Abstract
Amphipathic α-helices of exchangeable apolipoproteins have shown to play crucial roles in the formation of infectious hepatitis C virus (HCV) particles through the interaction with viral particles. Among the Flaviviridae members, pestivirus and flavivirus possess a viral structural protein Erns or a non-structural protein 1 (NS1) as secretory glycoproteins, respectively, while Hepacivirus including HCV has no secretory glycoprotein. In case of pestivirus replication, the C-terminal long amphipathic α-helices of Erns are important for anchoring to viral membrane. Here we show that host-derived apolipoproteins play functional roles similar to those of virally encoded Erns and NS1 in the formation of infectious particles. We examined whether Erns and NS1 could compensate for the role of apolipoproteins in particle formation of HCV in apolipoprotein B (ApoB) and ApoE double-knockout Huh7 (BE-KO), and non-hepatic 293T cells. We found that exogenous expression of either Erns or NS1 rescued infectious particle formation of HCV in the BE-KO and 293T cells. In addition, expression of apolipoproteins or NS1 partially rescued the production of infectious pestivirus particles in cells upon electroporation with an Erns-deleted non-infectious RNA. As with exchangeable apolipoproteins, the C-terminal amphipathic α-helices of Erns play the functional roles in the formation of infectious HCV or pestivirus particles. These results strongly suggest that the host- and virus-derived secretory glycoproteins have overlapping roles in the viral life cycle of Flaviviridae, especially in the maturation of infectious particles, while Erns and NS1 also participate in replication complex formation and viral entry, respectively. Considering the abundant hepatic expression and liver-specific propagation of these apolipoproteins, HCV might have evolved to utilize them in the formation of infectious particles through deletion of a secretory viral glycoprotein gene.
Author summary
The family Flaviviridae consists of 4 genera, namely Flavivirus, Pestivirus, Pegivirus, and Hepacivirus. Flaviviruses and pestiviruses can infect various species and tissues; however, infection of pegivirus and hepacivirus is observed in a strikingly restricted range of tissue and hosts. Although all the Flaviviridae viruses possess a similar genome structure, hepatitis C virus (HCV) from Hepacivirus encodes no secretory glycoprotein, such as Erns of pestivirus and NS1 of flavivirus. The apolipoproteins, one of the host secretory glycoproteins, play important roles in the formation of infectious HCV particles through the interaction with viral particles. The data presented here show that the host-derived apolipoproteins and viral-derived Erns and NS1 have overlapping roles in the maturation of infectious particles of Flaviviridae. Considering an abundant expression of apolipoproteins in the liver and their liver-specific propagation, HCV might have evolved to utilize the apolipoproteins in the formation of infectious particles through deletion of a gene encoding a secretory viral glycoprotein. The data of this manuscript also suggest that utilization of host factors in the viral life cycle is closely associated with the tissue- and species-specificities and evolution among Flaviviridae viruses.
Introduction
The family Flaviviridae consists of 4 genera, Flavivirus, Pestivirus, Pegivirus, and Hepacivirus. Flaviviridae viruses commonly possess a single-stranded positive-sense RNA encoding 2–4 structural proteins and 7–8 non-structural proteins [1]. The host range and tissue tropism of these proteins are strikingly different. The genus Flavivirus is composed of more than 50 species with a wide host range from reptiles to mammals; these mosquito- or tick-borne viruses include dengue virus (DENV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV) [2]. The genus Pestivirus comprises economically important pathogens of even-toed ungulate animals, including classical swine fever virus (CSFV), bovine viral diarrhea virus (BVDV), and border disease virus (BDV), which cause major losses in livestock farming [3]. In the case of hepatitis C virus (HCV) from the genus Hepacivirus, the species-specificity is restricted to humans and chimpanzees [4].
Although the genome structure of HCV resembles that of pestivirus, a structural protein Erns is missing in HCV [1, 5]. Erns has been shown to be essential for infectious particle formation [5]. Although Erns has no transmembrane domain, the C-terminal amphipathic α-helices are important for anchoring to the membrane of viral particles [5]. Interestingly, the genus Flavivirus also encodes a non-structural secretory protein NS1 that is translocated to the lumen of the endoplasmic reticulum (ER) as a dimer or hexamer, and a membrane-binding dimer is known to participate in formation of the viral RNA replication complex [6]. However, the functional role of the hexametric form of NS1 in viral life cycle is not yet well understood. A recent report suggests that NS1 is involved not only in viral RNA replication but also in particle formation [7, 8]. Because HCV does not encode genes for secretory proteins such as Erns and NS1, host factors are suggested to compensate for the role of viral secretory proteins in the life cycle of HCV. Previous studies have shown that exchangeable apolipoproteins including apolipoprotein E (ApoE) participate in the formation of infectious HCV particles [4, 9]. The amphipathic α-helices from the apolipoproteins have been shown to be important for production of the infectious HCV particles through the interaction with viral particles [10]. In addition, we recently reported that a cellular protein, hCAP18/LL-37 (CAMP), that contains amphipathic α-helices can compensate for the role of apolipoproteins in the formation of infectious HCV particles, indicating that expression of secretory membrane-binding proteins such as apolipoproteins and CAMP is essential for the maturation of HCV particles [11]. Because these host factors interact with the ER-derived membrane in a manner similar to Erns and NS1, we hypothesized that the roles of Erns and NS1 in infectious particle formation are similar to those of secretory membrane-binding host factors.
Here, we showed that Erns and NS1 could compensate for the roles of apolipoproteins in the formation of infectious particles of HCV. By using Erns-deleted CSFV generated by a reverse genetics system, we revealed that both exchangeable apolipoproteins and NS1 could partially compensate for the roles of Erns in the formation of infectious pestivirus particles. These results suggest that the host- and virus-derived secretory glycoproteins have overlapping roles in the viral life cycle of Flaviviridae, especially in maturation of infectious particles, while Erns and NS1 also participate in replication complex formation and viral entry, respectively. HCV might have evolved to utilize host factors including exchangeable apolipoproteins through the loss of a viral gene encoding secretory glycoproteins.
Results
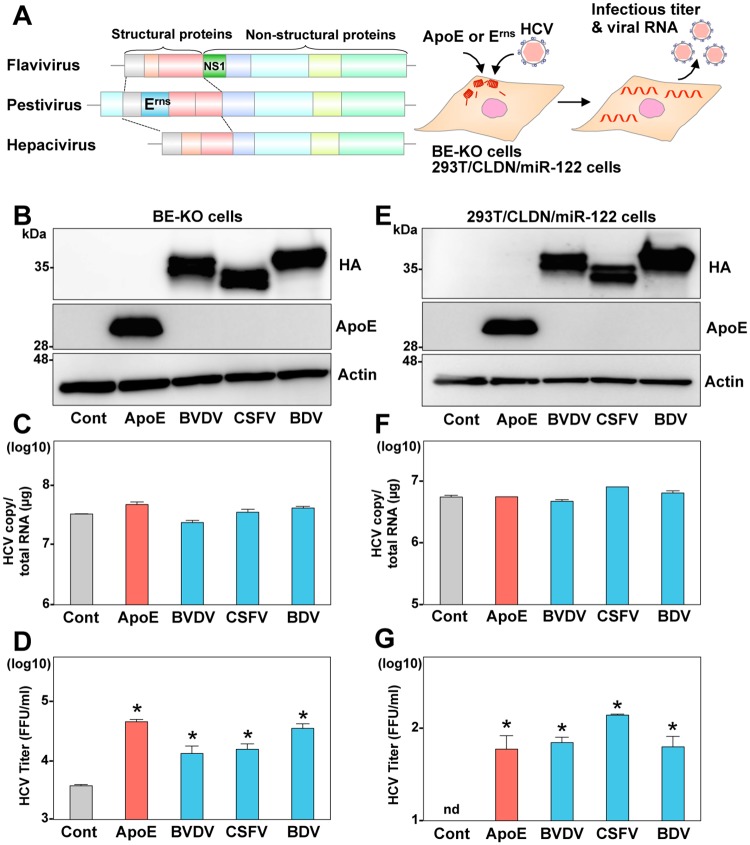
Erns can compensate for the role of apolipoproteins in particle formation of HCV
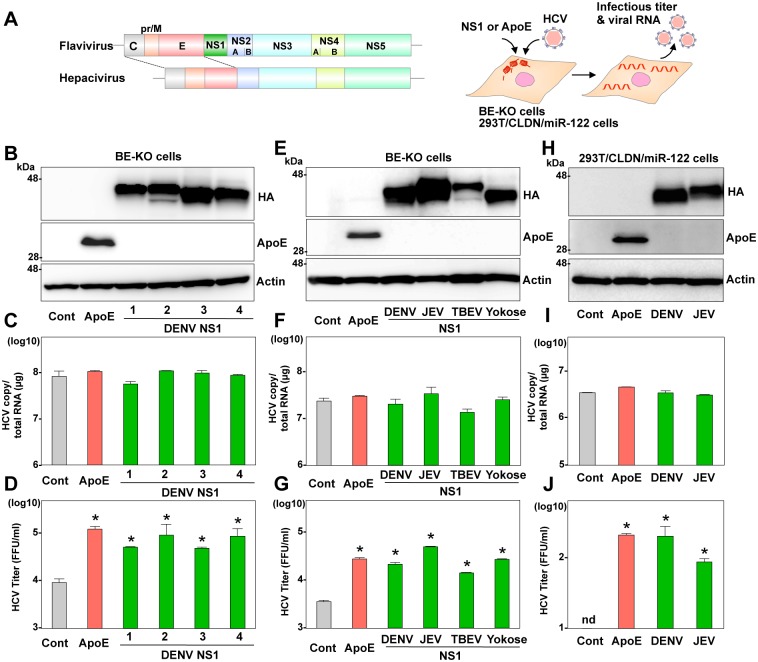
As previously reported, ApoB and ApoE double-knockout Huh7 (BE-KO) cells exhibited an impairment of infectious HCV particle formation, and exogenous expression of ApoE facilitated efficient particle formation (S1 Fig) [10]. To examine whether Erns of pestivirus can compensate for the role of apolipoproteins in the formation of infectious HCV particles, ApoE or HA-tagged Erns (HA-Erns) was lentivirally expressed in BE-KO cells and intracellular HCV RNA and infectious viral titers in the supernatants were determined at 72-h post-infection with HCV (Fig 1A–1D). Expression of HA-Erns derived from various pestiviruses, including BVDV, CSFV, and BDV, in BE-KO cells led to a significant recovery of the formation of HCV infectious particles to nearly the level in the control cells, while HCV RNA replication was not affected by the expression of these proteins. In addition, expression of HA-Erns in the BE-KO cells also enhanced infectious titers of Con1/JFH1 chimeric HCV-possessing genotype 1b structural proteins (S2 Fig), suggesting that Erns can compensate for the role of exchangeable apolipoproteins in the formation of infectious particles not only of genotype 2 but also of genotype 1 HCV. Reconstitution of a complete HCV propagation in non-hepatic cells has been accomplished in 293T cells [10]. Exogenous expression of Claudin1 (CLDN1), microRNA-122 (miR-122), and exchangeable apolipoproteins is required for entry, viral RNA replication, and particles formation, respectively. In 293T cells stably expressing CLDN1 and miR-122 (293T/CLDN/miR-122), expression of Erns facilitated the formation of infectious HCV particles to a similar extent as ApoE expression, but had no effect on the replication of viral RNA (Fig 1E–1G and S1 Fig). Next, to determine the effect of Erns expression on the entry and replication process of HCV, infectivity of HCV pseudotype particles (HCVpp) and replication of sub-genomic replicon (SGR) RNA were examined in the BE-KO cells expressing HA-Erns. Expression of either HA-Erns or ApoE in the BE-KO cells exhibited no effect on the entry of HCVpp (S3A Fig) or the colony formation of SGR RNA (S3B Fig). In addition, expression of Erns had no effect on the propagation of HCV in parental Huh7 cells (S4 Fig). These results suggest that Erns can compensate for the role of apolipoproteins in HCV propagation independent of entry and viral RNA replication.
Fig 1. Erns can compensate for the role of apolipoproteins in the infectious particle formation of HCV.
(A) Gene structures of flaviviruses, pestiviruses, and hepaciviruses, and the experimental procedure. (B) Expression of ApoE and HA-tagged Erns (HA- Erns) from BVDV, CSFV, and BDV was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at a multiple of infection (MOI) of 1 by qRT-PCR and focus-forming assay, respectively. (E) Expression of ApoE and HA-Erns in 293T/CLDN1/miR-122 cells was determined by immunoblotting analysis. Intracellular HCV RNA (F) and extracellular infectious titers (G) were determined at 72-h post-infection with JFH1 HCV at an MOI of 10 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
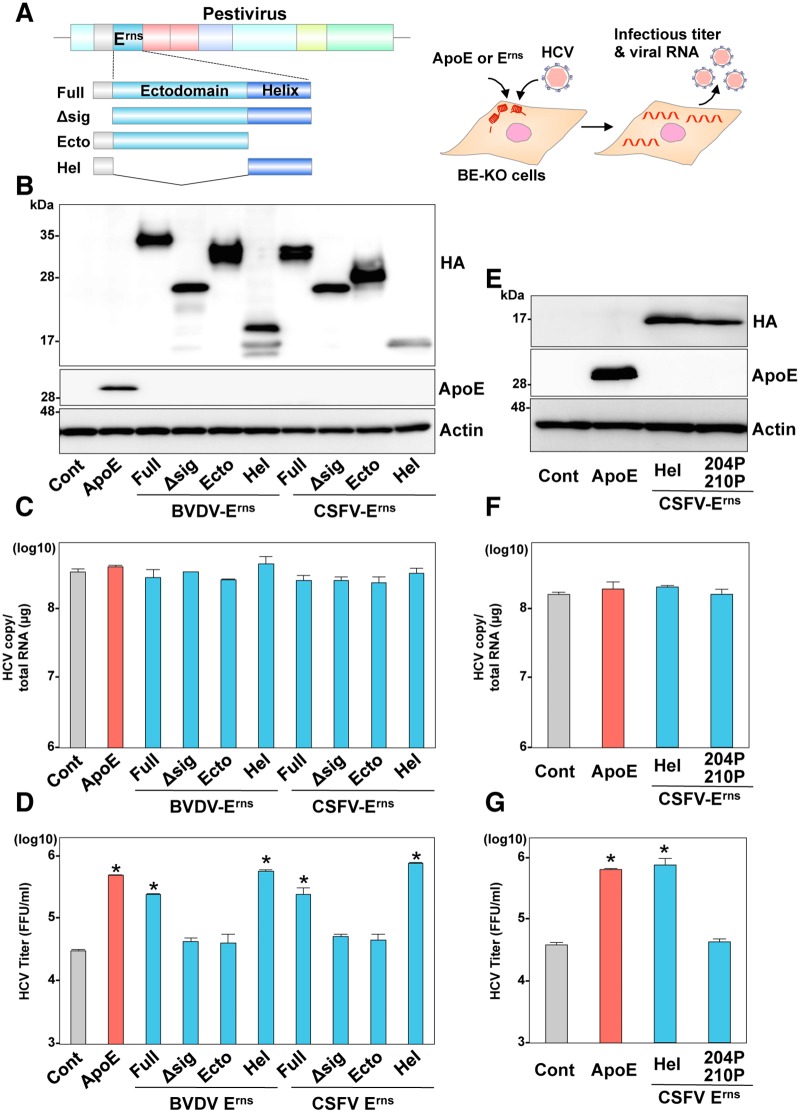
The C-terminal amphipathic α-helix of Erns is crucial for the formation of infectious HCV particles
The C-terminal α-helix domain of Erns is considered to participate in the formation of infectious particles by functioning as an anchor to viral particles [5, 12]. Thus, to examine whether the α-helices of Erns can compensate for the roles of exchangeable apolipoproteins in formation of infectious HCV particles, the BE-KO cells expressing deletion mutants of Erns derived from BVDV and CSFV were infected with HCV (Fig 2A). Expression of the HA-tagged either the full-length (Full) or truncated mutants lacking an ectodomain but carrying the C-terminal α-helices (Hel) of HA-Erns recovered the production of infectious particles in the BE-KO cells, but expression of the HA-Erns mutants carrying only an ectodomain (Ecto) or missing the signal sequence of the core protein (Δsig) did not recover the production, and none of the mutants affected the viral RNA replication at 72-h post-infection (Fig 2B–2D), suggesting that the amphipathic α-helices of Erns can compensate for the roles of apolipoproteins in HCV particle formation. In agreement with the previous report indicating that insertion of proline residues at positions 204 and 210 in the C-terminus impaired the amphipathic α-helical characteristics of Erns [13], transfection of an in vitro-transcribed mutant CSFV RNA with insertion of these proline residues in Erns (204P/210P) did not result in production of infectious CSFV in the porcine kidney-derived cell line, SK6 (S5 Fig). Next, to examine the roles of amphipathic α-helices of Erns in the infectious particle formation of HCV, the BE-KO cells expressing either the C-terminal α-helices (Hel) of CSFV HA-Erns or those carrying the insertion of the proline residues (204P/210P) were infected with HCV, and intracellular HCV RNA and infectious titers in the supernatants were determined at 72-h post-infection (Fig 2E–2G). Infectious titers of HCV in the BE-KO cells expressing the 204P/210P mutant were significantly lower than those in the BE-KO cells expressing either the C-terminal α-helices of HA-Erns or ApoE, while intracellular HCV RNA replication was comparable among these cells. These results strongly suggest that the amphipathic α-helices of Erns are crucial in allowing Erns to compensate for the role of apolipoproteins in the infectious particle formation of HCV in BE-KO cells.
Fig 2. The C-terminal amphipathic α-helix in Erns is important to compensation for the role of apolipoproteins in the infectious particle formation of HCV.
(A) The cDNA constructs for expression of HA-tagged Erns (HA-Erns) mutants; the full length of Erns with the signal sequence of the C-terminal 30 amino acids of core protein (Erns), and without the signal sequence (Δsig), the ectodomain of Erns with the signal sequence (Ecto), and the C-terminal amphipathic α-helix of Erns (Hel). (B) Expression of ApoE and the HA-Erns mutants was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. (E) The insertion of two proline residues (204P/210P) in the Hel mutant containing the C-terminal amphipathic α-helix of HA-Erns of CSFV was generated to examine the significance of the membrane-binding ability in the formation of HCV particles. Expression of Hel, Hel (204P/210P), and ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (F) and extracellular infectious titers (G) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
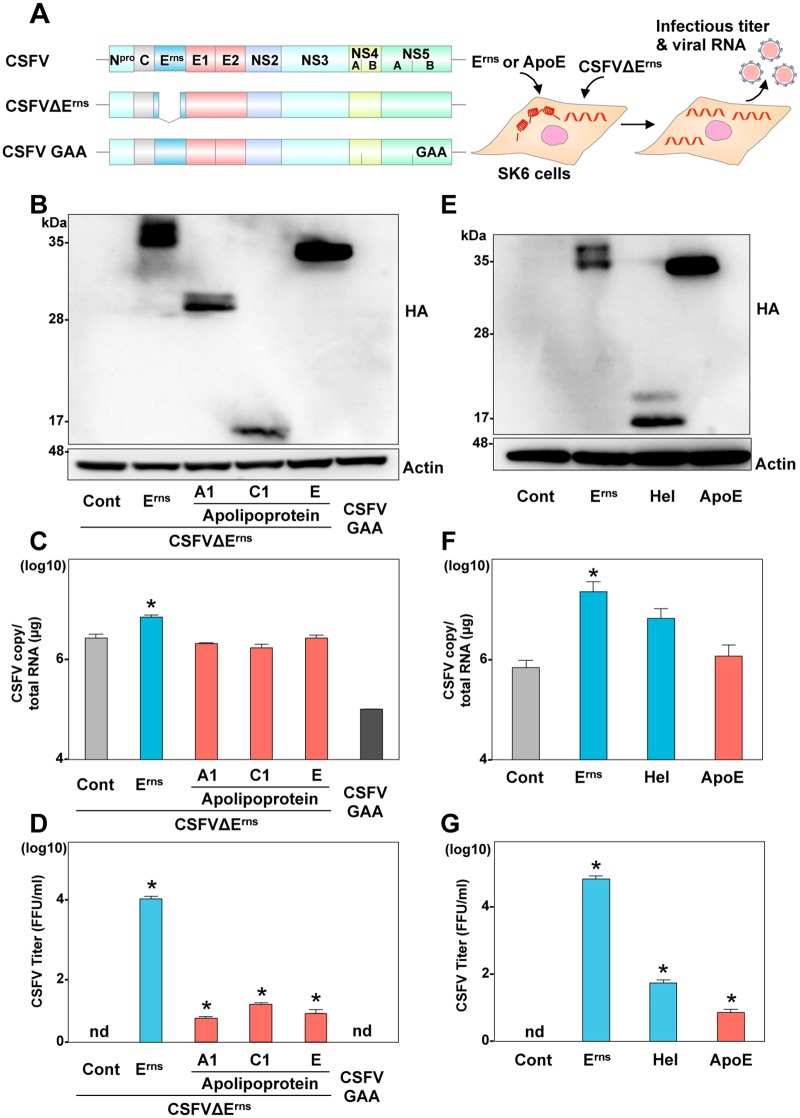
Apolipoproteins can partially compensate for the role of Erns in the formation of infectious particles of pestivirus
It has been well documented that trans-complementation of Erns can function in propagation of CSFV carrying a deletion of Erns in SK6 cells [14]. In the present study, therefore, we first confirmed that exogenous expression of secretory HA-Erns rescued the formation of infectious particles upon electroporation of the Erns-deleted CSFV (CSFVΔErns) RNA (Fig 3A) in SK6 cells (S6 Fig). To examine whether exchangeable apolipoproteins can compensate for the role of Erns in the production of infectious pestiviral particles, the CSFVΔErns RNA was electroporated into SK6 cells expressing either exchangeable apolipoproteins or HA-Erns, and intracellular viral RNA and infectious titers in the supernatants were determined (Fig 3A–3D). Surprisingly, the HA-tagged apolipoproteins, ApoA1, ApoC1, and ApoE, could rescue infectious particle formation of the incapable mutant CSFVΔErns. Next, to examine the role of amphipathic α-helices in the formation of pestivirus particles, either a full-length or a mutant lacking the ectodomain of HA-Erns (Hel) or HA-ApoE was expressed in SK6 cells, and intracellular viral RNA and infectious titers in the supernatants were determined at 72-h post-electroporation with the CSFVΔErns RNA (Fig 3E–3G). Although infectious particle formation in the supernatants of cells expressing HA-tagged either the C-terminal α-helices of Erns or ApoE was lower than that in the cells expressing the HA-tagged full-length Erns, substantial infectivity was recovered, suggesting that exchangeable apolipoproteins have a common role with the C-terminal amphipathic α-helices of Erns in the infectious particle formation of pestiviruses.
Fig 3. Exchangeable apolipoproteins can compensate for the role of Erns in the infectious particle formation of pestivirus.
(A) Schematics of the wild type, Erns deletion (CSFVΔErns), and polymerase dead (GAA) CSFV RNA, and the experimental procedure. (B) Expression of the full length of HA-tagged Erns (HA-Erns), HA-ApoA1, HA-ApoC1, and HA-ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. Intracellular CSFV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-electroporation with CSFVΔErns by qRT-PCR and focus-forming assay, respectively. (E) Expression of HA-Erns, Hel, and HA-ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. Intracellular CSFV RNA (F) and extracellular infectious titers (G) were determined at 72-h post-electroporation with CSFVΔErns by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
NS1 also has common function with apolipoproteins and Erns in infectious viral particle formation
Recently, it was shown that the secretory glycoprotein of flavivirus NS1 has dual functions in viral replication and infectious particle formation [7, 8]. Therefore, we considered that NS1 might also be capable of compensating for the function of exchangeable apolipoproteins and Erns in the particle formation of hepaciviruses and pestiviruses, respectively. Exogenous expression of the HA-tagged NS1 (HA-NS1) derived from DENV serotypes 1 to 4 rescued the infectious particle formation of HCV in the culture supernatants of the BE-KO cells at 72-h post-infection with HCV, while viral RNA replication was comparable among these cells (Fig 4A–4D). To analyze the role of NS1 in more detail, effects of exogenous expression of HA-NS1 derived from DENV, JEV, TBEV, and Yokose virus in the BE-KO cells on the infectious particle formation of HCV were determined. The formation of infectious particles of JFH1 virus (Fig 4E–4G) as well as Con1/JFH1 chimeric virus (S7 Fig) in the BE-KO cells was enhanced by the expression of HA-NS1 derived from these viruses, while the efficiencies of viral RNA replication were comparable. As seen in Erns expression, exogenous expression of HA-NS1 derived from DENV and JEV also facilitated the formation of infectious HCV particles in the 293T/CLDN-miR122 cells, while intracellular HCV RNA levels were not affected by the expression of HA-NS1 (Fig 4H–4J). In addition, expression of HA-NS1 exhibited no effect on the entry of HCVpp (S8A Fig) or replication of SGR RNA (S8B Fig). These results suggest that NS1 also possesses functions similar to those of exchangeable apolipoproteins in the particle formation of HCV.
Fig 4. Flavivirus NS1 can compensate for the role of apolipoproteins in the infectious particle formation of HCV.
(A) Gene structures of flavivirus and hepacivirus and the experimental procedure. (B) Expression of ApoE and HA-tagged NS1 (HA-NS1) from serotypes 1 to 4 of DENV was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. (E) Expression of ApoE and HA-NS1 from DENV, JEV, TBEV, and Yokose virus was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (F) and extracellular infectious titers (G) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. (H) Expression of ApoE and HA-NS1 from DENV and JEV in 293T/CLDN1/miR-122 cells was determined by immunoblotting analysis. Cells were infected with HCV at an MOI of 10, and intracellular HCV RNA (I) and infectious titers in the supernatants (J) at 72-h post-infection were determined by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
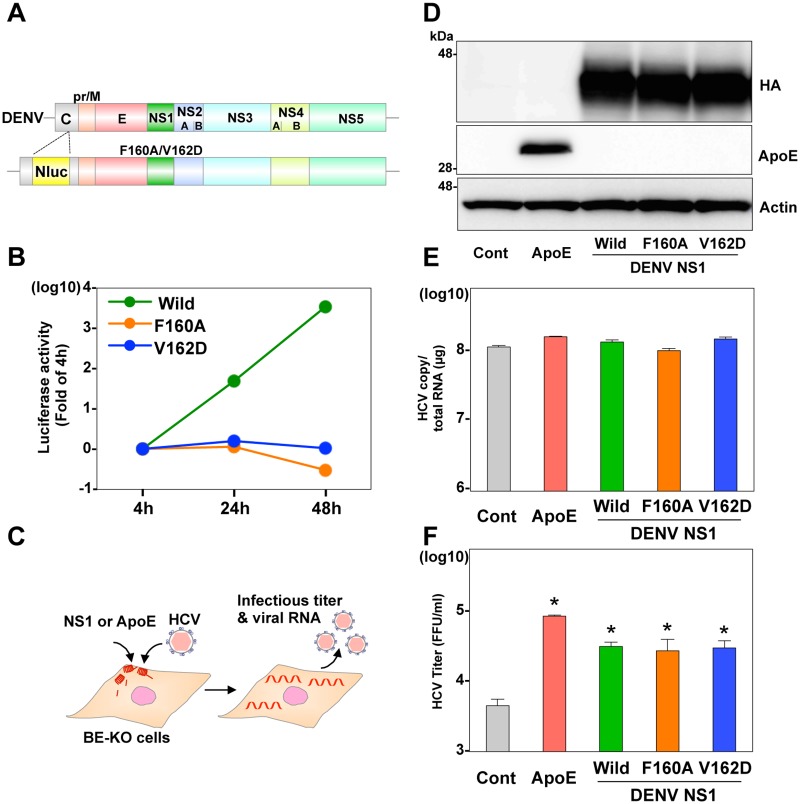
Previous reports have shown that mutations in the hydrophobic protrusion (F160A or V162D) of NS1 impaired viral RNA replication, but had no effect on the remodeling of liposome by incubation with recombinant NS1 [15]. To examine this issue, we first attempted to confirm that introduction of the mutations in the NS1 completely abolished the replication of DENV RNA, in contrast to the efficient replication of the wild type RNA (Fig 5A and 5B). To examine the role of membrane-binding ability of NS1 in the particle formation of HCV, infectious titers in the supernatants of the BE-KO cells exogenously expressing these HA-NS1 mutants were determined upon infection with HCV (Fig 5C–5F). The expression of HA-NS1 irrespective of the introduction of mutations rescued the formation of infectious HCV particles in the BE-KO cells, suggesting that NS1 can compensate for the roles of apolipoproteins in the formation of infectious HCV particles by different mechanisms of replication complex formation.
Fig 5. NS1 mutants in the hydrophobic protrusion deficient in viral replication can compensate for the role of ApoE in the infectious particle formation of HCV.
(A) Gene structure of a recombinant DENV with a luciferase gene and possessing a single amino-acid substitution in NS1 (F160A or V162D). (B) Luciferase activity was determined at 4, 24, and 48-h post-electroporation with the recombinant DENV RNA in BE-KO cells. (C) Experimental procedure. (D) Expression of ApoE and the wild type and mutant HA-tagged NS1 was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (E) and extracellular infectious titers (F) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
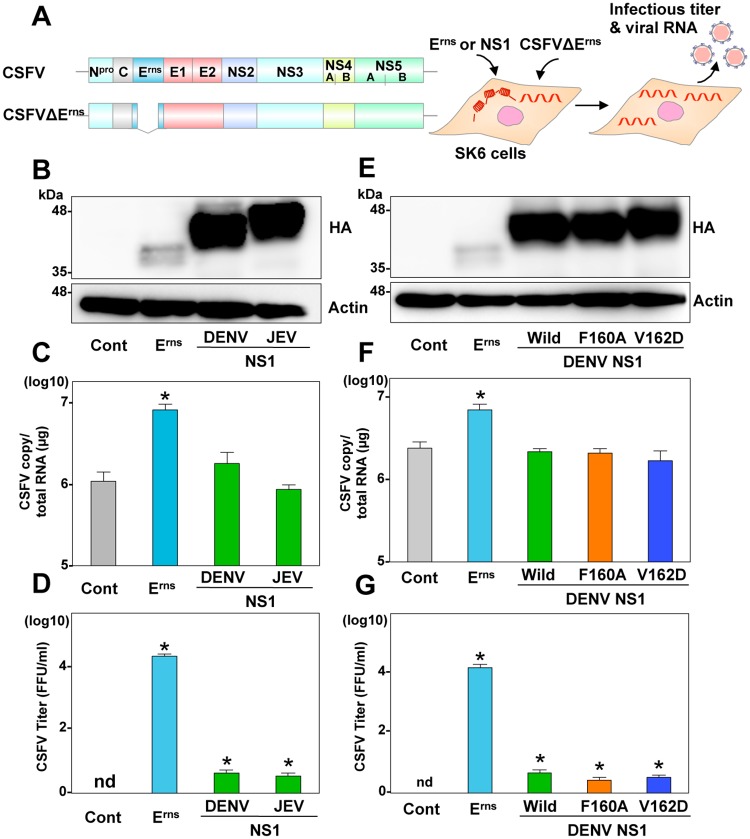
Next, to examine the role of NS1 in the particle formation of pestivirus, NS1 from DENV and JEV was expressed in SK6 cells electroporated with the CSFVΔErms RNA and intracellular viral RNA and infectious particle formations in the supernatants were determined (Fig 6A–6D). The HA-NS1 of DENV and JEV rescued the formation of infectious particles, but quite slightly less than 0.01% of rescue efficiency compared with the homologous Erns. These expressions exhibited no effect on viral RNA replication. In addition, expression of the HA-NS1 mutants (F160A or V162D) of DENV in cells transduced with the CSFVΔErns RNA also partially rescued the particle formation of CSFV (Fig 6E–6G), suggesting that NS1 can substantially but not completely function as the role of Erns in the infectious particle formation of pestiviruses. Taken together, these data suggest that NS1 participates in the infectious particle formation independent from the replication complex formation.
Fig 6. NS1 can compensate for the role of Erns in the infectious particle formation of CSFV.
(A) Schematics of the wild type and Erns deletion (CSFVΔErns) CSFV RNA, and the experimental procedure. (B) Expression of HA-tagged Erns (HA-Erns) and HA-NS1 from DENV and JEV was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. Intracellular CSFV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-electroporation with CSFVΔErns by qRT-PCR and focus-forming assay, respectively. (E) Expression of HA-Erns, and the wild type and mutant (F160A and V162D) HA-NS1 from DENV was determined by immunoblotting. Intracellular CSFV RNA (F) and extracellular infectious titers (G) were determined at 72-h post-electroporation with CSFVΔErns. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
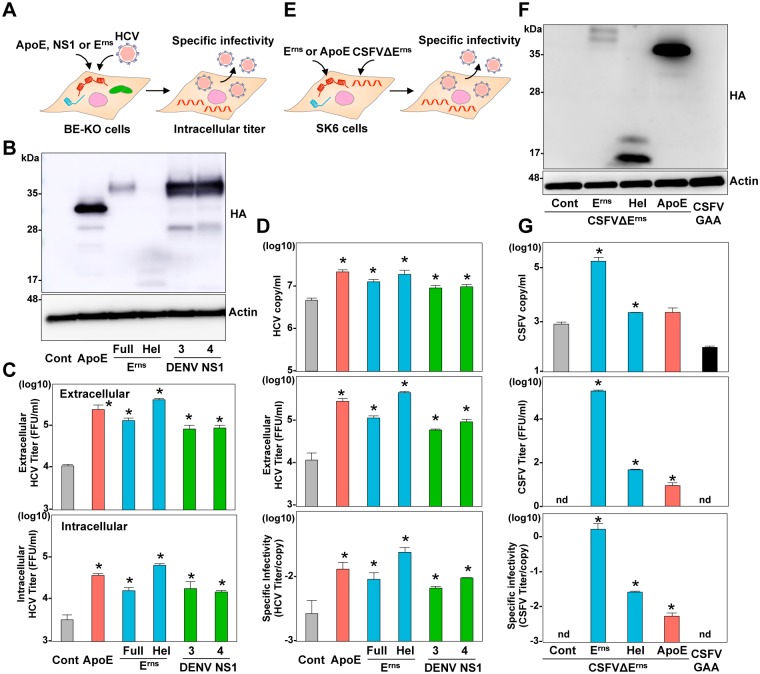
So far, we found that the secretory glycoproteins, ApoE, Erns and NS1, commonly participate in the formation of infectious HCV or pestivirus particles. To examine further detail of the roles of these secretory glycoproteins at the late stage of viral life cycle, intracellular infectivity and specific infectivity (infectious titers/viral RNA levels) in the supernatant were determined in BE-KO cells expressing HA-ApoE, HA-Erns, and Hel derived from CSFV, or HA-NS1 derived from DENV serotypes 3 and 4 at 72-h post-infection with HCV (Fig 7A and 7B). Both intracellular and extracellular infectious titers were enhanced by the expression of the proteins, suggesting that these viral glycoproteins participate in the intracellular particle formation (Fig 7C). In addition, specific infectivity of HCV was also enhanced by the expression of the viral proteins, indicating that these secretory glycoproteins participate in the maturation of infectious HCV particles (Fig 7D). Next to investigate the roles of these secretory glycoproteins in the maturation of pestivirus particles, CSFVΔErns RNA was electroplated into the SK6 cells expressing either HA-Erns, HA-Hel, or HA-ApoE, and specific infectivity in the supernatants was determined at 72-h post electroporation (Fig 7E and 7F). Specific infectivity of the non-infectious CSFVΔErns RNA was rescued by the exogenous expression of either Erns or ApoE, suggesting that the heterogenous secretory glycoproteins can partially but substantially compensate roles of Erns in maturation of the infectious particles of CSFV (Fig 7G).
Fig 7. ApoE, Erns, and NS1 participate in the infectious particle formation.
(A) Experimental procedure. (B) Expression of HA-tagged ApoE (HA-ApoE), the full length of HA-Erns, the C-terminal amphipathic α-helix of HA-Erns (Hel), and HA-NS1 from serotype 3 and 4 of DENV was determined by immunoblotting at 48-h post-transduction of lentiviruses into BE-KO cells. (C) Intracellular and extracellular infectious titers were determined at an MOI of 1 by a focus-forming assay. (D) Specific infectivity was calculated as extracellular infectious titers/extracellular HCV RNA copies in BE-KO cells expressing HA-ApoE, HA-Erns, Hel, and HA-NS1 at 72-h post-infection. (E) Experimental procedure. (F) Expression of the HA-tagged Erns, Hel, and ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. (G) Extracellular CSFV RNA and infectious titers were determined at 72-h post-electroporation with CSFVΔErns RNA by qRT-PCR and focus-forming assay, respectively. Specific infectivity was calculated as extracellular infectious titers/extracellular CSFV RNA copies in SK6 cells. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
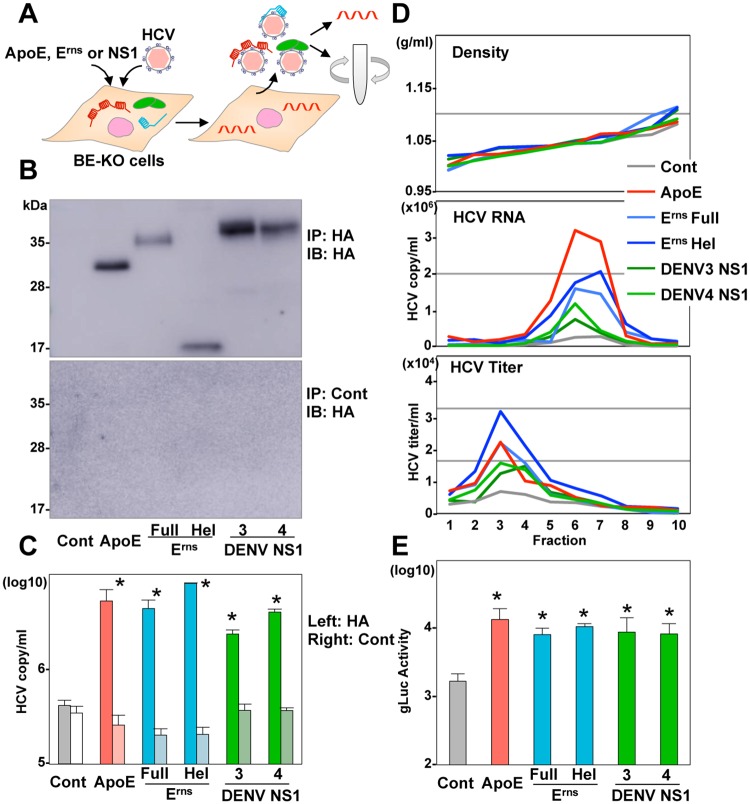
Finally, to characterize the viral particles produced in BE-KO cells expressing either ApoE, Erns, or NS1, association of the glycoproteins with viral particles was determined by immunoprecipitation and qRT-PCR. Large amounts of HCV RNA were recovered in the immunoprecipitates with an anti-HA antibody but not with a control antibody in BE-KO cells expressing HA-ApoE, HA-Erns, and HA-NS1, suggesting that not only ApoE but also Erns and NS1 directly bind to HCV particles (Fig 8A–8C). In addition, buoyant density gradient analysis revealed a similar distribution of viral RNA and infectious titers in the supernatants of BE-KO cells expressing either ApoE, Erns, Hel, or NS1 (Fig 8D). Furthermore, there was no significant difference in the physical properties of the pestivirus particles produced in SK6 cells expressing Erns and ApoE (S9 Fig). Next, to examine the role of the interaction of the glycoproteins with viral particles on the entry process, infectivity of trans-complemented HCV particles (HCVtcp) obtained from BE-KO cells expressing either ApoE, Erns, or NS1 was compared. Interestingly, infectivity of HCVtcp generated in BE-KO cells expressing either Erns or NS1 was comparable to that in those expressing ApoE (Fig 8E). Collectively, these results suggest that Erns and NS1 play similar roles with ApoE in the particle formation of HCV and CSFV.
Fig 8. Characterization of HCV particles produced in BE-KO cells expressing either ApoE, Erns or NS1.
(A) Experimental procedure. (B) HA-tagged complexes were immunoprecipitated from the supernatants of BE-KO cells expressing either HA-ApoE, HA-Erns, Hel, or HA-NS1 by using a control anti-IgG or anti-HA antibody. Expression of HA-ApoE, HA-Erns, Hel, or HA-NS1 in the immunoprecipitated samples was determined by immunoblotting. (C) The HCV RNA associated with ApoE, Erns, and NS1 was determined by qRT-PCR. (D) The supernatants of BE-KO cells expressing either HA-ApoE, HA-Erns, Hel, or HA-NS1 upon infection with HCV at an MOI of 1 were subjected to density gradient fractionation, and density (upper), HCV RNA levels (middle) and infectious titers (bottom) for each fraction were determined by qRT-PCR and focus forming assay, respectively. (E) Transcomplemented HCV particles (HCVtcp) were produced in BE-KO cells expressing either HA-ApoE, HA-Erns, HA-Hel or HA-NS1 at 72-h post-transfection with pCAGC-NS2/JFH1am and PHH SGR-JFH1/Gluc/NS3m. HCVtcp were inoculated into Huh7.5.1 cells, and gaussia luciferase (gLuc) activities were determined at 72-h post-infection. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
Discussion
In this study, we investigated the common roles of virus- and host-derived secretory membrane-binding proteins in the viral particle formation of viruses of the family Flaviviridae (S10 Fig). The viral secretory glycoproteins Erns and NS1 play important roles in the formation of infectious particles of pestiviruses and flaviviruses, respectively. In contrast, HCV utilizes host factors including exchangeable apolipoproteins [10]. The present data suggest that viral proteins were converged to host factors in the evolutional process of Flaviviridae viruses, and utilization of host factors in the viral life cycle is closely associated with the tissue- and species-specificities among Flaviviridae viruses.
Erns and NS1 are unique in pestiviruses and flaviviruses, respectively, and are enigmatic in Flaviviridae viruses in general, since their function has not been fully elucidated compared to other viral proteins such as core, envelope, protease, and polymerase [5]. Erns contains an amphipathic helix domain of 61 residues for membrane binding instead of a transmembrane [12]. Although the current study revealed that the C-terminal helix domain of Erns plays a crucial role in the infectious particle formation, the helix domains from exchangeable apolipoproteins and CAMP have been shown to play similar roles in particle formation [10, 11]. The effects of exogenous expression of the C-terminal α-helices of Erns on HCV and CSFV particle formation were comparable to those of ApoE; however, the absence of an ectodomain of Erns suppressed the efficiency of infectious particle formation of the pestivirus (Fig 3G), suggesting that the amphipathic helices of Erns possess a function in common with apolipoproteins but the ectodomain plays a different role in the life cycle of pestivirus. Initially, NS1 was considered to participate in assembly and release of flavivirus particles due to its localization in the ER lumen and secretion profile [16]. However, the introduction of reverse genetics into flavivirus research revealed that NS1 plays a crucial role in formation of the replication complex through its interaction with NS4A and NS4B in the ER lumen [17, 18]. A recent study on DENV showed that several mutations in NS1 impaired the production of infectious particles but had no effect on viral RNA replication [7]. In addition, transposon mutagenesis of the Zika virus genome exhibited the requirement of NS1 at the late stage of viral life cycle [8]. On the other hand, Akey et al. showed that NS1 mutants that are unable to generate replication complexes are nonetheless capable of remodeling the membranes of liposomes [15]. In this study, we showed that NS1 participates in the particle formation of HCV and CSFV, but not in the replication of viral RNA. Collectively, these data suggest that NS1 plays dual roles in the formation of replication complex and infectious particles through different mechanisms. Secretory glycoproteins were interacted with HCV particle and enhanced the infectious particle production, suggesting that viral NS1, Erns and host-derived secretory glycoproteins commonly have a function in, at least, virus particle maturation leading to support release and stability of the virus.
Flaviviruses and pestiviruses have a broad tissue range of infection, while hepaciviruses exhibit strong hepatic tropism [2–4]. Several host factors other than exchangeable apolipoproteins are also involved in the liver tropism of HCV infection [4, 19]. CLDN1 and miR-122, which participate in entry and replication of the viral genome, respectively, are mainly expressed in the liver. Considering the abundant expression of the exchangeable apolipoproteins in the liver, HCV might have evolved to utilize the apolipoproteins in the formation of infectious particles through deletion of the viral genome that encodes secretory glycoproteins, leading to acquire liver tropism as well as to utilize miR-122 and CLDN1. In contrast to hepaciviruses, pegiviruses exhibit PBMC tropism [20]. Recently, we reported that CAMP, which is highly expressed in bone marrow and in many cell types related to the immune system, plays a role similar to ApoE in the formation of infectious HCV particles [11]. It is feasible that an abundant expression of CAMP participates in the tropism of pegivirus infection and the lack of secretory viral proteins. In addition, pegiviruses have lost almost all their capsid genes in the course of evolution, suggesting that other host or exogenous factors in PBMC compensate for the role of the core protein in the formation of pegivirus particles [21]. Recently, Zhang et al. reported that a capsidless single-stranded RNA virus hijacks capsid proteins from another double-stranded RNA virus for its propagation [22]. Further studies are required to clarify whether endogenous or exogenous factors were utilized for the formation of pegivirus particles.
Previous reports have shown that amphipathic helices of exchangeable apolipoproteins play important roles in the formation of infectious HCV particles through their interaction with the viral membrane, and the association of the apolipoproteins with HCV particles has been detected in the sera of hepatitis C patients and culture supernatants [10, 23, 24]. The present study clearly indicated that the enhancement of infectious particle formation by the expression of apolipoproteins has similarity to the enhancement of infectious particle formation by Erns. In addition, infectivity of HCVtcp produced in BE-KO cells expressing either Erns or NS1 was comparable with that in those expressing ApoE. Interestingly, both ApoE and Erns bind to viral particles through their interaction with heparan sulfate, which is an entry factor for HCV and CSFV [25, 26]. DENV has also been shown to bind to the cell surface through the interaction of NS1 with heparan sulfate and to utilize laminin receptor for an efficient entry, as seen in the entry of pestiviruses through the interaction of Erns and the receptor [27–29]. Considering the interaction of these glycoproteins with surface proteins of plasma membrane, NS1 and Erns play a similar role with apolipoproteins in the entry of HCV. Recent reports showed that ApoE associated with viral particles enhances infectivity of HCV [30, 31], therefore, non-cell derived exchangeable apolipoproteins in the culture medium might participate in the enhancement of infectivity of HCV through the interaction with viral particles. In contrast, expression of ApoE and NS1 has shown to compensate the particle formation of pestivirus in trans, however, the efficiency of recovery was significantly low compared with that of Erns. The amphipathic helices of Erns retain a common function in particle formation with apolipoproteins but Erns might be evolved to optimize the structural element for an efficient replication of pestivirus.
In conclusion, we have shown that the membrane-binding glycoproteins, including NS1, Erns, and exchangeable apolipoproteins, play common roles in the life cycle of viruses in Flaviviridae. This may help to reveal new mechanisms in the tissue tropism and evolution of Flaviviridae viruses. However, further studies are needed to elucidate the biological significance of the enigmatic viral proteins Erns and NS1 in the viral life cycle.
Materials and methods
Plasmids
The cDNA clones of ApoE, pestiviral Erns from BVDV, CSFV, and BDV, flaviviral NS1 from JEV, DENV, TBEV, and Yokose virus, and AcGFP were inserted between the XhoI and XbaI sites of the lentiviral vector pCSII-EF-RfA by using Infusion technique, and the resulting plasmids were designated as pCSII-EF-ApoE, pCSII-EF-Erns, pCSII-EF-NS1, and pCSII-EF-GFP, respectively. The mutant cDNAs of Erns and NS1 were amplified by PCR and inserted into pCSII-EF. The plasmid pHH-JFH1 encodes a full-length cDNA of the JFH1 strain (GenBank accession number: AB047639) [32]. pHH-JFH1-E2p7NS2mt contains three adaptive mutations in pHH-JFH1, and pSGR-JFH1 encodes subgenomic cDNA of the JFH1 strain. The CSFV was derived from the full-length cDNA clone of the GPE− strain (GenBank accession number: D49533) [33]. The plasmids encoding CSFVΔErns, the 204P and 210P mutants of Erns and the polymerase dead (GAA) mutant of CSFV were constructed by using a KOD-Plus-Mutagenesis Kit (Toyobo) and the respective oligonucleotide primers. The plasmid pMW119-DV4 Nluc sec encodes a full-length infectious clone of the DENV serotype 4 H241 strain (GenBank accession number: AY947539) containing a NanoLuc-luciferase gene in-frame insertion into the viral C gene as described previously [34]. The cDNA clones encoding the viral sequence for transfection were flanked by a modified T7 promoter sequence at the 5′ end and NotI restriction site at the 3′ end. The plasmid pX330 encoding hCas9 and sgRNA was obtained from Addgene (Addgene plasmid 42230). The fragments of guided RNA targeting the ApoB and ApoE gene were inserted into the Bbs1 site of pX330 and designated pX330-ApoB and pX330-ApoE, respectively. The plasmids used in this study were confirmed by sequencing with an ABI 3130 genetic analyser (Life Technologies).
Cell lines
All cell lines were cultured at 37°C under the conditions of a humidified atmosphere and 5% CO2. The human hepatocellular carcinoma-derived Huh7, human embryonic kidney-derived 293T cells, and African green monkey kidney-derived VeroE6 cells were maintained in DMEM (Nakarai) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS). Huh7 cells were provided by the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan. 293T and Vero cells were obtained from the American Type Culture Collection. The Huh7-derived cell line Huh7.5.1 was kindly provided by F. Chisari. The porcine kidney-derived SK6 cells [35] were propagated in DMEM supplemented with 100U/ml penicillin, 100 μg/ml streptomycin, 5% BVDV antibody free FBS (Japan Bio Serum) and 5% horse serum (Life Technologies). Huh7 cells were transfected with pX330-ApoE and pX330-ApoB by Trans IT LT-1 (Mirus), and single cell clones were established. To screen for gene-knockout Huh7 cell clones, mutations in the target loci were determined by using a Surveyor assay (Transgenomic) according to the manufacturer’s protocol. Frameshift of the genes and deficiencies of protein expressions were confirmed by direct sequencing and immunoblotting analysis, respectively.
Antibodies
Mouse monoclonal antibodies to β-actin, dsRNA, and ApoE were purchased from Sigma, English & Scientific Consulting Kft, and Santa Cruz, respectively. Rabbit anti-CLDN1 and Alexa Flour (AF) 488-conjugated anti-rabbit IgG antibodies were purchased from Life Technologies. Rat anti-HA antibody was purchased from Roche Diagnostics. Rabbit anti-HCV NS5A antibody and mouse monoclonal antibody 46/1 against pestiviral NS3 were generated as described previously [36, 37].
In vitro transcription, RNA transfection, and colony formation
The plasmid pSGR-JFH1 digested with XbaI and blunted with mung bean exonuclease was transcribed in vitro by using a MEGAscript T7 kit (Life Technologies) according to the manufacturer's protocol. Capped and polyadenylated firefly luciferase (Fluc) RNAs were synthesized by using an mMESSAGE mMACHINE T7 Ultra kit (Life Technologies). The in vitro transcribed RNA (5 μg) was electroporated into cells at 5×106 cells/0.4 ml under conditions of 190 V and 950 μF using a Gene Pulser (BioRad) and plated on DMEM containing 10% FBS. The medium was replaced with fresh DMEM containing 10% FBS and 1 mg/ml G418 at 24-h post-transfection of transcribed RNA. The remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1-month post-electroporation.
Preparation of viruses
pHH-JFH1-E2p7NS2mt was introduced into Huh7.5.1 cells, HCVcc in the supernatant was collected after serial passages, and infectious titers were determined by a focus-forming assay and expressed in focus-forming units (FFU). All of the cDNA-derived CSFV viruses were rescued as described previously [33]. The supernatants were collected from the electroplated cells and infectious titers were determined by a focus-forming assay. The infectious DENV clones linearized with NotI were transcribed by using an mMESSAGE mMACHINE T7 Ultra Kit, and the in vitro transcribed RNA was electroporated as described above. The supernatants were collected from the electroplated cells and infectious titers were determined by a focus-forming assay. HCVpp bearing E1 and E2 glycoproteins of JFH1 were produced as described previously [38]. HCVtcp were generated as described previously [39].
Immunoblotting
Cells lysed on ice in lysis buffer (20 mM Tris-HCl [pH7.4], 135 mM NaCl, 1% Triton-X 100, 10% glycerol) supplemented with a protease inhibitor mix (Roche) were boiled in loading buffer and subjected to 5–20% gradient SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes (Millipore) and incubated with the appropriate antibodies. The immune complexes were visualized with SuperSignal West Femto substrate (Thermo Scientific) and detected by use of an LAS-4000 image analyser (Fujifilm).
Quantitative RT-PCR
For quantification of viral RNA copies, total RNA was extracted from cells by using a PureLink RNA Mini Kit (Life Technologies), and then first-strand cDNA synthesis and qRT-PCR were performed by using a TaqMan RNA-to-CT 1-step Kit and ViiA7 system (Life Technologies), respectively, according to the manufacturer’s protocols. HCV RNA was quantified by using primers for TaqMan PCR targeted to the noncoding region of HCV as described previously [36]. For quantification of CSFV RNA, the primer set reported by Hoffmann et al. was employed [40]. Fluorescent signals were determined by the ViiA7 system.
Immunoprecipitation
Culture supernatants of viral infected cells were incubated with anti-HA antibody beads or control IgG beads at 4°C for 2 h. The Sepharose beads were washed three times in PBS and RNAs were extracted using the Qiazol reagent (Qiagen). HCV-RNA associated with HA-tagged protein was detected by qRT-PCR as described as above.
Buoyant density fractionation
Culture supernatants of viral infected cell were concentrated by using Spin-X UF concentrators (Corning), applied to the top of a linear gradient formed from 10 to 40% Optiprep (Axis-Shield) in PBS, and spun at 36,000 rpm for 16-h at 4°C by using an SW41 rotor (Beckman Coulter). Aliquots of 10 or 12 consecutive fractions were collected from top to bottom, and the density, infectious titer, and viral RNA level were determined for each fraction.
Statistical analysis
The data for statistical analyses are the averages of two independent experiments. Results were expressed as the means ±standard deviations or standard errors. The significance of differences in the means was determined by Student's t-test.
Supporting information
(A) Experimental procedure. (B) Expression of CLDN1 and ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into BE-KO and 293T cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 (BE-KO cells) and 10 (293T cells) by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) Experimental procedure. Intracellular HCV RNA (B) and extracellular infectious titers (C) in BE-KO cells at 48-h post-transduction with lentiviruses were determined at 72-h post-infection with Con1/JFH1 chimeric HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) BE-KO cells expressing either ApoE or HA-tagged Erns (HA-Erns) were inoculated with pseudotype particles bearing HCV envelope proteins E1 and E2 (HCVpp) (left) or VSV-G protein (VSVpp) (right), and luciferase activity was determined at 48-h post-infection. (B) Subgenomic HCV replicon RNA of the JFH1 strain was electroporated into the BE-KO cells with/without expression of ApoE or HA-Erns by the lentiviral vectors, and the remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1-month post-electroporation after selection with 1 mg/ml of G418.
(TIF)
(A) Experimental procedure. (B) Expression of ApoE, HA-tagged NS1 (HA-NS1) from JEV and DENV, and HA-Erns from BVDV was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively.
(TIF)
(A) Schematics of CSFV of the wild type and mutants possessing insertion of proline (204P/210P) in Erns and replacement in polymerase dead (GAA), and the experimental procedure. (B) Intracellular CSFV RNA and extracellular infectious titers (C) were determined at 72-h post-electroporation with CSFV RNA by qRT-PCR and focus-forming assay, respectively.
(TIF)
(A) Schematics of the wild type and Erns deletion (CSFVΔErns) RNA, and the experimental procedure. (B) Expression of HA-tagged Erns (HA-Erns) with or without the signal sequence of the core protein (Erns or Δsig Erns) was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. (C) Extracellular infectious titer in cells expressing either HA-Erns or HA-Δsig Erns was determined at 72-h post-electroporation with CSFVΔErns by focus-forming assay.
(TIF)
(A) Gene structures of the flavivirus and hepacivirus and the experimental procedure. Intracellular HCV RNA (B) and extracellular infectious titers (C) were determined at 72-h post-infection with Con1/JFH1 chimeric HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) BE-KO cells expressing either ApoE or HA-tagged NS1 (HA-NS1) were inoculated with pseudotype particles bearing HCV envelope proteins E1 and E2 (HCVpp) (left) or VSV-G protein (VSVpp) (right), and luciferase activity was determined at 48-h post-infection. (B) Subgenomic HCV RNA replicon of the JFH1 strain was electroporated into the BE-KO cells with/without expression of ApoE or HA-NS1 by the lentiviral vectors, and the remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1-month post-electroporation after selection with 1 mg/ml of G418.
(TIF)
(A) Experimental procedure. (B) The SK6 cells expressing either HA-tagged Erns (HA-Erns, middle) or HA-ApoE (bottom) were electroporated with pestivirus RNA. At 72-h post-electroporation, the culture supernatants were subjected to density gradient fractionation, and viral RNA copies (Left panels) and infectious titers (Right panels) for each fraction were determined.
(TIF)
(TIF)
Acknowledgments
We are grateful to the Kenpoku Livestock Hygiene Service Center (Ibaraki, Japan) for viral genome of BDV. We thank M. Tomiyama and J Higuchi for her secretarial work and M. Ishibashi, M. Nagao, and Y. Sugiyama for their technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Science, Sports and Technology (MEXT http://www.mext.go.jp/) of Japan (16H06432, 16K21723, 16H06429, 15H04736, and 15K19110), by Health Sciences Research Grants (Research on Hepatitis, 16fk0210106h0001) from the Ministry of Health, Labor and Welfare of Japan and AMED (Japan Agency for Medical Research and Development http://www.amed.go.jp/), by JSPS KAKENHI (https://www.jsps.go.jp/english/e-grants/)Grant Number JP16J02628. TT is supported by JSPS Research Fellowships for young scientists (https://www.jsps.go.jp/english/e-grants/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lindenbach BD, Thiel H, Rice CM (2013) Flaviviridae Fields Virology, 6th Edition Knipe D M and Howley P M, Eds Lippincott-Raven Publishers, Philadelphia: 712–794. [Google Scholar]
- 2.Chanbers TJ, Hahn CS, Galler R, Rice CM (1990) Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44: 649–688. doi: 10.1146/annurev.mi.44.100190.003245 [DOI] [PubMed] [Google Scholar]
- 3.Schweizer M, Peterhans E (2014) Pestiviruses. Annu Rev Anim Biosci 2: 141–163. doi: 10.1146/annurev-animal-022513-114209 [DOI] [PubMed] [Google Scholar]
- 4.Fukuhara T, Matsuura Y (2013) Roles of miR-122 and lipid metabolism in HCV infection. J Gastroenterol 48(2): 169–176. doi: 10.1007/s00535-012-0661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tautz N, Tews BA, Meyers G (2015) The molecular biology of pestiviruses. Adv Virus Res 93: 47–160. doi: 10.1016/bs.aivir.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Rastogi M, Sharma N, Singh SK (2016) Flavivirus NS1: a multifaced enigmatic viral protein. Virol J 13: 131 doi: 10.1186/s12985-016-0590-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaturro P, Cortese M, Chatel-Chaix L, Fischl W, Bartenschlager R (2015) Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog 11: e1005277 doi: 10.1371/journal.ppat.1005277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulton BO, Sachs D, Schwarz MC, Palese P, Evans MJ (2017) Transposon mutagenesis of the Zika virus genome highlights regions essential for RNA replication and restricted for immune evasion. J Virol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Luo G (2009) Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol 83(24): 12680–12691. doi: 10.1128/JVI.01476-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuhara T, Wada M, Nakamura S, Ono C, Shiokawa M, Yamamoto S, et al. (2014) Amphipathic α-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog 10: e1004534 doi: 10.1371/journal.ppat.1004534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puig-Basagoiti F, Fukuhara T, Tamura T, Ono C, Uemura K, Kawachi Y, et al. (2016) Human cathelicidin for the role of apolipoproteins in the infectious particle formation of HCV. J Virol 90(19): 8464–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberle D, Muhle-Goll C, Burck J, Wolf M, Reiber S, Luy B, et al. (2014) Structure of the membrane anchor of pestivirus glycoprotein Erns, a long tilted amphipathic helix. PLoS Pathog 10: e1003973 doi: 10.1371/journal.ppat.1003973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle D, Oetter KM, Meyers G (2015) Lipid binding of the amphipathic helix serving as membrane anchor of pestivirus glycoprotein Erns. PLoS ONE 10: e0135680 doi: 10.1371/journal.pone.0135680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey CF, Bauhofer O, Ruggli N, Summerfield A, Hofmann MA, Tratschin JD (2006) Classical swine fever virus replicon particles lacking the Erns gene: a potential marker vaccine for intradermal application. Vet Res 37(5): 655–670. doi: 10.1051/vetres:2006028 [DOI] [PubMed] [Google Scholar]
- 15.Akey DL, Brown WC, Dutta S, Konwerski J, Jose J, Jurkiw TJ, et al. (2014) Flavivirus NS1 structures reveal surface for associations with membranes and the immune system. Science 343(6173): 881–885. doi: 10.1126/science.1247749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JM, Crooks AJ, Stephenson JR (1989) The synthesis and maturation of a non-structural extracellular antigen from tick-borne encephalitis virus and its relationship to the intracellular NS1 protein. J Gen Virol 70(2): 335–343. [DOI] [PubMed] [Google Scholar]
- 17.Varnavski AN, Khromykh AA (1999) Noncytopathic flavivirus replicon RNA-based system for expression and delivery of heterologous genes. Virology 255(2): 366–375. doi: 10.1006/viro.1998.9564 [DOI] [PubMed] [Google Scholar]
- 18.Lindenbach BD, Rice CM (1999) Genetic interaction of flavivirus nonstructural protein NS1 and NS4A as a determinant of replicase function. J Virol 73(6): 4611–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W, Qiu C, Biswas M, Jin J, Watkins SC, Montelaro RC, et al. (2008) Correlation of the tight junction-like distribution of claudin-1 to the cellular tropism of hepatitis C virus. J Biol Chem 283(13): 8643–8653. doi: 10.1074/jbc.M709824200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey AL, Lauck M, Mohns M, Peterson EJ, Beheler K, Brunner KG, et al. (2015) Durable sequence stability and bone marrow tropism in a macaque model of human pegivirus infection. Sci Transl Med 7(305): 305ra144 doi: 10.1126/scitranslmed.aab3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapleton JT, Foung S, Muefhoff AS, Bukh J, Simmonds P (2011) The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus pegivirus within the family flaviviridae. J Gen Virol 92(2): 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Hisano S, Tani A, Kondo H, Kanematsu S, Suzuki N. (2016) A capsidless ssRNA virus hosted by an unrelated dsRNA virus. Nat Microbiol 1: 1–6. [DOI] [PubMed] [Google Scholar]
- 23.Fukuhara T, Ono C, Puig-Basagoiti F, Matsuura Y (2015) Roles of lipoproteins and apolipoproteins in particle formation of hepatitis C virus. Trend Microbiol 23(10): 618–629. [DOI] [PubMed] [Google Scholar]
- 24.Hueging K, Weller R, Doepke M, Vieyres G, Todt D, Wolk B, et al. (2015) Several human liver cell expressed apolipoproteins complement HCV virus production with varying efficacy conferring differential specific infectivity to released viruses. PLoS ONE 10: e0134529 doi: 10.1371/journal.pone.0134529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulst MM, Gennip HGP, Moormann RJM (2000) Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein Erns. J Virol 74(20): 9553–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. (2012) Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol 86(13): 7256–7267. doi: 10.1128/JVI.07222-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinerk W, et al. (2007) Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog 3: e183 doi: 10.1371/journal.ppat.0030183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, He WR, Shen L, Dong H, Yu J, Wang X, et al. (2015) The laminin receptor is a cellular attachment receptor for classical swine fever virus. J Virol 89(9): 4894–4906. doi: 10.1128/JVI.00019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tio PH, Jong WW, Cardosa MJ (2005) Two dimensional VOPBA reveals laminin receptor (LAMR1) interaction with dengue virus serotypes 1, 2 and 3. Virol J 2: 25 doi: 10.1186/1743-422X-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankwitz D, Doepke M, Hueging K, Weller R, Bruening J, Behrendt P, et al. (2017) Maturation of secreted HCV particles by incorporation of secreted apoE protects from antibodies by enhancing infectivity. J Hepatol in press. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Li Y, Bi Y, Zhang H, Yao Y, Li Q, et al. (2017) Extracellular interactions between hepatitis C virus secreted apolipoprotein E. J Virol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masaki T, Suzuki R, Saeed M, Mori K, Matsuda M, Aizaki H, et al. (2010) Production of infectious hepatitis C virus by using RNA polymerase I-mediated transcription. J Virol 84(11): 5824–5835. doi: 10.1128/JVI.02397-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura T, Sakoda Y, Yoshino F, Nomura T, Yamamoto N, Sato Y, et al. (2012) Selection of classical swine fever virus with enhanced pathogenecity reveals synergistic virulence determinants in E2 and NS4B. J Virol 86(16): 8602–8613. doi: 10.1128/JVI.00551-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou G, Xu HY, Qing M, Wang QY, Shi PY (2011) Development and characterization of a stable luciferase dengue virus for high-throughput screening. Antiviral Res 91(1): 11–19. doi: 10.1016/j.antiviral.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 35.Kasza L, Shadduck JA, Christofinis GJ (1972) Establishment, viral susceptibility and biological characteristics of a swine kidney cell line SK-6. Res Vet Sci 13(1): 46–51. [PubMed] [Google Scholar]
- 36.Fukuhara T, Kambara H, Shiokawa M, Ono C, Katoh H, Morita E, et al. (2012) Expression of microRNA miR-122 facilitates an efficient replication in nonhepatic cells upon infection with hepatitis C virus. J Virol 86(15): 7918–7933. doi: 10.1128/JVI.00567-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameyama K, Sakoda Y, Tamai K, Igarashi H, Tajima M, Mochizuki T, et al. (2006) Development of an immunochromatographic test kit for rapid detection of bovine viral diarrhea virus antigen. J Virol Methods 138(1–2): 140–146. doi: 10.1016/j.jviromet.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 38.Bartosch B, Dubuisson J, Cosset FL (2003) Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med 197(5): 633–642. doi: 10.1084/jem.20021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki R, Saito K, Kato T, Shirakura M, Akazawa D, Ishii K, et al. (2012) Trans-complemented hepatitis C virus particles as a versatile tool for study of virus assembly and infection. Virology 432: 29–38. doi: 10.1016/j.virol.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann B, Beer M, Schelp S, Schirrmeier H, Depner K (2005) Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J Virol Methods 130(1–2): 36–44. doi: 10.1016/j.jviromet.2005.05.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Experimental procedure. (B) Expression of CLDN1 and ApoE was determined by immunoblotting at 48-h post-transduction of lentiviruses into BE-KO and 293T cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 (BE-KO cells) and 10 (293T cells) by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) Experimental procedure. Intracellular HCV RNA (B) and extracellular infectious titers (C) in BE-KO cells at 48-h post-transduction with lentiviruses were determined at 72-h post-infection with Con1/JFH1 chimeric HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) BE-KO cells expressing either ApoE or HA-tagged Erns (HA-Erns) were inoculated with pseudotype particles bearing HCV envelope proteins E1 and E2 (HCVpp) (left) or VSV-G protein (VSVpp) (right), and luciferase activity was determined at 48-h post-infection. (B) Subgenomic HCV replicon RNA of the JFH1 strain was electroporated into the BE-KO cells with/without expression of ApoE or HA-Erns by the lentiviral vectors, and the remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1-month post-electroporation after selection with 1 mg/ml of G418.
(TIF)
(A) Experimental procedure. (B) Expression of ApoE, HA-tagged NS1 (HA-NS1) from JEV and DENV, and HA-Erns from BVDV was determined by immunoblotting at 48-h post-transduction of lentiviruses into the BE-KO cells. Intracellular HCV RNA (C) and extracellular infectious titers (D) were determined at 72-h post-infection with JFH1 HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively.
(TIF)
(A) Schematics of CSFV of the wild type and mutants possessing insertion of proline (204P/210P) in Erns and replacement in polymerase dead (GAA), and the experimental procedure. (B) Intracellular CSFV RNA and extracellular infectious titers (C) were determined at 72-h post-electroporation with CSFV RNA by qRT-PCR and focus-forming assay, respectively.
(TIF)
(A) Schematics of the wild type and Erns deletion (CSFVΔErns) RNA, and the experimental procedure. (B) Expression of HA-tagged Erns (HA-Erns) with or without the signal sequence of the core protein (Erns or Δsig Erns) was determined by immunoblotting at 48-h post-transduction of lentiviruses into SK6 cells. (C) Extracellular infectious titer in cells expressing either HA-Erns or HA-Δsig Erns was determined at 72-h post-electroporation with CSFVΔErns by focus-forming assay.
(TIF)
(A) Gene structures of the flavivirus and hepacivirus and the experimental procedure. Intracellular HCV RNA (B) and extracellular infectious titers (C) were determined at 72-h post-infection with Con1/JFH1 chimeric HCV at an MOI of 1 by qRT-PCR and focus-forming assay, respectively. In all cases, asterisks indicate significant differences (* p < 0.01) versus the results of the control cells.
(TIF)
(A) BE-KO cells expressing either ApoE or HA-tagged NS1 (HA-NS1) were inoculated with pseudotype particles bearing HCV envelope proteins E1 and E2 (HCVpp) (left) or VSV-G protein (VSVpp) (right), and luciferase activity was determined at 48-h post-infection. (B) Subgenomic HCV RNA replicon of the JFH1 strain was electroporated into the BE-KO cells with/without expression of ApoE or HA-NS1 by the lentiviral vectors, and the remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1-month post-electroporation after selection with 1 mg/ml of G418.
(TIF)
(A) Experimental procedure. (B) The SK6 cells expressing either HA-tagged Erns (HA-Erns, middle) or HA-ApoE (bottom) were electroporated with pestivirus RNA. At 72-h post-electroporation, the culture supernatants were subjected to density gradient fractionation, and viral RNA copies (Left panels) and infectious titers (Right panels) for each fraction were determined.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.