expression in ECs (12). Therefore, blood flow would facilitate the continuous provision of circulatory S1P by limiting endothelial S1P degradation while allowing S1P release (Figure 2a). There are additional intracellular S1P degrading enzymes, namely S1P phosphatase 1 and 2 (57); however, their contribution to the S1P gradient is not yet clear. By contrast, extracellular S1P is dephosphorylated by three lipid phosphate phosphatases (LPP1–3) located on the cell surface (58). Among them, LPP3-dependent S1P degradation is essential for the maintenance of local perivascular S1P levels to support immune cells’ egress into circulation (59) Circulatory S1P is continuously degraded by cell-dependent degradation mechanisms (12). Thus, the balance between the continuous supply and clearance of S1P establishes the S1P gradients.
Embryonic S1P
During embryogenesis, blood S1P compartmentalization seems to differ from that of the postnatal period. RBC-specific Sphk1/Sphk2 double knockout embryos displayed almost complete reduction (>95%) of embryonic S1P (10). This study used Sphk1 RBC-specific knockout in the background of Sphk2 global knockout, which might mask the contribution of SphK2 in specific cell types to plasma S1P. Nevertheless, this observation suggests that RBCs are an exclusive source of embryonic S1P and that ECs begin to contribute to the blood S1P enrichment after vascular network maturation. Embryonic vascular structure is immature, with ECs undergoing dynamic processes of angiogenesis, remodeling, and pruning (60). The cellular or molecular mechanisms underlying this transition of circulating S1P sources are unknown. Embryonic and adult ECs may express different levels of factors involved in S1P production, secretion, and degradation. Furthermore, protein chaperones for embryonic S1P were not determined. It is noteworthy that albumin production becomes prominent only at late embryonic stages, whereas α-fetoprotein is another major plasma protein in embryos (61, 62). Although Apom gene expression is initiated at embryonic day 10 (E10) (63), its contribution to embryonic circulatory S1P is unclear. Either α-fetoprotein or ApoM might not be the sole protein chaperone in the embryos. This scenario is supported by the observation that Afp (the gene coding α-fetoprotein) or Apom knockout embryos normally develop (48, 64) unlike Sphk1/Sphk2 double knockouts (10, 65) or S1P receptor(s) knockout embryos (66–69). As mentioned above, S1P chaperones are more than just S1P carriers; they are thought to stabilize, extract, and direct specific S1P signaling functions. Therefore, embryonic stage-specific S1P chaperones might facilitate the unique regulation and function of embryonic circulatory S1P.
S1P in Lymph Circulation
The cellular source of lymph circulation is different from that of blood circulation. The main source of S1P in lymph is proposed to be lymphatic EC (LEC) (70) (Figure 1c). Distinct compartmentalization of lymph and blood S1P was inferred from the observation that adoptive transfer of wild-type bone marrow to S1Pless mice failed to restore lymph S1P levels (9). Indeed, LEC-specific Sphk1/Sphk2 knockout mice showed fiftyfold lower levels of S1P in lymph circulation with normal S1P levels in blood circulation (70). Global or EC-specific Spns2 knockout mice displayed substantial reduction in lymph S1P levels, suggesting that lymph S1P is secreted by SPNS2 from LECs (33). Accordingly, lymphocytes from EC-specific Spns2 knockout mice showed tenfold higher cell-surface S1P1 expression in lymph (33). This observation can be explained by their exposure to lower levels of extracellular S1P, which promotes receptor endocytosis.
The composition or function of S1P carrier(s) in lymph also might be different from those in blood. Lymph has a generally similar protein composition to that of blood plasma albeit with different concentrations (71). Indeed, ApoM is found in lymph at approximately half of the plasma levels (45). However, lymph S1P levels were not changed in Apom knockout mice (45). Therefore, ApoM might be a minor S1P chaperone in lymph. Future studies on lymph S1P chaperones may provide valuable insights into general roles of S1P chaperones in immune and lymphatic functions.
VASCULAR CENTRIC FUNCTIONS OF S1P RECEPTORS
At present, five receptors for S1P (S1P1–5) were identified. The differential but overlapping intra-cellular signaling pathways and expression patterns of each receptor enable S1P to exert its diverse and redundant functions (72–75). S1P1 exclusively couples with heterotrimeric Gαi/o proteins, whereas S1P2 and S1P3 couple with Gαi/o, Gαq, and Gα12/13, and S1P4 and S1P5 with Gαi/o and Gα12/13. Although S1P1–3 receptors are widely expressed and are the predominant S1P receptors in vascular cells, S1P4 and S1P5 expressions are more restricted mainly to the lymphoid and central nervous systems, respectively. Thus, studies in the cardiovascular system have focused on S1P1–3. ECs primarily express S1P1 and S1P3, whereas S1P2 and S1P3 are expressed on VSMCs. However, endothelial expression of S1P2 was also confirmed in larger vessels of normal tissue, lung microvasculature, and tumor and inflamed vasculature (76). Therefore, these receptors seem to have different functions depending on the vascular bed or physiological context as discussed below. For more detailed information on S1P receptor signaling and tissue/cell distributions, the reader is referred to other reviews (72–75).
Recycling and Degradation of S1P1
Among five S1P receptors, the trafficking regulation of S1P1 is well studied. After S1P stimulation, S1P1 is desensitized by phosphorylation of its C-terminal tail and subsequent internalization by β-arrestin- and clathrin-dependent mechanisms (77–79). Most of the internalized S1P1 is recycled back to the plasma membrane (77, 79). Only a small portion is targeted to lysosomal/proteasomal degradation (77, 79). Importantly, S1P1 is highly sensitive to internalization and desensitization compared with other GPCRs. This unique characteristic of S1P1 facilitates its distinctive roles in the immune cell movement in and out of the circulatory systems. Moreover, S1P1 cell surface level is an indicator of S1P exposure status to cells because S1P availability and S1P1 cell surface expression are correlated inversely (4, 33, 59, 70, 80).
Modulation of activated S1P1 fates, recycling, or degradation by pharmacological agents is a well-known mechanism applied to clinical practice. Fingolimod (FTY720/Gilenya), a structural analog of sphingosine, is phosphorylated by SphK2 to FTY720-P (20, 81, 82). FTY720 was approved as the first-line oral therapeutic for relapsing-remitting multiple sclerosis (MS) by the US Food and Drug Administration (83, 84). Although FTY720-P acts as a high-affinity agonist on S1P1 and S1P3–5 (85, 86), it also acts as a functional antagonist for S1P1. In contrast to S1P, FTY720-P induces persistent internalization (87). This is followed by polyubiquitinylation and degradation of S1P1, leading to a marked decrease in S1P1 expression (79, 88). This functional antagonism on S1P1 leads to the trapping of autoreactive lymphocytes in the lymphoid organs, away from the central nervous system, which seems to explain the clinical efficacy of FTY720 (89).
Innovative Reporter Systems for S1P1 Activation and S1P Availability in Vivo
Two unique reporter mouse models utilizing S1P1 were recently developed. Proia and colleagues used the “Tango” system developed by another team (90), which detects interactions between GPCR and β-arrestin. They adopted the Tango system to an in vivo context using mouse genetics (91). In this S1P1-green fluorescent protein (GFP) reporter mouse, a synthetic S1P1 signaling pathway was genetically engineered so that a transcription factor is released from activated S1P1 to turn on a nuclear-targeting GFP reporter gene. Therefore, the S1P1-GFP reporter mouse displays mapping of S1P1 activation status in vivo. S1P1-GFP reporter mice validated the high-activation status of S1P1 in previously expected cell types including ECs (91). In addition, they also revealed that endothelial S1P1 activation is locus- and flow-dependent (46). Nonetheless, Schwab and colleagues (92) aimed to define S1P geography in vivo. As mentioned above, researchers took advantage of the unique S1P1 trafficking properties to define local S1P availability by cell surface S1P1 levels. However, this approach is based on the assumption that total cellular S1P1 expression is similar among comparison groups. Moreover, the loss of cell surface S1P1 does not always indicate S1P1 activation because GPCRs can internalize even in the absence of their ligands. Moreover, we cannot determine S1P availability in areas where S1P 1 is not normally expressed. Schwab et al. (92) overcame these issues by designing a reporter mouse model in which GFP-tagged S1P1 and RFP-tagged S1P–unresponsive S1P1 mutants were overexpressed at equal levels. By checking the S1P1 trafficking status by ratiometric imaging measurements, they estimated relative S1P concentration in vivo. Using this system, they found that S1P concentration is kept low by the lipid phosphatase LPP3 in the splenic tissue of red pulp (92). This is unexpected because the red pulp, the area filled with RBCs, was previously assumed to be rich with S1P. These elegant reporter systems are expected to provide additional insights to understand the spatiotemporal control or S1P levels in complex tissue microenvironments.
Chaperone-Dependent Signaling of S1P1
The S1P1-dependent functions of S1P seem to be carrier dependent: Albumin-bound and HDL-bound S1P show situation-dependent signaling persistence or biased signaling. In cultured EC experiments, HDL-bound S1P displayed sustained S1P1 signaling and barrier activity longer than albumin-bound S1P (42–44). In addition, HDL-bound S1P may enhance the pulmonary barrier integrity more efficiently in vivo (48, 93), as discussed below. More recently, our laboratory reported the chaperone-dependent anti-inflammatory roles of S1P–S1P1 signaling (46). In human umbilical vein endothelial cells (HUVECs), HDL-bound S1P, but not albumin-bound S1P, suppressed cytokine–induced NF-κ activation and adhesion molecule expression (46). S1P induced rapid phosphorylation of ERK irrespective of its carrier (46). However, only HDL-bound S1P increased the association of S1P1 with β-arrestin 2 (46). Accumulating evidence now supports the idea that GPCRs can activate G protein-independent but β-arrestin-dependent intracellular signaling (94). Consistently, β-arrestin 2 knockdown ablated the anti-inflammatory effects of HDL-S1P in HUVECs (46). However, albumin-bound S1P, but not HDL-bound S1P, inhibited adenylyl cyclase, possibly by a Gαi/o-dependent pathway (46). These observations imply the existence of a biased-agonism depending on the different S1P chaperones. In addition, HDL-bound S1P, but not albumin-bound S1P, suppressed lymphopoiesis in the bone marrow, although it is not necessary for normal lymphocyte trafficking in vivo (45).
How does S1P bound to various chaperones facilitate differential signaling persistence or biased agonism? One of the possible mechanisms is that coreceptors for S1P1 might tether S1P–bound HDL in proximity to S1P1. Indeed, two of the HDL receptors, scavenger receptor-BI and endothelial lipase, were shown to be essential for HDL–induced and S1P–dependent cellular responses in cell culture experiments (42, 95). These tethering proteins might facilitate the more efficient delivery of S1P to S1P1. Moreover, this mode of signaling may lead to the differential fate of S1P1 after activation, internalization, recycling, or degradation. This hypothesis is supported by the aforementioned in vitro EC experiments. In these studies, S1P1 treated with HDL-bound S1P was more protected from the internalization or degradation than S1P1 treated with albumin-bound S1P (44, 46). However, there are other possible mechanisms, including HDL-dependent cholesterol uptake from cells and lipid microdomain modulation.
Blood Flow-Dependent Regulation of S1P1
Blood flow can increase transcription and protein levels of S1P1 (Figure 2b). S1P1 was shown to be induced by fluid shear in HUVECs (96). Accordingly, an increase of S1P1 protein and a response to S1P were observed in shear-treated HUVECs (97, 98). Flow-dependent regulation of S1P1 expression is seen in the retinal vascular plexus, where S1pr1 expression displayed high expression in the flow-positive, mature regions of the vascular network and low expression in the sprouting vascular front region (99). These results suggest that blood flow induces S1pr1 transcription via flow-regulated transcription factors. In lymphocytes, Kru¨ppel-like factor 2 (KLF2) is a critical transcription factor for S1pr1 expression (100–102). Notably, KLF2 is a major flow-regulated transcription factor (103, 104), coordinating approximately half of the gene expression programs evoked by shear stress (104). However, the detailed mechanisms by which endothelial S1P1 expression is regulated, including the involvement of KLF2, have not yet been addressed even though Klf2 and S1pr1 knockout mice showed similar vascular developmental anomalies (66, 105). As mentioned above, blood flow also downregulates S1P lyase transcripts. Therefore, future studies on the mechanistic basis for flow-mediated S1P1 and S1P lyase transcriptional regulations are needed to reveal one of the remarkable intersections of S1P and mechanotransduction systems.
Shear stress also enhances or even directly activates S1P1 signaling in ECs (Figure 2b). In the mouse aorta, S1P1 expression was observed on the plasma membrane of ECs in the descending aorta, which is subject to steady laminar shear stress (99). However, S1P1 is barely detected on the plasma membrane; instead, it is found intracellularly in the endocytotic vesicles in areas of turbulent shear (lesser curvature) of the aorta that is subjected to disturbed or turbulent flow (99). Concomitantly, in the observation of the aorta from S1P1-GFP-reporter mice, substantial numbers of ECs had nuclear GFP in turbulent shear areas but not in the laminar shear stress area (46). Taken together, these data suggest that disturbed flow enhances S1P1 activation and internalization in vivo. Shear stress might even directly activate S1P1 in a ligand-independent manner.
In HUVECs, laminar shear stress induces the adherens junction assembly and alignment of EC monolayers to the direction of flow (99). Laminar flow also activates ERK, Akt, and the endothelial isoform of nitric oxide (NO) synthase (eNOS) (99). Importantly, all of these flow-mediated effects were abolished by pharmacological or shRNA-mediated targeting of S1P1 (99). Moreover, the overexpression of an S1P nonbinding S1P1 mutant restored shear responsiveness, suggesting that S1P1 can work as a component of shear stress sensing machinery in a ligand-independent manner (99). What are the molecular mechanisms for the ligand-independent mechanosensory role of S1P1? It is conceivable that there mightbemechanosensitive coreceptors for S1P1.In this scenario, proper S1P1 expression might facilitate mechanosensory signaling by recruiting components of the mechanosensory complex (106). Alternatively, S1P1 might sense mechanical stimuli by itself, as is proposed for other GPCRs. If so, how can different stimuli to S1P1 (e.g., chaperone-bound S1P versus shear stress) be organized to address the physiological function of S1P1 in vivo? The answers await future studies, especially those searching for the binding partners of S1P1 or the combinatorial usage of pS1Pless and S1P1-GFP reporter mice.
SPHINGOSINE 1-PHOSPHATE IN VASCULAR DEVELOPMENT
S1P plays critical roles in embryonic vascular development. For example, genetic deletion of S1prI in mice caused embryonic lethality at E12.5 to E14.5 as a result of severe hemorrhage (66). Because EC-specific knockouts of S1pr1 phenocopied S1pr1 global knockouts, S1P1 function in the endothelial compartment seems to be critical for the regulation of embryonic vascular stability (67). On the contrary, no clear developmental defects were observed in either S1pr2 or S1pr3 single knockout embryos (68, 69, 107). However, simultaneous deletion of S1pr2 and S1pr3 resulted in approximately 50% embryonic hemorrhage and death at approximately E13.5 (68, 69). Moreover, embryos lacking all of the S1pr1–3 showed the most severe bleeding, which occurs much earlier than the S1pr1 single deletion (69). These results indicate the supportive and/or redundant roles of S1P2 and S1P3 in embryonic vascular development. Similar to the compound S1P receptor(s) knockout phenotypes, global or RBC-specific Sphk1/Sphk2 double-knockout embryos also displayed severe defects in vascular development (10, 65). Therefore, it is likely that circulatory S1P activation of S1P receptors is a fundamental event in early vascular development.
S1P1 as a Negative Regulator of Sprouting Angiogenesis
S1P1 inhibits angiogenic sprouting and thus influences the collective endothelial cell behavior during embryonic vascular development. In the murine retinal angiogenesis model, EC-specific deletion of S1pr1 leads to a hypersprouting phenotype: The vascular plexus becomes overly dense, resulting in inadequate growth of the vasculature into the retinal tissue (99, 108). The endothelial hypersprouting phenotypes are also observed in multiple locations of S1pr1 knockout embryos, including the hindbrain, neural tube, and limbs (108–110). It is notable that embryonic S1pr1 deletion leads to aortic hyperbranching (108). This is the most distinctive vascular phenotype of S1pr1 knockout embryos that was not reported in other gene mutants. However, this phenotype was recapitulated in RBC-specific Sphk1/Sphk2 double-knockout embryos (10). Together, the embryonic lethality of these mutants seems to bemainly due to exaggerated and ectopic endothelial sprouting.
S1P contributes to vascular stabilization by repressing the sprouting behavior of ECs after the initiation of blood flow. This is distinct from the well-documented roles of Notch signaling at the angiogenic front (60, 111). Indeed, S1pr1 deletion did not change the expression of Notch-target genes in embryonic microvessels or retinal tissues (99, 108). The aortic hyperbranching phenotype of S1pr1 knockout embryos was not observed in the EC-specific deletion of Notch target Rbp-j (108). Thus, the S1P1-dependent regulation of sprouting angiogenesis appears to be an independent pathway that is not epistatic to Notch signaling. Notably, the onset of the S1pr1-knockout phenotype occurred after the establishment of major vascular networks. Because S1P is compartmentalized in blood circulation, perfusion of the newly constructed vessels would initiate the S1P signaling.In this process, the RBC-dominant supply of embryonic blood S1P likely initiates S1P signaling after vascular perfusion. Furthermore, blood flow itself might support this process by enhancing expression and activity of S1P1.
The Role of Endothelial S1P1 in The Mural Cell Investment of Nascent Vasculature
Endothelial S1P1 regulates mural cell investment, another important step in vascular stabilization. In coculture experiments with murine embryonic ECs (MEECs) and mural cells, S1P induced mural cell binding with MEECs from wild-type but not from S1pr1 knockout embryos (112). Mechanistically, S1P induced the polarized distribution of N-cadherin on the apical EC surface, which should strengthen the EC-mural cell interactions (112). Consistently, the mislocalization of N-cadherin or mural cell coverage defects was observed in the dorsal aorta of S1pr1 knockout embryos (66, 67, 112). However, these mural cell coverage defects may be secondary to the hypersprouting phenotype described above. In contrast, pericyte-deficient mice displayed much milder and later onset vascular abnormalities (113, 114). Understanding of the postnatal function of S1P1 on mural cell investment still awaits further studies.
SPHINGOSINE 1-PHOSPHATE IN PHYSIOLOGICAL VASCULAR INTEGRITY
S1P is a major regulator of vascular barrier function. Many in vitro studies have shown that S1P induces barrier protection by the EC cytoskeleton and assembly of cell–cell junction proteins (115, 116). Indeed, circulatory S1P is essential for the basal vascular integrity (48, 117, 118). Endothelial S1P1 plays a major role in the physiological maintenance of barrier integrity (45, 88, 93, 99, 119, 120). By contrast, S1P2 is involved in barrier disruption in most vascular beds during anaphylaxis (25, 121–123), and in MS (124) and stroke (125). Here, we focus on and discuss the role of circulatory S1P in the physiological maintenance of vascular barrier integrity.
Maintenance of Endothelial Barrier Integrity by Blood S1P
Blood S1P plays a pivotal role in the maintenance of constitutive vascular integrity. Indeed, pS1Pless mice showed a basal vascular leak in the lung, which can be rescued by the restoration of plasma S1P levels (118). Basal pulmonary leakage phenotypes were observed even in mice showing partial (approximately 50%) reduction in blood S1P levels, namely Sphk1 (117) or Apom knockout mice (48). However, virtually no exaggerated pulmonary leakage was observed in EC-specific Spns2 knockout mice showing a 23% reduction of plasma S1P level (33). These data suggest that blood S1P regulates basal barrier integrity in a dose- and/or chaperone-dependent manner. This hypothesis is further supported by the observation that EC-specific S1pr1 knockout mice showed much higher pulmonary leakage than Apom knockout mice (45). It is also conceivable that S1P might regulate barrier integrity in a cellular source-dependent manner. RBCs supply circulatory S1P predominantly via SphK1. By contrast, the SPNS2-mediated S1P supply should be exclusively from ECs. Therefore, the S1P supply from RBCs might have a more significant role in endothelial barrier maintenance. Furthermore, HDL-bound S1P might have a larger contribution to the constitutive barrier integrity than albumin-bound S1P. This scenario is supported by cultured EC experiments as discussed above. Further studies are needed to elucidate the dose-, source-, and carrier-dependent role of circulatory S1P in barrier maintenance.
Among five S1P receptors, S1P1 plays a central role in the endothelial barrier homeostasis. Indeed, the pharmacological blockage of S1P1 induced pulmonary edema in mice (88, 119). Global or EC-specific deletion of S1pr1 increased vascular permeability under basal conditions in the colon, retina, and lung (45, 93, 99, 120). Moreover, basal leakage phenotypes of pS1Pless and Apom knockout mice were rescued by selective agonists for S1P1 (93, 118). Clinical use of FTY720 showed adverse events, including respiratory symptoms and macular edema (83, 126). Importantly, the FTY720–induced increase of lung vascular permeability was dramatically reduced in S1P1-S5A knockin mice in which genomic S1P1 is replaced with an internalization-defective mutant of S1P1 that resists FTY720–induced degradation (88). In accordance with the protection from pulmonary edema, S1P1 protein degradation by FTY720 was substantially ablated in the mutant lungs (88). Therefore, targeting lymphocyte S1P1 while sparing endothelial S1P1 may be a better therapeutic strategy for the control of various autoimmune diseases.
The Blood-Brain Barrier
The role of S1P in the physiological barrier function in tissues with a specialized vascular barrier (e.g., brain, spinal cord, retina, testis) is not known. The blood-brain barrier (BBB) is a highly selective permeability barrier that protects the central nervous system from variations in blood composition and toxins. In cultured human brain ECs, S1P enhanced the barrier integrity via S1P5 activation (127). However, S1P2 signaling rather diminished BBB integrity in human brain ECs (124). Using the in situ rodent brain perfusion model, the administration of S1P increased the brain uptake of substrates of P-glycoprotein, an ATP-driven drug efflux pump (128, 129). The effect is mediated by the S1P1-dependent rapid and reversible reduction of P-glycoprotein activity (128, 129). However, the in vivo role of S1P in physiological barrier function has not been studied. We have recently shown that Apom knockout mice have normal BBB integrity under physiological conditions (45). Therefore, unlike its role in normal vascular barrier integrity, the impact of ApoM-bound S1P on BBB maintenance appears dispensable. BBB is composed of ECs together with pericytes, astrocytes, and neurons. Hence, these components might promote BBB function through multiple S1P receptors and the potential local perivascular supply of S1P.
Barrier Maintenance in High Endothelial Venules by Locally Released S1P
In HEVs, postcapillary venules for lymphocytes homing to LNs and SLOs, endothelial barrier integrity is regulated by S1P in a unique manner. As described above, platelets do not contribute to plasma S1P levels. However, platelet-derived local S1P has a critical role in the maintenance of HEVs’ barrier integrity. Indeed, pS1Pless mice showed severe leakage and bleeding in LNs after an immune challenge (130). This barrier dysfunction was completely rescued by the daily transfusion of platelets from wild-type mice but not from pS1Pless mice (130). Mechanistically, extravasated platelets in the perivenular space of HEVs interact with the perivenular sleeve of fibroblastic reticular cells and release S1P (130). The locally released S1P maintains vascular en-dothelial (VE)-cadherin expression and tightens the HEVs’ barrier in an S1P1-dependent manner (130). Different vascular beds have distinct perivascular components and requirements for barrier maintenance. Thus, the perivascular local supply of S1P might facilitate the unique barrier regulation by cooperating with more exhaustive regulation through constitutive circulatory S1P.
VASCULAR TONE REGULATION BY SPHINGOSINE 1-PHOSPHATE
S1P potentially regulates vascular tone in both directions: vasodilation or vasoconstriction. Sphk1 or Sphk2 knockout mice showed normal mean arterial blood pressure (25). Thus, a partial reduction or increase in circulatory S1P levels is unlikely to affect homeostatic blood pressure. However, exogenous application of S1P induces vascular tone changes in arteries from various tissues. Therefore, it is conceivable that circulatory S1P controls local vascular tone in vivo. Indeed, S1pr2 knockout mice displayed a regional increase of blood flow (131), as discussed below. Generally, S1P induces vasodilation through S1P1 and S1P3 on ECs, by activation of eNOS and the production of NO, a strong vascular relaxant (132). In contrast, S1P causes vasoconstriction through S1P2 and S1P3 activation on VSMCs at higher concentrations, presumably under conditions of endothelial injury/damage (132). This dichotomy likely depends on the anatomical location of vascular beds and the pathophysiological context.
S1P1 As a Vasodilatory Receptor
S1P exerts vasodilative action by acting on S1P1, presumably by cooperating with flow-dependent mechanotransduction systems. Using cultured ECs and isolated blood vessels, many studies have shown that S1P induces a marked production of NO via activation of eNOS (133–136). Pharmacological or siRNA-mediated inhibition studies also have elucidated the key role of S1P1 in this process (137, 138). Indeed, the S1P1 antagonist substantially reduced basal eNOS activity, increased basal tone, and enhanced the vasoconstriction response to α-adrenergic stimulation in murine mesenteric arteries (138). Notably, flow-mediated vasodilation was blunted by an S1P1 antagonist (138). Thus, S1P1 activation is indispensable for the well-established vasodilatory role of blood flow. These data suggest cooperation between the aforementioned S1P1 signaling and mechanotransduction systems.
Bidirectional Roles of S1P2 and S1P3 in Vascular Tone Regulation
The role of S1P2 on vascular tone regulation seems to be either vasoconstrictive or vasodilatory, depending on the vascular beds or segments. A study with S1pr2-knockout mice elucidated a role of S1P2 in the homeostatic vascular tone (131). S1pr2 knockout mice display normal systolic blood pressure; however, they showed regional (renal and mesenteric artery) elevated blood flow and a decrease in vascular resistance (131). Vascular contractile response to α-adrenergic stimulation was also blunted in S1pr2 knockout mice both in vivo and ex vivo (131). Exogenous application of S1P also in duced vasoconstriction in perfused mouse lung models, which was blunted in S1pr2 knockout mice (139); S1P2 can also function as a vasodilatory receptor. In an isolated cerebral artery, S1P induced an exaggerated vasoconstriction in S1pr2 knockout mice compared with wild-type mice (140). Together, S1P2 function in vascular tone is bidirectional depending on the vascular beds. Notably, in renal microcirculation, S1P induced an S1P2-dependent vasoconstriction in afferent but not efferent arterioles (141). This observation indicates that S1P can regulate vascular tone in both a vascular bed-dependent and a segment-specific manner. S1pr2 is preferentially expressed in VSMCs in the vessel wall. However, some ECs in a variety of organs also express S1pr2 in vivo (76). Thus, the bidirectional or locus-dependent role of S1P2 would be explained by an expression balance between ECs and VSMCs among different vascular beds or segments. Further detailed S1P2 expression analysis or experiments using tissue-specific S1pr2 knockout mice will likely elucidate the context-dependent role of S1P2 in vascular tone regulation.
S1P2 is essential for proper maintenance of the auditory and vestibular systems by controlling blood flow in the cochlea. S1pr2 knockout mice exhibited profound hearing loss early in life with the progressive degeneration of auditory and vestibular organs (142–144). Notably, the earliest cellular lesions in the cochlea were found in the stria vascularis, a compartment that harbors the main vasculature of the inner ear (144). S1pr2 mRNA is abundantly expressed in stria vascu-laris (144), and S1pr2 knockout stria vascularis showed morphologic abnormalities of capillary dilation and distortion (144). In addition, S1P induced vasoconstriction in gerbil spiral modiolar arteries that directly supply blood to the vessels of the stria vascularis (145). Remarkably, the S1P– induced vasoconstriction was blunted by an S1P2 antagonist (144). Hence, the S1P2-mediated F egress was suggested by the observation vasoconstriction of modiolar arteries might be essential to protect strial capillary beds from high pressure. Importantly, Spns2 knockout mice clearly phenocopied the hearing loss and stria vas-cularis phenotypes of S1pr2 knockout mice (146). The hearing loss phenotype was observed in mice with neural crest-specific deletion of Spns2 but not in mice with EC-, LEC-, RBC-, or platelet-specific Spns2 deletion (146). Therefore, the local secretion of S1P rather than circulatory S1P seems to be important for maintenance of the auditory and vestibular systems. A recent report shows that S1PR2 is a causative gene for autosomal-recessive hearing impairment in consanguineous Pakistani families (147). A pharmacological agent that controls S1P2 activity may have a therapeutic benefit to prevent and/or treat hearing disorders not only by gene mutation but also by otoxic drugs, noise, and aging.
As is the case in S1P2, S1P3 activation induces either vasoconstriction or vasodilation, depending on the vascular beds. In a preconstructed thoracic artery, S1P induced vasodilation, which was blunted in S1pr3 knockout mice (148). In the same model, S1pr3 knockout thoracic artery showed a 60% reduction in vasorelaxation responses to HDL, suggesting that the vasoactive role of HDL is partially preserved by S1P3 (148). These effects of S1P or HDL were absent in Nos3 (the gene coding eNOS)-knockout mice, indicating the involvement of eNOS-dependent NO production (148). Therefore, S1P3 seems to work as a vasorelaxant receptor presumably cooperating with S1P1, although S1P3 activation induced vasoconstriction in murine isolated cerebral artery (140, 149). S1P3 may serve vasoconstrictive functions in humans. FTY720 administration is associated with an increase in blood pressure in human patients (83, 84). Remarkably, siponimod, an S1P3-sparing S1P1/5 selective agonist under clinical testing (150), had no significant effect on rat or human blood pressure (151). Thus, FTY720–induced hypertension is likely from the activation of S1P3.
TRAFFICKING OF IMMUNE CELLS AT THE TISSUE-CIRCULATORY INTERFACE
Enrichment of S1P in blood and lymph circulation is critical for immune cell trafficking. In lymphoid organs, tissue S1P levels are kept low by S1P lyase-dependent degradation (4, 53–55). Lymphocytes egress into blood or lymph circulation at egress portals by S1P1-dependent sensing of S1P gradients at the tissue-circulatory interface (89, 152). Indeed, lymphocyte egress was severely inhibited when circulatory S1P was depleted (9, 33, 70) or when S1P levels in lymphoid tissue were elevated (4, 53–55). As mentioned above, FTY720-P induces internalization/degradation of S1P1 in lymphocytes and thereby inhibits lymphocyte egress (89). The S1P1 receptor on circulating lymphocytes is mostly internalized and desensitized due to the high S1P concentration (153). The internalization/desensitization of S1P1 is critical for circulating lymphocytes to overcome the S1P–dependent attraction to blood and to home again to lymphoid tissues via other chemoattractant cues. Indeed, blood lymphocytes that lack GRK2 displayed a reduced ability to enter LNs, an ability that was restored in S1pr1-deficient mice (154). This S1P gradient-mediated lymphocyte trafficking strategy is also utilized for splenic B cell shuttling (155) and trafficking of the other immune cells, including dendritic cells (156), natural killer cells (157, 158), and hematopoietic stem cells (159) by utilizing S1P1 or other S1P receptor(s).
Microenvironmental Distribution of S1P at the Thymic-Vascular Interface
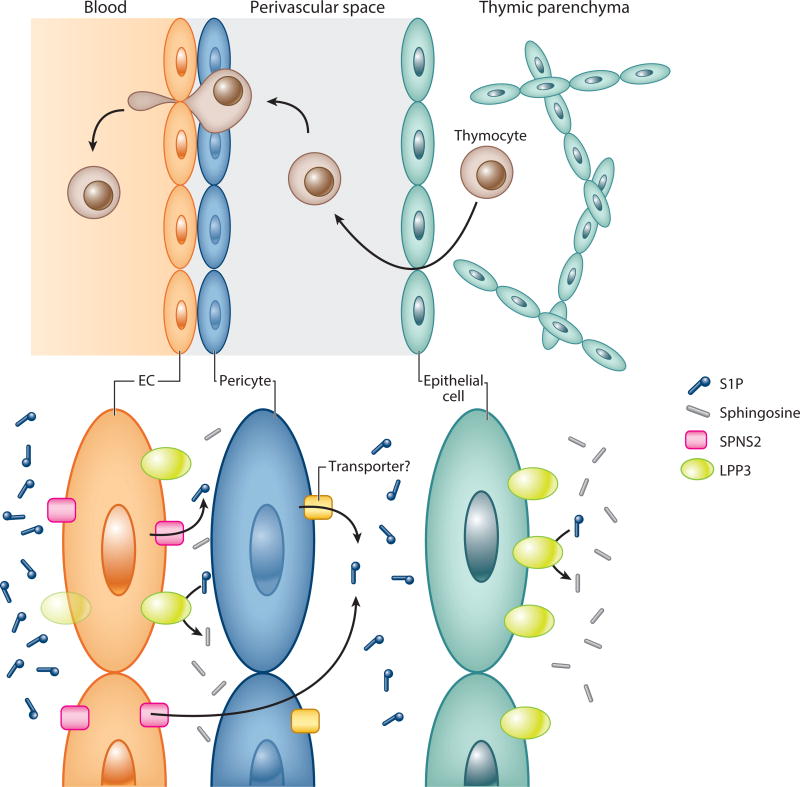
In addition to the steep S1P gradients at the tissue-vascular interface, local perivascular S1P heterogeneity may also be important for the efficient thymic egress of mature T lymphocytes. The contribution of blood S1P–independent thymic egress was suggested by the observation that the restoration of blood S1P failed to achieve full rescue of thymic egress in pS1Pless mice (9). Therefore, nonhematopoietic cell-intrinsic factors may facilitate S1P1-dependent thymocyte trafficking into the vascular system.
Neural crest-derived pericytes that ensheath blood vessels are proposed to be contributors. This hypothesis originated from the observation that overexpression of S1P1 in thymocytes leads to their perivascular accumulation in the thymus. Thus, it is likely that thymocyte S1P1 activation was ongoing at the region between the basement membrane of the blood vessels and the adjacent pericytes. This scenario was further supported by the analysis using neural crest-specific Sphk1/Sphk2 knockout mice (80). These mice had normal S1P concentration in blood and tissues. Nevertheless, they showed a significantly increased retention of mature thymocytes in the thymus (80). Consistently, S1P1 cell surface expression was slightly elevated in these mature thymocytes, indicating their reduced exposure to S1P (80). These results suggest that local S1P supply in the perivascular microenvironment might support the recruitment of thymocytes to the thymic-vascular interface.
In addition, ECs also might contribute to the perivascular control of S1P levels by supplying S1P to the abluminal side of thymic vasculature. This hypothesis originated from the analyses of EC-specific Spns2 knockout mice, which showed severe defects in thymic egress of lymphocytes (31). As discussed above, Spns2 knockout mice displayed a 50% reduction of blood S1P. However, this partial reduction cannot fully explain the total impairment of thymic egress. First, that amount of blood S1P was enough to stimulate lymphocyte S1P1 in vitro. Furthermore, normal thymic egress was observed in Sphk1 knockout mice showing a 50% reduction of blood S1P. Therefore, thymocytes can sense the S1P gradients at the thymic-vascular interface even if blood S1P level decreased ~50%. If so, how can their thymic egress defects be explained? One possible explanation is that ECs might release S1P to the abluminal side of thymic vasculature. As a result, thymocytes can be efficiently recruited to the thymic-vascular exit portals.
Moreover, epithelial and endothelial LPP3 may be important to generate and maintain proper S1P distribution in the perivascular microenvironment of the thymus. Global Ppap2b (the gene coding LPP3) knockout mice showed impaired thymic egress of lymphocytes (59). Importantly, the S1P levels in blood and whole thymic tissue were unchanged in Ppap2b knockout mice. Nevertheless, the thymocytes showed decreased S1P1 cell surface expression, indicating their exposure to high S1P levels. These data suggested that local but not global S1P levels might be elevated in the Ppap2b knockout thymus. The impaired thymic egress and decreased thymocyte S1P1 cell surface expression were also observed in epithelial cell-specific Ppap2b knockout mice. Because epithelial cells form a network throughout the thymus, LPP3-dependent S1P clearance around epithelial cells might modify perivascular S1P distribution. EC-specific Ppap2b knockout mice also displayed impaired thymic egress. Notably, LPP3 showed polarized localization toward the basolateral side of ECs (59). Therefore, endothelial LPP3 might maintain proper perivascular S1P distribution by finely tuning the S1P levels just at the thymus-blood interface, where thymocytes need a sharp gradient to egress into blood circulation.
Taken together, it is likely that a fine microenvironmental distribution of S1P is generated at the perivascular space by the cooperation of multiple cell types, such as ECs, pericytes, and epithelial cells. This S1P distribution pattern would facilitate efficient recruitment of thymocytes to the thymic-vascular interface and ultimately into the blood stream (Figure 3). However, the precise shape of the S1P distribution pattern is not yet elucidated. A related question is whether a similar strategy is applied in other vascular beds. The abluminal release of S1P by SPNS2 might provide specific egress cues for vascular bed- or segment-specific perivascular resident cells. This may also provide unique modulation of barrier integrity and vascular tone in vasculatures of different regions. In addition, given that S1P release is regulated by shear stress, region-specific differences in mechanical stimuli might facilitate flow-mediated fine-tuning of vascular function. The findings of microenvironmental S1P distribution also have highlighted the limitation of traditional S1P quantification systems. Novel reporter mice systems for in vivo mapping of S1P1 activation or S1P distribution will be needed to answer these questions.
Figure 3.
The thymic egress strategy and microenvironmental distribution of S1P. Newly matured thymocytes exit thymic parenchyma into perivascular spaces. Double basement membrane, one located in ECs and the other in epithelial cells, forms the unique space. After migrating to the perivascular spaces, thymocytes exit into the blood by sensing the sharp S1P gradient at the thymic-vascular interface. Pericytes are proposed to provide S1P in the perivascular space to support the recruitment of thymocytes. The pericytic S1P transporter is not yet determined. ECs might also contribute to S1P distribution at the perivascular space by secreting S1P to the abluminal side of thymic vasculature. Conversely, LPP3 localizes toward the basolateral side of ECs and finely tunes S1P levels at the thymus-blood interface. LPP3 is also expressed in epithelial cells, which would highlight the perivascular S1P compartmentalization or gradients. Abbreviations: EC, endothelial cell; LPP3, lipid phosphate phosphatase 3; S1P, sphingosine 1-phosphate; SPNS2, spinster homolog 2.
CONCLUSIONS AND FUTURE PERSPECTIVES
Recent studies have provided us with new insights into the generation and maintenance of circulatory S1P. With this in mind, we have now begun to appreciate the physiological significance of this simple lipid mediator in the closed circulatory system of vertebrates, such as the establishment and maintenance of stable vasculature and precise hematopoietic cell trafficking. Furthermore, the primordial role of circulatory S1P is highlighted by the tight link between S1P and blood flow. Therefore, we speculate that circulatory S1P confinement is a strategy of vertebrates to adapt to the closed circulatory system. Moreover, recent data on perivascular microenvironmental S1P distribution have led to a deeper understanding of S1P physiology in and around the tissue barriers. These findings generate new questions. For example, does S1P bound to different chaperones or from different sources have unique roles in vivo? What is the molecular basis for overlapping functional roles of S1P and shear stress signaling? Do different vascular beds have unique perivas-cular S1P distribution? To answer these questions, future studies are needed that combine genetic knockout models, as well as experiments that integrate biochemistry, biophysics, and biophotonics approaches. For example, novel in vivo reporter tools should help us by providing both spatial and temporal information on S1P distribution or S1P receptor activation. These studies are likely to provide significant physiological insights with potential implications for novel diagnostics and therapeutics.
Acknowledgments
This work is supported by NIH grants (HL67330, HL89934, CA77839, and HL117798) and a Transatlantic network grant to T.H. from the Fondation Leducq. K.Y. acknowledges support from the Japan Society for the Promotion of Science. We thank Yu Hisano and Eric Engelbrecht for a careful reading of the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kihara A. Sphingosine 1-phosphate is a key metabolite linking sphingolipids to glycerophospho-lipids. Biochim. Biophys. Acta. 2014;1841:766–72. doi: 10.1016/j.bbalip.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Hla T. Genomic insights into mediator lipidomics. Prostaglandins Other Lipid Mediat. 2005;77:197–209. doi: 10.1016/j.prostaglandins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–39. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 5.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta. 2008;1781:477–82. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Givens C, Tzima E. S1P1 bridges mechanotransduction and angiogenesis during vascular development. Dev. Cell. 2012;23:451–52. doi: 10.1016/j.devcel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu. Rev. Biomed. Eng. 2014;16:505–32. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 2007;8:1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 9.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–98. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Investig. 2014;124:4823–28. doi: 10.1172/JCI77685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–62. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–76. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997;121:969–73. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 14.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 15.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J. Biol. Chem. 1997;272:5291–97. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5’-phosphate binding domain exposed to the cytosol. Biochem. Biophys. Res. Commun. 2004;325:338–43. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Jonnalagadda D, Sunkara M, Morris AJ, Whiteheart SW. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim. Biophys. Acta. 2014;1841:1581–89. doi: 10.1016/j.bbalip.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urtz N, Gaertner F, von Bruehl ML, Chandraratne S, Rahimi F, et al. Sphingosine 1-phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ. Res. 2015;117:376–87. doi: 10.1161/CIRCRESAHA.115.306901. [DOI] [PubMed] [Google Scholar]
- 19.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004;279:52487–92. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 20.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–58. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 21.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Kharel Y, Mathews TP, Gellett AM, Tomsig JL, Kennedy PC, et al. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem. J. 2011;440:345–53. doi: 10.1042/BJ20110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharel Y, Raje M, Gao M, Gellett AM, Tomsig JL, et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J. 2012;447:149–57. doi: 10.1042/BJ20120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensken SC, Bode C, Nagarajan M, Peest U, Pabst O, Graler MH. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 2010;184:4133–42. doi: 10.4049/jimmunol.0903358. [DOI] [PubMed] [Google Scholar]
- 25.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J. Clin. Investig. 2010;120:1429–40. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–20. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharel Y, Morris EA, Congdon MD, Thorpe SB, Tomsig JL, et al. Sphingosine kinase 2 inhibition and blood sphingosine 1-phosphate levels. J. Pharmacol. Exp. Ther. 2015;355:23–31. doi: 10.1124/jpet.115.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc. Res. 2010;85:484–93. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–27. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 30.Osborne N, Brand-Arzamendi K, Ober EA, Jin SW, Verkade H, et al. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 2008;18:1882–88. doi: 10.1016/j.cub.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Investig. 2012;122:1416–26. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLOS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza A, Bréart B, Ramos-Perez WD, Pitt LA, Gobert M, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–10. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–11. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honig SM, Fu S, Mao X, Yopp A, Gunn MD, et al. FTY720 stimulates multidrug transporter-and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J. Clin. Investig. 2003;111:627–37. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. PNAS. 2006;103:16394–99. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 2006;47:614–21. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, et al. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 2007;103:2610–19. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 39.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010;285:10477–86. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 2011;286:1758–66. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode C, Sensken SC, Peest U, Beutel G, Thol F, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 2010;109:1232–43. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 2006;281:37457–67. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 43.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, et al. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J. Biol. Chem. 2008;283:25074–81. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 2012;287:44645–53. doi: 10.1074/jbc.M112.423426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523:342–46. doi: 10.1038/nature14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata N, Sato K, Kon J, Tomura H, Yanagita M, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000;352(Pt. 3):809–15. [PMC free article] [PubMed] [Google Scholar]
- 48.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. PNAS. 2011;108:9613–18. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christoffersen C, Nielsen LB, Axler O, Andersson A, Johnsen AH, Dahlbäck B. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 2006;47:1833–43. doi: 10.1194/jlr.M600055-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Kurano M, Tsukamoto K, Ohkawa R, Hara M, Iino J, et al. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 2013;229:102–9. doi: 10.1016/j.atherosclerosis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Sevvana M, Ahnström J, Egerer-Sieber C, Lange HA, Dahlbäck B, Muller YA. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J. Mol. Biol. 2009;393:920–36. doi: 10.1016/j.jmb.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 52.Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–38. [PubMed] [Google Scholar]
- 53.Weber C, Krueger A, Munk A, Bode C, Van Veldhoven PP, Gra¨ler MH. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J. Immunol. 2009;183:4292–301. doi: 10.4049/jimmunol.0901724. [DOI] [PubMed] [Google Scholar]
- 54.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, et al. Incomplete inhibition of sphingo-sine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLOS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bektas M, Allende ML, Lee BG, Chen W, Amar MJ, et al. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 2010;285:10880–89. doi: 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito K, Anada Y, Tani M, Ikeda M, Sano T, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 2007;357:212–17. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 57.Mandala SM. Sphingosine-1-phosphate phosphatases. Prostaglandins Other Lipid Mediat. 2001;64:143–56. doi: 10.1016/s0090-6980(01)00111-3. [DOI] [PubMed] [Google Scholar]
- 58.Pyne S, Long JS, Ktistakis NT, Pyne NJ. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem. Soc. Trans. 2005;33:1370–74. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- 59.Bre´art B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 2011;208:1267–78. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010;22:617–25. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand. J. Clin. Lab. Investig. 1956;8:174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 62.Tilghman SM, Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. PNAS. 1982;79:5254–57. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang XY, Jiao GQ, Hurtig M, Dong X, Zheng L, et al. Expression pattern of apolipoprotein M during mouse and human embryogenesis. Acta Histochem. 2004;106:123–28. doi: 10.1016/j.acthis.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Gabant P, Forrester L, Nichols J, Van Reeth T, De Mees C, et al. Alpha-fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. PNAS. 2002;99:12865–70. doi: 10.1073/pnas.202215399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005;25:11113–21. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000;106:951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–67. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 68.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 2002;277:25152–59. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 69.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004;279:29367–73. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 70.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloop CH, Dory L, Roheim PS. Interstitial fluid lipoproteins. J. Lipid Res. 1987;28:225–37. [PubMed] [Google Scholar]
- 72.Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphin-gosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 2011;111:6299–320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014;55:1596–608. doi: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Investig. 2015;125:1379–87. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, et al. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772–81. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 77.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 1999;10:1179–90. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watterson KR, Johnston E, Chalmers C, Pronin A, Cook SJ, et al. Dual regulation of EDG1/S1P(1) receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J. Biol. Chem. 2002;277:5767–77. doi: 10.1074/jbc.M110647200. [DOI] [PubMed] [Google Scholar]
- 79.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007;282:9082–89. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 80.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–35. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003;278:47281–90. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 82.Kharel Y, Lee S, Snyder AH, Sheasley-O’Neill SL, Morris MA, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 2005;280:36865–72. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 83.Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010;362:402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 84.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 85.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–49. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 86.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–57. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 87.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–53. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 88.Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, et al. Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J. Clin. Investig. 2011;121:2290–300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 90.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, et al. The genetic design of signaling cascades to record receptor activation. PNAS. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kono M, Tucker AE, Tran J, Bergner JB, Turner EM, Proia RL. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J. Clin. Investig. 2014;124:2076–86. doi: 10.1172/JCI71194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramos-Perez WD, Fang V, Escalante-Alcalde D, Cammer M, Schwab SR. A map of the distribution of sphingosine 1-phosphate in the spleen. Nat. Immunol. 2015;16:1245–52. doi: 10.1038/ni.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christensen PM, Liu CH, Swendeman SL, Obinata H, Qvortrup K, et al. Impaired endothelial barrier functioninapolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30:2351–59. doi: 10.1096/fj.201500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011;32:521–33. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tatematsu S, Francis SA, Natarajan P, Rader DJ, Saghatelian A, et al. Endothelial lipase is a critical determinant of high-density lipoprotein-stimulated sphingosine 1-phosphate-dependent signaling in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2013;33:1788–94. doi: 10.1161/ATVBAHA.113.301300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 1997;240:737–41. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 97.Hughes SK, Wacker BK, Kaneda MM, Elbert DL. Fluid shear stress modulates cell migration induced by sphingosine 1-phosphate and vascular endothelial growth factor. Ann. Biomed. Eng. 2005;33:1003–14. doi: 10.1007/s10439-005-5756-1. [DOI] [PubMed] [Google Scholar]
- 98.Aoki S, Osada M, Kaneko M, Ozaki Y, Yatomi Y. Fluid shear stress enhances the sphingosine 1-phosphate responses in cell-cell interactions between platelets and endothelial cells. Biochem. Biophys. Res. Commun. 2007;358:1054–57. doi: 10.1016/j.bbrc.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 99.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell. 2012;23:600–10. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, et al. Krüppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 101.Bai A, Hu H, Yeung M, Chen J. Krüppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J. Immunol. 2007;178:7632–39. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 102.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–30. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–63. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 104.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Investig. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J. Biol. Chem. 2001;276:33697–704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 108.Gaengel K, Niaudet C, Hagikura K, Laviña B, Muhl L, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell. 2012;23:587–99. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 109.Chae SS, Paik JH, Allende ML, Proia RL, Hla T. Regulation of limb development by the sphin-gosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis. Dev. Biol. 2004;268:441–47. doi: 10.1016/j.ydbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Ben Shoham A, Malkinson G, Krief S, Shwartz Y, Ely Y, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development. 2012;139:3859–69. doi: 10.1242/dev.078550. [DOI] [PubMed] [Google Scholar]
- 111.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 112.Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–45. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 114.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 115.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–12. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 116.Xiong Y, Hla T. S1P control of endothelial integrity. Curr. Top. Microbiol. Immunol. 2014;378:85–105. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, et al. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–97. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009;119:1871–79. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat. Chem. Biol. 2006;2:434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 120.Montrose DC, Scherl EJ, Bosworth BP, Zhou XK, Jung B, et al. S1P(1) localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J. Lipid Res. 2013;54:843–51. doi: 10.1194/jlr.M034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 2010;207:465–74. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olivera A, Dillahunt SE, Rivera J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol. Lett. 2013;150:89–96. doi: 10.1016/j.imlet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cui H, Okamoto Y, Yoshioka K, Du W, Takuwa N, et al. Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice. J. Allergy Clin. Immunol. 2013;132 doi: 10.1016/j.jaci.2013.07.026. 1205–14.e9. [DOI] [PubMed] [Google Scholar]
- 124.Cruz-Orengo L, Daniels BP, Dorsey D, Basak SA, Grajales-Reyes JG, et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J. Clin. Investig. 2014;124:2571–84. doi: 10.1172/JCI73408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim GS, Yang L, Zhang G, Zhao H, Selim M, et al. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat. Commun. 2015;6:7893. doi: 10.1038/ncomms8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 127.van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, et al. Sphingo-sine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J. Neuroinflamm. 2012;9:133. doi: 10.1186/1742-2094-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. PNAS. 2012;109:15930–35. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cartwright TA, Campos CR, Cannon RE, Miller DS. Mrp1 is essential for sphingolipid signaling to p-glycoprotein in mouse blood-brain and blood-spinal cord barriers. J. Cereb. Blood Flow Metab. 2013;33:381–88. doi: 10.1038/jcbfm.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–9. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R440–46. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 132.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res. 2009;82:212–20. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways byEDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001;276:12420–26. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 134.Morales-Ruiz M, Lee MJ, Zo¨llner S, Gratton JP, Scotland R, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Giprotein/phosphoinositide 3-kinase pathway in endothelial cells. J. Biol. Chem. 2001;276:19672–77. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 135.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 2001;276:31780–85. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 136.Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2045–52. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- 137.Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosaka H. Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br. J. Pharmacol. 2007;150:470–79. doi: 10.1038/sj.bjp.0707114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cantalupo A, Zhang Y, Kothiya M, Galvani S, Obinata H, et al. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat. Med. 2015;21:1028–37. doi: 10.1038/nm.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L137–45. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, et al. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br. J. Pharmacol. 2008;153:140–47. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guan Z, Singletary ST, Cook AK, Hobbs JL, Pollock JS, Inscho EW. Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J. Am. Soc. Nephrol. 2014;25:1774–85. doi: 10.1681/ASN.2013060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear. Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 143.Herr DR, Grillet N, Schwander M, Rivera R, Mu¨ller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J. Neurosci. 2007;27:1474–78. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 2007;282:10690–96. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 145.Scherer EQ, Lidington D, Oestreicher E, Arnold W, Pohl U, Bolz SS. Sphingosine-1-phosphate modulates spiral modiolar artery tone:Apotential role in vascular-based inner ear pathologies? Cardiovasc. Res. 2006;70:79–87. doi: 10.1016/j.cardiores.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 146.Chen J, Ingham N, Kelly J, Jadeja S, Goulding D, et al. Spinster homolog 2 (Spns2) deficiency causes early onset progressive hearing loss. PLOS Genet. 2014;10:e1004688. doi: 10.1371/journal.pgen.1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Santos-Cortez RL, Faridi R, Rehman AU, Lee K, Ansar M, et al. Autosomal-recessive hearing impairment due to rare missense variants within S1PR2. Am. J. Hum. Genet. 2016;98:331–38. doi: 10.1016/j.ajhg.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nofer JR, van der Giet M, To¨lle M, Wolinska I, von Wnuck Lipinski K, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004;113:569–81. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, et al. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur. J. Pharmacol. 2003;469:125–34. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- 150.Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br. J. Pharmacol. 2012;167:1035–47. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Legangneux E, Gardin A, Johns D. Dose titration of BAF312 attenuates the initial heart rate reducing effect in healthy subjects. Br. J. Clin. Pharmacol. 2013;75:831–41. doi: 10.1111/j.1365-2125.2012.04400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–33. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arnon TI, Xu Y, Lo C, Pham T, An J, et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Green JA, Suzuki K, Cho B, Willison LD, Palmer D, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat. Immunol. 2011;12:672–80. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Czeloth N, Bernhardt G, Hofmann F, Genth H, Fo¨rster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 2005;175:2960–67. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 157.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007;8:1337–44. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 158.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009;206:2469–81. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Massberg S, Schaerli P, Knezevic-Maramica I, Ko¨llnberger M, Tubo N, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]