Abstract
The posaconazole tablet formulation was developed to have improved bioavailability compared to the oral suspension. Here, we compared posaconazole plasma concentration (PPC) with the posaconazole oral suspension versus the tablet in Korean patients undergoing remission induction chemotherapy for hematologic malignancies. PPC was measured at 3, 8, and 15 days of treatment with the oral suspension (174 patients) or the tablet (40 patients). At all time-points, mean PPC was significantly higher with the tablet compared to the oral suspension. Our findings suggest that posaconazole tablets generate an optimal PPC earlier and in more patients than the oral suspension among Korean patients.
Keywords: Posaconazole, Antifungal agents, Tablet, Dosage forms
Following its initial approval as an oral suspension formulation in 2006, posaconazole has been widely used as a prophylactic antifungal agent in chemotherapy for patients with hematologic malignancies [1,2]. For seven years, posaconazole was only available as an oral suspension. However, this formulation has unpredictable bioavailability that is affected by many variables, including meals and acid suppression therapy [3,4], potentially leading to sub-therapeutic plasma concentrations and breakthrough fungal infections [5,6,7]. To improve bioavailability, a posaconazole tablet formulation was developed, and was approved in 2013 [8,9]. The posaconazole tablet is formulated with a pH-dependent polymer matrix generated using hot-melt extrusion technology [10]. It shows more consistent absorption and bioavailability regardless of food intake, and it is not affected by gastric acid suppression therapy [8]. However, the bioavailability of the posaconazole tablet is not yet established in Korean patients. The aim of the present study was to compare posaconazole plasma concentration (PPC) following use of the posaconazole tablet or oral suspension among Korean patients with hematologic malignancies.
We conducted a prospective study at two university-affiliated hospitals in South Korea (Seoul National University Hospital, Seoul, and Seoul National University Bundang Hospital, Seongnam) between September 2014 and February 2017. Our study included patients of > 18 years old who underwent remission induction chemotherapy for acute myeloid leukemia (AML) or myelodysplastic syndrome, and who received posaconazole as a prophylactic antifungal agent. We excluded patients who refused to participate in or who discontinued posaconazole during chemotherapy. We obtained informed written consent from every patient, and this study was approved by the Institutional Review Boards of both study hospitals (IRB No. 1407-183-599).
Since posaconazole tablets were not available in Korea prior to September 2016, the patients who enrolled before September 2016 received the posaconazole oral suspension and those who enrolled after September 2016 received the posaconazole tablets. We planned to increase the dosage strategies from 200 mg tid to 200 mg qid in the oral suspension group [11], and from 300 mg qd to 400 mg qd in the tablet group if PPC was low (<400 ng/mL on day 3 or <500 ng/mL on day 8). In this study, all patients with low PPC were in the oral suspension group. PPC after increasing administration was excluded from the analysis.
PPC was measured by liquid chromatography-tandem mass spectrometry on Day 3, Day 8, and Day 15 [4]. We considered a PPC of ≥700 ng/mL to be optimal for antifungal prophylaxis, based on Food and Drug Administration (FDA) pharmacokinetic data that shows a higher incidence of breakthrough invasive fungal infections in patients with a Cavg of <700 ng/mL [12,13,14]. We also examined patient baseline characteristics and adverse events. Baseline characteristics were assessed within three days after starting drugs and adverse events were assessed twice a week by infection specialist during the entire study period by using the National Cancer Institute’s common terminology criteria for adverse events [15].
Baseline characteristics were described using standard descriptive summary statistics (means, standard deviations, and percentages). We performed univariate analysis to test for between-group differences. We utilized Student’s t-test and the chi-square test or Fisher’s exact test, depending on the variable type. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA).
Our study enrolled a total of 174 patients who received the posaconazole oral suspension between September 2014 and August 2016, and 40 patients who received the posaconazole tablet between September 2016 and April 2017. The mean age was 55.5 years in the oral suspension group, and 53.9 years in the tablet group (P = 0.585). In both groups, most enrolled patients were undergoing treatment for AML (89.1% of the oral suspension group and 92.5% of the tablet group). The two groups did not differ in baseline characteristics, except body weight (Table 1). Patients were able to use H2 blockers and proton pump inhibitors if the attending physicians determined that such treatment was essential for patient care.
Table 1. Baseline characteristics of the study groups.
| Characteristic | Oral suspension (n = 174) | Tablet (n = 40) | P-value |
|---|---|---|---|
| Age, years | 55.5 ± 13.4 | 53.9 ± 17.0 | 0.585 |
| Male sex, n (%) | 96 (55.2) | 23 (57.5) | 0.789 |
| Height, cm | 163.8 ± 8.7 | 164.4 ± 9.2 | 0.705 |
| Weight, kg | 63.1 ± 11.7 | 67.7 ± 14.7 | 0.034 |
| AML diagnosis, n (%) | 155 (89.1) | 37 (92.5) | 0.773 |
| Oral mucositis grade ≥2, n (%) | 5 (2.9) | 1 (2.5) | 1.000 |
| Diarrhea grade ≥2, n (%) | 8 (4.6) | 0 (0) | 0.357 |
| Acid-lowering agent use | |||
| PPI, n (%) | 7 (4.0) | 3 (7.5) | 0.401 |
| H2 blocker, n (%) | 23 (13.2) | 1 (2.5) | 0.055 |
AML, acute myeloid leukemia; PPI, proton pump inhibitor.
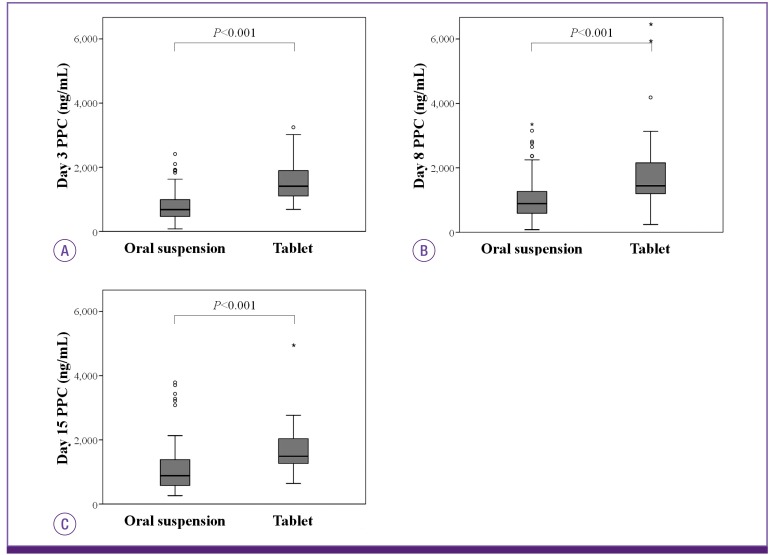
The mean PPC on all study days was significantly higher in the tablet group than in the oral suspension group. On Day 3, the mean PPC was 769.5 ± 427.4 ng/mL in the oral suspension group and 1,560.6 ± 668.3 ng/mL in the tablet group (P <0.001). On Day 8, the mean PPC was 1,015.6 ± 631.9 ng/mL in the oral suspension group and 1866.5 ± 1278.3 ng/mL in the tablet group (P <0.001). On day 15, the mean PPC was 1,099.4±752.4 ng/mL in the oral suspension group and 1693.4 ± 805.0 ng/mL in the tablet group (P <0.001) (Figure 1). On Day 3, Day 8, and Day 15, respectively, we found optimal PPC (≥700 ng/mL) in 47.3%, 63.2%, and 66.3% of patients in the oral suspension group and in 96.6%, 94.9%, and 96.8% of patients in the tablet group.
Figure 1.
Comparison of posaconazole plasma concentration at Day 3 (A), Day 8 (B), and Day 15 (C) between the oral suspension group and tablet group.
PPC, posaconazole plasma concentration.
There were several adverse events during the study period. A total of 81 patients experienced diarrhea (63 cases of grade 1, and 18 cases of grade ≥2), but only one case of grade 1 diarrhea in the oral suspension group was considered to be related to posaconazole administration. In addition, liver function abnormalities were observed in 61 patients (45 cases of grade 1, and 16 cases of grade ≥2), and all of them were related to other medical conditions such as parenteral nutrition, acute cholecystitis, multi-organ failure, and other medication rather than posaconazole administration.
Our present results showed that the posaconazole tablet formulation had better bioavailability than the oral suspension in Korean patients. Also, there was no correlation between adverse events and PPC, similar to previous studies [16,17]. Previous studies in other countries show that 91-97% of patients taking the posaconazole tablet achieve optimal PPC [18,19,20], which is similar to our present findings in Korean patients.
The unpredictable bioavailability of the posaconazole oral suspension necessitates frequent therapeutic drug monitoring (TDM). However, many hospitals cannot frequently examine PPC. Therefore, the improved bioavailability of the posaconazole tablet is a major advantage in that it may alleviate the need for frequent TDM [14,18]. Additionally, many patients with hematologic malignancies experience gastrointestinal issues, such as mucositis and diarrhea, due to chemotherapy. In contrast to the oral suspension, the posaconazole tablet can be administrated with acid suppression therapy without reducing bioavailability [14], which is a great advantage since many hematologic malignant patients require acid suppression therapy.
Our present study had several limitations. First, the study included only a small number of cases. However, the results were statistically significant despite the low statistical power. Second, the oral suspension group included patients whose frequency of posaconazole administration was adjusted after TDM during the study period. In these cases, the PPC measurements attained after changing dose strategies were excluded from analysis, potentially introducing selection bias. However, the excluded cases showed low PPC in the oral suspension group and, thus, the bias would not affect our conclusion that the posaconazole tablet was better than the oral suspension. Finally, patients in each group were enrolled in different periods. Despite these limitations, our present findings are important, as this is the first prospective study to analyze the posaconazole tablet in a Korean population.
In conclusion, among our population of Korean patients with hematologic malignancies, we found that the tablet group attained optimal PPC earlier and in more patients compared to the oral suspension group, without an increase of adverse events. The more consistent bioavailability of the posaconazole tablet demonstrated in the present study supports the possibility that routine TDM may not be necessary in patients taking the posaconazole tablet. Our findings suggest that the posaconazole tablet should be the preferred posaconazole formulation among patients with hematologic malignancy in Korea.
Acknowledgement
Our research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2053474).
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 2.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 3.Torres HA, Hachem RY, Chemaly RF, Kontoyiannis DP, Raad II. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5:775–785. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 4.Chae H, Cho SY, Yu H, Cha K, Lee S, Kim M, Kim Y, Kim YJ, Kim HJ, Lee DG. Determination of posaconazole concentration with LC-MS/MS in adult patients with hematologic malignancy. Clin Chim Acta. 2015;450:220–226. doi: 10.1016/j.cca.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother. 2012;56:5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoenigl M, Raggam RB, Salzer HJ, Valentin T, Valentin A, Zollner-Schwetz I, Strohmeier AT, Seeber K, Wölfler A, Sill H, Krause R. Posaconazole plasma concentrations and invasive mould infections in patients with haematological malignancies. Int J Antimicrob Agents. 2012;39:510–513. doi: 10.1016/j.ijantimicag.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Kang SH, Kim HS, Bae MN, Kim J, Yoo JY, Lee KY, Lee DG, Kim HJ. Fatal breakthrough mucormycosis in an acute myelogenous leukemia patient while on posaconazole prophylaxis. Infect Chemother. 2015;47:49–54. doi: 10.3947/ic.2015.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarascio AJ, Slain D. Review of the new delayed-release oral tablet and intravenous dosage forms of posaconazole. Pharmacotherapy. 2015;35:208–219. doi: 10.1002/phar.1533. [DOI] [PubMed] [Google Scholar]
- 9.McKeage K. Posaconazole: a review of the gastro-resistant tablet and intravenous solution in invasive fungal infections. Drugs. 2015;75:397–406. doi: 10.1007/s40265-015-0348-3. [DOI] [PubMed] [Google Scholar]
- 10.Krishna G, Ma L, Martinho M, Preston RA, O'mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single-and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67:2725–2730. doi: 10.1093/jac/dks268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park WB, Cho JY, Park SI, Kim EJ, Yoon S, Yoon SH, Lee JO, Koh Y, Song KH, Choe PG, Yu KS, Kim ES, Bang SM, Kim NJ, Kim I, Oh MD, Kim HB, Song SH. Effectiveness of increasing the frequency of posaconazole syrup administration to achieve optimal plasma concentrations in patients with haematological malignancy. Int J Antimicrob Agents. 2016;48:106–110. doi: 10.1016/j.ijantimicag.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Jang SH, Colangelo PM, Gobburu JV. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88:115–119. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 13.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiederhold NP. Pharmacokinetics and safety of posaconazole delayed-release tablets for invasive fungal infections. Clin Pharmacol. 2015;8:1–8. doi: 10.2147/CPAA.S60933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. NIH publication No. 09-5410. National Cancer Institute; 2009. [Google Scholar]
- 16.Moton A, Krishna G, Wang Z. Tolerability and safety profile of posaconazole: evaluation of 18 controlled studies in healthy volunteers. J Clin Pharm Ther. 2009;34:301–311. doi: 10.1111/j.1365-2710.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 17.Catanzaro A, Cloud GA, Stevens DA, Levine BE, Williams PL, Johnson RH, Rendon A, Mirels LF, Lutz JE, Holloway M, Galgiani JN. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin Infect Dis. 2007;45:562–568. doi: 10.1086/519937. [DOI] [PubMed] [Google Scholar]
- 18.Pham AN, Bubalo JS, Lewis JS., 2nd Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses. 2016 doi: 10.1111/myc.12452. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jiménez JL, Candoni A, Raad I, Laverdiere M, Langston A, Kartsonis N, Van Iersel M, Connelly N, Waskin H. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71:718–726. doi: 10.1093/jac/dkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58:6993–6995. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]