Abstract
Objectives
To define whether adults with a Fontan circulation, who have life-long venous congestion and limited cardiac output, have impaired glomerular filtration rate (GFR) or elevated urinary biomarkers of kidney injury.
Methods
We measured circulating cystatin C and creatinine (n=70) and urinary creatinine, albumin, kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and N-acetyl glucosaminidase (NAG)(n=59) in ambulatory adult Fontan patients and 20 age- and sex- matched controls. Urinary biomarkers were normalized to urine creatinine concentration. Survival free from non-elective cardiovascular hospitalization was compared, by estimated GFR and urinary biomarker levels using survival analysis.
Results
Cystatin C GFR was lower in the Fontan group compared with controls (114.2±22.8 vs. 136.3±12.8mL/min/1.73m2, p<0.0001); GFR<90mL/min/1.73m2 in 14.3% vs. 0% of controls. Albumin-to-creatinine ratio (ACR), KIM-1 and NAG were elevated compared with controls; ACR=23.2 [7.6–38.3] vs. 3.6 [2.5–5.7]mg/g, p<0.0001; NAG=1.8 [1.1–2.6] vs. 1.1 [0.9–1.6]U/g, p=0.02; KIM-1=0.91 [0.52–1.45] vs. 0.33 [0.24–0.74]ng/mg, p=0.001. Microalbuminuria, ACR>30mg/g, was present in 33.9% of the Fontan patients but in none of the controls.
Over median 707 [IQR 371–942] day follow-up, 31.4% of patients had a clinical event. Higher KIM-1 and NAG were associated with higher risk of non-elective hospitalization or death (HR/+1SD=2.1, 95%CI=1.3–3.3, p=0.002; HR/+1SD=1.6, 95%CI=1.05–2.4, p=0.03, respectively); cystatin C GFR was associated with risk of the outcome (HR/+1SD=0.66, 95%CI=0.48–0.90, p=0.009) but creatinine-based GFR was not (HR/+1SD=0.91, 95%CI=0.61–1.38, p=0.66). Neither ACR nor NGAL were associated with events.
Conclusions
The Fontan circulation is commonly associated with reduced estimated GFR and evidence for glomerular and tubular injury. Those with lower cystatin C GFR and tubular injury are at increased risk of adverse outcomes.
Keywords: biomarker, Fontan procedure, congenital heart disease, chronic kidney disease, urine
INTRODUCTION
Interactions between the heart, blood vessels and kidneys play a central role in the pathophenotype of heart failure (HF) in adults with acquired heart disease. Cardio-renal syndrome refers to a wide range of bi-directional adverse effects of primary heart or kidney disease on the other organ.[1] Chronic heart disease, for example, can cause progressive chronic kidney dysfunction because of adverse hemodynamics (e.g., low cardiac output and high venous pressure) with related neurohormonal, inflammatory and immunologic effects. In patients with either acute or chronic HF, kidney dysfunction is associated with adverse outcomes.[2, 3] Clinical care focuses mainly on one dimension of kidney function, glomerular filtration rate (GFR). Equations that incorporate measurement of circulating creatinine concentration are commonly used to estimate GFR, but this approach is limited in scope and can be misleading without understanding the larger clinical context.[3, 4] Other methods, including measurement of cyststin C, may improve GFR estimation and better predict outcomes.[5, 6]
The Fontan procedure separates the pulmonary and systemic circulations in patients born with functional single ventricle congenital heart disease. Systemic venous return is directed to the pulmonary circulation without the benefit of a subpulmonary ventricle. Cyanosis and ventricular volume overload are alleviated at the expense of chronically elevated systemic venous pressure and limited stroke volume augmentation. These consequences are presumably associated with ongoing end organ damage. While research has focused on liver fibrosis and protein losing enteropathy, there is preliminary evidence for important adverse renal effects; one small study found that chronic glomerular injury, evidenced by microalbuminuria, was present in 9 of 21 patients with a Fontan circulation.[7]
While eGFR provides a view of one important aspect of kidney function, alternative circulating and urine biomarkers have been identified to characterize glomerular, tubular and interstitial injury.[8, 9] Albuminuria, for example, suggests injury to the glomerular filtration barrier, though can be related to proximal tubular dysfunction.[10] Biomarkers of tubulointerstitial injury include kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and N-acetyl glucosaminidase (NAG).[8] Elevations in these urinary biomarkers are independently associated with disease severity and prognosis, and may distinguish underlying pathophysiology in adults with HF due to acquired heart disease.[11, 12, 13, 14]
Given the presence of limited cardiac output augmentation and elevated venous pressure in the Fontan circulation, we hypothesized that reduced eGFR and tubulointerstitial injury would be common and that markers of kidney dysfunction would be associated with adverse outcomes.
METHODS
Study Sample
We enrolled outpatients ≥18 years-old who had previously undergone a Fontan procedure and consented to participate between February 2012 and June 2014 at Boston Children’s Hospital or Brigham and Women’s Hospital.[15] None of the patients enrolled had been hospitalized in the prior 30 days. Exclusion criteria included 2-ventricle repair or cardiac transplantation subsequent to Fontan procedure, known kidney disease or recent creatinine>2 mg/dL. Control subjects were selected by frequency matching for 10-year age group and sex for each of the Fontan groups (those with urine samples and those with blood samples). Controls were self-reported non-smokers without diabetes mellitus or known kidney or cardiovascular disease recruited via 2 different methods: A) postings on the Boston Children’s Hospital website and B) directly approached people who accompanied a patient to a clinical visit in the adult congenital heart disease clinic. Only 6 of the selected control subjects provided both urine and blood samples; therefore each group of 20 controls includes 6 in common and 14 additional control subjects with either blood or urine specimens. The study was approved by Boston Children’s Hospital’s Institutional Review Board and informed consent was obtained from all subjects; there was a formal reliance agreement between Partners/Brigham and Women’s and Boston Children’s Hospital Institutional Review Boards.
Biomarker Assessment
Urine collected from spontaneous clean catch voids underwent centrifugation at 4,000 rpm for 5 minutes at 4°C. Urine supernatant was aliquoted into 2 mL cryostorage tubes and frozen at −80°C; assays were performed on samples with no prior freeze–thaw cycles. At the time of assay, samples were thawed, vortexed and centrifuged at 14,000 rpm at 4°C and the supernatant was transferred to another tube.
Urine biomarkers were measured as previously described.[9, 16]. Briefly, KIM-1 and NGAL were measured using microbead-based assays and NAG was measured using an enzymatic assay (Roche Diagnostics, Risch-Rotkreuz, Switzerland). Creatinine and albumin were measured using a Randox Daytona analyzer. All samples were measured in duplicate and the intra- and inter-assay coefficients of variation were <10% for all assays. A small number of measurements were below the reported lower limit of detection for each given assay (n=1 NGAL<0.53 ng/mL; n=3 NAG<0.2U/L) and these values were set to the lower limit of detection.
Blood collected via peripheral venipuncture in an EDTA tube was processed to plasma within 30 minutes and frozen at −80⁰C. Cystatin C was measured with an enzyme-linked immunoassay (R&D Systems, Inc., Minneapolis, MN). Reported inter-assay coefficient of variation of 7.0, 5.0 and 5.9% at mean values of 17.2, 30.8 and 60.9 ng/mL respectively. Other laboratory testing, including serum creatinine, was performed on fresh processed samples by a Clinical Laboratory Improvement Amendments-certified laboratory (Laboratory Corporation of America, Burlington, NC). Glomerular filtration rate was estimated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations using creatinine alone, cystatin C alone, and the combination of creatinine and cystatin C (referred to through the text, respectively, as: GFRCr, GFRCysC, GFRCysC+Cr).[6, 17]
There was no association between cystatin C and storage time (β=−0.075 ng/mL/day, p=0.30, adjusting for control status). This was also true for urine creatinine and log transformed urine albumin, NAG, NGAL and KIM-1 (β=+0.016 mg/dL/day, p=0.74; β=+0.001 log mg/dL/day, p=0.18; β<0.0001 log mU/mL/day, p=0.99; β=+0.00075 log ng/mL/day, p=0.45; and β=−0.00027 log pg/mL/day, p=0.67, respectively, all adjusting for control status).
Clinical and Outcomes Assessment
Demographic data, underlying cardiac diagnosis, prior interventions, medication use, and comorbidities were extracted from medical records, along with clinical laboratory testing and cardiac imaging within 2 years of sample collection and invasive hemodynamic data within 5 years. Data on systemic ventricular function and valve regurgitation severity were extracted from clinical reports. The combined outcome of interest was time to first non-elective cardiovascular hospitalization or death. Non-elective cardiovascular hospitalization was defined as overnight admission for HF, arrhythmia or symptoms of arrhythmia, thrombotic or embolic events, cerebral hemorrhage or a specific Fontan-related reason (i.e., ascites, protein losing enteropathy, plastic bronchitis).
Statistical Analyses
The 2-sided unpaired Student’s t- or Wilcoxon rank sums test, as appropriate for distribution, was used to compare continuous variables. Fisher’s exact test was used to analyze categorical variables between groups stratified by urine biomarker level. Continuous variables are presented as mean±SD for normally distributed variables and median [25th–75th percentile] for non-normally distributed variables. Urine biomarkers were expressed as a ratio to urinary creatinine concentration and these creatinine-normalized values were natural log transformed because of the skewed distribution of these variables. The Pearson product-moment correlation coefficient was used to analyze the correlation between each log transformed urine biomarker and specific clinical and biomarker covariates of interest. For this analysis, non-normally distributed variables were log transformed. The Log Rank test was used to perform univariate survival analysis comparing survival times for those above with those below the median value for each urine biomarker and also comparing those with decreased and normal eGFR by the various estimating methods. Cox regression was used to adjust for potential confounding variables. Time-to-event analyses were conducted from the date of biospecimen collection to the date of the first clinical event (non-elective cardiovascular hospitalization or death), with censoring of event-free individuals at the most recent clinical follow up date when event status was known. The proportional hazards assumption was tested for each model both graphically (plots of Martingale and deviance residuals by value of each predictor; cumulative Martingale residuals over time; log(−log(survival) versus log(time)) and formally (adding a time dependent covariate; Kolmogorov-type supremum test based on 1,000 simulated replications). There was no evidence this assumption was violated. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Software, La Jolla, CA). A 2-sided p value <0.05 was considered statistically significant.
RESULTS
Clinical characteristics of Fontan patients in the study sample (for n=59 with urine biomarker data; results for the n=70 with blood creatinine and cystatin C were similar and are not presented) are shown in Table 1. When compared to age- and sex-frequency matched controls, Fontan patients tended to be shorter (p=0.04) but were similar in terms of weight and body mass index(Supplemental Table 1).
Table 1. Baseline demographic, biomarker and clinical data for patients with a Fontan circulation aged 18 to 54 years.
Demographic, biomarker and clinical characteristics for n=59 patients with a Fontan circulation with available urine biomarker data; data on glomerular filtration rate are for n=70 patients with available data on circulating creatinine and cystatin C. Continuous variables are presented in the left column as mean±SD or median [25th–75th percentile] while categorical variables are presented in the right column as n (%).
| Mean±SD or median [25th–75th percentile] | N (%) | |
|---|---|---|
| Age, y | 30.7±9.8 | |
| Male | 35 (59.3%) | |
| Height, cm | 168.3±8.9 | |
| Weight, kg | 70.6±15.7 | |
| BMI, kg/m2 | 24.8±4.2 | |
| Oxygen saturation, % | 94.0±3.8 | |
| GFRCr, mL/min/1.73m2 | 106.2±22.9 | |
| GFRCysC, mL/min/1.73m2 | 114.3±22.7 | |
| GFRCys+Cr, mL/min/1.73m2 | 115.7±20.7 | |
| Urine biomarkers | ||
| Creatinine, mg/dL | 118 [68.8–157.6] | |
| Albumin, mg/dL | 24.8 [4.5–58.3] | |
| KIM-1, pg/mL | 921.7 [426.3–1640.6] | |
| NGAL, ng/mL | 25.9 [11.5–76.2] | |
| NAG, mU/mL | 1.9 [1.0–3.2] | |
| ACR, mg Albumin/g creatinine | 23.2 [7.4–38.3] | |
| KIM-1, ng/mg creatinine | 0.9 [0.5–1.5] | |
| NGAL, ng/mg creatinine | 21.7 [9.5–81.0] | |
| NAG, U/g creatinine | 1.8 [1.1–2.6] | |
| Diagnosis | ||
| Tricuspid Atresia | 22 (37.3%) | |
| Double-Inlet LV | 14 (23.7%) | |
| HLHS | 11 (18.6%) | |
| Unbalanced AV Canal | 3 (5.1%) | |
| Double-Outlet RV | 2 (3.4%) | |
| Other | 7 (11.9%) | |
| Ventricular Morphology | ||
| Right | 15 (25.4%) | |
| Left | 43 (72.9%) | |
| Other | 1 (1.7%) | |
| Heterotaxy | 5 (8.5%) | |
| Fontan Type, current | ||
| Atrio-Pulmonary | 10 (17.0%) | |
| Lateral Tunnel | 36 (34.0%) | |
| Atrio-Ventricular or Bjork | 2 (3.4%) | |
| Extracardiac | 11 (18.6%) | |
| Patent fenestration | 8 (13.6%) | |
| NYHA functional class I | 43 (72.9%) | |
| History of PLE | 3 (5.1%) | |
| Clinical ascites | 7 (11.9%) | |
| Clinical arrhythmia | 34 (57.6%) | |
| Medication | ||
| ACEi/ARB | 33 (55.9%) | |
| Beta-blocker | 16 (27.1%) | |
| Digoxin | 17 (28.8%) | |
| Anti-arrhythmic | 10 (7.0%) | |
| Aspirin | 32 (54.2%) | |
| Warfarin | 28 (47.5%) | |
| Loop diuretic | 20 (33.9%) | |
| Potassium-sparing diuretic | 12 (20.3%) | |
| Thyroid replacement | 6 (10.2%) | |
| Systemic Ventricular Function | ||
| Normal | 28 (49.1%) | |
| Mildly depressed | 24 (42.1%) | |
| Moderately or severely depressed | 5 (8.8%) | |
| Systemic AVV Regurgitation | ||
| None | 16 (28.1%) | |
| Mild | 35 (61.4%) | |
| Moderate or Severe | 6 (10.5%) | |
| Aortic Regurgitation | ||
| None | 33 (57.9%) | |
| Mild | 21 (36.8%) | |
| Moderate or Severe | 3 (5.3%) |
KIM1-1: kidney injury molecule 1; NGAL: neutrophil gelatinase-associated lipocalin; NAG: N-acetyl glucosaminidase; ACR: albumin-to-creatinine ratio; GFRCr, GFRCysC, GFRCysC+Cr: estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration equations using creatinine, cystatin C, and cystatin C+creatinine, respectively;[6] BMI, body mass index; LV, left ventricle; HLHS, hypoplastic left heart syndrome; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVV, atrioventricular valve; NYHA, New York Heart Association; PLE, protein losing enteropathy.
Cystatin C, creatinine and Estimated GFR
Cystatin C
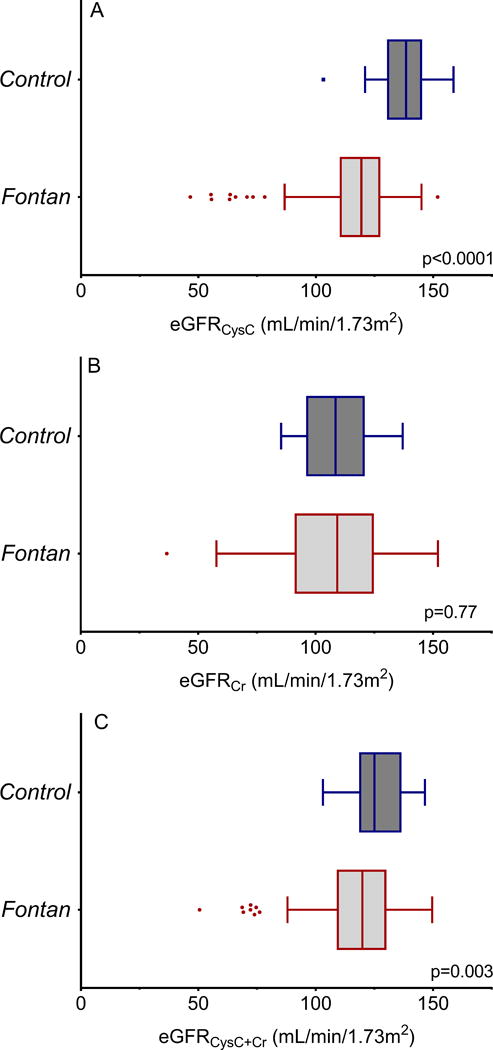
Cystatin C levels were higher in Fontan patients compared with controls (745.7±189.4 vs 566.7±85.3 ng/mL, p<0.0001) and GFRCysC was lower (114.3±22.7 vs. 136.3±12.8 mL/min/1.73m2, p<0.0001; Figure 1A). GFRCysC was <90mL/min/1.73m2 in 12.9% (9/70) of the Fontan subjects, but in none of the controls. There were 3 Fontan subjects (4.3%) with GFRCysC<60 mL/min/1.73m2.
Figure 1.
Creatinine
There was no difference in serum creatinine between the Fontan subjects and controls (0.87±0.20 vs 0.88±0.15 g/dL, p=0.86) and GFRCr was similar (106.2±22.9 vs. 107.4±14.4 mL/min/1.73m2, p=0.77; Figure 1B). GFRCr was <90 mL/min/1.73m2 in 22.9% (16/70) of the Fontan subjects, and 15% (3/20) of the controls; of note, GFRCr was >85 mL/min/1.73m2 in all 3 controls with GFRCr<90 mL/min/1.73m2 while 11 of 16 Fontan subjects with GFRCr<90 mL/min/1.73m2 had GFRCr<85 mL/min/1.73m2. There were 2 Fontan subjects (2.9%) with GFRCr<60 mL/min/1.73m2.
Cystatin C and Creatinine
GFRCysC+Cr, based on the CKD-EPI equations, was lower in the Fontan group (115.7±20.7 vs. 126.7±11.3 mL/min/1.73m2, p=0.003; Figure 1C). Estimated GFRCysC+Cr was <90mL/min/1.73m2 in 12.9% (the same subjects classified as GFR<90 by the cystatin C only equation) of the Fontan subjects, and none of the controls. Only 1 Fontan subject (2.9%) had GFRCysC+Cr<60 mL/min/1.73m2.
Urine Biomarkers
Urine albumin-to-creatinine ratio (ACR), NAG and KIM-1 concentrations were significantly higher in the Fontan group than in the control group (ACR 23.2 [7.6–38.3] vs. 3.6 [2.5–5.7] mg/g, Wilcoxon rank sums p<0.0001; NAG 1.8 [1.1–2.6] vs. 1.1 [0.9–1.6] U/g, p=0.02; KIM-1 0.91 [0.52–1.45] vs. 0.33 [0.24–0.74] ng/mg, p=0.001; Figure 2). There was no significant difference in urinary NGAL concentration between Fontan patients and controls (26.5 [9.6–81.0] vs. 57.8 [14.1–152.6] ng/mg, p=0.13; Figure 2).
Figure 2.
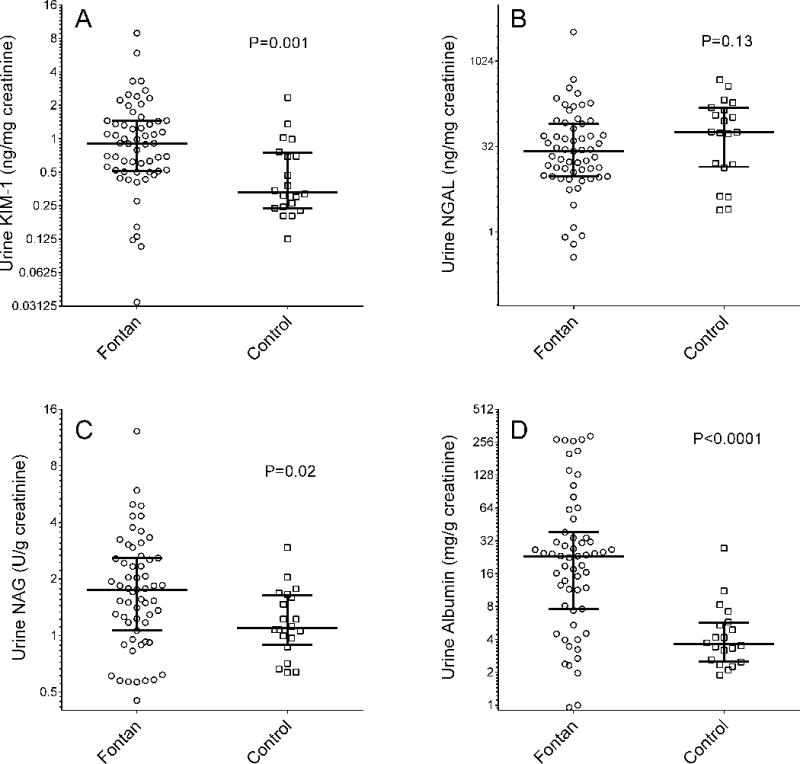
Patients were then stratified according to the median value of each urine biomarker (Supplemental Table 2). Those with higher KIM-1 or NAG tended to be older and there was a female preponderance in the higher NGAL group. There were no statistically significant relationships between the proportion of patients with any specific adverse clinical characteristic (e.g., NYHA FC>1, arrhythmia) though the few patients with uncommon adverse consequences of the Fontan circulation (e.g., ascites, PLE) tended to have higher KIM-1 and NAG.
Urine biomarker levels were not associated with underlying CHD diagnosis, ventricular morphology, Fontan type, ventricular function or valve regurgitation. Those with NAG levels above the median were more likely to be taking digoxin and all patients on thyroid replacement had KIM-1 levels above the median; otherwise, there was no relationship between urine biomarker level and medication use.
Correlations between Estimated GFR, Urine Biomarkers and Clinical Variables
There was modest correlation between the 4 urine biomarkers, with the strongest relationship being between KIM-1 and NAG (r=0.48, p=0.0002; partial correlation using linear regression adjusting for urine creatinine to avoid spurious correlation).[18] Interestingly, higher systolic blood pressure tended to be associated with lower KIM-1 and higher GFRCysC, though the association was weak (Table 2). Older age correlated modestly well with higher KIM-1 and NAG, but was more strongly associated with lower GFRCysC. Oxygen saturation was not correlated with any urine biomarker, but correlated directly with lower GFRCysC. Platelet count was associated with KIM-1 (r=0.30, p=0.03). There was little relationship between urine biomarker level and common liver tests. The two exceptions were that higher ACR was associated with higher serum albumin (r=0.28, p=0.03) and higher KIM-1 was associated with lower AST (r=−0.29, p=0.03). High sensitivity C-reactive protein was inversely associated with GFRCysC (r=−0.32, p=0.008) and directly associated with NAG (r=0.38, p=0.003). Uric acid level was inversely associated with GFRCysC (r=−0.38, p=0.001) but was not urine biomarker levels. Only 20 patients had BNP measured for clinical indications; in this subset, there was no significant relationship between log transformed BNP and any urine biomarker, while higher BNP was associated with lower GFRCysC (r=−0.59, p=0.006). We repeated these analyses for correlations between GFRCr and the variables described above for GFRCysC; the correlations were weaker for the creatinine based GFR estimates.
Table 2. Correlations between urine biomarkers, estimated GFR, and clinical data for 59 patients with a Fontan circulation.
Correlation between each urine biomarker, cystatin C GFR, age, body mass index, blood pressure and oxygen saturation. Correlations significant with P value <0.05 are presented in bold.
| Log ACR | Log KIM-1 | Log NGAL | Log NAG | GFRCysC | |
|---|---|---|---|---|---|
| Log ACR | – | 0.34 | −0.01 | 0.37 | 0.18 |
| Log KIM-1 | – | – | 0.27 | 0.48 | −0.25 |
| Log NGAL | – | – | – | 0.05 | −0.24 |
| Log NAG | – | – | – | – | −0.24 |
| Age | 0.09 | 0.26 | −0.04 | 0.37 | −0.59 |
| BMI | −0.14 | 0.01 | −0.03 | −0.04 | −0.11 |
| Systolic BP | −0.06 | −0.30 | −0.13 | −0.18 | 0.21 |
| O2 saturation | −0.02 | −0.01 | −0.11 | −0.16 | 0.27 |
| Platelet count | −0.02 | 0.30 | 0.23 | 0.14 | −0.01 |
| Albumin | 0.28 | −0.22 | −0.16 | −0.13 | 0.26 |
| Alkaline phos. | 0.05 | −0.05 | −0.10 | 0.11 | −0.30 |
| AST | 0.04 | 0.29 | 0.17 | 0.15 | 0.04 |
| ALT | 0.08 | −0.17 | −0.02 | −0.15 | 0.19 |
| Total bilirubin | 0.00 | −0.14 | −0.05 | −0.12 | 0.19 |
| MELD-XI | −0.07 | −0.09 | −0.02 | −0.16 | −0.21 |
| Log hsCRP | −0.03 | 0.18 | −0.19 | 0.38 | −0.32 |
| Uric acid | 0.02 | −0.00 | 0.07 | 0.06 | −0.38 |
The Pearson correlation coefficient is presented for relationships between age, BMI, blood pressure, saturation and each urine biomarker (normalized to urinary creatinine concentration and log transformed). However, due to potential spurious correlation in the relationships between calculated variables sharing the same denominator,[18] relationships between urine biomarkers reflect the type II partial correlation coefficient from a linear regression model with a log transformed urine biomarker as the dependent variable, another log transformed urine biomarker as the dependent variable adjusting for urine creatinine concentration. Correlations between urine biomarkers include the subset of patients with available urine data (n=59), while those for cystatin C GFR include all subjects with available data (n=69 or 70/70 for all biomarkers except platelets and alkaline phosphatase where data were available for only n=67 and 63/70, respectively). Data on oxygen saturation were available for n=54 and 64 patients for the urine subset and blood subset, respectively.
ACR: albumin-to-creatinine ratio; KIM1-1: kidney injury molecule 1; NGAL: neutrophil gelatinase-associated lipocalin; NAG: N-acetyl glucosaminidase; GFRCysC: estimated glomerular filtration rate, CKD-EPI cystatin C equation; BMI: body mass index; BP: blood pressure; O2: oxygen; Alkaline phos.: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MELD-XI: Model of End-Stage Liver Disease Excluding INR; hsCRP: high sensitivity C-reactive protein.
Higher GFRCysC was associated with higher % predicted peak VO2 (n=60, r=0.41, p=0.0009) and lower VE:VCO2 slope (r=−0.31, p=0.02). Higher log ACR was associated with higher peak VO2 (n=51, r=0.32, p=0.02) and lower VE:VCO2 slope (r=0.31, p=0.03). The was no relationship between any of the other urine biomarkers and either peak VO2 or VE:VCO2 slope.
Outcomes Analysis
Median follow-up duration was 707 [371–942] days. There were 22 events overall: 19 non-elective cardiovascular hospitalizations (2 with subsequent death) and 3 deaths without preceding non-elective hospitalization. Median time to event was 388.5 [161–653] days. Of the 19 first non-elective hospitalizations, 10 were primarily for arrhythmia (most atrial), 6 for HF including ascites and 3 were for other reasons: hypotension and dizziness, cerebrovascular accident and hemoptysis. Three of the deaths were due to sudden death of unknown cause, 1 was pulseless electrical activity in the context of elective cardioversion of atrial arrhythmia and progressive HF and 1 was due to cardiac arrest during a hospitalization for sepsis due to cellulitis. Among the subset of 59 subjects with urine collected, follow-up time was similar and there were 20 events overall including all 5 deaths.
GFR Estimates
Cystatin C
Higher GFRCysC was associated with lower risk of incident events (HR per 1SD=0.66, 95%CI 0.48–0.90) and this relationship was similar after adjustment for age or NYHA functional class (Table 3). The subset of 9 patients with GFRCysC< 90 mL/min/1.73m2 were at substantially increased risk of adverse outcomes (HR=3.25, 95%CI 1.26–8.40).
Table 3. Hazard ratios for non-elective cardiovascular hospitalization or death using various approaches to estimate glomerular filtration rate in adults with a Fontan circulation.
Hazard ratios with 95% confidence intervals for glomerular filtration rate estimated using 3 different equations, univariate and adjusting for age or NYHA FC. Results for GFRCysC+Cr <90 mL/min/1.73m2 are not presented because the results are identical to those for GFRCysC<90 mL/min/1.73m2.
| Univariate | Age Adjusted | NYHA FC Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | ||
| GFRCysC | per +1SD | 0.66 | 0.48–0.90 | 0.009 | 0.68 | 0.44–1.04 | 0.08 | 0.68 | 0.49–0.95 | 0.02 |
| <90 mL/min/1.73m2 | 3.25 | 1.26–8.40 | 0.010 | 2.60 | 0.83–8.18 | 0.10 | 2.80 | 1.06–7.45 | 0.04 | |
| GFRCr | per +1SD | 0.91 | 0.61–1.38 | 0.66 | 1.40 | 0.75–2.62 | 0.29 | 0.98 | 0.63–1.52 | 0.93 |
| <90 mL/min/1.73m2 | 1.87 | 0.76–4.65 | 0.18 | 1.41 | 0.51–3.89 | 0.51 | 2.05 | 0.85–4.94 | 0.28 | |
| GFRCysC+Cr | per +1SD | 0.75 | 0.52–1.07 | 0.11 | 0.88 | 0.53–1.46 | 0.62 | 0.78 | 0.54–1.14 | 0.21 |
GFRCr, GFRCysC, GFRCysC+Cr: estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration equations using creatinine, cystatin C, and cystatin C+ creatinine, respectively; NYHA FC: New York Heart Association functional class; SD: standard deviation.
Creatinine
There was no apparent relationship between GFRCr and risk of incident events. Adjustment for age or NYHA functional class did not importantly affect this result. Concordantly, the 16 patients with GFRCr<90 mL/min/1.73m2 were not at appreciably higher risk of adverse outcome (Table 3).
Cystatin C and Creatinine
There was a trend towards lower risk of incident events with higher GFRCysC+Cr. Since the same patients were classified as having GFR<90 mL/min/1.73m2 by both the cystatin C only equation and the combined equation, survival analysis was identical.
Urine Biomarkers
KIM-1
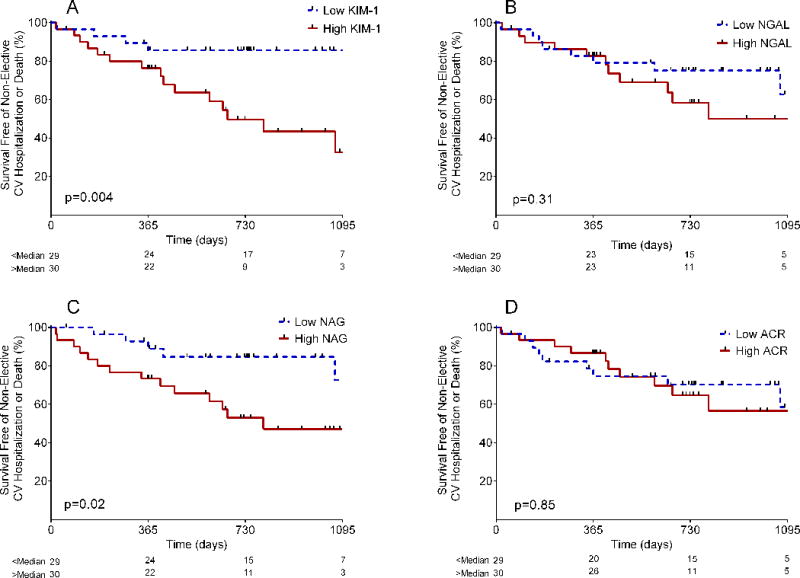
Higher KIM-1 was associated with greater hazard for the combined outcome, whether expressed as a log transformed continuous variable or dichotomized by median value (Table 4; Figure 3). This relationship was unaffected by adjustment for age, NYHA FC, or GFRCysC (Table 4).
Table 4. Associations between various urine biomarkers and incident non-elective cardiovascular hospitalization or death in adults with a Fontan circulation.
Hazard ratios with 95% confidence intervals for urine biomarkers, all normalized to urine creatinine concentration and natural log transformed, univariate and adjusting for age, NYHA FC, or GFRCysC.
| Univariate | Age Adjusted | NYHA FC Adjusted | GFRCysC Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | ||
| KIM-1 | per +1SD | 2.1 | 1.3–3.3 | 0.002 | 2.0 | 1.2–3.2 | 0.004 | 2.1 | 1.3–3.3 | 0.003 | 2.0 | 1.2–3.4 | 0.007 |
| >median | 4.5 | 1.5–13.5 | 0.004 | 3.7 | 1.2–11.7 | 0.02 | 4.5 | 1.5–13.5 | 0.008 | 3.7 | 1.2–11.5 | 0.02 | |
| NAG | per +1SD | 1.6 | 1.05–2.4 | 0.03 | 1.4 | 0.9–2.2 | 0.20 | 1.5 | 0.98–2.3 | 0.06 | 1.4 | 0.9–2.0 | 0.13 |
| >median | 3.2 | 1.2–9.0 | 0.03 | 2.6 | 0.9–7.7 | 0.08 | 3.0 | 1.1–8.5 | 0.03 | 2.7 | 0.93–7.6 | 0.07 | |
| NGAL | per +1SD | 1.0 | 0.6–1.8 | 0.91 | 1.4 | 0.9–2.3 | 0.16 | 1.3 | 0.8–2.2 | 0.27 | 1.3 | 0.8–2.0 | 0.38 |
| >median | 1.1 | 0.4–3.3 | 0.80 | 1.8 | 0.7–4.7 | 0.21 | 1.8 | 0.7–4.7 | 0.20 | 1.8 | 0.7–4.7 | 0.21 | |
| ACR | per +1SD | 1.1 | 0.6–2.1 | 0.74 | 0.9 | 0.5–1.5 | 0.70 | 1.0 | 0.6–1.8 | 0.88 | 1.1 | 0.7–1.9 | 0.67 |
| >median | 1.2 | 0.4–3.5 | 0.74 | 1.0 | 0.4–2.5 | 0.96 | 1.2 | 0.5–2.9 | 0.76 | 1.4 | 0.6–3.7 | 0.47 | |
GFRCysC,: estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration equations using cystatin C; NYHA FC: New York Heart Association functional class.
Figure 3.
NAG
Higher NAG was also associated with greater risk for the combined outcome. This relationship was independent of NYHA functional class (when expressed as a dichotomous variable) but was attenuated somewhat by adjustment for age or GFRCysC (Figure 3; Table 4).
NGAL and ACR
Neither NGAL nor ACR was associated with incident events when analyzed as continuous variables. This remained the case when categorized by median value and after adjustment for age, GFRCysC, or NYHA functional class (Figure 3; Table 4).
DISCUSSSION
This study demonstrates that, compared with age- and sex- matched controls, adults with a Fontan circulation tend to have: (1) reduced GFR as estimated by circulating cystatin C, (2) a higher prevalence of microalbuminuria consistent with glomerular injury, and (3) elevated urinary biomarkers indicative of tubular injury. Reduced GFRCysC, elevated urinary KIM-1 and elevated urinary NAG were each associated with an increased incidence of the composite end-point of non-elective cardiovascular hospitalization or death.
Interaction between the heart, vasculature and kidneys is central to the pathophysiology of HF. There is a wide spectrum of pathologic cardio-renal interactions, often referred to under the umbrella term cardio-renal syndrome. It is well demonstrated that acutely or chronically low cardiac output (CO) causes reduced renal perfusion and, potentially, renal ischemia. More recent studies have detailed the key contribution of elevated right atrial pressure and venous congestion to predict worsening glomerular filtration rate. In patients with systolic HF, both low cardiac output and high right atrial pressures are associated with impaired renal function and increased mortality.[19, 20] Diastolic HF (normal LVEF and CO), however, is also associated with significant renal insufficiency and increased mortality, further suggesting venous congestion plays a central role in kidney dysfunction in HF.[2]
We report the presence of impaired GFR in patients with a Fontan circulation relative to normal controls. All the methods to estimate GFR that we analyzed suggest that more than 1 in 10 of these patients had reduced GFR (<90 mL/min/1.73m2 in 12.9%, 12.9% and 22.9% using cystatin C, combined and creatinine estimates, respectively) and that a small subset (~3–4%) had GFR<60 mL/min/1.73m2. These values likely underestimate the prevalence of importantly low GFR since we excluded patients with known clinical kidney disease. A recent study of patients following Fontan palliation, including mainly pediatric patients, similarly reported that 10.3% of the 68 patients had GFR<90 mL/min/1.73m2.[21] While each estimating equation suggested a similar proportion of patients with reduced GFR, cystatin C estimates suggested an overall distribution shift toward lower GFR while the creatinine-based approach did not. It is unclear why the creatinine-based GFR estimates were lower than those based on cystatin C. One might have expected the converse since patients with a Fontan circulation have lower lean muscle mass then matched controls.[22] It may be that features of the Fontan circulation other than GFR affect cystatin C levels; chronic systemic inflammation is one possible explanation. While prior large studies including a wide spectrum of congenital heart disease diagnoses reported an association between creatinine-based GFR estimates and mortality,[23] only cystatin C estimates of GFR were associated with increased risk of incident adverse outcomes in this study focusing on patients with a Fontan circulation. As has been suggested in broader epidemiologic studies,[24] this may suggest that cystatin C provides a more accurate or otherwise informative estimate. Additional study is needed to assess the relative accuracy and prognostic significance of different GFR estimates in the Fontan population.
Our findings support previous reports that microalbuminuria or at least trace dipstick proteinuria is prevalent in patients with a Fontan circulation.[7, 21] This is concerning in the context of extensive evidence that microalbuminuria predicts adverse cardiovascular outcomes in patients with various underlying disease as well as in the general population. Albuminuria is generally associated with the presence of renal endothelial or glomerular dysfunction. We were, therefore, surprised by the lack of association between urine microalbuminuria and adverse outcomes in this study. Small sample size and relatively few events could explain the finding, though we were able to demonstrate a higher incidence of adverse outcomes for patients with elevated concentrations of 2 other urine biomarkers. The association between the presence of microalbuminuria and better aerobic capacity was also unexpected. Microalbuminuria can be a normal finding in some healthy people after intense exercise, though the mechanism has not been well defined. Frequent and intense aerobic activity could cause microalbuminuria in patients with a Fontan circulation in the context of exaggerated increases in venous pressure and limited cardiac output.[25] It is plausible that microalbuminuria might indeed identify chronic episodic renal ischemia and congestion in a subset of active, fit patients, but the benefit of regular exercise outweighs collateral kidney injury. In any case, microalbuminuria does not appear to strongly predict disease severity or prognosis.
Conversely, 2 markers of tubular injury, KIM-1 and NAG, were predictive of adverse events. KIM-1 is a membrane bound protein, and metalloproteinase dependent processes cleave the ectodomain into the urine and extracellular spaces following injury.[8] NAG originates in cellular lysosomes and its large size precludes glomerular filtration; NAG therefore only appears in the urine with proximal tubule injury and loss of lysosomal integrity.[8] Population based studies have reported higher levels of various urine biomarkers, including KIM-1, predict adverse outcomes including incident kidney disease, cardiovascular events and death.[26, 27] Studies in adults with chronic HF have demonstrated increased levels of urinary biomarkers suggestive of both glomerular and tubular injury, and have shown such elevation to be associated with adverse outcomes.[12, 13, 14]
There was no difference in urinary NGAL between the Fontan and control groups, in contrast to the findings on ACR, KIM-1, and NAG. One reason may be the distinct anatomic source of NGAL compared with the other markers. NGAL is present in low concentrations in various biological fluids, including serum and cerebrospinal fluid.[28] While urinary KIM-1 and NAG derive from the proximal tubule, urinary NGAL is synthesized in the distal nephron (loop of Henle and distal tubules), and possibly through glomerular filtration of circulating NGAL.[28]. Interestingly, urinary KIM-1 and NAG appear to parallel the severity of venous congestion in adults with HF and levels respond to acute diuresis; this is not true for NGAL.[11] The selective elevation in KIM-1 and NAG, therefore, may correspond to the mechanism and location of renal injury (i.e., elevated venous pressure, proximal tubule). The lack of NGAL elevation, however, could alternatively be explained by the study design. Women comprised a slightly larger proportion of the control group relative to the Fontan group; the difference was not statistically significant but could bias towards lower NGAL in the Fontan group since women have higher urine NGAL levels than men.[29]. There are few published data on the effect of storage at −80C on NGAL, and none extend >6 months.[30] There was no linear relationship between storage time and urinary NGAL in our study sample, but this does not exclude idiosyncratic time-related declines.
Limitations
The small number of events precludes comprehensive multivariable adjustment, and our findings may be attributable to confounding. The age- and sex- matched comparison group represents a convenience sample and may not derive from an equivalent at-risk population as the Fontan subjects. While these results suggest GFR, urinary KIM-1 and urinary NAG are associated with adverse outcome in Fontan patients, the sample size is relatively small and further study is required to validate these findings and define whether this association is additive to information provided by alternative biomarkers and other clinical variables already in use.
We did not measure GFR directly. No method based on an isolated concentration of any molecule provides precise GFR estimates within the range of values seen in this study (e.g., MDRD and CKD-EPI estimates based on creatinine each have 95% limits of agreement >±30 for GFR>90 mL/min/1.73m2).[17] While the better predictive power of cystatin C estimates provides some support for its applicability in these patients, it is unknown how well any estimate performs relative to directly measured GFR in the Fontan population. Further study with larger sample size and specialized methods (e.g., iohexol clearance) will be required to determine whether one or another method is particularly appropriate in these patients.
Conclusions
Adults with a Fontan circulation often have impaired GFR and glomerular and tubular injury as evidenced by higher urinary albumin, KIM-1 and NAG. Lower GFRCysC as well as higher urinary KIM-1 and NAG were predictive of adverse outcomes.
Supplementary Material
Key Questions.
What is already known about this subject?
Patients with a single ventricle Fontan circulation have elevated venous pressure and limited cardiac output. Little is known about kidney function in this population. Research on adults with congenital heart disease, only a subset of whom have a Fontan circulation, suggests reduced glomerular filtration rate is relatively common and associated with adverse outcomes. One small study of the Fontan circulation reported a high prevalence of albuminuria.
What does this study add?
This study suggests that adults with a single ventricle Fontan circulation have (1) impaired glomerular filtration rate, as estimated by circulating cystatin C, (2) cystatin C estimates of glomerular filtration rate are associated with adverse outcomes while creatinine-based estimates are not, (3) there is active glomerular and tubular injury as evidenced by elevated urinary biomarkers, and (4) a urinary biomarker profile consistent with proximal tubular injury is associated with adverse outcomes.
How might this impact on clinical practice?
These results suggest that further study is merited on whether cystatin C rather than creatinine may be preferable to estimate glomerular filtration rate in this population. The urine biomarker findings raise the possibility that such testing may help identify patients with disadvantageous hemodynamics and worse prognosis.
Acknowledgments
We are grateful to Justin Owumi for his work on this project.
Funding sources: This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. ARO and MJL are supported by the Dunlevie Family Fund.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
Footnotes
The authors have no competing interests/conflicts of interest pertinent to the topic of this manuscript.
Author contributions:
All authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data as recommended by ICMJE. All authors have reviewed, provided critical input and given final approval of the manuscript.
In addition, specifically:
ARO planned, conducted and supervised data collection for the study and performed the analysis and drafted the manuscript. He is responsible for the overall content of the manuscript.
FRB planned and conducted the study.
FRM assisted in planning the study and in analyzing and interpreting the data.
BL conducted the study, designed the database used and performed detailed quality control.
EL drafted parts of the manuscript, assisted in data interpretation and provided detailed review of the manuscript.
MJL provided guidance on study design and conduct and assisted in interpreting the results.
VS performed parts of the study including the urine assays and provided guidance on data interpretation.
SSW was involved in planning the study and analyzing and interpreting the data.
Contributor Information
Fernando R Baraona, Email: fbaraona@med.puc.cl.
Finnian R Mc Causland, Email: fmccausland@partners.org.
Brittani Loukas, Email: brittani.loukas@chboston.org.
Elizabeth Landzberg, Email: eil2108@cumc.columbia.edu.
Michael J Landzberg, Email: mike.landzberg@chboston.org.
Venkata Sabbisetti, Email: vsabbisetti@partners.org.
Sushrut S. Waikar, Email: swaikar@partners.org.
References
- 1.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.McAlister FA, Ezekowitz J, Tonelli M, et al. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–9. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 3.Testani JM, Coca SG, Shannon RP, et al. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. European journal of heart failure. 2011;13:1224–30. doi: 10.1093/eurjhf/hfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta–analysis. Am J Kidney Dis. 2002;40:221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 6.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anne P, Du W, Mattoo TK, et al. Nephropathy in patients after Fontan palliation. Int J Cardiol. 2009;132:244–7. doi: 10.1016/j.ijcard.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 8.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29:1301–11. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule–1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. Journal of the American Society of Nephrology: JASN. 2014;25:2177–86. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol. 2012;2012:481520. doi: 10.1155/2012/481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damman K, Ng Kam Chuen MJ, MacFadyen RJ, et al. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57:2233–41. doi: 10.1016/j.jacc.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Damman K, Masson S, Hillege HL, et al. Tubular damage and worsening renal function in chronic heart failure. JACC Heart failure. 2013;1:417–24. doi: 10.1016/j.jchf.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32:2705–12. doi: 10.1093/eurheartj/ehr190. [DOI] [PubMed] [Google Scholar]
- 15.Opotowsky AR, Baraona FR, Owumi J, et al. Galectin–3 Is Elevated and Associated with Adverse Outcomes in Patients with Single Ventricle Fontan Circulation. Journal of the American Heart Association. 2016;5 doi: 10.1161/JAHA.115.002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–8. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J–H. Spurious correlation between ratios with a common divisor. Statistics & Probability Letters. 1999;44:383–6. [Google Scholar]
- 19.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 20.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S, Ruebner RL, Furth SL, et al. Assessment of Kidney Function in Survivors Following Fontan Palliation. Congenit Heart Dis. 2016 doi: 10.1111/chd.12358. [DOI] [PubMed] [Google Scholar]
- 22.Cordina R, O’Meagher S, Gould H, et al. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart. 2013;99:1530–4. doi: 10.1136/heartjnl-2013-304249. [DOI] [PubMed] [Google Scholar]
- 23.Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–8. doi: 10.1161/CIRCULATIONAHA.107.734921. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–43. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navaratnam D, Fitzsimmons S, Grocott M, et al. Exercise-Induced Systemic Venous Hypertension in the Fontan Circulation. Am J Cardiol. 2016;117:1667–71. doi: 10.1016/j.amjcard.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 26.Driver TH, Katz R, Ix JH, et al. Urinary kidney injury molecule 1 (KIM-1) and interleukin 18 (IL-18) as risk markers for heart failure in older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014;64:49–56. doi: 10.1053/j.ajkd.2014.01.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Seaghdha CM, Hwang SJ, Larson MG, et al. Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. Journal of the American Society of Nephrology: JASN. 2013;24:1880–8. doi: 10.1681/ASN.2013010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. Journal of the American Society of Nephrology: JASN. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 29.Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–3. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 30.Haase-Fielitz A, Haase M, Bellomo R. Instability of urinary NGAL during long-term storage. Am J Kidney Dis. 2009;53:564–5. doi: 10.1053/j.ajkd.2009.01.009. author reply 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


