Abstract
Background
No effective pharmacotherapies exist for the treatment of crack cocaine use disorders. Emerging data suggests that cannabinoids may play a role in reducing cocaine-related craving symptoms. This study investigated the intentional use of cannabis to reduce crack use among people who use illicit drugs (PWUD).
Methods
Data were drawn from three prospective cohorts of PWUD in Vancouver, Canada. Using data from participants reporting intentional cannabis use to control crack use, we used generalized linear mixed-effects modeling to estimate the independent effect of three predefined intentional cannabis use periods (i.e., before, during and after first reported intentional use to reduce crack use) on frequency of crack use.
Results
Between 2012 and 2015, 122 participants reported using cannabis to reduce crack use, contributing a total of 620 observations. In adjusted analyses, compared to before periods, after periods were associated with reduced frequency of crack use (Adjusted Odds Ratio [AOR] = 1.89, 95% Confidence Interval [CI]: 1.02–3.45), but not the intentional use periods (AOR= 0.85, 95% CI: 0.51–1.41). Frequency of cannabis use in after periods was higher than in before periods (AOR = 4.72, 95% CI: 2.47–8.99), and showed a tendency to lower frequency than in intentional cannabis use periods (AOR = 0.56, 95% CI: 0.32–1.01).
Conclusions
A period of intentional cannabis use to reduce crack use was associated with decreased frequency of crack use in subsequent periods among PWUD. Further clinical research to assess the potential of cannabinoids for the treatment of crack use disorders is warranted.
Keywords: cannabis, crack cocaine, people who use drugs, Δ9-tetrahydrocannabinol, cannabidiol, cannabinoids
1. Introduction
According to recent estimates, there are between 14 and 21 million current users of cocaine worldwide, of whom approximately seven million have a cocaine use disorder (Degenhardt et al., 2014; United Nations Office on Drugs and Crime, 2014). A substantial proportion of cocaine use is thought to occur in the form of crack cocaine, particularly among marginalized populations in urban settings in North and South America (Fischer, Cruz, Bastos, & Tyndall, 2013). Crack cocaine use, in turn, is associated with a number of health-, social-, and legal-related harms (DeBeck, Kerr, et al., 2009; DeBeck, Small, et al., 2009; Degenhardt et al., 2011; Fischer, Blanken, et al., 2015; Fischer & Coghlan, 2007; Fischer et al., 2013; Fischer, Powis, Firestone Cruz, Rudzinski, & Rehm, 2008; Shannon et al., 2008). Unfortunately, despite the substantial public health and social challenges posed by crack use, no effective pharmacotherapy exists for the treatment of crack cocaine use disorders, and the long-term effectiveness of available psychosocial interventions is limited (Fischer, Blanken, et al., 2015). Therefore, there is a critical need for continuous research on innovative therapeutic approaches for crack use disorders.
A growing body of evidence has described the key role of the human endocannabinoid system (ECBS) in the neurobiological adaptations and behavioral processes underlying substance use disorders (Prud’homme, Cata, & Jutras-Aswad, 2015). While data from small observational studies and animal models suggest that some cannabinoids may be effective in reducing craving — one of the major predictors of crack cocaine use (Paliwal, Hyman, & Sinha, 2008) — more robust data is currently lacking (Dreher, 2002; Fischer, Kuganesan, et al., 2015). Thus, the objective of this study was to investigate the potential impact of the intentional use of cannabis to reduce crack use on the subsequent crack use frequency in a community-recruited sample of people who use illicit drugs (PWUD) in Vancouver, Canada.
2. Materials and methods
2.1. Study design, procedures and population
Data for this study were drawn from three open and ongoing prospective cohorts of PWUD with harmonized procedures for recruitment, follow-up and data collection, in Vancouver, Canada. These include the Vancouver Injection Drug Users Study (VIDUS); the AIDS Care Cohort to E valuate exposure to Survival Services (ACCESS); and the At-Risk Youth Study (ARYS). VIDUS began recruitment in 1996, and ARYS and ACCESS in 2005. Individuals are recruited through snowball sampling and extensive street outreach in the city’s Downtown Eastside and Downtown South neighborhoods, both urban areas with high levels of illicit drug use, homelessness and marginalization. Where possible, all information gathering procedures are conducted in the same way regardless of a participant’s cohort membership to allow for analysis of merged data of studies focusing on outcomes and behaviors that cut across cohorts, as is the case of the present analysis. In brief, to be eligible, individuals need to reside within the greater Vancouver region and have used illicit drugs (other than cannabis) in the previous month. In addition, each cohort has specific inclusion criteria. VIDUS consists of HIV-negative adults (≥ 18 years) who injected drugs in the month prior to enrolment, ACCESS of HIV-positive adults, and ARYS of street-involved youth (14–26 years old.) Recruitment and study procedures for the three studies have been described in detail previously (Strathdee et al., 1998; Wood et al., 2008; Wood, Stoltz, Montaner, & Kerr, 2006). After providing written informed consent, at baseline and semi-annually thereafter, participants completed an interviewer-administered questionnaire, provided blood for HIV/HCV serological testing, and were examined by a study nurse who provides basic medical care and referrals to additional health services where appropriate. The questionnaire elicited data on socio-demographic characteristics, drug use patterns, health care access and utilization, including HIV and addiction care, as well as other relevant exposures. Participants received a $30 honorarium at each study visit. The VIDUS, ACCESS and ARYS studies have received ethical approval by the University of British Columbia/Providence Health Care Research Ethics Board.
For the present study, data from the three cohorts were combined to achieve sufficient power to examine the potential impact of intentional cannabis use on frequency of crack use. The analytic sample was restricted to participants who reported intentional use of cannabis to reduce their use of crack at least once during the study period. Specifically, individuals were included if they answered yes to the question “In the last 6 months, did you substitute one drug for another in order to control or slow down your use”, and indicated that they were using cannabis to reduce their use of crack. As this question was added to the questionnaires in June 2012, we considered all observations collected between this date and May 2015. Of note, measurements of crack cocaine use were longitudinal and fully distinct and independent from measurements of cannabis use, and the “substitution” question was systematically asked after these assessments.
2.2. Measures
The primary outcome of interest was the self-reported frequency of crack use in the six-month period prior to each study interview. At each semi-annual follow-up interview, participants were asked to estimate their crack use since the last visit, using six predefined frequency categories: (1) ≥ daily, (2) 2–3 times a week, (3) about once a week, (4) 1–3 times a month, (5) less than once a month, and (6) no use. We defined a reduction in use as a change from one frequency category to any other lower frequency category.
The primary explanatory variable was the cannabis use period. Three periods were defined: (1) before, observations before the first report of intentional cannabis use to reduce crack use, (2) during, interview-periods where the participant reported using cannabis to control the use of crack, and (3) after, observations after the first report of intentional cannabis use where no intentional use was reported.
We also considered a set of socio-demographic variables that were hypothesized to potentially confound the relationship between intentional cannabis use and crack use. Time-fixed variables of interest at baseline included: gender (male versus non-male); ancestry (Caucasian versus non-Caucasian); highest educational attainment (high school or postsecondary education vs. less than high school completion). Time-varying variables (updated at each semi-annual follow-up) included age (per year older), place of residency (Downtown Eastside, one of the largest open drug scenes in North America, versus other neighborhoods), and seeking treatment for crack cocaine.
2.3. Statistical analyses
As a first step, we compared characteristics of participants stratified by daily crack cocaine use in the last 6 months prior to their first interview in the study period (e.g., June 2012). We used the Pearson’s chi-squared (or Fisher’s exact test in the presence of small cell counts) for categorical variables, and the Wilcoxon rank sum test for continuous variables. Next, we examined the frequency of crack and cannabis use in each of the three cannabis use periods. Then, we estimated the bivariable relationship between the primary explanatory variable (i.e., cannabis use period, using the before period as the reference category) and each secondary covariate on frequency of crack use. We used generalized linear mixed-effects models (GLMM), treating the frequency of crack use as an ordinal outcome, and incorporating random intercepts to account for repeated measurements from the same participants over time. The proportional odds assumption was checked using the score test. This approach (i.e., ordinal outcome, random intercept) also allows for an estimation of the odds of changes in the frequency of crack cocaine use, regardless of the frequency each participant started with. As we modeled lower frequency of crack use, Odds Ratio (OR) >1 means decreased frequency of use compared to the “before” period, while OR <1 means increased frequency of use. To assess the independent effect of the cannabis use period on reduced crack cocaine use, we then fit a fixed multivariable model with the main explanatory variable and all secondary covariates that were associated with the outcome in bivariable analysis at a p-value <0.10. In addition, we forced into the multivariable model a variable representing calendar year of the interview to control for the cohort effect, and a variable representing cohort designation to control for possible heterogeneity of effect across cohorts.
Finally, to explore changes in cannabis use coinciding with the three cannabis use periods, we built an analogous 6-level cannabis use frequency variable and followed a similar approach as described above. The only difference is that for cannabis use, we modeled higher frequency of use instead of reduced frequency (i.e., OR>1 = higher frequency of cannabis use, and OR<1 = lower frequency). All statistical analyses were performed using the SAS software version 9.4 (SAS, Cary, NC), and two-sided p-values <0.05 were considered statistically significant.
3. Results
Between June 2012 and May 2015, 837 VIDUS, 670 ACCESS, and 493 ARYS participants completed at least one follow-up interview. Of these, 122 participants (49 VIDUS, 51 ACCESS, and 22 ARYS participants) reported intentional use of cannabis to reduce crack use at least once and were thus included in the present analysis, contributing to a total of 620 observations. The median duration of follow-up per participant during the study period was 29.1 (Inter-Quartile Range [IQR]: 24.0–30.1) months, resulting in a total of 268.1 person-years of follow up. Baseline characteristics of study participants stratified by ≥daily crack use in the last six months are shown in Table 1. The median age was 46 years (IQR: 34–53), 89 (73.0%) were male, and 51 (41.8%) HIV-positive. The majority of participants reported intentional use of cannabis to control crack only once (88, 72.1%), 26 (21.3%) reported intentional use of cannabis in two periods, and 8 (6.6%), 3 times or more.
Table 1.
Baseline characteristics of 122 illicit drug users who ever intentionally used cannabis to reduce crack cocaine use, stratified by daily crack cocaine use in the last six months
| Characteristic | Total (%) (n = 122) |
≥ Daily crack cocaine use
|
p — value | |
|---|---|---|---|---|
| Yes (%) (n = 45) | No (%) (n = 77) | |||
| Socio-demographic characteristics | ||||
| Age (med, IQR) | 46 (34–53) | 45 (34–54) | 47 (35–52) | 0.853£ |
| Male gender | 89 (73.0) | 29 (64.4) | 60 (77.9) | 0.106 |
| Caucasian ethnicity | 61 (50.0) | 21 (46.7) | 40 (52.0) | 0.574 |
| High school education or higher* | 59 (48.4) | 23 (51.1) | 36 (46.8) | 0.691 |
| Residency in the Downtown Eastside* | 68 (55.7) | 27 (60.0) | 41 (53.3) | 0.469 |
| Seek treatment for crack* | 22 (18.0) | 11 (24.4) | 11 (14.3) | 0.159 |
| Frequency of cannabis use* | ||||
| Did not use | 17 (13.9) | 5 (11.1) | 12 (15.6) | 0.837€ |
| Less than once a month | 6 (4.9) | 1 (2.2) | 5 (6.5) | |
| 1–3 times a month | 8 (6.6) | 3 (6.7) | 5 (6.5) | |
| About once a week | 10 (8.2) | 5 (11.1) | 5 (6.5) | |
| 2–3 times a week | 23 (18.9) | 9 (20.0) | 14 (18.2) | |
| ≥ Daily | 58 (47.5) | 22 (48.9) | 36 (46.7) | |
Refers to the 6-month period prior to the interview
Wilcoxon rank sum test
Fisher’s exact test
Figure 1 presents the frequencies of crack cocaine (panel A) and cannabis use (Panel B) in each of the three cannabis use periods (i.e., before, during, after). As shown in this figure, “2–3 times a week” and “≥daily” frequencies categories of crack use experienced the largest reductions between the before and after period.
Figure 1.
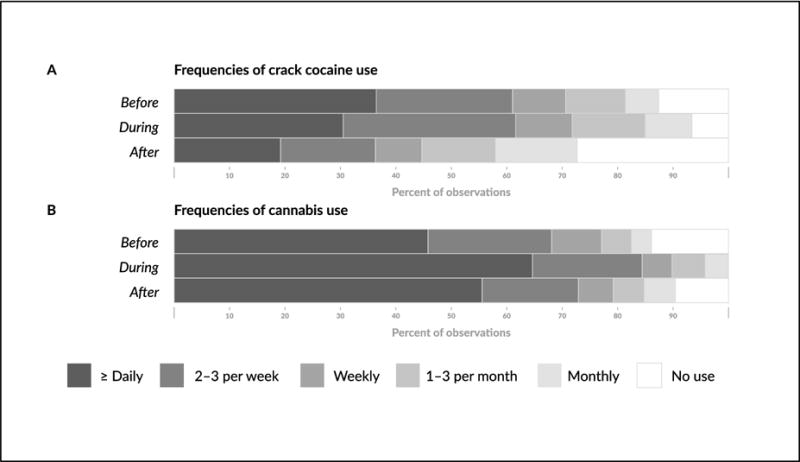
Frequencies of crack cocaine (Panel A) and cannabis use (Panel B) in each of the cannabis use periods.
As indicated in Table 2, in unadjusted analysis, compared to the before period, the odds of reduced frequency of crack use was higher in the after period (Odds Ratio [OR] = 3.93, 95% Confidence interval [CI] 2.57–5.99) but not during intentional cannabis use periods (OR = 1.14, 95% CI 0.73–1.77). In a multivariable GLMM, the after period remained positively associated with lower frequency of crack use (Adjusted Odds Ratio [AOR] = 1.89, 95%CI: 1.01–3.45).
Table 2.
Bivariable and multivariable generalized linear mixed-effects analyses of reduced frequency of crack cocaine use among 122 illicit drug users reporting intentional use of cannabis to reduce crack use, Vancouver, Canada (2012–2015)
| Variable | Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95% CI)‡€ | p-value |
|---|---|---|---|---|
| Primary variable of interest | ||||
| Cannabis use period* | ||||
| Before | 1.00 | 1.00 | ||
| During | 1.14 (0.73–1.77) | 0.579 | 0.85 (0.51–1.41) | 0.524 |
| After | 3.93 (2.57–5.99) | <0.001 | 1.89 (1.01–3.45) | 0.043 |
|
| ||||
| Covariates | ||||
| Age (per year older)£† | 0.97 (0.95–1.00) | 0.048 | 0.93 (0.89–0.97) | 0.002 |
| Male gender† | 2.58 (1.27–5.25) | 0.009 | 3.63 (1.70–7.73) | <0.001 |
| Caucasian ethnicity | 1.35 (0.71–2.56) | 0.359 | ||
| High school education or higher | 0.87 (0.46–1.65) | 0.668 | ||
| Residency in the Downtown Eastside£† | 0.42 (0.26–0.69) | <0.001 | 0.51 (0.31–0.85) | 0.009 |
| Seek treatment for crack£† | 0.31 (0.17–0.54) | <0.001 | 0.34 (0.19–0.62) | <0.001 |
Intentional use of cannabis to reduce crack use
Refers to the 6-month period prior to the interview
p-value <0.10 in bivariable analysis and included in the multivariable model
Only the list of variables included in the multivariable model is presented in this column
Estimates also adjusted for calendar year of the interview and cohort designation
Table 3 presents the unadjusted and adjusted GLMM analyses of the relationship between the cannabis use period and frequency of cannabis use. In adjusted analyses, compared to the before period, both the intentional use (AOR = 4.72, 95% CI 2.47–8.99) and the after periods (AOR = 2.63, 95% CI 1.21–5.74) were associated with higher frequency of cannabis use. In addition, cannabis use in the after periods showed a tendency to lower frequency than during the intentional use period (AOR = 0.56, 95% CI: 0.32–1.01, p=0.052), data not shown).
Table 3.
Bivariable and multivariable generalized linear mixed-effects analyses of higher frequency of cannabis use among 122 illicit drug users reporting intentional use of cannabis to reduce crack use, Vancouver, Canada (2012–2015)
| Variable | Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95% CI) ‡€ | p-value |
|---|---|---|---|---|
| Primary variable of interest | ||||
| Cannabis use period* | ||||
| Before | 1.00 | 1.00 | ||
| During | 5.48 (3.10–9.68) | <0.001 | 4.72 (2.47–8.99) | <0.001 |
| After | 2.66 (1.61–4.40) | <0.001 | 2.63 (1.21–5.74) | 0.015 |
|
| ||||
| Covariates | ||||
| Age (per year older)£† | 0.95 (0.91–0.99) | 0.010 | 0.97 (0.91–1.04) | 0.437 |
| Male gender | 1.30 (0.45–3.73) | 0.624 | ||
| Caucasian ethnicity | 0.88 (0.35–2.24) | 0.992 | ||
| High school education or higher | 1.11 (0.59–2.06) | 0.753 | ||
| Residency in the Downtown Eastside£ | 1.69 (0.85–3.36) | 0.132 | ||
| Seek treatment for crack£ | ||||
Intentional use of cannabis to reduce crack use
Refers to the 6-month period prior to the interview
p-value <0.10 in bivariable analysis and included in the multivariable model
Only the list of variables included in the multivariable model is presented in this column
Estimates also adjusted for calendar year of the interview and cohort designation
4. Discussion
In this longitudinal study, we observed that a period of self-reported intentional use of cannabis to control crack use was associated with subsequent periods of reduced use of crack among a community-recruited sample of PWUD in Vancouver, Canada. Interestingly, it was only the period after such intentional use of cannabis that was positively associated with decreased frequency of crack use, while the frequency of crack use during periods of intentional cannabis use was not statistically different to the before period. Consistent with the substitution hypothesis (Lau et al., 2015), the frequency of cannabis use increased both during and after the first reported intentional use of cannabis to reduce crack use. However, while frequencies of cannabis use were higher in the after periods compared to baseline levels (i.e., before periods), they show a decreasing trend than during periods where cannabis use was reported as a means to reduce crack use.
Our findings are in line with previous results from preliminary observational studies including a small case-series study from Brazil that followed 25 treatment-seeking individuals with crack use disorders who reported using cannabis to reduce cocaine-related craving symptoms. Over a nine-month follow-up period, the majority (68%) stopped using crack. Further, similar to our study, cannabis use peaked during the first three months of follow-up, with only occasional use of cannabis reported in the remainder six months of follow-up (Labigalini, Rodrigues, & Da Silveira, 1999). Qualitative studies among crack users in Jamaica and Brazil also indicate that cannabis is frequently used as a self-medication strategy to reduce craving and other undesirable effects of crack (e.g., feelings of paranoia and anxiety) which in turn results in decreased cocaine-seeking behaviour (and associated illicit or endangering activities to procure the drug) and use of crack (Dreher, 2002; Goncalves & Nappo, 2015). Conversely, other studies have revealed that long-term cannabis dependence might increase cocaine craving and risk of relapse among individuals with poly-drug substance use disorders (Aharonovich et al., 2005; Fox, Tuit, & Sinha, 2013; Viola et al., 2014), suggesting that patterns of cannabis use and dependence, and the timing of self-medication with cannabis might play a role in explaining the different outcomes across studies. In addition, qualitative research may help to better understand motivations and expectations of cannabis use among crack users.
Emerging pre-clinical and clinical data provide biological plausibility for the findings of the present analysis. Specifically, Parker et al. demonstrated that both Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the two primary cannabinoids found in the cannabis plant, facilitated extinction of cocaine-induced conditioned place preference (CPP) in rat models, a suggested proxy for craving (Parker, Burton, Sorge, Yakiwchuk, & Mechoulam, 2004). CBD was also found to persistently disrupt the reconsolidation of cocaine- and opioid-related memory (de Carvalho & Takahashi, 2016), and to reduce cue-induced heroin-seeking behavior in rats, with effects lasting for at least two weeks after CBD administration (Ren, Whittard, Higuera-Matas, Morris, & Hurd, 2009). Based on this encouraging animal data, clinical trials exploring the potential role of CBD in addiction treatment have started to arise. Consistent with results from pre-clinical studies, findings from pilot human trials have indicated that single doses of 400 or 800 mg of CBD for three consecutive days were well tolerated and effective in decreasing cue-induced and general craving, as well as anxiety among heroin-dependent individuals, with persistent effects of up to seven days (Hurd et al., 2015; Manini et al., 2015). Although no clinical studies have yet been conducted among individuals with cocaine use disorders, taken together, findings from this study and others (Dreher, 2002; Goncalves & Nappo, 2015; Hurd et al., 2015; Labigalini et al., 1999; Manini et al., 2015; Parker et al., 2004; Prud’homme et al., 2015; Ren et al., 2009) support calls to further investigate the therapeutic potential of cannabinoids to attenuate craving and other cocaine-cessation symptoms (e.g., anxiety), and thus lower rates of relapse (Fischer, Kuganesan, et al., 2015).
Both temporality and a plausible biological pathway support a potential causal relationship between self-medication with cannabis and reduced crack use. However, findings from the present study should be interpreted in light of a number of limitations. First, the three parents cohorts from where our data were drawn are not random samples; therefore our results may not be generalizable to the larger population of drug users in Vancouver or other settings. That said, socio-demographic characteristics of our study sample are similar to epidemiological data of crack use across Canada, including socio-economic marginalization and high prevalence of HIV (Fischer & Coghlan, 2007; Fischer et al., 2013). Second, as we did not have access to a comparison group of individuals trying to reduce their use of crack through a different modality, we cannot exclude the possibility that the observed reductions were the product of a different exposure (e.g., cognitive strategies). Third, the use of observational data with non-random assignment to the exposure of interest further degrades our ability to assign causality. Fourth, drug use patterns assessed in this analysis relied on self-reported data, which might be subject to social-desirability bias and underreporting. However, previous research has shown PWUD’s reports of drug use to be reliable (De Irala, Bigelow, McCusker, Hindin, & Zheng, 1996; Langendam, van Haastrecht, & van Ameijden, 1999). Further, we have no reason to believe that the magnitude of this potential bias, if any, will differ among cannabis substitution periods. Fifth, our study instrument does not collect information on the specific composition of the cannabis used (e.g., concentrations of THC and CBD) or craving measures, and thus we are not able to attribute the observed effects to a specific cannabinoid or dosage, or mechanism of action.
5. Conclusions
In summary, we found that a period of intentional cannabis use to reduce use of crack was associated with subsequent reductions in the frequency of crack cocaine use among PWUD in Vancouver, Canada. Although more research is needed to confirm these results, findings from the present analysis are in line with previous observational and preclinical data suggesting that cannabinoids may play a role in attenuating cocaine-related craving symptoms, and thus reduce crack use or relapse. Given the substantial global burden of morbidity and mortality attributable to crack cocaine use disorders alongside a lack of effective pharmacotherapies, we echo calls for rigorous experimental research on cannabinoids as a potential treatment for crack cocaine use disorders (Fischer, Kuganesan, et al., 2015).
Highlights.
We investigated the impacts of intentional cannabis use to control crack use
Periods after intentional cannabis use were associated with decreased crack use
Frequencies of cannabis use were higher during intentional use periods with a decreasing tendency in after periods
Acknowledgments
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We would specifically like to thank: Deborah Graham, Tricia Collingham, Carmen Rock, Jennifer Matthews, Steve Kain, Cody Callon, Benita Yip and James Nakagawa for their research and administrative assistance.
Funding sources: The study is supported by the US National Institutes of Health (U01-DA038886 and R01-DA021525). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine, which supports EW. MES is supported by a Michael Smith Foundation for Health Research (MSFHR) postdoctoral fellowship award and a Canada Addiction Medicine Research Fellowship (US National Institute on Drug Abuse, R25-DA037756). M-JM is supported in part by the US National Institutes of Health (R01-DA021525), a Scholar Award from MSFHR and a New Investigator Award from the Canadian Institutes of Health Research (CIHR). KD is supported by a MSFHR/St. Paul’s Hospital Foundation-Providence Health Care Career Scholar Award and a CIHR New Investigator Award. Didier Jutras-Aswad holds a Fonds de Recherche du Québec — Santé (FRQS, Québec, Canada) clinical researcher career award. JM is supported by the British Columbia Ministry of Health and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse (NIDA) at the US National Institutes of Health (NIH). JM has also received financial support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President’s Emergency Plan for AIDS Relief (PEPfAR), UNICEF, the University of British Columbia, Simon Fraser University, Providence Health Care and Vancouver Coastal Health Authority. KH is supported by a CIHR New Investigator Award (MSH-141971). The funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: MES, MJM and TK designed the study. HD conducted the statistical analysis. MES wrote the initial draft. All authors contributed to the interpretation of the findings, the critical revision of the manuscript for intellectual content, and approved submission of the final manuscript.
Conflict of interest: The University of British Columbia has received unrestricted funding from NG Biomed, Ltd., an applicant to the Canadian federal government for a licence to produce medical cannabis, to support M-JM’s research. JM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. All other authors declare no conflict of interest.
References
- Aharonovich E, Liu X, Samet S, Nunes E, Waxman R, Hasin D. Postdischarge cannabis use and its relationship to cocaine, alcohol, and heroin use: a prospective study. Am J Psychiatry. 2005;162(8):1507–1514. doi: 10.1176/appi.ajp.162.8.1507. [DOI] [PubMed] [Google Scholar]
- de Carvalho CR, Takahashi RN. Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict Biol. 2016 doi: 10.1111/adb.12366. [DOI] [PubMed] [Google Scholar]
- De Irala J, Bigelow C, McCusker J, Hindin R, Zheng L. Reliability of self-reported human immunodeficiency virus risk behaviors in a residential drug treatment population. Am J Epidemiol. 1996;143(7):725–732. doi: 10.1093/oxfordjournals.aje.a008806. [DOI] [PubMed] [Google Scholar]
- DeBeck K, Kerr T, Li K, Fischer B, Buxton J, Montaner J, Wood E. Smoking of crack cocaine as a risk factor for HIV infection among people who use injection drugs. CMAJ. 2009;181(9):585–589. doi: 10.1503/cmaj.082054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Small W, Wood E, Li K, Montaner J, Kerr T. Public injecting among a cohort of injecting drug users in Vancouver, Canada. J Epidemiol Community Health. 2009;63(1):81–86. doi: 10.1136/jech.2007.069013. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, Vos T. The global epidemiology and burden of psychostimulant dependence: findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Singleton J, Calabria B, McLaren J, Kerr T, Mehta S, Hall WD. Mortality among cocaine users: a systematic review of cohort studies. Drug Alcohol Depend. 2011;113(2–3):88–95. doi: 10.1016/j.drugalcdep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Dreher M. Crack heads and roots daughters: The therapeutic use of cannabis in Jamaica. Journal of Cannabis Therapeutics. 2002;2(3–4):121–133. [Google Scholar]
- Fischer B, Blanken P, Da Silveira D, Gallassi A, Goldner EM, Rehm J, Wood E. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: a comprehensive narrative overview of English-language studies. Int J Drug Policy. 2015;26(4):352–363. doi: 10.1016/j.drugpo.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Fischer B, Coghlan M. Crack use in North American cities: the neglected ‘epidemic’. Addiction. 2007;102(9):1340–1341. doi: 10.1111/j.1360-0443.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Cruz MS, Bastos FI, Tyndall M. Crack across the Americas — a massive problem in continued search of viable answers: exemplary views from the North (Canada) and the South (Brazil) Int J Drug Policy. 2013;24(6):631–633. doi: 10.1016/j.drugpo.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Fischer B, Kuganesan S, Gallassi A, Malcher-Lopes R, van den Brink W, Wood E. Addressing the stimulant treatment gap: A call to investigate the therapeutic benefits potential of cannabinoids for crack-cocaine use. Int J Drug Policy. 2015;26(12):1177–1182. doi: 10.1016/j.drugpo.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Fischer B, Powis J, Firestone Cruz M, Rudzinski K, Rehm J. Hepatitis C virus transmission among oral crack users: viral detection on crack paraphernalia. Eur J Gastroenterol Hepatol. 2008;20(1):29–32. doi: 10.1097/MEG.0b013e3282f16a8c. [DOI] [PubMed] [Google Scholar]
- Fox HC, Tuit KL, Sinha R. Stress system changes associated with marijuana dependence may increase craving for alcohol and cocaine. Hum Psychopharmacol. 2013;28(1):40–53. doi: 10.1002/hup.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JR, Nappo SA. Factors that lead to the use of crack cocaine in combination with marijuana in Brazil: a qualitative study. BMC Public Health. 2015;15:706. doi: 10.1186/s12889-015-2063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M, Jutras-Aswad D. Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurotherapeutics. 2015;12(4):807–815. doi: 10.1007/s13311-015-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigalini E, Jr, Rodrigues LR, Da Silveira DX. Therapeutic use of cannabis by crack addicts in Brazil. J Psychoactive Drugs. 1999;31(4):451–455. doi: 10.1080/02791072.1999.10471776. [DOI] [PubMed] [Google Scholar]
- Langendam MW, van Haastrecht HJ, van Ameijden EJ. The validity of drug users’ self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol. 1999;28(3):514–520. doi: 10.1093/ije/28.3.514. [DOI] [PubMed] [Google Scholar]
- Lau N, Sales P, Averill S, Murphy F, Sato SO, Murphy S. A safer alternative: Cannabis substitution as harm reduction. Drug Alcohol Rev. 2015;34(6):654–659. doi: 10.1111/dar.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, Hurd YL. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015;9(3):204–210. doi: 10.1097/ADM.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93(3):252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175(3):360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Prud’homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Subst Abuse. 2015;9:33–38. doi: 10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29(47):14764–14769. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K, Rusch M, Morgan R, Oleson M, Kerr T, Tyndall MW. HIV and HCV prevalence and gender-specific risk profiles of crack cocaine smokers and dual users of injection drugs. Subst Use Misuse. 2008;43(3–4):521–534. doi: 10.1080/10826080701772355. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. Vienna: United Nations; 2014. [Google Scholar]
- Viola TW, Tractenberg SG, Wearick-Silva LE, Rosa CS, Pezzi JC, Grassi-Oliveira R. Long-term cannabis abuse and early-onset cannabis use increase the severity of cocaine withdrawal during detoxification and rehospitalization rates due to cocaine dependence. Drug Alcohol Depend. 2014;144:153–159. doi: 10.1016/j.drugalcdep.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, Montaner JS. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. doi: 10.1186/1477-7517-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


