Abstract
Background
G-CSF-stimulated hematopoietic progenitor cells (HPCs) collected by apheresis have become the predominant graft source for HPC transplantation in adults. Among healthy allogeneic donors, demographic characteristics (age, sex, BMI) and baseline hematologic counts affect HPC mobilization, leading to variability in CD34+ apheresis yields. Racial differences in HPC mobilization are less well characterized.
Methods
We retrospectively analyzed data from 1,096 consecutive G-CSF-stimulated leukapheresis procedures in healthy allogeneic African American (AA) or Caucasian donors.
Results
In a multivariate analysis, after adjusting for age, sex, BMI, baseline platelet and MNC counts, and daily G-CSF dose, peak CD34+ cell mobilization was significantly higher among AAs (n=215) than Caucasians (n=881) (123 ± 87 vs 75 ± 47 cells/uL; p<0.0001). A ceiling effect was observed with increasing G-CSF dose (10 vs 16 mcg/kg/day) in AAs (123 ± 88 vs 123 ± 87) but not in Caucasians (74 ± 46 vs 93 ± 53, p<0.001). In AA donors, presence of sickle cell trait (SCT, n=41) did not affect CD34+ mobilization (peak CD34+ 123 ± 91 vs 107 ±72 cells/uL, HbAS vs HbAA, p=0.34). Adverse events were minimal and similar across race.
Conclusions
AAs demonstrated significantly better CD34 mobilization responses to G-CSF than Caucasians. This was independent of other demographic and hematologic parameters. Studying race-associated pharmacogenomics in relation to G-CSF may improve dosing strategies. Adverse event profile and CD34 mobilization were similar in AA donors with and without SCT. Our findings suggest that it would be safe to include healthy AA donors with SCT in unrelated donor registries.
Keywords: Apheresis, Race, African American, CD34, Mobilization, Hematopoietic Progenitor Cells, Sickle Cell Trait, Duffy antigen
INTRODUCTION
Hematopoietic progenitor cells (HPCs) collected by apheresis of G-CSF-stimulated donors have surpassed bone marrow as the graft source of choice for hematopoietic stem cell transplantation in adults1. Peripheral blood HPC grafts are currently used successfully to treat hematologic and non-hematologic disorders, both benign and malignant2-4. Compared to bone marrow aspiration, their relative ease of collection by apheresis and the abundance of CD34+ cell yields make them the preferred source. However, significant variability in HPC yields has been reported even among healthy allogeneic donors. Demographic characteristics such as female sex, advancing donor age, lower body mass index (BMI), lower baseline platelet counts and lower G-CSF dose are known to be negatively correlated with CD34+ cell mobilization5,6.
Racial differences in G-CSF-mediated HPC mobilization are less well characterized. Physiologically, lower absolute neutrophil counts (ANC) are observed in African Americans (AAs) compared to Caucasians. Benign ethnic neutropenia (BEN) is described in about 5% of healthy AAs and is characterized by a decrease in granulocytes and monocytes with minimal differences in other white blood cell (WBC) subsets7,8 Postulated mechanisms for this phenomenon include a decreased stem cell reserve or fewer G-CSF receptors per cell among AA subjects. An association with the Duffy blood group antigen null phenotype, seen in 67% of AAs, and a consequent decrease in chemokine-mediated leukocyte recruitment has also been proposed9-11. Cord blood units collected from AA donors are reported to have lower total nucleated counts (TNC) and CD34+ cell counts12. Further, studies show relatively decreased leukocyte demargination and corticosteroid-mediated leukocyte egress in healthy AA adults13,14. Paradoxically, two recent clinical studies noted equivalent or increased G-CSF- stimulated HPC yields among AAs compared to other races5,15.
Healthy AAs also have lower hemoglobin and MCV compared to their Caucasian counterparts16. Low MCV and iron deficiency among healthy donors, which may not affect stem cell mobilization, have been implicated in poor collection efficiencies (due to device-related abnormalities in cell separation mechanics), thus affecting final yields17.
The effect of sickle cell trait (SCT) among AA HPC donors was evaluated in a small study which showed a trend towards better peripheral blood mobilization but poorer apheresis collection efficiencies in sickle trait versus non-sickle trait AA subjects. This resulted in similar CD34+ cell apheresis yields among the two groups. Additionally, no significant adverse events were reported among AAs with or without SCT during the process of G-CSF-stimulation and HPC collection18. Despite these data, healthy AA donors who screen positive for SCT are currently excluded from unrelated donor registries.
Our primary objective was to compare G-CSF-stimulated CD34+ cell mobilization and HPC apheresis yields among healthy AA compared with Caucasian donors. Further, we evaluated the role of physiologic interracial differences, including that of sickle cell trait, in HPC mobilization and apheresis collection outcomes.
MATERIALS AND METHODS
Study subjects
We retrospectively analyzed 1,096 consecutive healthy allogeneic related and unrelated first-time HPC apheresis donors who self-characterized their race as African American or Caucasian. Given the possibility of biased results due to significant heterogeneity within the following groups, healthy donors who described their race as Hispanic, Asian, Pacific Islander, mixed and/or other were excluded. All donors were 14 years of age or older and were either healthy siblings enrolled in institutional transplant protocols or unrelated healthy volunteers enrolled in the National Marrow Donor Program (NMDP) or the Department of Transfusion Medicine’s research apheresis protocols. Donors underwent G-CSF (filgrastim, Neupogen, Amgen, Thousand Oaks, CA) stimulated HPC collection by apheresis from April 1999 to May 2013. An unstimulated leukapheresis procedure for lymphocyte collection (lymphapheresis) was performed in the 7 days preceding G-CSF administration in 336 subjects. Informed consent was obtained in accordance with the Helsinki Declaration and our Institutional Review Board–approved transplantation and research apheresis protocols. Donor demographic data at the time of HPC collection, including age, sex, weight and height, were obtained by medical record review.
HPC mobilization, collection, and cryopreservation
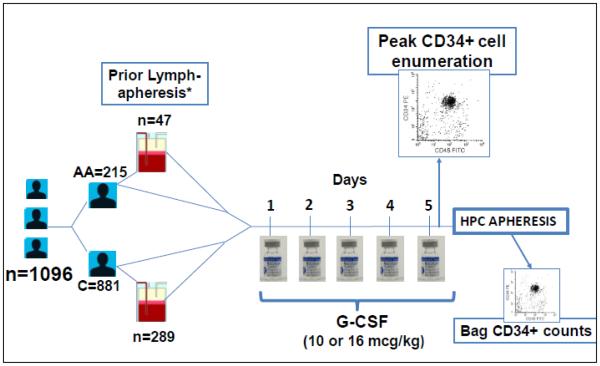
Subcutaneous injections of G-CSF were administered for 5 consecutive days at a daily dose of 10-16 mcg/kg, with the fifth dose given at least two hours prior to the start of the HPC apheresis procedure. The actual dose administered was obtained from a review of pharmacy and nursing records. Apheresis procedures were performed on the CS-3000 Plus continuous-flow apheresis device (Fenwal Division, Baxter, Deerfield, IL) or a COBE Spectra Apheresis device (Terumo BCT, Lakewood, CO) using prophylactic intravenous calcium infusions as previously described19. CD34+ collection efficiencies were similar using the two devices in our center. Volume processed per procedure ranged from 6 to 33 liters (L) for HPC collections (mean ± SD, 19 ± 5 L), depending on the immediate pre-apheresis CD34+ cell count and the targeted cell dose, and from 3 to 25 L (11 ± 3 L) for lymphapheresis procedures. HPC and unstimulated leukapheresis components were cryopreserved in plasma with 5% dimethyl sulfoxide, 6.5% pentastarch, and 4% human albumin per institutional operating procedures. Details of the study design are shown in Figure 1.
Figure 1. Study design.
*Within 7 days before HPC collection. AA=African American, C=Caucasian. Peak CD34+ cell enumeration was performed as a stat pre-apheresis flow cytometry assay with results known within 3 hours of starting the procedure.
Laboratory data
Complete blood count (CBC) including a differential and RBC indices were obtained at baseline, i.e. prior to G-CSF administration or, in patients who underwent lymphapheresis collections, prior to lymphapheresis, and were repeated on the day of collection, immediately prior to and following apheresis. Serology records for ABO, Rh and Duffy red cell phenotype were gathered from the Department of Transfusion Medicine database. Donor hemoglobin electrophoresis data were collected from all AA and selected Caucasian subjects at baseline to determine the presence of sickle cell and/or thalassemia traits. CD34+ cell quantitation was performed on peripheral blood immediately pre-apheresis (2 hours after the 5th dose of G-CSF), post-apheresis, and on the apheresis product by flow cytometry as previously described.20 Flow cytometric techniques did not change significantly during the 14 year period covered in this review.
Statistical analysis
The total mononuclear cell count was calculated as the sum of lymphocyte and monocyte counts reported on the CBC differential. Collection efficiencies were calculated using the formula21:
Summary statistics were calculated for all numerical data. Two-tailed unpaired Student t-tests were used to compare groups of two with a presumed normal distribution. Analysis of variance was used to compare more than 2 groups. Categorical variables were compared using a 2-tailed Fisher exact test. Multivariate analyses were performed using stepwise forward logistic regression, based on parameters having significance in univariate analysis, using a commercial statistics program (JMP, Version 7, SAS Institute Inc., Cary, NC). Results are given as the mean ± SD. A p value of <0.01 was considered significant.
RESULTS
Donor demographics
All AA (n=215) and Caucasian (n=881) donors with complete data sets were included. Sex ratio was similar among the two groups (45 vs 52% male; p=0.09). AAs were younger (39 vs 43 years, p=0.001) and had greater weight (86 vs 81 kg, p=0.001) and BMI (30 vs 27; p<0.0001) than Caucasians. The total daily dose of G-CSF was greater in AAs than Caucasians (920 vs 850 mcg, p<0.0001) but the G-CSF dose/kg was similar in the 2 groups (Table 1).
Table 1.
Donor demographics
| African Americans |
Caucasians | p-value* | |
|---|---|---|---|
| N (%) | 215 (20) | 881 (80) | |
| Male (%) | 97 (45) | 458 (52) | 0.09 |
| Age (yrs) | 39 ± 13 | 43 ± 14 | 0.001 |
| Height (cm) | 171 ± 11 | 172 ± 10 | 0.22 |
| Weight (kg) | 86 ± 19 | 81 ± 19 | 0.001 |
| BMI | 30.4 ± 7.2 | 26.8 ± 6.1 | <0.0001 |
| Total G-CSF dose (mcg/d) | 920 ± 196 | 850 ± 205 | <0.0001 |
| G-CSF dose per kg (mcg/kg/d) | 10.9 ± 1.7 | 10.6 ± 1.6 | 0.03 |
P<0.01 considered significant
Donor race, CD34+ mobilization, and HPC apheresis cell yields
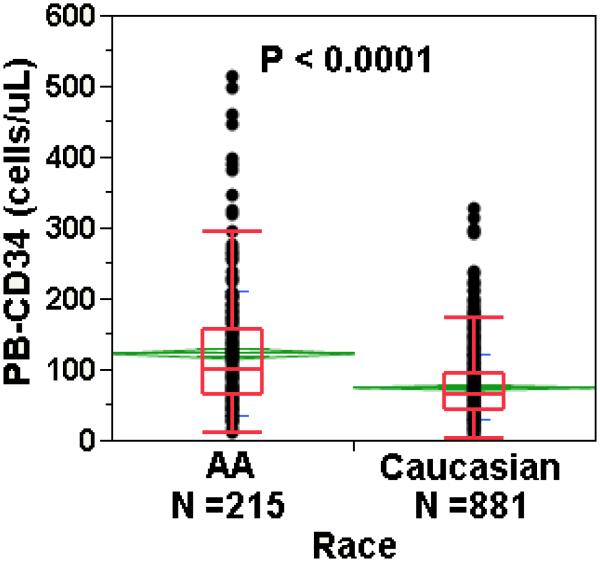
African Americans mobilized significantly better than Caucasians with mean peak circulating CD34+ counts of 123 vs 75 cells/uL (p<0.0001) (Figure 2). CD34+ apheresis yield was also significantly greater in AAs than Caucasians (51 ± 35 vs 32 ± 21 × 106 cells per liter processed, p <0.0001), consistent with higher pre-apheresis counts. Apheresis collection efficiency was similar in the two racial groups (AAs, 64%; Caucasians, 62%; p=0.11). Lymphapheresis within the 7 days prior to starting G-CSF was associated with significantly improved CD34+ cell mobilization; however, the effect did not differ by race (Figure S1).
Figure 2. CD34+ cell mobilization responses in African Americans vs Caucasians.
G-CSF-assisted HPC mobilization was significantly better in African Americans than Caucasians. PB=peripheral blood. The whisker-plot box contains the middle 25 to 75% of the data, with median shown by the line inside the box, and top and bottom lines outside the box demarcating the lowest 10% and highest 90% of the data. The mean is represented by the line through the center of the diamond, the upper and lower boundaries of the diamond showing the standard deviation.
In a univariate analysis of factors associated with higher peripheral blood CD34+ counts, three factors were overwhelmingly correlated with better peak CD34 mobilization: higher total G-CSF dose, AA race, and greater BMI, followed by higher baseline platelet and MNC counts, prior lymphapheresis, and male sex. After adjustment for total GCSF dose, AA race was the single parameter most strongly correlated with peak PB-CD34+ mobilization. In multivariate stepwise analysis, after total G-CSF dose and race were included in the model, donor BMI lost much of its contribution. Baseline platelet and MNC counts remained highly correlated, and after they were introduced into the model, prior lymphapheresis, male sex, and younger age remained significantly correlated with peak PB-CD34 counts (Table 2). To ensure that confounding factors were not introducing bias, the analysis was repeated by forcing all other parameters into the multivariate regression model and retaining race until the end; AA race still remained a significant predictor of better CD34 mobilization.
Table 2.
Regression analysis of factors associated with higher CD34+ cell counts
| Univariate Analysis | p-value | Multivariate analysis (after adjusting for total GCSF dose) |
p-value |
|---|---|---|---|
| Total G-CSF dose | <10−24 | African American race | <10−19 |
| African American race | < 10−23 | Baseline platelet count | <10−17 |
| Higher BMI | <10−23 | Baseline MNC count | <10−9 |
| Higher baseline platelet count | <10−14 | Prior lymphapheresis | 0.0003 |
| Higher baseline MNC count | <10−10 | Male gender | 0.0003 |
| Prior lymphapheresis | 0.0004 | Younger age | <0.001 |
| Male gender | 0.0009 | Higher BMI | 0.003 |
| Younger age | 0.08 |
African Americans were significantly less likely than Caucasians to be poor mobilizers and significantly more likely to be super-mobilizers. A pre-apheresis CD34+ cell count of < 20/uL was seen in 1.4% vs 6.1%, and a CD34+ cell count of > 120/uL in 39.1% vs 13.5% of AA versus Caucasian donors, respectively (p <0.001 for both comparisons) (Table 3).
Table 3.
Effect of race on pre-apheresis blood CD34+ cell count
| Pre-apheresis blood CD34+ cell count/uL |
Caucasian donors |
African American donors |
||
|---|---|---|---|---|
| N | % | N | % | |
| < 20 | 54 | 6.1 | 3 | 1.4 |
| 20-30 | 74 | 8.4 | 5 | 2.3 |
| 31-50 | 162 | 18.4 | 23 | 10.7 |
| 51-80 | 256 | 29.1 | 51 | 23.7 |
| 81-120 | 216 | 24.5 | 49 | 22.8 |
| >120 | 119 | 13.5 | 84 | 39.1 |
|
| ||||
| Total | 881 | 100 | 215 | 100 |
Effect of G-CSF on laboratory parameters
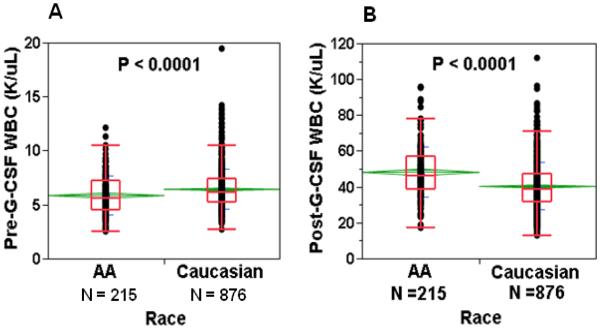
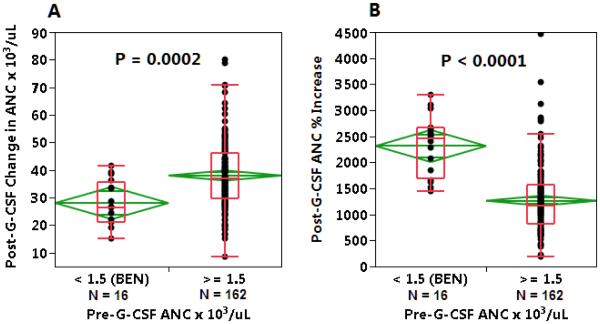
Hemoglobin and mean corpuscular volume were significantly lower among AAs than Caucasians, both at baseline and after G-CSF administration. Platelet counts were similar between the two groups, and showed a similar degree of decline following G-CSF administration. AAs had lower baseline ANC (3.4 vs 4.0 × 103 cells/uL, p<0.001) than Caucasians, but demonstrated significantly higher peak WBC and MNC counts after G-CSF administration (Table 4, Figure 3). Among AA donors with BEN, defined as an ANC < 1.5 × 103/uL, G-CSF stimulation resulted in a significantly higher percentage ANC increase compared to donors with pre-G-CSF ANC in the normal range (mean 23-fold increase in ANC among BEN AA donors (n=17) vs 13-fold increase in ANC among other AAs (n=161), p<0.0001) (Figure 4). In AA donors with known Duffy phenotype, Duffy antigen expression did not affect CD34 mobilization (peak CD34 counts 114 ± 81 vs 134 ± 85 cells/uL, Fya-b- (n=49) vs Fya+ and/or Fyb+ (n=20), p=0.4). Baseline ANC was similar in AA donors with and without Duffy antigen expression.
Table 4.
Effect of G-CSF on laboratory parameters
| African Americans |
Caucasians | p-value | |
|---|---|---|---|
| Baseline platelets (103/uL) | 260 ± 65 | 251 ± 58 | 0.06 |
| Baseline MNC (103/uL) | 2.46 ± 0.67 | 2.48 ± 0.69 | 0.8 |
| Baseline Hb (g/dL) | 13.4 ± 1.4 | 14.2 ± 1.3 | <0.0001 |
| Baseline MCV (fL) | 85.5 ± 6.3 | 89.6 ± 4.5 | <0.0001 |
| Baseline RDW (%) | 13.1 ± 1.7 | 12.3 ± 1.2 | <0.0001 |
|
| |||
| Post-GCSF platelets (103/uL) | 248 ± 144 | 231 ± 56 | 0.09 |
| Post-GCSF MNC (103/uL) | 6.7 ± 2.1 | 5.8 ± 1.8 | <0.0001 |
| Post-GCSF Hb (g/dL) | 12.8 ± 1.5 | 13.4 ± 1.3 | <0.0001 |
| Post-GCSF MCV (fL) | 85.8 ± 6.1 | 90.2 ± 4.6 | <0.0001 |
| Post-GCSF RDW (%) | 13.0 ± 1.8 | 12.4 ± 1.3 | 0.0008 |
Figure 3. Effect of G-CSF on changes in WBC counts in African Americans vs Caucasians.
A. Baseline WBC was significantly lower in African American than Caucasian donors. B. Paradoxically, WBC counts following G-CSF were significantly greater in African Americans than Caucasians. See legend to Figure 2.
Figure 4. Effect of G-CSF in African Americans with benign ethnic neutropenia.
A. ANC increase following G-CSF administration was greater in African American donors without than with benign ethnic neutropenia (BEN, defined as baseline ANC <1.5 × 103/uL). B. However, percent ANC increase after G-CSF was significantly greater in AA subjects with BEN. See legend to Figure 2.
G-CSF dose and CD34+ mobilization in African Americans and Caucasians
When stratified by G-CSF dose, at higher doses (16 mcg/kg/d), the difference in mobilization responses between the two groups was less apparent (peak CD34+ counts 123 vs 93 cells/uL, AA (n=33) vs Caucasian (n=73), p=0.07) than at lower doses (10 mcg/kg/d), where peak CD34 counts were 123 vs 74 cells/uL, AA (n=182) vs Caucasian (n=808), p<0.0001 (Table S1). Higher G-CSF doses resulted in better CD34+ mobilization in Caucasian but not in AA donors. Mean peak CD34+ counts following G-CSF 16 mcg/kg vs 10 mcg/kg were 123 vs 123 cells/uL (p=0.5) in AAs and 94 vs 74 cells/uL (p<0.001) in Caucasians, respectively.
Effect of sickle cell trait on CD34+ cell mobilization and HPC apheresis yields
African Americans with sickle cell trait (SCT) received significantly higher total G-CSF dose as well as G-CSF dose/kg, by protocol design, related to transplant preparative regimens in those who had siblings with sickle cell disease3. Despite this increased dose, in AA donors with known HbS status, the presence of SCT had no effect on CD34+ mobilization (peak CD34+ counts 123 ± 91 vs 107 ± 72 cells/uL, HbAS (n=41) vs HbAA (n=84), p=0.34). Although MCV was lower among AAs with SCT, collection efficiency was similar among AAs with and without SCT (Table S2).
Adverse events
No significant difference in the incidence of severe adverse events (AE ≥ grade 3 by CTCAE criteria22) was seen among AA vs Caucasian donors. Among AA donors with SCT, one subject experienced a grade >3 SAE with diffuse body pain on days 4 and 5 of G-CSF administration, requiring hospitalization. This patient had a history of rheumatoid arthritis, requiring opiates at baseline. Among AA donors without SCT, one subject was hospitalized overnight (grade >3 SAE) for bleeding from a central venous catheter site. Accurate assessment of grades 1 and 2 AEs was unavailable due to inconsistent data collection in our retrospective study. NSAID and/or opiate requirement was similar among SCT vs non-SCT AA donors. SCT donors did not demonstrate significant elevations in serum creatinine or transaminases compared to their non-SCT AA counterparts following G-CSF administration. None of 41 HPC components from SCT donors congealed upon thaw.
DISCUSSION
Our study demonstrates that healthy African American donors are characterized by significantly more robust CD34+ mobilization responses to G-CSF than Caucasian donors. This effect was independent of age, gender, BMI, presence of hemoglobin S, and other variables, and occurred despite physiologically lower neutrophil counts among AAs than Caucasians prior to G-CSF stimulation. Other investigators failed to find such robust differences between AA and Caucasian donors, but those studies were smaller and did not take all relevant variables into account in a structured multivariate analysis.15 A recent study demonstrated findings similar to our study with regard to racial discrepancies in CD34+ cell mobilization. However, collection efficiencies were dependent on donor BMI and sex22. In our experience, pre-apheresis CD34+ cell counts were the most important predictors of CD34 yields per liter processed after adjusting for donor demographics, G-CSF dose per kg, laboratory parameters, and procedure related variables.
Benign ethnic neutropenia has been associated with the lack of Duffy antigen expression on red blood cells, which is found in 67% of AAs but is rare in Caucasians. Duffy antigen is a chemokine receptor which can inhibit leukocyte migration9. Variability in expression of Duffy antigen thus might be a plausible explanation for the marked difference in HPC mobilization between AA and Caucasian subjects. However, we found no significant differences in HPC mobilization among AA donors with or without Duffy expression on their blood cells. Surprisingly, we found a marked enhancement in neutrophil mobilization in response to G-CSF in AA donors with benign ethnic neutropenia vs those with normal baseline ANC counts.
Our analysis was limited by small sample size, and our understanding of the actual mechanisms underlying this racial variation in HPC mobilization is speculative. Genome-wide association studies have identified variants in the cmpl gene among AAs23,24. MPL is the platelet and megakaryocyte receptor for thrombopoietin (TPO), an essential regulator of megakaryocyte differentiation and platelet production. TPO is also known to regulate the HSC niche25. It is possible that MPL variants among AAs may mediate differential responses to G-CSF stimulated stem cell egress from the marrow niche.
A ceiling effect in response to increased doses of G-CSF (>10 mcg/kg) was seen in AAs but not in Caucasians, suggesting that dose titration based on race might be used to optimize HPC yields. From a clinical standpoint, mobilization failures were more common in Caucasians and may result in the need for second day apheresis collections. Preemptive application of knowledge about racial differences in HPC mobilization may help transplant clinicians plan apheresis collection schedules and use resources more effectively, avoiding overcollection in some cases, and averting the need for additional collections in others. The identification of a subgroup of donors more likely to yield robust CD34+ cell collections may also help narrow the choice of donors from unrelated registries. Donors may also be counselled prior to donation on what to expect based on their demographic characteristics.
Lymphapheresis within the 7 days preceding G-CSF administration was found to enhance CD34+ mobilization and increase HPC apheresis yield. Platelet depletion during the prior lymphapheresis procedure may have resulted in a TPO-mediated increase in progenitor cells common to both megakaryocytes and HPCs. The increase was not significant if lymphapheresis was performed greater than 7 days prior to GCSF administration, suggesting a transient HPC stimulation effect. Interestingly, a marginal decrease in platelet counts was observed following G-CSF administration, suggesting a competitive “steal” of common progenitors towards HPC production. The effects of lymphapheresis and the lowering of platelet counts with G-CSF were independent of race.
CD34+ cell collection efficiency was correlated in prior studies with iron deficiency and low MCV and was related to abnormal cell separation mechanics during apheresis17. In our cohort, AAs demonstrated significantly lower MCV and hemoglobin levels than Caucasians both at baseline and following G-CSF administration; however, mean CD34+ collection efficiency was similar in both groups. It is likely that the value of the MCV is less important than the cause of a low MCV in terms of impact on apheresis device performance. Iron deficiency is associated with the presence of red cells of highly variable size, as reflected in an elevated red cell distribution width (RDW). We have found that a high RDW in the presence of a low MCV is more likely to be associated with impaired leukapheresis collection efficiency than a low MCV alone. Sickle trait subjects had a normal RDW, thus explaining the lack of impact of the low MCV on CD34+ collection efficiency.
Common adverse events due to G-CSF injections include headaches, bone pain, myalgias, and insomnia. Occasionally, more severe adverse events such as splenic rupture, myocardial infarction and arrhythmias have been reported in healthy donors. Adverse events in our donor cohort were generally of mild to moderate severity and were similar to those reported in prior studies25,26. In contrast, adverse effects of G-CSF can be significant in patients with sickle cell anemia and include cases of life-threatening sickling crisis26-29. One small randomized trial found that G-CSF mobilization and HPC apheresis were as safe in donors with sickle cell trait as they were in AA non-trait donors18. Yet donors with sickle cell trait are excluded from participation in the NMDP registry, based largely on a single case of G-CSF-associated multiorgan failure in a patient with compound heterozygous sickle cell/β+ thalassemia, which, unlike SCT, is a form of sickle cell disease.30 Since sickle cell trait is present in up to 10% of AAs, eliminating these individuals also negatively impacts the unrelated donor pool. Our data include the largest number of consecutive AA donors with sickle cell trait yet reported to undergo G-CSF-assisted HPC collection, and demonstrate no differences in occurrence of severe adverse clinical events, efficacy of CD34 mobilization, efficiency of CD34 collection, or product loss during cryopreservation and thaw, compared with HPC donations from non-trait AA donors.
In conclusion, racial differences in G-CSF-mediated CD34+ cell mobilization are a novel clinical finding and occur in a direction paradoxical to that predicted by known physiologic mechanisms. Further evaluation of race-associated genetic polymorphisms in relation to G-CSF pharmacokinetics may help improve G-CSF dosing strategies. Clinically, identifying donors at risk for either poor or exceptionally good mobilization may help transplant teams plan ahead and allocate resources appropriately. Finally, the absence of significant adverse clinical events or deleterious changes in product quality among donors with sickle cell trait suggests that these individuals may safely be included in unrelated donor registries.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Charles Bolan, MD, for a critical review of the manuscript. We thank the staff of the Dowling Apheresis Clinic at the NIH Department of Transfusion Medicine, including Karen Diggs, S. De Gladden, Tracey Chinn, Sara Ramirez, and Tamsen Swiegart for their expert care of donors during apheresis, and Sarah Pogue and Tanya Scinto, for their care and coordination of the NMDP donors. We also thank the clinical support staff of Hematology Branch, NHLBI and the sickle cell team at NIDDK for their management of donors. This work was supported by the intramural research programs of the NHLBI, NIDDK and the Clinical Center, NIH.
Footnotes
Disclaimers: The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Conflicts of Interest: None
Sources of Support: The authors are federal employees. There were no non-federal sources of support.
REFERENCES
- 1.Neben S, Marcus K, Mauch P. Mobilization of hematopoietic stem and progenitor cell subpopulations from the marrow to the blood of mice following cyclophosphamide and/or granulocyte colony-stimulating factor. Blood. 1993;81:1960–7. [PubMed] [Google Scholar]
- 2.Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials J Clin Oncol. 2005;23:5074–87. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, Childs RW, Rodgers GP, Powell JD, Tisdale JF. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AJ. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–8. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 5.Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ, Fowler DH, Bishop MR, Kang EM, Malech HL, Dunbar CE, Khuu HM, Wesley R, Yau YY, Bolan CD. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuya H, Watanabe T, Nakagawa R, Watanabe H, Okamoto Y, Onishi T, Abe T, Kawano Y, Kagami S, Takaue Y. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox Sang. 2005;89:229–35. doi: 10.1111/j.1423-0410.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 7.Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch Intern Med. 1991;151:501–5. [PubMed] [Google Scholar]
- 8.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–92. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Grann VR, Ziv E, Joseph CK, Neugut AI, Wei Y, Jacobson JS, Horwitz MS, Bowman N, Beckmann K, Hershman DL. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2008;143:288–93. doi: 10.1111/j.1365-2141.2008.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezvani K, Flanagan AM, Sarma U, Constantinovici N, Bain BJ. Investigation of ethnic neutropenia by assessment of bone marrow colony-forming cells. Acta Haematol. 2001;105:32–7. doi: 10.1159/000046530. [DOI] [PubMed] [Google Scholar]
- 11.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma B. Ethnic differences in plasma levels of interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF) Transl Res. 2007;149:10–4. doi: 10.1016/j.trsl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Ballen KK, Kurtzberg J, Lane TA, Lindgren BR, Miller JP, Nagan D, Newman B, Rupp N, Haley NR. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–75. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Bain BJ, Phillips D, Thomson K, Richardson D, Gabriel I. Investigation of the effect of marathon running on leucocyte counts of subjects of different ethnic origins: relevance to the aetiology of ethnic neutropenia. Br J Haematol. 2000;108:483–7. doi: 10.1046/j.1365-2141.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 14.Mason BA, Lessin L, Schechter GP. Marrow granulocyte reserves in black Americans. Hydrocortisone-induced granulocytosis in the "benign" neutropenia of the black. Am J Med. 1979;67:201–5. doi: 10.1016/0002-9343(79)90391-7. [DOI] [PubMed] [Google Scholar]
- 15.Carilli AR, Sugrue MW, Rosenau EH, Chang M, Fisk D, Medei-Hill M, Williams K, Wiggins L, Wingard JR. African American adult apheresis donors respond to granulocyte-colony-stimulating factor with neutrophil and progenitor cell yields comparable to those of Caucasian and Hispanic donors. Transfusion. 2012;52:166–72. doi: 10.1111/j.1537-2995.2011.03253.x. [DOI] [PubMed] [Google Scholar]
- 16.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106:740–5. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TF, Chen SH, Yang SH, Su YC, Chu SC, Li DK. Poor harvest of peripheral blood stem cell in donors with microcytic red blood cells. Transfusion. 2013;53:91–5. doi: 10.1111/j.1537-2995.2012.03751.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang EM, Areman EM, David-Ocampo V, Fitzhugh C, Link ME, Read EJ, Leitman SF, Rodgers GP, Tisdale JF. Mobilization, collection, and processing of peripheral blood stem cells in individuals with sickle cell trait. Blood. 2002;99:850–5. doi: 10.1182/blood.v99.3.850. [DOI] [PubMed] [Google Scholar]
- 19.Bolan CD, Cecco SA, Wesley RA, Horne M, Yau YY, Remaley AT, Childs RW, Barrett AJ, Rehak NN, Leitman SF. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42:935–46. doi: 10.1046/j.1537-2995.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Moncada V, Bolan C, Yau YY, Leitman SF. Analysis of PBPC cell yields during large-volume leukapheresis of subjects with a poor mobilization response to filgrastim. Transfusion. 2003;43:495–501. doi: 10.1046/j.1537-2995.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 21.Bolan CD, Carter CS, Wesley RA, Yau YY, Barrett AJ, Childs RW, Read EJ, Leitman SF. Prospective evaluation of cell kinetics, yields and donor experiences during a single large-volume apheresis versus two smaller volume consecutive day collections of allogeneic peripheral blood stem cells. Br J Haematol. 2003;120:801–7. doi: 10.1046/j.1365-2141.2003.04157.x. [DOI] [PubMed] [Google Scholar]
- 22.Hsu JW, Wingard JR, Logan BR, Chitphakdithai P, Akpek G, Anderlini P, Artz AS, Bredeson C, Goldstein S, Hale G, Hematti P, Joshi S, Kamble RT, Lazarus HM, O'Donnell PV, Pulsipher MA, Savani BN, Schears RM, Shaw BE, Confer DL. Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant. 2015;21:165–71. doi: 10.1016/j.bbmt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moliterno AR, Williams DM, Gutierrez-Alamillo LI, Salvatori R, Ingersoll RG, Spivak JL. Mpl Baltimore: a thrombopoietin receptor polymorphism associated with thrombocytosis. Proc Natl Acad Sci U S A. 2004;101:11444–7. doi: 10.1073/pnas.0404241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auer PL, Johnsen JM, Johnson AD, Logsdon BA, Lange LA, Nalls MA, Zhang G, Franceschini N, Fox K, Lange EM, Rich SS, O'Donnell CJ, Jackson RD, Wallace RB, Chen Z, Graubert TA, Wilson JG, Tang H, Lettre G, Reiner AP, Ganesh SK, Li Y. Imputation of exome sequence variants into population- based samples and blood-cell-trait-associated loci in African Americans: NHLBI GO Exome Sequencing Project. Am J Hum Genet. 2012;91:794–808. doi: 10.1016/j.ajhg.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M, Horwitz EM. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–49. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnamurti L, Abel S, Maiers M, Flesch S. Availability of unrelated donors for hematopoietic stem cell transplantation for hemoglobinopathies. Bone Marrow Transplant. 2003;31:547–50. doi: 10.1038/sj.bmt.1703887. [DOI] [PubMed] [Google Scholar]
- 27.Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, Lazarus HM, Waller EK, Seftel M, Stroncek DF, Lopez AM, Maharaj D, Hematti P, O'Donnell PV, Loren AW, Leitman SF, Anderlini P, Goldstein SC, Levine JE, Navarro WH, Miller JP, Confer DL. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121:197–206. doi: 10.1182/blood-2012-03-417667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzhugh CD, Hsieh MM, Bolan CD, Saenz C, Tisdale JF. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy. 2009;11:464–71. doi: 10.1080/14653240902849788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motulsky AG. Frequency of sickling disorders in U.S. blacks. N Engl J Med. 1973;288:31–3. doi: 10.1056/NEJM197301042880108. [DOI] [PubMed] [Google Scholar]
- 30.Grigg AP. Granulocyte colony-stimulating factor-induced sickle cell crisis and multiorgan dysfunction in a patient with compound heterozygous sickle cell/beta+ thalassemia. Blood. 2001;97:3998–9. doi: 10.1182/blood.v97.12.3998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.