Abstract
Organ transplant recipients (OTR) have a substantially elevated risk of squamous cell skin carcinoma (SCSC), largely attributed to immunosuppressive medications used to prevent graft rejection, although data to support the role of newer drugs on SCSC risk are sparse. We investigated the association between immunosuppressive medications and SCSC risk among cardiac and renal transplant recipients in the SCOT cohort study. Incident cases were ascertained through medical record review after self-report of skin biopsy (N=170). Controls without SCSC (N=324) were matched to cases on: gender, age, race, transplant year, hospital, donor type, organ transplanted, and time between transplant and interview. Conditional logistic regression was used to evaluate the association between specific medications and SCSC. Users of the older anti-metabolite azathioprine were more than twice as likely to develop SCSC (OR=2.69; 95%CI 1.23–5.84) compared to non-users. In contrast, the newer anti-metabolite preparations (i.e., mycophenolic acid [MPA]) were associated with lower SCSC risk (OR=0.43; 95%CI 0.27–0.66). This inverse association between MPA and SCSC persisted among OTR with no history of azathioprine use, even after adjustment for simultaneous use of the calcineurin inhibitor tacrolimus (OR=0.50; 95%CI 0.31–0.81). Our data suggest that the increased risk of SCSC historically associated with azathioprine is not seen in OTR prescribed newer regimens, including MPA and tacrolimus.
Keywords: skin cancer, organ transplant, immunosuppression, non-melanoma skin cancer, immunosuppressive medications
Introduction
The risk of squamous cell skin carcinoma (SCSC) is greatly elevated among organ transplant recipients (OTR) compared to the general population.(1–6) Data from a study of over 2,000 renal and cardiac transplant recipients reported that approximately 10% of transplant recipients developed at least one SCSC post-transplant, with at least 70% of SCSC patients developing an additional skin tumor during in the following 5 years.(7) SCSC is therefore a relatively common post-transplant complication.
Damage from ultraviolet light (UV) exposure is the central, preventable etiologic agent in skin cancer, and transplant recipients with a history of severe sunburn and markers of UV damage develop excess SCSC on sun-exposed areas.(8, 9) Immunosuppression is also a key cofactor, with elevated skin cancer risk in both OTR receiving immunosuppressive medications to prevent graft rejection and HIV/AIDS patients.(10, 11) Organ transplant registry studies have demonstrated a dose-response relationship between years of immunosuppression post-transplant and elevated skin cancer risk.(12–14) Longer duration of induced immunosuppression post-transplant necessarily results in not just years of a depressed immune response but also a longer duration of exposure to potentially carcinogenic transplant-related medications.(15–17)
Azathiopine (AZA) is a purine analogue (anti-metabolite) that interrupts rapidly dividing cells, including synthesis of immune cells. It was one of the first drugs used to maintain graft function by lowering immune response to post-transplant regimens. (18) Its ability to interrupt DNA synthesis can inhibit repair of cells damaged by UV exposure.(19–23) Experimental data have demonstrated that exposure to AZA in UV-treated mice leads to the development of skin tumors,(24) and case reports from as early as the late 1960’s documented the occurrence of various cancers in OTRs treated with AZA mono-therapy.(25–27)
Cyclosporine, a calcineurin inhibitor, is another medication used to prevent graft rejection post-transplant. It was introduced in the 1980’s to supplement AZA-based regimens, and like AZA has neoplastic properties.(28) Cyclosporine can disrupt nucleotide excision repair and impact photoproduct removal systems that lead to increased sensitization to UV damage.(17, 29) Experiments in mice treated with cyclosporine have illustrated carcinogenic properties of the drug,(30, 31) including tumor development after UV exposure.(24) Cyclosporine treatment has also been associated with higher rates of skin cancer among transplant patients in a dose-dependent manner.(32, 33)
The skin cancer risk profile of drugs introduced to post-transplant medication regimens in more recent years have not been well characterized. Mycophenolic mofetil and mycophenolate sodium are nucleotide inhibitors introduced in 1995 and 2004, respectively, to replace the carcinogenic AZA, but limited data provide conflicting evidence for a role of these mycophenolic acid preparations (MPAs) in overall cancer risk.(34–37) Likewise, tacrolimus is a calcineurin inhibitor introduced to replace cyclosporine in 1994. Data from a meta-analysis of randomized trials comparing overall cancer rate according to choice of calcineurin inhibitor demonstrated no difference between tacrolimus and cyclosporine,(38) but more recent data from a clinical trials database suggest lower skin cancer risk associated with tacrolimus.(16)
Prescription of AZA plus cyclosporine versus MPA plus tacrolimus represents two distinct immunosuppressive eras. This changing clinical practice warrants an evaluation of the association between newer immunosuppressive medications and SCSC risk, particularly a direct comparison to older drugs that carefully matches on transplantation era and post-transplant follow-up. We therefore investigated the role of immunosuppressive transplant-related medication use, including clinically relevant drug combinations prescribed in the 2000’s, in relation to the development of SCSC within a nested case-control study from the Seattle-based Skin Cancer after Organ Transplant (SCOT) cohort study.
Methods
Study Population
Details of cohort eligibility and recruitment have been published.(39) Briefly, the SCOT study enrolled renal and cardiac transplant recipients who were at least 18 years of age at receipt of their first kidney, kidney/pancreas, or heart transplant between 1995 and 2010 at one of three transplant centers in Seattle (n=2,004). Transplant recipients had to have an intact graft for at least 3 months. Eligible recipients were approached between 2008 and 2012 for study recruitment and mailed a baseline questionnaire. Individuals who self-reported any skin biopsy occurring between their time of transplant and the baseline questionnaire were considered potential SCSC cases. Review of pathology reports was conducted for those reporting a skin biopsy to identify incident, clinically confirmed SCSC cases.
Of the 196 confirmed cases of SCSC in the SCOT cohort, 172 (88%) were enrolled into a nested case-control study, and data for 170 of these cases were available for this analysis. Retrospective cohort members without an SCSC diagnosis between the time of transplant and the baseline questionnaire were eligible to be selected as controls. Controls were matched to cases on the following when possible: time between transplant and baseline questionnaire, organ transplanted (kidney, kidney/pancreas, heart), gender, age at transplant (+/− 5 years), year of transplant (+/− 2 years), transplant hospital, donor type (living vs. deceased), and race (white vs. non-white). Approximately 2 controls were selected for each SCSC case identified for a total of 337 controls recruited (81% participation rate). Data for 324 controls were available for this analysis.
Exposure Assessment
After enrollment into the nested case-control study, participants completed a more detailed, in-person interview that collected information on demographics, medical history, sun exposure, smoking, sexual history, family history of cancer, and medication use, including use of immunosuppressive transplant medications. Each participant detailed transplant medications taken between one year prior to the date of transplant and the date of the in-person interview. For all medication questions, duration of medication use was calculated using the reported age the medication was first taken and age the medication was stopped. Since we focus on SCSC diagnosed after transplant and prior to the in-person interview, only medications used prior to the diagnosis of SCSC or the comparable reference date for matched controls were considered to be etiologically relevant and therefore included in the analysis. Additionally, only steroid use that was specifically noted as being transplant related was included in analyses. Both Myfortic (mycophenolate sodium) and Cellcept (mycophenolate mofetil) were categorized as mycophenolic acid (MPA), the active ingredient in both drugs. Cellcept users represented 93.7% of the MPA group.
Statistical Analyses
Multivariable conditional logistic regression, conditioning on the matched sets of cases and controls, was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between SCSC risk and use of immunosuppressive, transplant-related medications in the etiologically relevant time window. Due to the potential for residual confounding within 5-year age strata and inexact matching on transplant hospital, conditional logistic regression models included covariates for age at transplant (continuous value) and transplant hospital.
Associations between specific transplant-related medications and SCSC risk were investigated. Nearly every organ transplant recipient reported use of at least one transplant-related medication; primary odds ratios for individual medications compared participants whose medication use history included the specific medication versus participants whose medication use history did not include the specific medication, but included other medications. Finally, regression models were considered with restriction to the more severe outcome of multiple (2 or more) SCSCs diagnosed.
Results
Renal and renal/pancreas transplants were more common in this nested case-control study, and only 19% of the participants received a cardiac transplant. Despite matching in 5-year age intervals between cases and controls, cases tended to be older: 30.6% of individuals diagnosed with SCSC were ≥ 62 years of age at transplant, as compared to only 21.6% of individuals who did not develop SCSC. Cases were also more likely to have a family history of skin cancer (cases: 34.1%; controls: 21.3%) and to have a prior history of precancerous skin lesions (cases: 48.8%; controls: 24.7%). As expected, cases were also more likely than controls to self-report a skin type that severely burned and/or blistered after sun exposure (cases: 33.6%; controls: 21.8%). (Table 1)
Table 1.
SCOT Nested Case Control Study Population Characteristics, by Case Status
| Cases (n=170) | Matched a Controls (n=324) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Organ Transplanted | ||||
| Renal Only | 124 | 72.9 | 242 | 74.7 |
| Cardiac | 35 | 20.6 | 61 | 18.8 |
| Renal/Pancreas | 11 | 6.5 | 21 | 6.5 |
| Age at Transplant | ||||
| ≤48 years | 39 | 22.9 | 89 | 27.5 |
| 49–54 years | 43 | 25.3 | 79 | 24.4 |
| 55–61 years | 36 | 21.2 | 86 | 26.5 |
| ≥62 years | 52 | 30.6 | 70 | 21.6 |
| Body Mass Index (kg/m 2) | ||||
| <25.0 | 66 | 38.8 | 119 | 36.7 |
| 25.0 – 29.9 | 76 | 44.7 | 139 | 42.9 |
| ≥30.0 | 28 | 16.5 | 66 | 20.4 |
| Family History of Skin Cancer c | ||||
| No | 112 | 65.9 | 255 | 78.7 |
| Yes | 58 | 34.1 | 69 | 21.3 |
| Reported White or Caucasian Race | ||||
| No | 8 | 4.7 | 39 | 12.0 |
| Yes | 162 | 95.3 | 285 | 88.0 |
| History of Actinic or Solar Keratosis | ||||
| No | 87 | 51.2 | 244 | 75.3 |
| 1 | 11 | 6.5 | 19 | 5.9 |
| 2–4 | 26 | 15.3 | 26 | 8.0 |
| 5–10 | 21 | 12.4 | 23 | 7.1 |
| >10 | 25 | 14.7 | 12 | 3.7 |
| Tendency to Sunburn after Initial Sun Exposure | ||||
| Severe Burn with Blistering | 12 | 7.1 | 14 | 4.4 |
| Painful Burn Followed by Peeling | 45 | 26.5 | 56 | 17.4 |
| Mild Burn Followed by degree of Tanning | 87 | 51.2 | 163 | 50.6 |
| Tan without any Sunburn | 26 | 15.3 | 89 | 27.6 |
| Skin Tone after Prolonged Sun Exposure | ||||
| Very Brown and Deeply Tanned | 28 | 16.5 | 108 | 33.5 |
| Moderately Tanned | 79 | 46.5 | 135 | 41.9 |
| Mildly Tanned, Tendency to Peel | 49 | 28.8 | 62 | 19.3 |
| Burned Only, Freckled, or no Tan | 14 | 8.2 | 17 | 5.3 |
| Donor Type b | ||||
| Living, Related | 32 | 25.8 | 55 | 22.7 |
| Living, Unrelated | 26 | 21.0 | 44 | 18.2 |
| Deceased | 66 | 53.2 | 143 | 59.1 |
Controls were matched to cases on the following: time between transplant and the baseline interview, organ transplant type (kidney, kidney/pancreas, heart), gender, age at transplant (+/− 5 years), year of transplant (+/− 2 years), transplant hospital, donor type (living vs. deceased), and race (white vs. non-white)
Family history defined as reported occurrence of skin cancer in any family member
Numbers and percentages reported out of patients with a renal transplant (n=398)
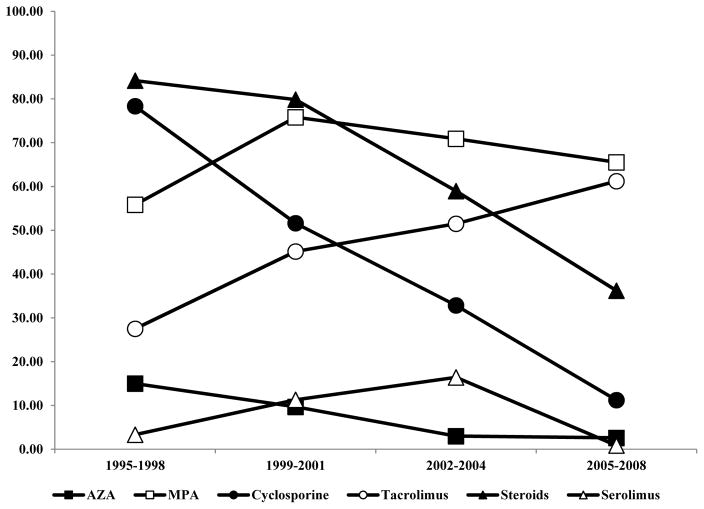
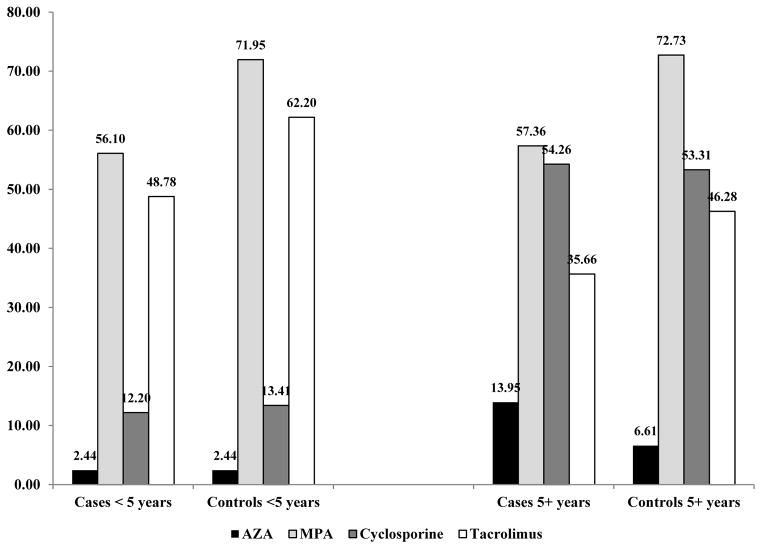
Among the 494 OTRs included in this nested case-control study, the immunosuppressive medications used post-transplant most commonly included one of the nucleotide inhibitors (MPA: 67.2%; AZA: 7.5%), and one of the calcineurin inhibitors (tacrolimus: 46.4%; cyclosporine: 43.5%). Patterns of immunosuppressive, transplant-related medication use differed according to the year of transplant. Study participants whose transplant occurred in earlier years (1995–1998) were more likely to report use of AZA, cyclosporine, and steroids compared with participants transplanted in later years (2005–2008), who were more likely to be prescribed tacrolimus and MPA. (Figure 1) The average time between transplant and date of SCSC diagnosis among the cases was 8.6 years (SD=3.7 years; median=9.0 years). Both cases and controls with a longer average time between transplant and diagnosis were more likely to report the use of drugs commonly prescribed in earlier years (i.e., AZA and cyclosporine). (Figure 2)
Figure 1.
Frequency of immunosuppressive medications in study participants, according to year of transplant
Figure 2.
Frequency of immunosuppressive medications in study participants, according to case status and time between transplant and diagnosis/reference
The first objective of this study was to determine whether MPA had the same cancer profile as AZA. We found that these agents were significantly associated with SCSC in opposite directions (Table 2). Participants with immunosuppressive medication regimens that included AZA were more than twice as likely to develop SCSC compared to participants who did not take AZA (OR=2.69; 95% CI 1.23–5.84), even after accounting for factors that influenced drug prescription patterns such as organ transplanted, transplant year, and transplant hospital. In contrast to AZA, participants reporting use of MPA had a lower risk of SCSC (OR=0.43; 95% CI 0.27–0.66). This lower likelihood of SCSC associated with prescription of MPA after transplant was present both when evaluating cases diagnosed in the first 5 years after transplant and cases diagnosed during prolonged post-transplant follow-up. (Figure 2)
Table 2.
Association between Immunosuppressive Medications and SCSC Risk
| Cases | Controls | OR | 95% CI | |
|---|---|---|---|---|
| AZA | ||||
| No | 151 (88.8) | 306 (94.4) | 1.00 | Referent |
| Yes | 19 (11.2) | 18 (5.6) | 2.69 | 1.23–5.84 |
| MPA | ||||
| No | 73 (42.9) | 89 (27.5) | 1.00 | Referent |
| Yes | 97 (57.1) | 235 (72.5) | 0.43 | 0.27–0.66 |
| Cyclosporine | ||||
| No | 95 (55.9) | 184 (56.8) | 1.00 | Referent |
| Yes | 75 (44.1) | 140 (43.2) | 0.97 | 0.60–1.55 |
| Tacrolimus | ||||
| No | 104 (61.2) | 161 (49.7) | 1.00 | Referent |
| Yes | 66 (38.8) | 163 (50.3) | 0.68 | 0.44–1.05 |
| Sirolimus | ||||
| No | 158 (92.9) | 295 (91.1) | 1.00 | Referent |
| Yes | 12 (7.1) | 29 (9.0) | 0.96 | 0.47–1.99 |
| Steroid | ||||
| No | 62 (36.5) | 111 (34.3) | 1.00 | Referent |
| Yes | 108 (63.5) | 213 (65.7) | 0.95 | 0.62–1.46 |
Odds Ratio Estimates are generated from conditional logistic regression models that condition on the matched sets and also included the following covariates: age at transplant and transplant hospital
Because the association between MPA and SCSC risk may simply reflect the inclusion of higher-risk AZA users in the referent group for non-MPA, we evaluated the association between MPA and SCSC risk restricted to non-AZA users (N=457). Non-AZA users reporting use of MPA (N=320) continued to have a substantially lower risk of SCSC (OR=0.48; 95% CI 0.30–0.78) compared to those not prescribed MPA (N=137), even after adjusting for tacrolimus use (OR=0.50; 0.31–0.81). Compared to MPA users, non-users reported substantially less use of calcineurin inhibitors (48.9% vs. 96.6%), but higher use of sirolimus (12.4% vs. 7.2%); however, sirolimus users in both groups was quite low (N=17 vs. N=23).
The second objective was to determine whether the choice of calcineurin inhibitor also impacted SCSC risk. Overall, reported tacrolimus use was associated with a non-significant lower SCSC risk (OR=0.68; 95% CI 0.44–1.05) compared to participants reporting no tacrolimus use. However, a direct comparison of study participants reporting use of tacrolimus but no cyclosporine to those reporting use of cyclosporine but no tacrolimus pointed to no difference in SCSC development according to calcineurin inhibitor choice (OR=1.02; 95% CI 0.55–1.89). The majority of tacrolimus users also reported use of MPA (75.1%); when restricting to these MPA users, we still observed no difference in SCSC risk in a direct comparison of tacrolimus versus cyclosporine (OR=0.90; 95% CI 0.38–2.14).
For other medications of interest, we observed no association with SCSC risk in our study population (sirolimus: OR=0.96; 95% CI 0.47–1.99, steroids: OR=0.95; 95% CI 0.62–1.46). We also examined effect estimates restricted to OTRs with a more severe outcome, diagnosis with multiple SCSC primaries (including both synchronous and metachronous primaries, Table 3). The effect estimate for MPA was similar for this more severe outcome (OR=0.50; 95% CI 0.28–0.90). AZA also remained statistically significantly associated with an altered risk of multiple SCSCs, with an over 3-fold increased risk of ≥2 SCSCs associated with the inclusion of AZA in the patient drug regimen (OR=5.17; 95% CI 1.54–17.3).
Table 3.
Association between Immunosuppressive Medications and Risk of Multiple SCSC
| Cases with ≥2 SCSC | Controls | OR | 95% CI | |
|---|---|---|---|---|
| AZA | ||||
| No | 78 (87.6) | 306 (94.4) | 1.00 | Referent |
| Yes | 11 (12.4) | 18 (5.6) | 5.17 | 1.54–17.3 |
| MPA | ||||
| No | 35 (39.3) | 89 (27.5) | 1.00 | Referent |
| Yes | 54 (60.7) | 235 (72.5) | 0.50 | 0.28–0.90 |
| Cyclosporine | ||||
| No | 40 (44.9) | 184 (56.8) | 1.00 | Referent |
| Yes | 49 (55.1) | 140 (43.2) | 1.22 | 0.67–2.23 |
| Tacrolimus | ||||
| No | 56 (62.9) | 161 (49.7) | 1.00 | Referent |
| Yes | 33 (37.1) | 163 (50.3) | 0.74 | 0.39–1.41 |
Odds Ratio Estimates are generated from conditional logistic regression models that condition on the matched sets and also included the following covariates: age at transplant and transplant hospital
Numbers and percentages for cases reported out of study participants with non-missing information on number of multiple SCSC (N=132)
Discussion
We observed a difference in the risk of SCSC according to the type of transplant-related, immunosuppressive medication used. AZA, a UV-sensitizing DNA synthesis inhibitor initially introduced in the 1960’s, was confirmed to be associated with more than twice the risk of developing SCSC. In a new finding, the anti-metabolite MPA was associated with a substantially lower risk of SCSC when compared with AZA. A transition in calcineurin inhibitors, from cyclosporine to tacrolimus over time was not associated with a significantly altered risk of SCSC in this study population.
Both AZA and cyclosporine are classified by the International Agency for Research on Cancer as group I carcinogens, but whether newer medication combinations impact skin cancer risk differently than these older drugs has received less attention. Our data suggest that the anti-metabolite prescribed is the most important transplant-related factor relating to skin cancer risk among OTR, with the historically elevated SCSC risk associated with AZA not observed among users of MPA. In contrast to AZA, use of MPA as the initial immunosuppressive therapy has not been consistently linked to increased skin cancer risk in kidney transplant recipients.(34, 36) Furthermore, our findings are consistent with three recent reports demonstrating a lower frequency of cancer in direct comparisons of MPA to AZA users.(35, 37, 40) One of these reports evaluated skin cancer as a distinct outcome from overall cancer, and as in this study, found that SCSC risk was associated with the choice of nucleotide inhibitor rather than calcineurin inhibitor.(37) Importantly, our report considers this association in the context of the clinical practice of multi-drug regimens and provides evidence that the inverse association of MPA with SCSC risk is independent of the prescription of maintenance calcineurin inhibitor therapy (i.e., tacrolimus).
We further considered the possibility that lower SCSC rates associated with MPA may be attributable not to a unique, protective effect of MPA but rather the fact that patients prescribed MPA are less likely to use AZA, a known carcinogen. In the SCOT study, only 16 of 374 MPA users (4.3%) reported AZA use. We found that even when restricting to OTRs without a history of AZA use, participants using MPA had a lower risk of SCSC than those not using this newer nucleotide inhibitor.
Although this is a biologically plausible finding considering laboratory data documenting anti-carcinogenic properties of the anti-metabolite,(41, 42) more sensitive data on MPA dose administered will be needed from future studies to convincingly demonstrate that MPA is in fact protective against SCSC rather than simply being less harmful than AZA. The amount of medication administered is important; previous work demonstrated elevated skin cancer risk at 3 years post-transplant among heart recipients associated with higher cumulative doses of total immunosuppressive medication.(43) If the conventional dose of administered drugs has substantially changed over time (i.e., lower doses of both nucleotide and calcineurin inhibitors), or if non-MPA users were receiving higher doses of other immunosuppressive medications, this may have contributed to lower rates of SCSC associated with regimens containing the newer anti-metabolite.
Among the non-MPA users, approximately half (48.9%) reported use of a calcineurin inhibitor, although whether dose was elevated to account for the lack of MPA use is unknown. This proportion of calcineurin inhibitor use was lower than the prevalence in the overall study population. Patients receiving neither nucleotide nor calcineurin inhibitors (i.e., not receiving a standard regimen) may have been different in ways that may have increased susceptibility to skin cancer development (i.e., poor drug reactions or serious co-morbidities).
Only a small proportion of the study participants, including non-MPA users, reported receipt of sirolimus (non-MPA users=12.4%; N=17), precluding further evaluation of this medication. Switching to sirolimus after receipt of previous immunosuppressive medications has been associated with a reduced risk of skin cancer progression and occurrence,(44, 45) although a recent population-based registry study reported no statistically significant reduction in skin cancer risk associated with reported sirolimus use.(46)
Comparisons of cyclosporine versus tacrolimus in recent years have not demonstrated consistent differences in skin cancer risk dependent upon which calcineurin inhibitor is included in an immunosuppressive regimen. Despite some evidence of a lower rate of both overall and skin cancer development in tacrolimus users compared to cyclosporine users,(16, 47) the majority of data suggest that there is no substantial difference in the cancer profile between these two drugs.(38, 48, 49) This makes sense in light of similar mechanisms of action between cyclosporine and tacrolimus.(50) This also agrees with our data, which found that SCSC risk did not differ according to whether a participant had a history that included use of either tacrolimus or cyclosporine, both overall and when restricting to OTRs using either calcineurin inhibitor in combination with MPA.
Taken together, our results indicate that neither of the newer medications (i.e., MPA or tacrolimus) is associated with the substantial increase in SCSC risk that was observed for older medications such as AZA. Recent time trend data provide some support for this assertion, with a lower cumulative risk of SCSC post-transplant observed in years during which MPA and tacrolimus use were common, compared to years characterized by AZA and cyclosporine use.(51) However, it cannot be ruled out that changes in the recommended immunosuppressive medication dose may have substantially changed over time and contributed to lower rates of SCSC in recent years, independent of which medication was prescribed.
Our study was able to match for factors likely to influence both post-transplant outcome and the length of treatment with immunosuppressive medications, including time since transplant, treatment hospital, transplant year, and organ type. Another strength of this study was the follow-up of all self-reported skin biopsies with confirmation of case reports through examination systematic centralized review of pathology reports. Limitations of the study should also be considered in the interpretation of our results. Cohort participants who failed to report receipt of skin biopsy, had clinically undiagnosed cancers, or who died prior to the start of study recruitment may represent misclassified non-cases, leading to attenuated risk estimates. Future studies should evaluate more sensitive measures of transplant medication use collected prospectively, including information on dosage from the pharmacy records, as evidence of a dose-response relationship. This might lend plausibility to the finding in our study that MPA is associated with a decreased risk of SCSC.
Several of the immunosuppressive medications prescribed to transplant recipients impacted the development of skin cancer in this patient population. The transition from AZA to MPA was shown to be a beneficial one for transplant recipients, decreasing their risk of being diagnosed with SCSC. MPA can induce apoptosis of activated T-cells, decrease recruitment of leukocytes, and reduce tissue damage by reducing oxidation.(52) Our data suggest that patients on MPA and not exposed to AZA are at a reduced risk of SCSC. Larger prospective studies with additional follow-up are needed to establish if an excess risk of SCSC persists in OTR not exposed to AZA.
Supplementary Material
Abbreviations
- SCOT
Skin Cancer after Organ Transplant
- SCSC
squamous cell skin carcinoma
- OTRs
organ transplant recipients
- AZA
azathioprine
- UV
ultraviolet
- MPA
mycophenolic acid
Footnotes
Disclosures. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. Journal of the American Academy of Dermatology. 2002 Jul;47(1):1–17. doi: 10.1067/mjd.2002.125579. quiz 8–20. [DOI] [PubMed] [Google Scholar]
- 2.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010 Aug;10(8):1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. Epub 2010/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 3.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. The New England journal of medicine. 2003 Apr 24;348(17):1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 4.Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010 Sep;6(9):511–9. doi: 10.1038/nrneph.2010.102. Epub 2010/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011 Nov 2;306(17):1891–901. doi: 10.1001/jama.2011.1592. Epub 2011/11/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. International journal of cancer Journal international du cancer. 2013 Mar 15;132(6):1429–38. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 7.Euvrard S, Kanitakis J, Decullier E, Butnaru AC, Lefrancois N, Boissonnat P, et al. Subsequent skin cancers in kidney and heart transplant recipients after the first squamous cell carcinoma. Transplantation. 2006 Apr 27;81(8):1093–100. doi: 10.1097/01.tp.0000209921.60305.d9. [DOI] [PubMed] [Google Scholar]
- 8.de Vries E, Arnold M, Altsitsiadis E, Trakatelli M, Hinrichs B, Stockfleth E, et al. Potential impact of interventions resulting in reduced exposure to ultraviolet (UV) radiation (UVA and UVB) on skin cancer incidence in four European countries, 2010–2050. The British journal of dermatology. 2012 Aug;167( Suppl 2):53–62. doi: 10.1111/j.1365-2133.2012.11087.x. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich C, Schmook T, Sachse MM, Sterry W, Stockfleth E. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2004 Apr;30(4 Pt 2):622–7. doi: 10.1111/j.1524-4725.2004.30147.x. Epub 2004/04/06. eng. [DOI] [PubMed] [Google Scholar]
- 10.Cobucci RN, Saconato H, Lima PH, Rodrigues HM, Prudencio TL, Junior JE, et al. Comparative incidence of cancer in HIV-AIDS patients and transplant recipients. Cancer Epidemiol. 2012 Jan 9; doi: 10.1016/j.canep.2011.12.002. Epub 2012/01/13. Eng. [DOI] [PubMed] [Google Scholar]
- 11.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 12.van Leeuwen MT, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ. 2010;340:c570. doi: 10.1136/bmj.c570. Epub 2010/02/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller B, Braathen LR, Marti HP, Hunger RE. Skin cancers in renal transplant recipients: a description of the renal transplant cohort in Bern. Swiss medical weekly. 2010;140:w13036. doi: 10.4414/smw.2010.13036. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Non-melanoma skin cancer risk in the Queensland renal transplant population. The British journal of dermatology. 2002 Nov;147(5):950–6. doi: 10.1046/j.1365-2133.2002.04976.x. [DOI] [PubMed] [Google Scholar]
- 15.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004 Jun 27;77(12):1777–82. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 16.Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transplant international : official journal of the European Society for Organ Transplantation. 2006 Aug;19(8):607–20. doi: 10.1111/j.1432-2277.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 17.Thoms KM, Kuschal C, Oetjen E, Mori T, Kobayashi N, Laspe P, et al. Cyclosporin A, but not everolimus, inhibits DNA repair mediated by calcineurin: implications for tumorigenesis under immunosuppression. Experimental dermatology. 2011 Mar;20(3):232–6. doi: 10.1111/j.1600-0625.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 18.Halloran PF. Immunosuppressive drugs for kidney transplantation. The New England journal of medicine. 2004 Dec 23;351(26):2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 19.Harwood CA, Attard NR, O’Donovan P, Chambers P, Perrett CM, Proby CM, et al. PTCH mutations in basal cell carcinomas from azathioprine-treated organ transplant recipients. British journal of cancer. 2008 Oct 21;99(8):1276–84. doi: 10.1038/sj.bjc.6604665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrett CM, Walker SL, O’Donovan P, Warwick J, Harwood CA, Karran P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. The British journal of dermatology. 2008 Jul;159(1):198–204. doi: 10.1111/j.1365-2133.2008.08610.x. [DOI] [PubMed] [Google Scholar]
- 21.Brem R, Li F, Karran P. Reactive oxygen species generated by thiopurine/UVA cause irreparable transcription-blocking DNA lesions. Nucleic acids research. 2009 Apr;37(6):1951–61. doi: 10.1093/nar/gkp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Graaf YG, Rebel H, Elghalbzouri A, Cramers P, Nellen RG, Willemze R, et al. More epidermal p53 patches adjacent to skin carcinomas in renal transplant recipients than in immunocompetent patients: the role of azathioprine. Experimental dermatology. 2008 Apr;17(4):349–55. doi: 10.1111/j.1600-0625.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 23.Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. The Journal of laboratory and clinical medicine. 2001 Jan;137(1):14–20. doi: 10.1067/mlc.2001.111469. [DOI] [PubMed] [Google Scholar]
- 24.Kelly GE, Meikle W, Sheil AG. Effects of immunosuppressive therapy on the induction of skin tumors by ultraviolet irradiation in hairless mice. Transplantation. 1987 Sep;44(3):429–34. doi: 10.1097/00007890-198709000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplantation proceedings. 1969 Mar;1(1):106–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Scharf J, Nahir M, Eidelman S, Jacobs R, Levin D. Carcinoma of the bladder with azathioprine therapy. Jama. 1977 Jan 10;237(2):152. [PubMed] [Google Scholar]
- 27.Schneck SA, Penn I. De-novo brain tumours in renal-transplant recipients. Lancet. 1971 May 15;1(7707):983–6. doi: 10.1016/s0140-6736(71)91384-5. [DOI] [PubMed] [Google Scholar]
- 28.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999 Feb 11;397(6719):530–4. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 29.Kuschal C, Thoms KM, Boeckmann L, Laspe P, Apel A, Schon MP, et al. Cyclosporin A inhibits nucleotide excision repair via downregulation of the xeroderma pigmentosum group A and G proteins, which is mediated by calcineurin inhibition. Experimental dermatology. 2011 Oct;20(10):795–9. doi: 10.1111/j.1600-0625.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 30.Masuhara M, Ogasawara H, Katyal SL, Nakamura T, Shinozuka H. Cyclosporine stimulates hepatocyte proliferation and accelerates development of hepatocellular carcinomas in rats. Carcinogenesis. 1993 Aug;14(8):1579–84. doi: 10.1093/carcin/14.8.1579. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, Martin MS, Pelletier H, Lagadec P, Martin F. Effects of cyclosporin A on progressive and regressive tumors induced by two cancer lines derived from a single colon carcinoma chemically induced in the rat. Immunobiology. 1989 Feb;178(4–5):401–15. doi: 10.1016/S0171-2985(89)80062-2. [DOI] [PubMed] [Google Scholar]
- 32.Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998 Feb 28;351(9103):623–8. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 33.McGeown MG, Douglas JF, Middleton D. One thousand renal transplants at Belfast City Hospital: post-graft neoplasia 1968–1999, comparing azathioprine only with cyclosporin-based regimes in a single centre. Clinical transplants. 2000:193–202. [PubMed] [Google Scholar]
- 34.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004 Jun;4(6):905–13. doi: 10.1111/j.1600-6143.2004.00450.x. Epub 2004/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 35.David KM, Morris JA, Steffen BJ, Chi-Burris KS, Gotz VP, Gordon RD. Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clinical transplantation. 2005 Apr;19(2):279–85. doi: 10.1111/j.1399-0012.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 36.Marcen R, Galeano C, Fernandez-Rodriguez A, Jimenez-Alvaro S, Teruel JL, Rivera M, et al. Effects of the new immunosuppressive agents on the occurrence of malignancies after renal transplantation. Transplantation proceedings. 2010 Oct;42(8):3055–7. doi: 10.1016/j.transproceed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Molina BD, Leiro MG, Pulpon LA, Mirabet S, Yanez JF, Bonet LA, et al. Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplantation proceedings. 2010 Oct;42(8):3001–5. doi: 10.1016/j.transproceed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. The Cochrane database of systematic reviews. 2005;4:CD003961. doi: 10.1002/14651858.CD003961.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Madeleine MM, Johnson LG, Daling JR, Schwartz SM, Carter JJ, Berg D, et al. Cohort Profile: The Skin Cancer After Organ Transplant Study. Int J Epidemiol. 2012 Nov 21; doi: 10.1093/ije/dys179. Epub 2012/11/23. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Dec;5(12):2954–60. doi: 10.1111/j.1600-6143.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 41.Tressler RJ, Garvin LJ, Slate DL. Anti-tumor activity of mycophenolate mofetil against human and mouse tumors in vivo. International journal of cancer Journal international du cancer. 1994 May 15;57(4):568–73. doi: 10.1002/ijc.2910570421. [DOI] [PubMed] [Google Scholar]
- 42.Williams RH, Lively DH, DeLong DC, Cline JC, Sweeny MJ. Mycophenolic acid: antiviral and antitumor properties. The Journal of antibiotics. 1968 Jul;21(7):463–4. doi: 10.7164/antibiotics.21.463. [DOI] [PubMed] [Google Scholar]
- 43.Fortina AB, Piaserico S, Caforio AL, Abeni D, Alaibac M, Angelini A, et al. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Archives of dermatology. 2004 Sep;140(9):1079–85. doi: 10.1001/archderm.140.9.1079. [DOI] [PubMed] [Google Scholar]
- 44.Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. Bmj. 2014;349:g6679. doi: 10.1136/bmj.g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salgo R, Gossmann J, Schofer H, Kachel HG, Kuck J, Geiger H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010 Jun;10(6):1385–93. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 46.Yanik EL, Gustafson SK, Kasiske BL, Israni AK, Snyder JJ, Hess GP, et al. Sirolimus use and cancer incidence among US kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(1):129–36. doi: 10.1111/ajt.12969. [DOI] [PubMed] [Google Scholar]
- 47.Marcen R, Pascual J, Tato AM, Teruel JL, Villafruela JJ, Fernandez M, et al. Influence of immunosuppression on the prevalence of cancer after kidney transplantation. Transplantation proceedings. 2003 Aug;35(5):1714–6. doi: 10.1016/s0041-1345(03)00669-9. [DOI] [PubMed] [Google Scholar]
- 48.Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997 Aug 15;64(3):436–43. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 49.Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998 Aug 27;66(4):493–9. doi: 10.1097/00007890-199808270-00014. [DOI] [PubMed] [Google Scholar]
- 50.Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003 Aug 15;76(3):597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 51.Bannon FJ, McCaughan JA, Traynor C, O’Brien K, Gavin AT, Maxwell AP, et al. Surveillance of nonmelanoma skin cancer incidence rates in kidney transplant recipients in Ireland. Transplantation. 2014 Sep 27;98(6):646–52. doi: 10.1097/TP.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 52.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000 May;47(2–3):85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.