Abstract
The lateral habenula (LHb) is an epithalamic brain region implicated in aversive processing via negative modulation of midbrain dopamine (DA) and serotonin (5-HT) systems. Given the role of the LHb in inhibiting DA and 5-HT systems, it is thought to be involved in various psychiatric pathologies, including drug addiction. In support, it has been shown that LHb plays a critical role in cocaine- and ethanol-related behaviors, most likely by mediating drug-induced aversive conditioning. In our previous work, we showed that LHb lesions increased voluntary ethanol consumption and operant ethanol self-administration and blocked yohimbine-induced reinstatement of ethanol self-administration. LHb lesions also attenuated ethanol-induced conditioned taste aversion suggesting that a mechanism for the increased intake of ethanol may be reduced aversion learning. However, whether afferents to the LHb are required for mediating effects of the LHb on these behaviors remained to be investigated. Our present results show that lesioning the fiber bundle carrying afferent inputs to the LHb, the stria medullaris (SM), increases voluntary ethanol consumption, suggesting that afferent structures projecting to the LHb are important for mediating ethanol-directed behaviors. We then chose two afferent structures as the focus of our investigation. We specifically studied the role of the inputs from the lateral hypothalamus (LH) and ventral pallidum (VP) to the LHb in ethanol-directed behaviors. Our results show that the LH-LHb projection is necessary for regulating voluntary ethanol consumption. These results are an important first step towards understanding the functional role of afferents to LHb with regard to ethanol consumption.
Keywords: lateral habenula, lateral hypothalamus, ventral pallidum, ethanol, aversion
1. Introduction
The lateral habenula (LHb) is an epithalamic brain structure that plays an important role in aversion-driven learning and behavior [1]. Neurons within the LHb show increased and decreased firing in response to aversive and rewarding stimuli, respectively [2, 3]. The LHb modulates consumption of ethanol and cocaine, as well as drug-induced aversive conditioning [4–6]. LHb lesions increase voluntary ethanol consumption in an intermittent-ethanol access (IEA) paradigm and reduce ethanol-induced conditioned taste aversion (CTA) [4]. Further, increased firing in LHb neurons is important for expression of ethanol-induced CTA [7]. LHb lesions also block yohimbine-induced reinstatement of cocaine- and ethanol-seeking, suggesting that LHb modulates stress-induced drug-seeking [4, 8]. While these experiments demonstrate a role for the LHb in mediating drug-related behaviors, whether afferent input to the LHb is also required has not been established.
The LHb receives major afferents from limbic forebrain, basal ganglia and cortical structures through the stria medullaris (SM) [9, 10]. While there are several candidate structures that may provide relevant inputs to the LHb for ethanol-directed behaviors, including orbitofrontal cortex [11], ventral tegmental area [12], entopeduncular nucleus [13], we chose to focus on the lateral hypothalamus (LH) and ventral pallidum (VP) [9, 14, 15]. Inputs from these brain regions to the LHb are candidate pathways mediating ethanol-directed behaviors, as both the LH and VP have been implicated in regulating appetitive and consummatory behaviors. The LH regulates many reward-related behaviors [16, 17], including ethanol consumption [18–20]. For example, administration of D1 agonists and D2 antagonists into the LH increases ethanol intake, whereas administration of D1 antagonists, D2 agonists, or opioid agonists into LH reduces ethanol intake [18] [19]. Further, recent evidence shows that optogenetic inhibition of the LH-LHb produces real-time preference and acutely increases consumption of a palatable liquid [21]. Similarly, the VP regulates ethanol consumption and ethanol-seeking [22–24] and is crucial for ethanol reinforcement [25] and reinstatement of drug-seeking [26, 27]. Opioid signaling in the VP has been implicated in these effects, as mu opioid receptor signaling in the VP decreases ethanol intake [24]. Together this evidence suggests that LH-LHb and VP-LHb pathways may regulate ethanol-directed behaviors.
In the present study, we first sought to determine whether afferents to the LHb are required for ethanol-directed behaviors, and then to understand the specific role of afferents from the LH and the VP to the LHb in these behaviors. We first lesioned the SM, interrupting the major source of afferents to the LHb, and examined voluntary ethanol consumption using the IEA paradigm. Bilateral SM lesion resulted in significantly higher ethanol intake during IEA compared to that measured in control rats. To begin identifying the specific afferent brain regions mediating this effect, we next studied the role of LH and VP afferents to the LHb in ethanol-directed behaviors. In separate experiments, we used a disconnection procedure to interrupt communication between these afferent structures and the LHb and investigated voluntary ethanol consumption, operant ethanol self-administration, and yohimbine-induced reinstatement of ethanol self-administration. We also investigated alterations in CTA and taste preference as possible mechanisms underlying the increase in ethanol consumption as we did in Haack, Sheth et al, 2014 [4]. Our results suggest that the LH-LHb projection plays a significant role in regulating voluntary ethanol consumption, whereas the VP-LHb projection does not.
2. Materials and Methods
2.1. General methods
We conducted four distinct experiments to evaluate the role of afferent input to the LHb in regulating ethanol-directed behaviors.
In Experiment 1, we sought to determine if afferents to the LHb played any role in regulating ethanol intake. In this experiment, we bilaterally lesioned the SM and studied the effects of this lesion on voluntary ethanol intake, compared to animals receiving sham lesions.
In Experiment 2, we performed a preliminary anatomical study to ensure that a disconnection strategy [28–31] could effectively be used to interrupt communication between afferent structures and the LHb. This strategy is predicated on predominantly ipsilateral connectivity between afferent regions and the target structure. We thus used retrograde tracing to directly investigate the distribution of ipsilateral vs. contralateral projections from the LH and the VP to the LHb and to target LH and VP lesions to regions that showed maximum retrograde labeling after CTb was injected into the LHb. Further, we examined c-Fos activation in the LH and VP after exposure to the adrenergic system stressor, yohimbine and also quantified LH and VP neurons that expressed c-Fos and projected to the LHb. This was done in order to identify neurons that might contribute to yohimbine-induced reinstatement examined in experiments 3 and 4.
In Experiments 3 and 4, we sought to characterize the role of two major afferent inputs to the LHb - the LH (Experiment 3) and the VP (Experiment 4) - in ethanol-directed behaviors. In each of these experiments, we used a functional disconnection strategy to interrupt communication between these structures and the LHb, comparing the effects of ipsilateral vs. contralateral lesions of these structures (see experimental details below) on voluntary ethanol intake, operant ethanol self-administration, and yohimbine-induced reinstatement in control and functionally disconnected groups. Further, we investigated the effect of LH-LHb disconnection on ethanol-induced CTA and taste preference.
2.2. Subjects
Adult male Long-Evans rats were used as subjects in all experiments (400–450g at receipt, Charles-River, Wilmington, MA). Experiments were approved by the University of Utah Animal Care and Use Committee and carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition). A total of 89 rats were used in these studies.
A total of 22 rats (9 sham- and 13 SM-lesioned) were used in Experiment 1. All of these rats were used in measuring voluntary intake during IEA.
A total of 6 rats were used in anatomical and c-Fos studies conducted in Experiment 2.
A total of 32 rats (16 with unilateral ipsilateral lesions of the LH and LHb and 16 with unilateral contralateral lesions of these structures) were used in Experiment 3. Five ipsi- and 4 contralaterally-lesioned rats had to be eliminated due to misplacement of the LH or LHb lesion, resulting in a sample size of 11 ipsi- and 12 contralaterally-lesioned rats for final analysis in the IEA paradigm, taste preference, operant ethanol self-administration, extinction and yohimbine-induced reinstatement of ethanol self-administration experiments. For the CTA experiment, one contralaterally-lesioned rat had to be eliminated from CTA analysis due to profound neophobia to the saccharin solution, resulting in a final sample size of 11 ipsi- and 11 contralaterally-lesioned rats in the CTA paradigm.
A total of 29 rats (14 with unilateral ipsilateral lesions of the VP and LHb and 15 with unilateral contralateral lesions of these structures) were used in Experiment 4. Four ipsi- and four contralaterally-lesioned rats were excluded from analysis due to poor health or misplacement of the lesion, resulting in a final sample size of 10 ipsi- and 11 contralaterally-lesioned rats in the IEA paradigm, taste preference, operant ethanol self-administration and extinction of ethanol self-administration experiments. One ipsi- and one contralaterally-lesioned rat did not reach the extinction criterion and hence were not tested for yohimbine-induced reinstatement, resulting in a final group size of nine ipsi- and 10 contralaterally-lesioned rats in the reinstatement analysis.
Rats were single-housed in Plexiglas tub cages and maintained on a 12-hour (h) light/dark cycle. Ad libitum access to food and water was available throughout all experimental procedures. All procedures occurred during the light cycle, with lights on at 6 AM.
2.3. Drugs
Ethanol (Decon Labs, King of Prussia, PA) was prepared in filtered tap water (20% v/v) for the IEA paradigm and in physiological saline for the CTA paradigm. Saccharin and quinine solutions used in the taste preference experiments were prepared in distilled water. Yohimbine used in reinstatement and c-Fos activation experiments was prepared in distilled water. Ibotenic acid (10 μg/μl) used for excitotoxic lesions was prepared in physiological saline. All drugs were purchased from Sigma-Aldrich (St. Louis, MO).
2.4. Characterization of anatomical inputs from the VP and LH to the LHb
The disconnection paradigm used to study afferent input to the LHb requires a predominance of ipsilateral afferent input (see details in section 2.5 below) [28–32]. To confirm the ipsilateral predominance of the LH-LHb and VP-LHb pathways, and to determine the location of the inputs within each structure for precise lesion targeting, we studied LH and VP projections to the LHb using the retrograde tracer, cholera-toxin b (CTb). Further, double immunoprocessing for c-Fos and CTb following yohimbine injection was conducted to identify neurons that are recruited by yohimbine that may be important for ethanol reinstatement produced by yohimbine.
Surgery was conducted with the rats under isoflurane anesthesia (5% induction, 2% maintenance). Neo-Predef (a topical anesthetic), buprenorphine (0.06 mg/kg, intraperitoneal, IP), and penicillin (3 × 108 units/kg, intramuscular [IM]) were also administered to provide analgesia and prevent infection. Rats were placed in a flat-skull position in a stereotaxic apparatus, the skull exposed, and burr holes drilled above the target regions.
CTb (40 nl, 1% concentration, List Biological Laboratories) was injected unilaterally into the LHb (coordinates: −3.6 AP, −5.1 DV, 0.7 ML mm from bregma) during stereotaxic surgery (see [33]), and the animals allowed one week to recover before examining the effects of yohimbine on the induction of c-Fos. Animals were then injected with vehicle or yohimbine (2.5mg/kg, IP) and returned to their homecages for 2.5 hours to correspond with the timing of maximal c-Fos activation that would be seen during the reinstatement test used in experiment 3 and 4. Animals were then deeply anesthetized using sodium pentobarbital (140 mg/kg), and transcardially perfused with saline, followed by 4 % paraformaldehyde. The brains were then removed, cyroprotected in 20% sucrose and sectioned on a cryostat (40 μm) before being immunostained for both CTb and c-Fos [33, 34].
Briefly sections were rinsed in 50% ethanol, 3 % hydrogen peroxide, and 5% normal horse serum (NHS) in phosphate buffer (PB, 0.1M, pH=7.4). Sections were then incubated at room temperature in rabbit anti-c-Fos (1:1000, Santa Cruz Biotechnology) and goat anti-CTb (1:10 000, List Biologicals), followed by biotinylated donkey anti-rabbit immunoglobulin (1:1000, Jackson ImmunoResearch Laboratories), all in 2% NHS and 0.2% triton X-10 in PB for 24 hr. To reveal c-Fos black reaction product, a nickel intensified diaminobenzidine (DAB) reaction was carried out following incubation in avidin-biotin complex reagent (1:170, Vectastain Elite, 2 hr, Vector Laboratories, USA). Specifically, sections were incubated in a solution containing 0.5 % DAB, 0.04% nickel sulfate, 0.2 % ammonium chloride and 0.2% D-glucose (pH: 6.0), which was catalyzed by glucose oxidase (735 units/ml, 10 min). To reveal a brown reaction product for CTb, sections were then incubated in biotinylated donkey anti-goat immunoglobulin (1:1000, Jackson ImmunoResearch Laboratories), and the DAB reaction was repeated without nickel intensification.
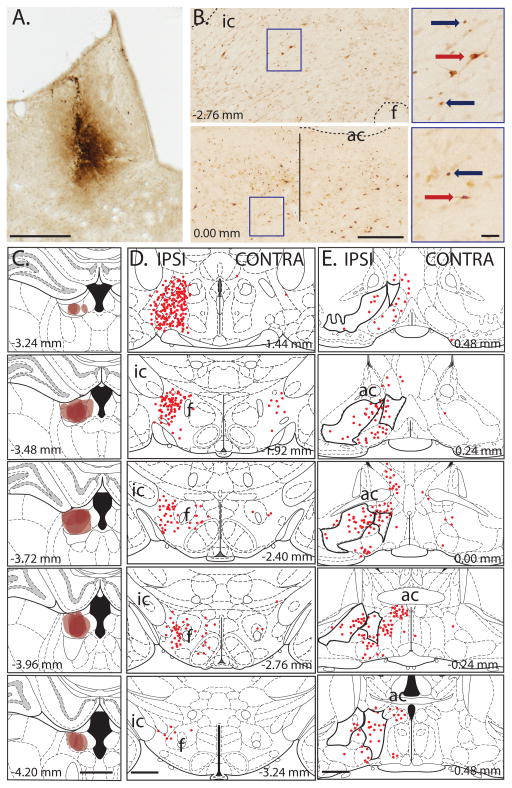
Sections were mounted on gelatin-coated slides, cleared in xylene and coverslipped before quantifying c-Fos and CTb using a light-microscope (Leica, DFC300FX). Counts were made of the LH and VP ipsilateral to the CTb injection site, according to boundaries defined in a rat brain atlas (Paxinos & Watson, 2006). Total counts of CTb-positive cell bodies in each afferent structure were averaged across three sections for each animal (for LH: between −1.92 and −2.40 mm, and for VP: between 0.36 and −0.24 mm from bregma), and then averaged for each group. CTb labeling was depicted on brain atlas templates for all 6 animals [35], where each CTb-labeled neuron was represented as a brown dot (Fig. 1).
Fig. 1. Cholera Toxin b (CTb) labeling.
(A) Photomicrograph of representative CTb injection site in LHb (scale bar: 0.5 mm). (B) Photomicrographs showing LH (top panel) and VP (bottom panel) (scale bar: 0.25 mm). Vertical line indicates approximate boundary between VP and more medial structures. Right panels show higher magnification images of CTb (red arrow) and c-Fos (blue arrow) following yohimbine injection for areas indicated by blue box (scale bar: 50 μm). Note that there was more c-Fos expression following yohimbine than vehicle injection for the LH, but there is no co-localization of c-Fos and CTb. (C) CTb injection sites in the LHb (n=6; scale bar: 1 mm). (D) CTb retrograde-labeling of neurons within the LH that project to LHb are represented as red circles. There were more projection neurons on the same side (IPSI) of the brain as the CTb injection site than on the alternate side (CONTRA). The neurons shown for ‘IPSI’ are those that would be ‘disconnected’ in our lesion study (scale bar: 1 mm). (E) CTb retrograde-labeling of neurons within the VP and lateral preoptic areas (indicated by black outlined areas) that project to LHb are represented as red circles (scale bar: 1 mm). For all panels numbers show distance from bregma. (ic: internal capsule, ac:anterior commissure, f:fornix)
2.5. Stereotaxic lesions
Lesions of all brain structures were performed with rats under isoflurane anesthesia (5% induction, 2% maintenance). Analgesia and procedures to prevent infection were as described above.
For LH-LHb and VP-LHb disconnection studies, unilateral lesions of each brain structure were made, with lesions of distinct brain structures made either ipsi-(control) or contralateral to each other (functional disconnection). Ipsilateral lesions of the afferent structure (LH or VP) and LHb preserves functional communication of these structures in the unlesioned hemisphere. Experiment 2 confirmed a predominance of ipsilateral input to the LHb in both the LH-LHb and VP-LHb pathways [9, 14]. Thus, contralateral lesions of the afferent structure (LH or VP) and the LHb interrupt functional communication between these brain regions.
Electrolytic lesions were used to interrupt the SM and ablate the LHb (as previously performed recently by us and others [4, 7, 36–39]). For SM lesions, bilateral electrolytic lesions were produced by passing current (0.5 mA, 20 sec) through a stainless steel electrode (AM Systems, Sequim, WA) at the following coordinates: AP: −1.8mm, ML: ±0.9 mm, DV: −5.5 mm relative to bregma [35]. For sham lesions, electrodes were lowered 1 mm dorsal to the coordinates, but no current was passed. Electrolytic lesions of the LHb were produced by passing current (0.5 mA, 20 sec) through a stainless steel electrode (AM Systems, Sequim, WA) at the following coordinates: AP: −3.7 mm, ML: 0.7 mm and DV: −5.6 mm [35].
Excitotoxic lesions were used to ablate the LH and VP while sparing fibers of passage. LH and VP lesions were produced by infusing ibotenic acid (0.6 μl of 10 μg/μl) through a 31-gauge injector connected to polyethylene tubing (PE50) connected to a 1-μl glass Hamilton syringe (Reno, NV) on a micro-infusion pump (Harvard Apparatus 2000, Holliston, MA). For each infusion, a volume of 0.6 μl was injected over 4 minutes, and the injector was left in place for an additional 2 minutes to allow diffusion of the drug. LH coordinates were: AP: −2.2 mm, ML: 1.7 mm, DV: −9.1 mm. VP coordinates were: AP: 0.0 mm, ML: 1.8 mm, DV: −8.4 mm, relative to bregma [35]. The hemisphere lesioned was counterbalanced across rats. Chlorodiazepoxide (0.5 ml of 10 mg/ml, IP) was injected after excitotoxic lesions to prevent seizure activity.
2.6. Intermittent ethanol access (IEA) and taste preference
Voluntary ethanol consumption was monitored for 8 weeks using a two-bottle choice IEA paradigm for experiments 1, 3 and 4. In this paradigm, a single bottle containing 20% v/v ethanol was made available in the home cage on alternate weekdays (Monday, Wednesday, and Friday) [4]. Filtered tap water was always available in a separate bottle in the home cage.
Rats were exposed to the IEA paradigm for two months, over a total of 24 drinking sessions. Total ethanol and water consumed, as well as ethanol preference relative to water [ethanol intake/total fluid intake (water +ethanol intake)] was calculated for each ethanol drinking session. Ethanol intake and ethanol preference were averaged for each week. Food was available ad lib and food intake was monitored weekly for all rats. Rats were weighed weekly.
Given that an increase in preference for ethanol could result from alterations in taste sensitivity [40], we examined taste preference following IEA. Here, water intake was compared to that of saccharin and quinine. Quinine intake was measured first (6 consecutive daily sessions) followed by saccharin intake (a further 6 sessions). Each session consisted of a 48-h period in which two bottles were provided in the home-cage. One bottle contained distilled water, and the second bottle contained either quinine or a saccharin solution. Tastant concentrations increased across sessions [0.001, 0.003, 0.01, 0.03, 0.1 and 0.3 mM concentrations for successive quinine sessions; 0.01, 0.05, 0.1, 0.5, 1 and 5 mM concentrations for successive saccharin sessions]. Consumption was recorded at 24-h intervals, and the bottle position was switched at this time to minimize side preferences. Quinine and saccharin preference for each concentration (i.e. each 48-h period) was calculated by averaging the intake for the two 24-h periods and then dividing by the average total fluid intake.
2.7. Operant responding for ethanol
Operant responding for ethanol was investigated in LH-LHb (Experiment 3) and VP-LHb (Experiment 4) ipsi- and contralaterally-lesioned rats following the IEA procedure. Training occurred in eight standard Med Associates chambers (St. Albans, VT). In training sessions, responding on the active lever resulted in retraction of the lever, extinguishing of the cue light, activation of the syringe pump, and delivery of ethanol in the receptacle. Initially, only an active lever was present during training and every lever response was reinforced with 0.1 ml of 20% ethanol (FR-1 schedule). After an initial overnight session, rats were trained daily for 60 min each session until they responded at stable levels (less than 20% variability across two sessions). All rats reached this criterion within 4 sessions. Then, the reinforcement schedule was set at FR-3 (i.e. every third lever press was reinforced). After stable responding on this paradigm was attained, the inactive lever was introduced. Responses on this lever were recorded, but had no programmed consequences. All rats were trained on this final paradigm for 11 sessions. The final measure of operant responding for ethanol was taken as the average number of active lever presses across the last three sessions. We confirmed that all ethanol was consumed by dabbing a paper towel in the magazine after each operant session. For all rats, all of the ethanol was always consumed.
2.8. Extinction and reinstatement of ethanol self-administration
After operant training, extinction of operant responding for ethanol was examined in LH-LHb (Experiment 3) and VP-LHb (Experiment 4) ipsi- and contralaterally-lesioned rats. Extinction sessions were identical to training sessions except that the syringe containing the ethanol was removed from the syringe pump. Thus, responding on the active lever resulted in retraction of the lever, extinguishing of the cue light, and activation of the syringe pump, but no ethanol delivery. Extinction sessions were conducted daily. Once responding was reduced to a total of 15 or fewer active lever presses for three consecutive sessions, the rats were tested for yohimbine-induced reinstatement. In Experiment 3, LH-LHb ipsilaterally-lesioned rats reached the extinction criterion after 7.4±0.1 sessions and LH-LHb contralaterally-lesioned rats reached the criterion after 7.5±0.1 sessions (p>0.05, no significant difference). In Experiment 4, all rats reached the extinction criterion by 7 extinction sessions.
Yohimbine (2 mg/kg) or vehicle solution was administered (IP) 30 min prior to testing responding in extinction (90-min test session [8]). To minimize variability in response rates, 4 reinstatement sessions were conducted in each animal (two after yohimbine injection and two after vehicle injection), in counter-balanced order across rats. Each test session was separated by an extinction session to ensure extinction of operant responding. For each rat, responses were then averaged across each of the two tests.
2.9. Ethanol-induced conditioned taste aversion (CTA)
As increase in preference for ethanol could also result from differences in learning about the aversive consequences of ethanol consumption, we also examined CTA [4, 41, 42]. LH-LHb ipsi- and contralaterally-lesioned rats were tested in a CTA paradigm (Experiment 3) [43]. Rats were initially water deprived for 24 hours, and then received 20 min access to tap water in their home-cage for three days (days 1–3). On day 4, rats were given access to saccharin (0.125% in tap water) for 20 min in their home cages, and consumption of that saccharin was measured. Rats were then divided into four groups balanced for baseline saccharin consumption: ipsilateral lesion-vehicle injection (n=5), ipsilateral lesion-ethanol (n=6), contralateral lesion-vehicle (n=7) and contralateral lesion-ethanol (n=5). Rats then received a single injection of either 1.5g/kg body weight of 20% v/v ethanol (IP) or an equivalent volume of vehicle saline solution after access to saccharin. On the two days following injection (days 5–6), rats received 20-min access to tap water in their home cages. This three-day cycle (saccharin access followed immediately by injection, followed by two days of water access not paired with injection) was repeated three times (i.e. three conditioning trials). Then, CTA was extinguished by exposing the rats to the same cycle but saccharin access was no longer paired with an injection. This three-day cycle continued until saccharin consumption returned to pre-CTA levels.
2.10. Verification of lesions
Lesion sites were verified after testing. Rats were deeply anesthetized with sodium pentobarbital (140 mg/kg) and transcardially perfused with saline, followed by 4 % paraformaldehyde. The brains were removed, cryoprotected and then cut on a freezing microtome (45-μm thickness). For electrolytic lesions of SM, free-floating sections were examined under a microscope. For lesions of LHb and LH or VP, brain sections were immuno-stained for neuronal nuclei (NeuN) based on a previously published protocol [44]. Lesions were verified using a light microscope, and plotted on templates modified from a rat brain atlas [35].
2.12. Statistical analyses
Voluntary ethanol consumption, ethanol preference, water intake and total fluid intake during IEA were analyzed using 2-way repeated-measures (RM) analysis of variance (ANOVA) with lesion condition (sham/lesioned for SM experiments, and ipsi/contra lesion placement in LH-LHb and VP-LHb experiments) and time as the two factors. Taste preference, extinction of ethanol-self-administration and yohimbine-induced reinstatement were also analyzed using 2-way RM ANOVA with lesion condition as one factor and tastant concentration, extinction session or drug (vehicle or yohimbine) as the second factor. CTA results were analyzed using 3-way RM ANOVA with lesion condition and drug as between factors, and trial as within factor. Differences between groups for the average of the last three operant training sessions were analyzed using t-tests. Finally, the c-Fos and CTb counts were analyzed using 2-way RM ANOVA with region (LH or VP) and injection (yohimbine or vehicle) as the two factors. All significant interactions were further examined using simple effect analysis. We corrected for multiple comparisons by employing the False Discovery Rate method [45]. JMP Pro 11 (SAS Institute Inc., Cary, NC) and Prism 7 (Graphpad Software, La Jolla, CA) were the statistical softwares used for analyses. Analyses were considered significant for p<0.05. Data are shown as mean ± S.E.M.
3. Results
3.1. CTb retrograde labeling and c-Fos double labeling after yohimbine exposure
Fig. 1 shows representative CTb injection sites and CTb retrograde labelling (Figs 1A–B), as well as CTb injection sites in LHb and retrograde labelling mapped for each animal for LH and VP (Figs 1C–E). CTb retrograde labeling was most dense in the anterior, rather than posterior, portions of the LH and in the medial rather than lateral portion of the VP (Fig. 1). Given that we did not stain for substance P [46–48], we cannot specifically determine whether CTb was located in the VP or medially adjacent structures such as lateral preoptic area, substantia innominata or stria terminalis. In any case, double labelling of CTb neurons within this basal forebrain region were low so further inquiry as to the exact location of CTb was not conducted (Fig. 1E for approx. location of CTb determined by using major landmarks and stereotaxic atlas). While few VP or LH inputs to the LHb showed c-Fos activation by yohimbine administration, yohimbine-induced c-Fos was present in both the LH and the VP (Table 1). The number of c-Fos-positive cells was greater following yohimbine versus control treatment (F (1,4)=11.90, p=0.03) and for the LH versus VP (F(1,4)=102.01, p<0.01). Importantly, there was also a treatment by region interaction (F(1,4)=27.04, p=0.01). Post-hoc analysis revealed that there was greater number of c-Fos-positive cells following yohimbine treatment compared to control treatment in LH (F(1,4)=15.75, p=0.03), but not in VP (F(1,4)=11.90, p=0.09). Analysis of double-labeled neurons confirmed that there were no differences between treatment groups and regions and no interaction (F(1,4)<0.50, p>0.52 for all contrasts), indicating that neither LH nor VP afferents to the LHb were activated by yohimbine injection and thus suggesting that these inputs to the LHb are not important for yohimbine induced-reinstatement (see below).
Table 1.
Mean ±S.E.M counts of CTb-, c-Fos- and Fos/CTb-labeled neurons per section from control and yohimbine (YOH)-treated animals. Asterisks indicate significant difference between treatment groups, p<0.05
| LH | VP | |||
|---|---|---|---|---|
| control | YOH | control | YOH | |
| CTb | 13.5±6.7 | 9.1 ±4.1 | 4.8 ±2.3 | 3.6 ±1.8 |
| c-Fos | 7.7±0.8 | 25.3±4.3* | 2.3 ±0.2 | 8.3 ±2.6 |
| Fos/CTb | 0.1 ±0.1 | 0.1 ±0.1 | 0.0 ±0.0 | 0.1 ±0.1 |
3.2. Verification of lesions
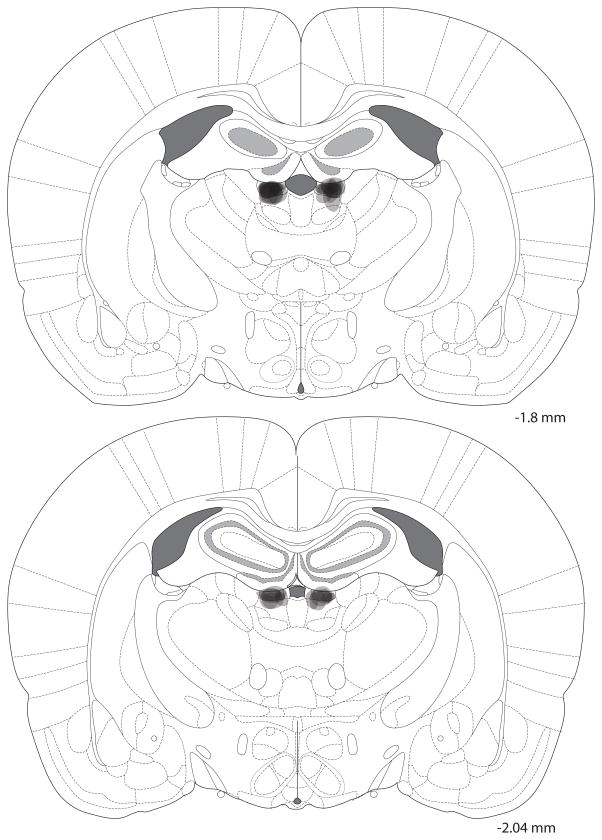
SM lesions for experiment 1 are shown in Fig. 2. Specifically, the extent of holes to the tissue is shown for each animal. It is likely that cell loss was larger than the indicated region extending ventrally into the thalamus, but this could not be confirmed without Nissl staining.
Fig. 2. SM lesions.
Lesion sites were centered on the SM. Semi-transparent shading shows the extent of holes of electrolytic lesions overlaid for each rat, so the darkest areas indicate areas of greatest damage. For some rats, damage did extend ventrally into the thalamus. Numbers to the right indicate anterioposterior position relative to bregma.
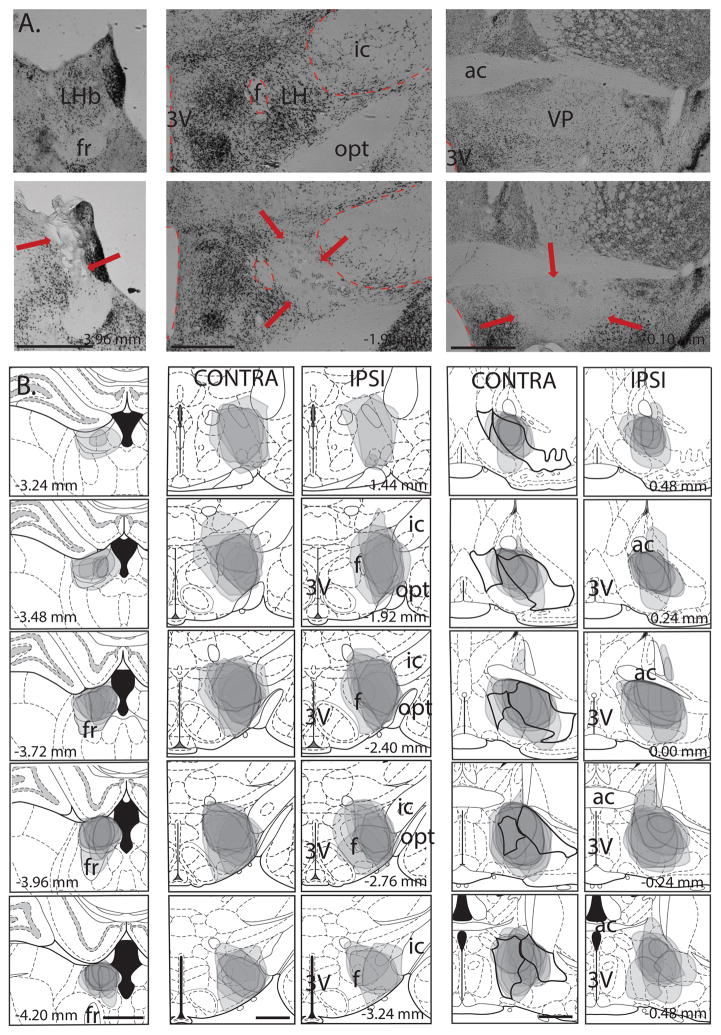
LHb, VP, and LH lesions in rats used in Experiments 3 and 4 are shown in Fig. 3 (grey outlines). It can be seen that LH lesions ablated the anterior rather than posterior portion of the LH, and the VP lesions targeted the medial rather than the lateral part of the central VP (preserving both the anterior and posterior poles); consistent with the regions of maximal CTb labeling for these structures (Fig. 1). While the lesion sites were largely confined to the LH, rarely extending into zona incerta, the VP lesions sometimes included the lateral portion of ventral preoptic area, the substantia innominata and nucleus of diagonal band.
Fig. 3. LHb, LH and VP lesion placements.
(A) Photomicrographs of representative sham (top row) and lesions (bottom row) for LHb (left panels), LH (middle panels) and VP (right panels). Note the considerably reduced NeuN staining within the boundaries of the LHb, LH and VP lesions (indicated by red arrows). (B) Lesion placements centered on LHb for the contralaterally-lesioned group in the LH-LHb disconnection study (left panels). Lesion placements in LH for contra- and ipsilaterally-lesioned groups for the LH-LHb study (n=12 and 11 for contra and ipsi groups respectively; middle panels). Lesion placements in VP for contra- and ipsilaterally-lesioned groups for the VP-LHb study (n=11 and 10 for contra and ipsi groups respectively; right panels). For all panels numbers show distance from bregma, and scale bars at 1mm. (ac: anterior commissure, f: fornix, fr: fasciculus retroflexus, ic: internal capsule, LH: lateral hypothalamus, LHb: lateral habenula, opt: optic tract, VP: ventral pallidum, 3V: 3rd ventricle).
3.3. Effects of SM lesion on intermittent ethanol access (Experiment 1)
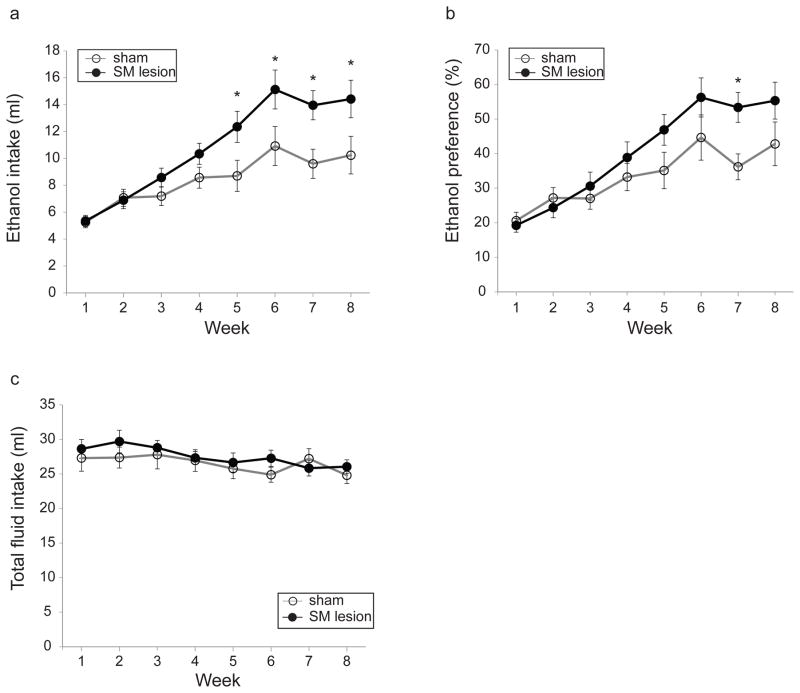
Ethanol intake progressively increased across eight weeks of IEA in both sham- and SM-lesioned rats (Fig. 4a; significant main effect of week, F(2.6,51.3)=27.9, p<0.0001). However, the SM-lesioned rats escalated their ethanol intake faster than sham-lesioned rats, and reached higher levels of ethanol intake at the end of 8 weeks (Fig. 4a; significant interaction of lesion condition and week, F(2.6,51.3)=3.3, p<0.05). Simple effect analysis showed that SM-lesioned rats drank more ethanol during weeks 5 and through 8 (p<0.05 for all comparisons). There was also a significant ethanol preference (vs. water) for the SM- versus sham-lesioned group (Fig. 4b; significant main effect of week, F(3.2,65)=33.8, p<0.0001, significant interaction of lesion condition and week, F(3.2,65)=3.4, p<0.05). Simple effect analysis showed that SM-lesioned rats had a higher preference for ethanol at week 7 (p<0.01 at week 7). Total fluid intake (ethanol plus water) was not statistically different between sham- and SM-lesioned groups, (Fig. 4c; no significant main effect of lesion condition, F(1,20)=0.4, p=0.54; no significant interaction of lesion condition and week, F(4.6,93.1)=1.0, p=0.4).
Fig. 4. Effect of stria medullaris (SM) lesions on voluntary ethanol consumption.
(a) SM lesions increase voluntary ethanol consumption in the intermittent ethanol access (IEA) paradigm. Sham- and SM-lesioned rats are indicated by open and closed circles, respectively, unless otherwise indicated. (b) SM lesions increase preference for ethanol in the IEA paradigm. (c) Total fluid intake does not differ between the two groups. Asterisk indicates significance (p<0.05).
Finally, the average body weight did not differ between the two groups during IEA (data not shown, no significant main effect of lesion condition, F(1,20)=0.3, p=0.56, significant main effect of week; F(1.5,30.2)=401.8, p<0.0001; marginally significant interaction of lesion condition and week, F(1.5,30.2)=3.6, p=0.05. However, there were no significant effects detected with simple effect analysis (p>0.05 for all comparisons).
3.4. Effects of LH-LHb disconnection on intermittent ethanol access (Experiment 3)
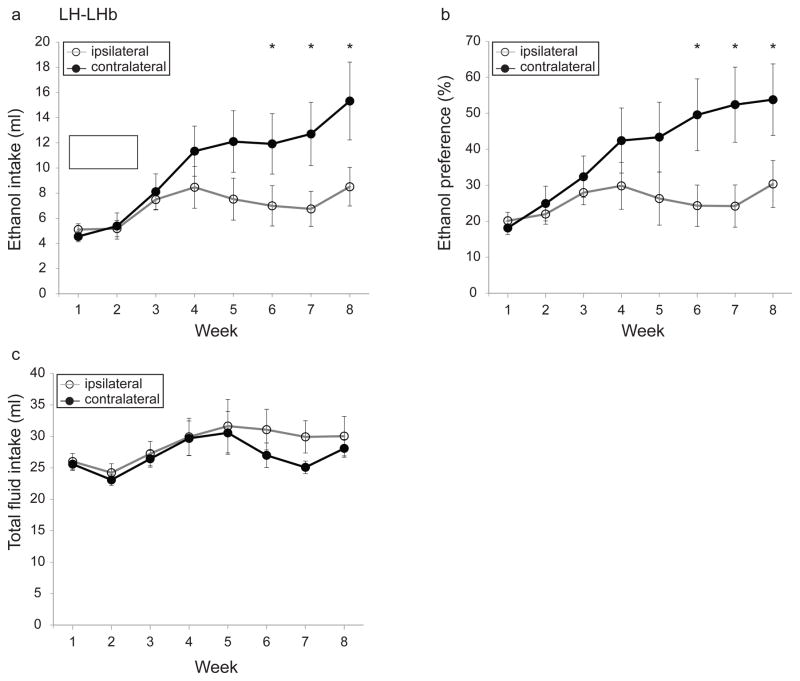
Ethanol intake increased progressively for rats with ipsilateral and contralateral LH-LHb lesions (Fig. 5a; significant main effect of week, F(1.8,37.9)=11.7, p<0.0005). However, contralaterally-lesioned rats consumed significantly more ethanol than ipsilaterally-lesioned rats overall (Fig. 5a; significant interaction of lesion condition and week, F(1.8,37.9)=3.8, p<0.05. Simple effect analysis revealed significance from week 5 through week 8, p<0.05 for weeks 5–7 and p<0.005 for week 8). Contralaterally-lesioned rats also had a higher ethanol preference than ipsilaterally-lesioned rats (Fig. 5b; significant interaction of lesion condition and week, F(2.3,48.7)=3.9, p<0.05; significant main effect of week, F(2.3,48.7)=7.4, p<0.00. Simple effect analysis demonstrated significance from week 6 through week 8, p<0.01 for all comparisons). Total fluid intake (ethanol plus water) did not differ between the two groups (Fig. 5c; no significant interaction of lesion condition and week, F(2.4,50.8)=0.7, p=0.54 and no significant main effect of lesion, F(1,21)=0.5, p=0.5).
Fig. 5. Effect of disconnection of the lateral hypothalamus-lateral habenula (LH-LHb) projection on voluntary ethanol consumption.
Disconnection of the LH-LHb input (a) increases voluntary ethanol consumption, (b) increases preference in the IEA paradigm, (c) does not alter total fluid intake. Open and closed circles represent ipsi- and contralaterally-lesioned rats, respectively. Asterisk indicates significance (p<0.05).
Finally, there were no statistically significant differences in body weight or food intake over the duration of IEA, comparing ipsi- and contralaterally-lesioned rats (data not shown; body weight, no significant interaction of lesion condition and week, F(1.5,32.1)=0.3, p=0.67, no significant main effect of lesion condition, F(1,21)=0.08, p=0.77 but main effect of week, F(1.5,32.1)=169.9, p<0.0001; food intake, no significant interaction of lesion condition and week, F(2.8,59.9)=1.8, p=0.16; and no significant main effect of lesion condition, F(1,21)=0.01, p=0.9 but main effect of week, F(2.8,59.9)=3, p<0.05).
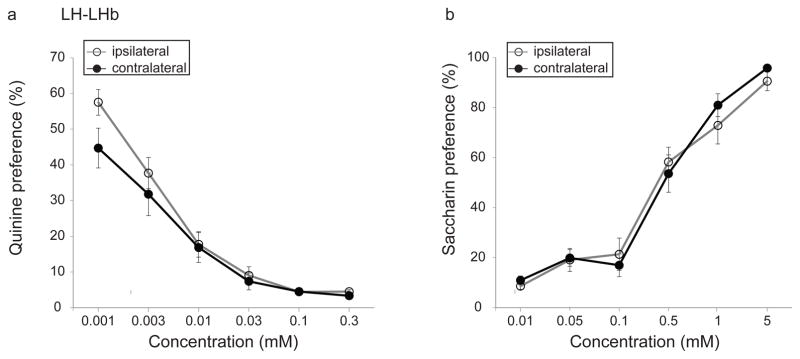
3.5. Effects of LH-LHb disconnection on taste preference
We evaluated taste preference in LH-LHb lesioned rats to investigate whether taste preference influenced ethanol intake in the IEA paradigm. Quinine preference decreased for both groups with increasing quinine concentration (Fig. 6a; significant main effect of quinine concentration, F(3.1,65.2)=82.2, p<0.0001). Lesion condition did not affect quinine preference (no significant interaction of lesion condition and quinine concentration, F(3.1,65.2)=1.3, p=0.26; no significant main effect of lesion condition, F(1,21)=1.5, p=0.23). Saccharin preference increased with increasing saccharin concentration for both groups, but again lesion condition had no effect on saccharin preference (Fig. 6b; significant main effect of saccharin concentration, F(3.6,75.9)=126.2, p<0.0001; no significant interaction of lesion condition and saccharin concentration, F(3.6,75.9)=0.68, p=0.59; and no significant main effect of lesion condition, F(1,21)=0.1, p=0.75).
Fig. 6. Effect of disconnection of the lateral hypothalamus-lateral habenula (LH-LHb) projection on taste preference.
Disconnection of the LH-LHb input does not change (a) quinine aversion or (b) saccharin preference.
3.6. Effects of LH-LHb disconnection on operant ethanol-self-administration, extinction and yohimbine-induced reinstatement of ethanol-self-administration
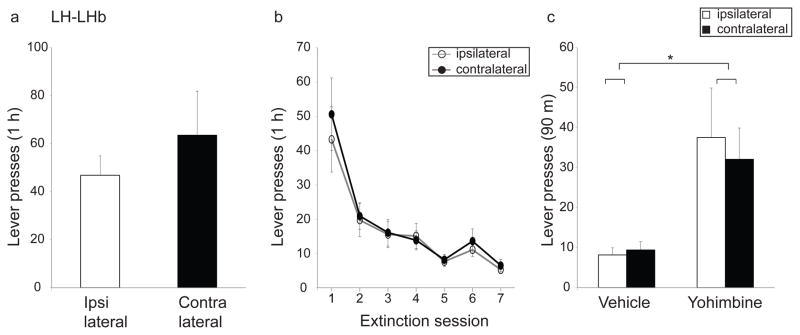
Rats with ipsilateral vs. contralateral LH-LHb lesions did not differ in the number of lever presses performed (Fig. 7a; t=−0.83, p=0.21) or in the amount of ethanol consumed (1.6 ± 0.27 ml for ipsi and 2.2 ± 0.56 ml for contra) during operant self-administration, or in the rate of extinction of this response (Fig. 7b; main effect of extinction session, F(2,42.7)=21.7, p<0.0001; no significant effect of lesion condition, F(1,21)=0.2, p=0.67; no significant interaction of lesion condition and extinction session, F(2,42.7)=0.2, p=0.81). In addition, we tested yohimbine-induced reinstatement of ethanol self-administration. Both the ipsi- and contralaterally-lesioned rats showed yohimbine-induced reinstatement of lever pressing (Fig. 7c; significant main effect of treatment, F(1,21)=15.1, p<0.001). The magnitude of reinstatement did not differ between the two groups (no significant main effect of lesion condition, F(1,21)=0.07, p=0.79; no significant interaction of lesion condition and treatment, F(1,21)=0.2; p=0.62).
Fig. 7. Effect of disconnection of the lateral hypothalamus-lateral habenula (LH-LHb) projection on operant ethanol behaviors.
Disconnection of the LH-LHb input does not alter (a) operant ethanol self-administration, (b) rate of extinction of ethanol self-administration or (c) yohimbine-induced reinstatement of ethanol self-administration. Ipsi- and contralaterally-lesioned rats are shown as open and closed bars respectively in (a) and (c) and as open and closed circles in (b). Asterisk indicates main effect of drug (yohimbine) in (c), (p<0.05).
3.7. Effects of LH-LHb disconnection on ethanol-induced CTA
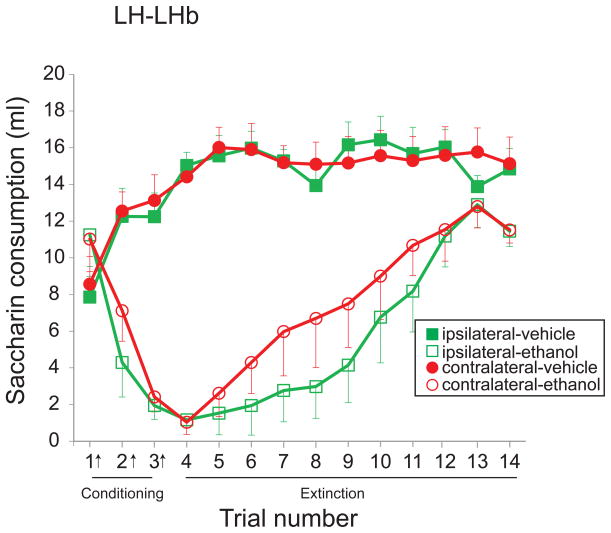
Ethanol injection conditioned a robust aversion to saccharin in both ipsi- and contralaterally-lesioned rats (Fig. 8; significant main effect of ethanol treatment, F(1,18)=46.6, p<0.0001; significant main effect of trial, F(9.3,167.5)=17.8, p<0.0001; significant interaction of ethanol treatment and trial, F(9.3,167.5)=23.3, p<0.0001). There was no difference between groups in conditioning or extinction of CTA (Fig. 8; no significant main effect of lesion condition, F(1,18)=0.6, p=0.46; no significant interaction of ethanol treatment and lesion condition, F(1,18)=0.4, p=0.55; no significant interaction of trial and lesion condition, F(9.3,167.5)=0.5, p=0.85; no significant 3-way interaction, F(9.3,167.5)=0.9, p=0.54).
Fig. 8. Effect of disconnection of the lateral hypothalamus-lateral habenula (LH-LHb) projection on ethanol-induced conditioned taste aversion (CTA).
Disconnection of the LH-LHb input does not alter ethanol-induced CTA. Ipsi- and contralaterally-lesioned rats show robust conditioned aversion to ethanol and similar rates of extinction of CTA. Arrows (x-axis) indicate trials in which saccharin consumption was paired with ethanol injection. Closed and open symbols indicate treatment with vehicle and ethanol respectively. Squares represent ipsi- and circles represent contralaterally-lesioned rats.
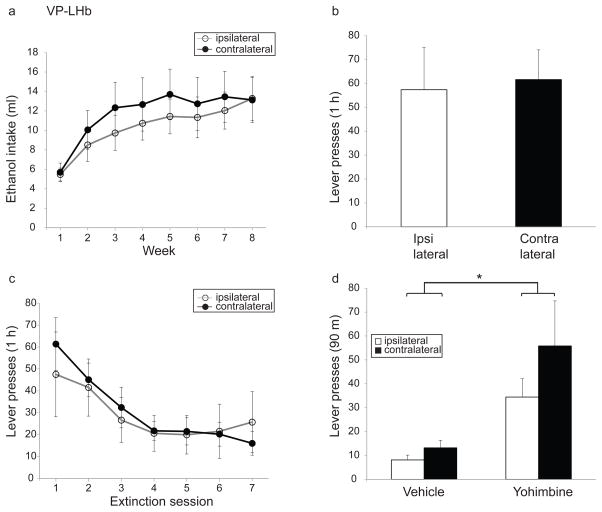
3.8. Effects of VP-LHb disconnection on intermittent ethanol access, operant ethanol-self-administration, extinction of ethanol-self-administration, yohimbine-induced reinstatement of ethanol self-administration and taste preference (Experiment 4)
During IEA, rats with ipsilateral and contralateral VP-LHb lesions progressively increased ethanol intake (Fig. 9a; significant effect of week, F(2.9,55)=17.7, p<0.0001). However, there was no difference between the lesion groups (Fig. 9a, no significant main effect of lesion condition, F(1,19)=0.3, p=0.57; no significant interaction of lesion condition and week F(2.9,55)=0.4, p=0.74).
Fig. 9. Effect of disconnection of the ventral pallidum-lateral habenula (VP-LHb) projection on ethanol-directed behaviors.
Disconnection of the VP-LHb input does not alter (a) ethanol consumption in IEA paradigm (b) operant ethanol self-administration (c) rate of extinction of ethanol self-administration or (d) yohimbine-induced reinstatement of ethanol self-administration. Ipsi- and contralaterally-lesioned rats are represented as open and closed circles, respectively in (a) and (c) and as open and closed-bars, respectively in (b) and (d).
There were no statistically significant differences between the ipsi- and contralaterally-lesioned groups in the number of lever presses made during operant ethanol self-administration (Fig. 9b; t=−0.2, p=0.85), the rate of extinction of ethanol self-administration (Fig. 9c; significant main effect of extinction session, F(2.5,48.3)=10.8, p<0.0001; no significant main effect of lesion condition, F(1,19)=0.03, p=0.86; no significant interaction of lesion condition and extinction session, F(2.5,48.3=0.7, p=0.5) or yohimbine-induced reinstatement of ethanol self-administration (Fig. 9d; significant main effect of treatment, F(1,17)=10.6, p<0.005; no significant main effect of lesion condition, F(1,17)=1.4, p=0.25; no significant interaction of lesion condition and treatment, F(1,17)=0.6, p=0.45).
4. Discussion
The LHb plays an important role in voluntary ethanol consumption, operant ethanol self-administration and yohimbine-induced reinstatement of ethanol self-administration [4]. However, afferents to the LHb needed to mediate these effects is not fully understood. In the present study, we therefore first examined the effects of lesion of the SM, the major afferent bundle carrying LHb inputs, on ethanol-directed behaviors. We found that lesion of the SM resulted in a significant increase in voluntary ethanol consumption in the IEA paradigm, suggesting that one or more inputs to LHb are important in regulating this behavior. Further, there was a marginal interaction of SM lesion and week on body weight, but no significant effects with simple effect analysis. Since there was no difference in food intake between sham and SM-lesioned rats, it is possible that SM-lesioned rats utilize the calories derived from ethanol more than sham-lesioned rats given that the former group had higher ethanol consumption [49]. We next explored the role of the LH and VP, as both of these brain regions provide major afferents to the LHb and have been implicated in regulating appetitive and consummatory behaviors. We found that disconnection of the LH-LHb projection increased voluntary ethanol intake without altering operant ethanol self-administration, or yohimbine-induced reinstatement of ethanol self-administration. Further, LH-LHb disconnection had no effect on taste preference or CTA, suggesting that alterations to neither taste nor aversion learning account for the increased consumption of ethanol by these animals. Disconnection of the VP-LHb did not alter any of the ethanol-directed behaviors. Thus, the LH provides important afferent information to the LHb in regulating voluntary ethanol intake, whereas the VP does not appear to.
4.1. Effects of disconnection of the LH-LHb projection on ethanol-related behaviors
Our results show that contralaterally-lesioned rats drank more ethanol and had a higher ethanol preference in the IEA paradigm than did ipsilaterally-lesioned rats in the LH-LHb group (Figs. 5a and 5b). In agreement, a recent study reported that optogenetic inhibition of the LH-LHb projection resulted in an increase in consumption of a palatable calorically dense liquid, indicative of a role of this projection in consummatory behaviors [21]. Further, previous experiments have shown that the LH plays a crucial role in voluntary ethanol intake. For example, DA and opioid transmission in the LH have been shown to regulate ethanol consumption [18, 19]. Interestingly, the role of the LH in ethanol intake reported in these studies differs from that observed in our study. These studies showed that pharmacological [18, 19] modulations of LH acutely altered ethanol consumption. However, our results indicate that the effect of disconnection of the LH-LHb pathway on ethanol intake emerges over time, rather than being an acute effect, suggestive of a learning mechanism. We have previously reported a similar pattern of increased escalation of ethanol intake over time after LHb lesions [4]; the present results suggest that afferents from the LH may be particularly important in mediating this learned behavior.
We previously showed that LHb lesion results in both escalation of voluntary ethanol intake and attenuation of ethanol-induced aversive conditioning in a CTA paradigm [4]. Further, a recent study showed that optogenetic stimulation of glutamatergic neurons in the LH projecting to the LHb is aversive and produces conditioned-place avoidance [21], suggesting that the LHb might have also have a role in conditioned behavior more generally. Thus, we speculated that reduced ethanol-induced aversion learning may contribute to increased voluntary ethanol intake. However, the present findings show that disconnection of the LH-LHb pathway did not alter CTA (Fig. 8), suggesting that a different mechanism may contribute to escalation of voluntary ethanol intake. The nature of this mechanism, however, is presently unclear. The LH plays an important role in consummatory behavior and maintenance of body weight. However, ipsi- and contralaterally-lesioned rats had very similar standard chow intake and weights during IEA, suggesting that increased ethanol intake in the contralaterally-lesioned group was independent of general effects on consummatory behavior. A speculative possibility is that altered anxiety levels may have contributed to differences in ethanol intake. The LH and LHb both play important roles in regulating anxiety, although a specific role for the LH-LHb projection in regulating anxiety-related behaviors has not been established [8, 50, 51]. Given that anxiety is an important factor in controlling ethanol intake [52, 53], it is possible that disconnection of the LH-LHb pathway alters anxiety levels, which then increases ethanol intake over time.
Disconnection of the LH-LHb pathway had no consequences on operant ethanol self-administration, extinction of ethanol self-administration, or yohimbine-induced reinstatement of ethanol self-administration. These results were surprising in light of evidence demonstrating a broad role for the LH in learning about and responding to reward-associated stimuli, including ethanol [54, 55]. Specifically, the LH is required for context-induced reinstatement of extinguished ethanol self-administration, as well as reinstatement of ethanol self-administration after punishment-imposed abstinence from ethanol intake [54, 56]. Further, the projection from the nucleus accumbens shell to the LH is critical in extinction of ethanol self-administration [17]. There are two important differences between our current study and the previous literature that may account for the divergent results. First, our study used chronic lesions as opposed to the acute pharmacological manipulations used by the previous studies [17, 54, 56]. Second, previous studies selectively looked at the effects of LH manipulation on ethanol-related behaviors, whereas our study looks specifically at the LH-LHb projection in ethanol-related behaviors. It is possible that LH mediates effects on extinction and reinstatement of ethanol self-administration through efferent targets other than the LHb [17]. Together, these studies suggest that although the LH is crucial for operant ethanol-directed behaviors, it does not mediate these effects through the LHb.
The results of our c-Fos study also support the idea that while the LH may be important for yohimbine-induced reinstatement, the LHb does not mediate its effects. Here, we found that the LHb projection neurons from the LH were not recruited by yohimbine injection (i.e. they did not express c-Fos), in line with the failure of LH-LHb disconnection to affect yohimbine-induced reinstatement in the current study. However, we demonstrated that total c-Fos was increased in LH following yohimbine, suggesting that the LH is responsive to yohimbine (and thus possibly also involved in reinstatement), but that the direct outputs of the LH are independent of LHb. These findings confirm earlier studies wherein the LH and a range of other forebrain structures involved in stress-related responses express c-Fos following exposure to yohimbine [57–59]. To our knowledge this is the first time the VP has been investigated, and we found that it was not significantly recruited by yohimbine.
It was surprising that while the LH-LHb disconnection escalates voluntary ethanol consumption it fails to have an effect on ethanol self-administration. Along similar lines, we have shown that lesion of the rostromedial tegmental nucleus (RMTg, a major efferent target of the LHb) increases voluntary ethanol consumption but does not affect ethanol self-administration [41]. One possible mechanism for these observations is that distinct neural circuits underlie voluntary intake and operant self-administration, and that the LHb is a node in both circuits, but the LH (and RMTg) are only nodes of the circuit underlying voluntary intake [41]. We note that there is a precedent for a dissociation of the neuromodulatory signals underlying consummatory vs. appetitive behaviors, with opioidergic and dopaminergic signaling, respectively, thought to contribute to these two types of behaviors [60].
4.2. Effects of disconnection of the VP-LHb projection on ethanol-related behaviors
In addition to the LH-LHb projection, we investigated the role of the VP-LHb projection in ethanol-directed behaviors, because of strong evidence that reward-related signals can be relayed to the LHb via inhibitory inputs from the VP [15]. This finding, coupled with results supporting the role of opioid transmission in the VP in mediating ethanol reward [24] and its involvement in reinstatement of drug-seeking [26, 27], made the VP a good candidate for mediating the effects of the LHb on ethanol-directed behaviors. However, we found that the disconnection of the VP-LHb projection produced no significant alterations in voluntary ethanol consumption, operant ethanol-self-administration, extinction of ethanol self-administration or yohimbine-induced reinstatement. In agreement with no effect of VP-LHb disconnection on yohimbine-induced reinstatement, we reported lack of CTb and c-Fos double-labeled neurons in the VP following yohimbine injection.
4.3. Afferent inputs to the LHb mediating ethanol-directed behaviors
As noted above, we have previously shown that the LHb mediates multiple facets of ethanol-related behaviors, including voluntary ethanol consumption, operant ethanol self-administration, yohimbine-induced reinstatement of ethanol self-administration and ethanol-induced CTA [4]. In the present study, we show that disconnection of the LH-LHb increases voluntary ethanol consumption, suggesting a critical role for this input to the LHb in controlling ethanol intake. On the other hand, the input from the VP to the LHb does not seem to be crucial in this regard.
Our results show that disconnection of the LH-LHb and VP-LHb projections do not cause significant changes in operant ethanol self-administration and yohimbine-induced reinstatement of ethanol self-administration, nor are these pathways recruited by exposure to yohimbine. These results contrast with those produced by lesions of the LHb, which increase operant ethanol self-administration and block yohimbine-induced reinstatement of ethanol self-administration [4]. These results suggest that LH and VP afferents to the LHb are not necessary for mediating these behaviors. With regard to operant ethanol self-administration, it is possible that the input from the ventral tegmental area (VTA) to the LHb is crucial, given that this input provides both reward- and aversion-related signals to the LHb [61, 62] and that the VTA has been previously implicated in ethanol self-administration [63, 64]. A potential afferent candidate for mediating effects of the LHb on yohimbine-induced reinstatement is the bed nucleus of the stria terminalis (BNST) [65], given its role in yohimbine-induced reinstatement of cocaine self-administration and stress-induced behaviors [65, 66]. Future studies should investigate the role of other major afferents to the LHb in regulating ethanol self-administration and yohimbine-induced reinstatement of ethanol self-administration.
4.4. Methodological considerations
The current study relies heavily on lesion techniques. While most of the lesions were well-targeted, some damage to the surrounding areas could not be avoided. For instance, in SM lesions, it is possible that cell loss extended ventrally into regions of the thalamus, including the anterior paraventricular nucleus of thalamus (aPVT) and the mediodorsal thalamus (MDT). aPVT has been shown to be involved in voluntary ethanol consumption [67]. Further, the MDT plays a crucial role in learning, specifically action-outcome associations and has been implicated in the transition from goal-directed to habitual reward seeking [68, 69]. Thus it is possible that cell loss in the aPVT and MDT may have contributed to the effects of the SM lesions on voluntary ethanol consumption in the IEA paradigm. Interestingly, PVT lesions cause significantly more weight gain as compared to controls [70], which may have contributed to the marginal effect of SM lesion on weight gain.
For the LH and VP disconnection, lesions were targeted within each structure to an area of maximum CTb retrograde labeling to the LHb. With regard to the LH, greater CTb retrograde labeling was observed in the anterior versus the posterior half of the LH, in agreement with a previous retrograde tracer study [21]. Our lesions targeted the anterior LH for most cases, while the posterior portion of the LH was relatively preserved (i.e. from approximately −3.24 mm to −4.36 mm from bregma). Therefore, it is not clear whether the posterior portion of LH also acts via LHb to regulate ethanol intake. The LH contains intermingled groups of neurons that differ in their projections and density across the LH, and subserve different motivational functions [71]. For example, the neurons that produce orexin, a neuropeptide attributed a special role in reward seeking, are located in the anterior sub-region of the LH [72]. Functional differences have been identified across the anterior-posterior extent of LH in early sleep studies [73, 74], but to our knowledge this has not been systematically investigated for reward-related function. In any case, disconnection of the LH from LHb in the current study increased ethanol intake demonstrating the importance of the LH to LHb projection in reward seeking.
With regard to the VP, CTb labeling was most dense in the medial part of VP, corresponding with the ventral medial VP (vmVP) and dorsal lateral VP (dlVP), consistent with previous tracing studies [75] (see [76] for description of subdivisions of VP). While there was some damage to surrounding structures such as lateral preoptic area, substantia innominata that also project to the LHb, we did not observe significant effects of our disconnection on ethanol-directed behaviors, suggesting that the VP is not necessary for mediating LHb effects on these behaviors. However, our lesions were centered on vmVP and dlVP leaving other subregions of the VP partly preserved, including the lateral part of the posteriorly located ventral lateral VP (vlVP) and the rostral pole of VP (rVP) so we cannot rule out these sub-divisions acting via LHb to regulate ethanol behaviors. Although the targeted vmVP and dlVP have well established roles in drug-administration, further investigation is required to determine whether the rVP or vlVP share similar roles [76]. Of interest, is that while lesions and GABA-related activation and inactivation, on feeding and liking reactions are consistent across these sub-regions of the VP, differences are revealed when opioid receptor agonists are used [77–79]. Specifically, DAMGO administered to anterior VP decreases liking reactions and decreases self-stimulation of LH (termed the ‘disgust generating zone’), while DAMGO into posterior VP increases liking reactions and increases self-stimulation of LH (termed the ‘hedonic hotspot’)[79, 80]. Thus, chemical disconnection of the VP from LHb using DAMGO may reveal a role for the VP-LHb pathway in ethanol-related behaviors.
Conclusions
This study used SM lesions to demonstrate that afferent input to the LHb is required to mediate LHb effects on ethanol-directed behavior. Using a disconnection approach, we showed that LH is one of the relevant inputs and that VP is not for mediating LHb effects on ethanol-related behaviors. Further investigation is required to determine whether other LHb afferents may also play a role. Finally, it was shown that disconnection of LH from LHb did not affect taste preference or CTA, suggesting these are not the mechanisms underlying escalated intake of ethanol observed in the LH-LHb disconnected rats. Future studies are required to examine the exact mechanism underlying this phenomenon.
Highlights.
Lesioning the stria medullaris increases voluntary ethanol consumption
Disconnection of the lateral hypothalamus to lateral habenula projection increases voluntary ethanol consumption.
Disconnection of the ventral pallidum to lateral habenula projection does not alter ethanol-directed behaviors.
Acknowledgments
Funding: Funding support was provided by the National Institutes Health under award MH094870
Footnotes
Conflict of interests: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503–13. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12(1):77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haack AK, et al. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9(4):e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo W, et al. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2015 doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jhou TC, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33(17):7501–12. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandon S, Keefe KA, Taha SA. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion. J Physiol. 2016 doi: 10.1113/JP272994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill MJ, et al. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–9. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173(1):123–46. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- 10.Vadovicova K. Affective and cognitive prefrontal cortex projections to the lateral habenula in humans. Front Hum Neurosci. 2014;8:819. doi: 10.3389/fnhum.2014.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolls ET. The roles of the orbitofrontal cortex via the habenula in non-reward and depression, and in the responses of serotonin and dopamine neurons. Neurosci Biobehav Rev. 2017;75:331–334. doi: 10.1016/j.neubiorev.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Yoo JH, et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:13697. doi: 10.1038/ncomms13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meye FJ, et al. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19(8):1019–24. doi: 10.1038/nn.4334. [DOI] [PubMed] [Google Scholar]
- 14.Poller WC, et al. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Hikosaka O. Diverse sources of reward value signals in the basal ganglia nuclei transmitted to the lateral habenula in the monkey. Front Hum Neurosci. 2013;7:778. doi: 10.3389/fnhum.2013.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aston-Jones G, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30(13):4626–35. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YW, et al. Differential role of D1 and D2 receptors in the perifornical lateral hypothalamus in controlling ethanol drinking and food intake: possible interaction with local orexin neurons. Alcohol Clin Exp Res. 2014;38(3):777–86. doi: 10.1111/acer.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YW, et al. Opioids in the perifornical lateral hypothalamus suppress ethanol drinking. Alcohol. 2013;47(1):31–8. doi: 10.1016/j.alcohol.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abulseoud OA, et al. Lateral hypothalamic kindling induces manic-like behavior in rats: a novel animal model. Int J Bipolar Disord. 2014;2(1):7. doi: 10.1186/s40345-014-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatakis AM, et al. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J Neurosci. 2016;36(2):302–11. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoo AT, et al. Role of the striatopallidal pathway in renewal and reacquisition of alcohol seeking. Behav Neurosci. 2015;129(1):2–7. doi: 10.1037/bne0000036. [DOI] [PubMed] [Google Scholar]
- 23.Perry CJ, McNally GP. A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci. 2013;38(5):2762–73. doi: 10.1111/ejn.12283. [DOI] [PubMed] [Google Scholar]
- 24.Kemppainen H, et al. Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin Exp Res. 2012;36(2):286–93. doi: 10.1111/j.1530-0277.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- 25.Melendez RI, et al. Involvement of the mesopallidal dopamine system in ethanol reinforcement. Alcohol. 2004;32(2):137–44. doi: 10.1016/j.alcohol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 26.McFarland K, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Bossert JM, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32(14):4982–91. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci. 1993;5(7):968–75. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 32.Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behav Neurosci. 2002;116(2):267–75. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 33.Furlong TM, et al. The role of prefrontal cortex in predictive fear learning. Behav Neurosci. 2010;124(5):574–86. doi: 10.1037/a0020739. [DOI] [PubMed] [Google Scholar]
- 34.Furlong TM, et al. The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: central circuitry mediating sympathoexcitation and baroreflex resetting. Eur J Neurosci. 2014;39(9):1429–38. doi: 10.1111/ejn.12521. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 36.Song M, et al. Lesions of the lateral habenula facilitate active avoidance learning and threat extinction. Behav Brain Res. 2017;318:12–17. doi: 10.1016/j.bbr.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Motohashi N, MacKenzie ET, Scatton B. Functional mapping of the effects of lesions of the habenular nuclei and their afferents in the rat. Brain Res. 1986;397(2):265–78. doi: 10.1016/0006-8993(86)90628-1. [DOI] [PubMed] [Google Scholar]
- 38.Heldt SA, Ressler KJ. Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion. Brain Res. 2006;1073–1074:229–39. doi: 10.1016/j.brainres.2005.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci. 2004;19(9):2551–60. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 40.Blednov YA, et al. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7(1):1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheth C, et al. Lesion of the rostromedial tegmental nucleus increases voluntary ethanol consumption and accelerates extinction of ethanol-induced conditioned taste aversion. Psychopharmacology (Berl) 2016;233(21–22):3737–3749. doi: 10.1007/s00213-016-4406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav Pharmacol. 2013;24(5–6):363–74. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- 43.Rinker JA, et al. Exposure to nicotine during periadolescence or early adulthood alters aversive and physiological effects induced by ethanol. Pharmacol Biochem Behav. 2011;99(1):7–16. doi: 10.1016/j.pbb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128(1):107–19. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 45.Benjamini Y, HY Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995 [Google Scholar]
- 46.Root DH, et al. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2013;521(3):558–88. doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahler SV, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17(4):577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard JM, et al. Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions. Neuron. 2016;90(6):1165–73. doi: 10.1016/j.neuron.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguiar AS, V, Da-Silva A, Boaventura GT. Can calories from ethanol contribute to body weight preservation by malnourished rats? Braz J Med Biol Res. 2004;37(6):841–6. doi: 10.1590/s0100-879x2004000600009. [DOI] [PubMed] [Google Scholar]
- 50.Hakvoort Schwerdtfeger RM, Menard JL. The lateral hypothalamus and anterior hypothalamic nucleus differentially contribute to rats’ defensive responses in the elevated plus-maze and shock-probe burying tests. Physiol Behav. 2008;93(4–5):697–705. doi: 10.1016/j.physbeh.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Kim SY, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–23. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spanagel R, et al. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122(4):369–73. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 53.Henniger MS, et al. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26(6):729–36. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 54.Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29(5):1331–42. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 56.Marchant NJ, et al. A critical role of lateral hypothalamus in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci. 2014;34(22):7447–57. doi: 10.1523/JNEUROSCI.0256-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53(4):275–83. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 58.Funk D, et al. Effects of pharmacological stressors on c-fos and CRF mRNA in mouse brain: relationship to alcohol seeking. Neurosci Lett. 2008;444(3):254–8. doi: 10.1016/j.neulet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138(1):235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 60.Kelley AE, et al. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 61.Stamatakis AM, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80(4):1039–53. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Root DH, et al. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci. 2014;34(42):13906–10. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauser SR, et al. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336(3):857–65. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czachowski CL, Delory MJ, Pope JD. Behavioral and neurotransmitter specific roles for the ventral tegmental area in reinforcer-seeking and intake. Alcohol Clin Exp Res. 2012;36(10):1659–68. doi: 10.1111/j.1530-0277.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494(1):75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213(1):19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20(3):469–81. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur J Neurosci. 2003;18(5):1286–94. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- 69.Hogarth L, et al. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- 70.Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851(1–2):66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- 71.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 73.Lin JS, et al. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479(2):225–40. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 74.Sakai K, MEMJSL, Zhang JG, Vanni-Mercier G. The posterior hypothalamus in the regulation of wakefulness and paradoxical sleep. The Diencephalon and Sleep. 1990 [Google Scholar]
- 75.Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct. 2013;218(5):1133–57. doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- 76.Root DH, et al. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho CY, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur J Neurosci. 2014;40(10):3556–72. doi: 10.1111/ejn.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 79.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25(38):8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32(12):1305–14. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]