Abstract
Background
1,3-Butadiene (BD) is an important carcinogen in tobacco smoke that undergoes metabolic activation to DNA-reactive epoxides. These species can be detoxified via glutathione conjugation and excreted in urine as the corresponding N-acetylcysteine conjugates. We hypothesize that single nucleotide polymorphisms in BD-metabolizing genes may change the balance of BD bioactivation and detoxification in White, Japanese American, and African American smokers, potentially contributing to ethnic differences in lung cancer risk.
Methods
We measured the levels of BD metabolites, 1- and 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene (MHBMA) and N-acetyl-S-(3,4-dihydroxybutyl)-L-cysteine (DHBMA), in urine samples from a total of 1,072 White, Japanese American, and African American smokers and adjusted these values for body mass index, age, batch, and total nicotine equivalents. We also conducted a genome wide association study to identify genetic determinants of BD metabolism.
Results
We found that mean urinary MHBMA concentrations differed significantly by ethnicity (p = 4.0 × 10−25). African Americans excreted the highest levels of MHBMA followed by Whites and Japanese Americans. MHBMA levels were affected by GSTT1 gene copy number (p < 0.0001); conditional on GSTT1, no other polymorphisms showed a significant association. Urinary DHBMA levels also differed between ethnic groups (p = 3.3 ×10−4), but were not affected by GSTT1 copy number (p = 0.226).
Conclusions
GSTT1 gene deletion has a strong effect on urinary MHBMA levels, and therefore BD metabolism, in smokers.
Impact
Our results show that the order of MHBMA levels among ethnic groups is consistent with their respective lung cancer risk and can be partially explained by GSTT1 genotype.
Keywords: 1, 3-Butadiene; race/ethnicity; urinary metabolites; smoking; mass spectrometry
Introduction
Cigarette smoking is a leading cause of lung cancer (1), with 14–28% of male smokers and 13–28% of female smokers above the age of 35 at risk for developing the disease in the United States (1). Smoking is responsible for 87% and 70% of lung cancer deaths in men and in women, respectively (2). However, the risk for the development of lung cancer in smokers varies greatly between ethnic groups, with African American and Native Hawaiian smokers having the highest risk, followed by White, Japanese American, and Latino smokers (3). These ethnic differences remain after adjustment for reported smoking history (3). While the exact origins of the pronounced ethnic differences in smoking-induced lung cancer risk remain to be established, the frequencies of genetic polymorphisms in xenobiotic metabolism genes differ significantly between racial groups, potentially affecting the extent of carcinogen bioactivation to DNA-reactive species (4–11).
The mechanism of smoking-induced lung cancer involves irreversible binding of metabolically activated tobacco carcinogens to DNA, forming covalent DNA adducts which cause mutations in critical genes (12). Cigarette smoke contains 69 known carcinogens, including 1,3-butadiene (BD) (20–75 mg and 205–360 mg per cigarette in mainstream and side stream smoke, respectively) (13,14). BD is a multi-site carcinogen in laboratory rats and mice (15–18). Epidemiological studies have uncovered an association between occupational exposure to BD and the development of leukemia and lymphoma in humans, leading to its classification as a Group 1 agent by IARC and as a known human carcinogen by the National Toxicology Program (19–26).
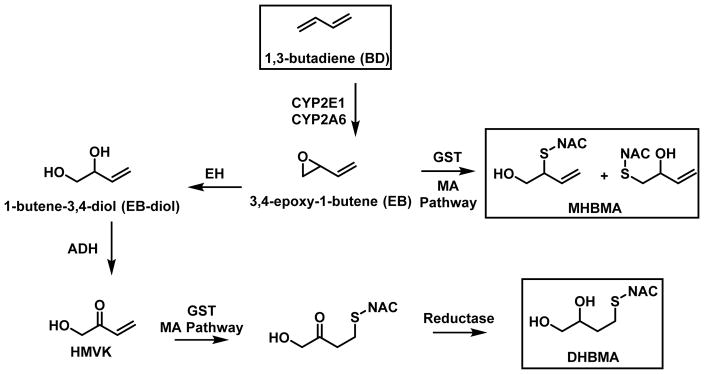
BD is metabolically activated to several DNA reactive species, including 3,4-epoxybut-1-ene (EB), 1,2,3,4-diepoxybutane (DEB), hydroxymethylvinyl ketone (HMVK), and 3,4-epoxy-1,2-butanediol (EBD) (Scheme 1) (27–29). Epoxidation of BD to EB is catalyzed by cytochrome P450 monooxygenases 2E1 and 2A6 (CYP2E1 and 2A6) (30). Epoxide hydrolase (EH)-mediated hydrolysis of EB gives rise to 1-butene-3,4-diol (EB-diol), which is subsequently converted to HMVK by alcohol dehydrogenase (ADH) (29,31). Alternatively, EB can be further epoxidized by CYP2E1 to 1,2,3,4-diepoxybutane (DEB) (28), which in turn can be hydrolyzed to epoxy-1,2-butanediol (EBD) (32,33).
Scheme 1.
Metabolism and Detoxification of BD
Analysis of urinary BD-mercapturic acids can be employed to monitor human exposure to BD and the extent of its bioactivation to electrophilic species. EB, HMVK, EBD, and DEB can be conjugated with glutathione (GSH) and further processed via the mercapturic acids pathway to form 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene and 1-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene) (together referred to as MHBMA, Scheme 1), N-acetyl-S-(3,4-dihydroxybutyl)-L-cysteine (DHBMA), 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane (THBMA) and 1,4-bis-(N-acetyl-L-cystein-S-yl)butane-2,3-diol (bis-BDMA), respectively (Scheme 1) (34–39). It has been proposed that glutathione S-transferases theta 1 and mu 1 (GSTT1 and GSTTM1) can catalyze this reaction (40,41), however, direct biochemical evidence for their involvement was not provided. Urinary concentrations of MHBMA and DHBMA are elevated in smokers as compared to nonsmokers and decrease upon smoking cessation; of the two, MHBMA is more strongly associated with smoking (35,42,43).
Significant interspecies differences in response to butadiene have been observed, with laboratory mice being significantly more sensitive than rats towards BD-induced cancer (16,17). Mice developed lung tumors following BD exposure to as low as 6.25 ppm, while rats developed only minor tumors at BD exposures as high as 1000 ppm (16,17). These differences are thought to be a result of more efficient bioactivation of BD to EB and DEB and less efficient detoxification of BD-epoxides in mice (27,44–46). Similarly, the balance of BD bioactivation and detoxification in a given individual is likely to be dependent upon competing enzymatic reactions mediated by CYP2E1, CYP2A6, EH, ADH, and GST proteins (Scheme 1). Specifically, single nucleotide polymorphisms (SNPs) in genes encoding for butadiene metabolism genes may affect the metabolic pathways of BD in smokers and workers occupationally exposed to BD, potentially modifying lung cancer risk (47–50).
The main goals of the present study were to compare urinary excretion of butadiene metabolites MHBMA and DHBMA in a large cohort of African American, White, and Japanese American smokers and to examine the associations between urinary MHBMA and DHBMA excretion and specific genetic variants via a large scale genome wide association study (GWAS). By identifying variants associated with MHBMA and DHBMA excretion, we can begin to establish the mechanisms by which differences in BD metabolism may modify the risk of smoking-induced lung cancer.
Experimental
Materials
MHBMA, DHBMA, 2H6-MHBMA, and 2H7-DHBMA were purchased from Toronto Research Chemicals (Toronto, Canada). Recombinant human GSTT1 and GSTT2 were purchased from MyBioSource (San Diego, CA). LC/MS grade formic acid was obtained from Sigma Aldrich (St. Louis, MO), and LC/MS grade water and acetonitrile were acquired from Fisher Scientific (Pittsburgh, PA). All other reagents were purchased from Sigma Aldrich (St. Louis, MO). Oasis HLB 96 well plates were procured from Waters Corporation (Milford, MA).
Study Population
Subjects for this study were participants in the Multiethnic Cohort Study (MEC), which consists of 215,251 men and women from five ethnic groups: Whites, African Americans, Hawaiians, Japanese Americans, and Latinos (3). Participants, aged 45–75 years old, from Hawaii and California enrolled in the MEC between 1993 and 1996 by completing a detailed questionnaire that outlined dietary habits, demographic factors, education level, occupation, personal behavior, prior medical conditions, and family history of cancer.
This specific study employed urine samples from Japanese American, African American and white individuals who were current smokers at time of urine collection and had no personal history of cancer. Blood and first morning urine samples were collected from participants in California; blood and an overnight urine sample were collected from participants in Hawaii. All urine was kept on ice until processing; aliquots were stored at −20 °C until analysis.
Data Collection
A total of 1,072 samples were analyzed for MHBMA and DHBMA (n= 327 African Americans, 396 Whites, and 349 Japanese Americans). MHBMA and DHBMA concentrations were adjusted for age, sex, total nicotine equivalents (TNE), and in some cases, for urinary creatinine. The methods of measuring creatinine and TNE (the sum of nicotine, cotinine, 3′-hydroxycotinine and their glucuronides, and nicotine N-oxide) have been previously described (7,51,52).
HPLC-ESI--MS/MS Analysis of MHBMA and DHBMA in Human Urine
Urinary concentrations of MHBMA and DHBMA were determined using previously published HPLC-ESI--MS/MS methods described in Supplement S1 (9,53). Samples that showed no MHBMA or DHBMA signal were assigned a value corresponding to the limit of detection divided by 2 (0.1 ng/mL urine for MHBMA [9 samples] and 2.5 ng/mL urine for DHBMA [2 samples]).
Sixteen sets of quality control samples (48 samples total) were included in the analyses. These positive controls were used to account for inter-batch variation. When necessary, data were adjusted for batch variations using the values for urinary MHBMA and DHBMA concentration in these samples. Overall, the mean coefficient of variation for these replicates was 8.87% and 8.49% for MHBMA and DHBMA, respectively.
In addition to calculating urinary MHBMA and DHBMA concentrations, a metabolic ratio DHBMA [MHBMA / (MHBMA + DHBMA)] was calculated. For this investigation, the metabolic ratio can be representative of a fraction of non-hydrolyzed EB and may provide an understanding into the extent of metabolic activation and detoxification of BD in a given individual.
Genotyping and Quality Control
DNA was extracted from blood leukocytes using a QiaAmp DNA blood extraction kit (Qiagen, Valencia, CA). Samples were genotyped using the Illumina Human1M-Duo BeadChip (1,199,187 SNPs) as described previously (54). The genotyping quality control consisted of removing individual samples with ≥2% of genotypes not called, removing SNPs ≤98% call rate and known duplicate samples, excluding samples with close relatives (as determined by estimated IBD status), and samples with conflicting or indeterminate sex. Imputation was performed using SHAPEIT and IMPUTE2 to a reference panel from the 1000 Genomes Project (1KGP; March, 2012) (55,56). We included SNPs with an IMPUTE2 info score of ≥0.30 and minor allele frequency (MAF) >1% in any MEC ethnic group. A total of 11,892,802 SNPs/indels with a frequency >1% in any single ethnic population (1,131,426 genotyped and 10,761,376 imputed) were included in the analysis.
GSTT1 and GSTM1 gene copy number assays were run using TaqMan copy number assays Hs00010004_cn Hs02575461_cn, respectively. All assays were run on the 7900HT FAST Real-Time System (Life Technologies, Carlsbad, CA). SNPs were called using the TaqMan Genotyper software, and copy number calls were determined using the CopyCaller v2.0 software (Life Technologies, Carlsbad, CA). Approximately 5% blind duplicate samples were included for quality control. Genotyping of the GSTT1 and GSTM1 deletion polymorphisms was successful in1,009, and 1,068 individuals, respectively. For the purposes of this study, the deletion or null genotype is represented as (0/0), one copy of the gene is represented as (1/0), and two copies of the gene is represented as (1/1).
Statistical Methods
Association of each variant with geometric means of MHBMA, DHBMA or metabolic ratio DHBMA [MHBMA / (MHBMA + DHBMA)] was evaluated using linear regression models, with adjustment for age, sex, race, TNE, BMI, and the first 10 principal components. Principal components were estimated using 19,059 randomly selected autosomal SNPs with frequency ≥ 2% in the combined multiethnic sample (57). A p-value cut-off of 5 × 10−8 was used for genome-wide significance. In regions with multiple associated variants, conditional models were used to evaluate individual signals at p < 5 × 10−8. In a like manner, ethnic-specific analyses were performed in each of the three individual populations. Percentage variation of MHBMA, DHBMA or the metabolic ratio was assessed using R2 values. To further assess associations with variants located in the deleted region, analyses among subjects homozygous for the GSTT1 non-null alleles were performed. We further examined associations of MHBMA, DHBMA, and metabolic ratio with variants in candidate gene regions known to be involved in butadiene metabolism and DNA repair (e.g. EPHX1, CYP2E1, and RAD51).
GSTT1 and GSTT2 catalyzed conjugation of EB with glutathione
3,4-epoxybut-1-ene (EB; final concentration: 2 mM) was incubated with glutathione (GSH; final concentration: 5 mM) in the presence or in the absence of GSTT1 or 2 (0, 2.5, or 5 μg) in 0.1 M phosphate buffer, pH 7.4 (50 μL total volume). The mixtures were allowed to incubate for 2 hours at 37 °C and quenched with 15% (w/w) trichloroacetic acid (50 μL). Reaction mixtures were filtered using Amicon Ultra Centrifugal Filters (0.5 mL, 10K; EMD Millipore, Billerica, Massachusetts), and the filters were subsequently washed with 100 μL water.
HPLC-ESI+-MS/MS analyses were performed using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA) coupled to an Agilent 1100 Series LC/MSD Trap SL (Agilent Technologies, Santa Clara, CA). An Agilent Zorbax SB300 C18 column (150 × 0.5 mm, 5 μm) column was maintained at 25 °C and eluted with 15 mM ammonium acetate (A) and methanol (B) with a linear gradient of (time, %B): 0–20 min, 2 to 20% B. Under these conditions, the epoxybutene-glutathione conjugate (EB-GSH) eluted at approximately 3.8 minutes. Extracted ion chromatograms of the conjugate, m/z 317.2, were used to determine the fold increase in EB-GSH formation as compared to the corresponding non-ezymatic reaction.
Results
A total of 1,072 smokers (327 African Americans, 349 Japanese Americans, and 396 Whites) were included in the analysis (Table 1). Overall, there were significant differences in smoking habits between these groups, with Whites smoking the greatest numbers of cigarettes per day (CPD), followed by Japanese Americans and African Americans (Table 1). Racial/ethnic differences were observed also for TNE, with, however, a different ordering: African American smokers had the highest levels, followed by Whites and Japanese Americans (Table 1). These trends with respect to CPD and TNE were the same when the smokers were categorized by sex. Significant differences were also seen in the creatinine levels between the groups, with African Americans having much higher levels than Japanese Americans and Whites (Table 1). Because of this large variability, MHBMA and DHBMA levels can appear artificially low for African Americans when adjusted for creatinine (Table S1). Therefore, unadjusted values (ng/mL urine) were employed in our final analyses.
Table 1.
Summary of study population stratified by race/ethnicity and sex.
| Median [Interquartile Range] | |||
|---|---|---|---|
|
| |||
| African Americans | Japanese Americans | Whites | |
|
|
|
|
|
| All | n = 327 | n = 349 | n = 396 |
| Age (years) | 64 [59–69] | 63 [59–69] | 62 [59–68] |
| BMI (kg/m2) | 26.9 [23.2–30.7] | 24.3 [21.9–26.6] | 24.8 [22.0–28.1] |
| Creatinine (mg/dL) | 88 [54–138] | 54 [33–81] | 53 [33–83.2] |
| Cigarettes per day | 10 [5–18] | 13 [10–20] | 17 [10–20] |
| Total nicotine equivalents (nmol/mL) | 44.2 [26.8–73.8] | 26.5 [15.4–41.2] | 35.8 [21.9–60.8] |
| Males | n = 94 | n = 181 | n = 169 |
| Age (years) | 63 [58–66] | 63 [59–68] | 62 [59–67] |
| BMI (kg/m2) | 25.7 [23.0–28.2] | 25.0 [23.0–26.9] | 25.8 [23.3–27.9] |
| Creatinine (mg/dL) | 124.5 [81.5–165.1] | 66 [40–95] | 71.0 [46.3–104.0] |
| Cigarettes per day | 10 [6–20] | 15 [10–20] | 20 [12.5–20] |
| Total nicotine equivalents (nmol/mL) | 54.4 [29.2–95.5] | 29.3 [17.9–45.6] | 39.9 [24.6–73.0] |
| Females | n = 233 | n = 168 | n = 227 |
| Age (years) | 65 [60–71] | 64 [59–70.5] | 62 [58–69] |
| BMI (kg/m2) | 27.5 [23.6–31.6] | 23.4 [20.6–26.5] | 24.0 [21.0–28.3] |
| Creatinine (mg/dL) | 79 [50–126] | 44.5 [28.0–63.5] | 46 [29–65] |
| Cigarettes per day | 10 [5–15] | 10 [7.5–20] | 15 [7–20] |
| Total nicotine equivalents (nmol/mL) | 41.3 [26.1–65.3] | 22.5 [13.0–35.0] | 31.2 [20.2–50.5] |
Geometric means for urinary concentrations of MHBMA and DHBMA in African American, Japanese American, and White smokers (ng/ml urine) are given in Table 2. Urinary levels of MHBMA were significantly different between the three ethnic groups overall (p = 4.0 × 10−25) in in both genders (males: p = 7.5 × 10−11, females: p = 1.7 × 10−15). African American smokers excreted the highest amounts of MHBMA (3.3 ng/mL urine), followed by White (5.7 ng/mL urine) and Japanese American smokers (3.3 ng/mL urine). Urinary levels of DHBMA also differed across ethnic groups (p = 3.3 × 10−4), with African Americans excreting the highest amounts of the metabolite, followed by White and Japanese American smokers (362.0, 294.6, and 292.7 ng/mL urine, respectively). These overall differences less significant in males (p = 0.07) as compared to females (p = 4.1 × 10−13). However, DHBMA levels in African American males were significantly higher than in White males (p < 0.05). Similarly, female African American smokers excreted significantly higher amounts of DHBMA than White females (p < 0.05). The ethnic differences for the metabolic ratio (calculated as MHBMA / (MHBMA + DHBMA)) were statistically significant (p = 1.7 × 10−14) and followed the same overall trend as MHBMA (Table 2). Ethnic differences in the extent of metabolic activation and detoxification of BD were observed for each sex (males: p = 2.2 × 10−6, females: p = 2.8 × 10−9). For both sexes, the metabolic ratio was significantly lower in Japanese Americans as compared to Whites (p < 0.05), while no differences were observed for other ethnic groups.
Table 2.
Geometric means (95% confidence limits) for urinary MHBMA and DHBMA by race/ethnicity and sex. Values are given in ng/mL urine.
| All | African Americans | Japanese Americans | Whites | p-value b | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Geometric means a | (95% CL)a | Geometric means a | (95% CL)a | Geometric means a | (95% CL)a | Geometric means a | (95% CL)a | ||
|
| |||||||||
| All | |||||||||
| N | 1072 | 327 | 349 | 396 | |||||
|
|
|||||||||
| MHBMA | 4.8 | (4.5–5.2) | 6.4 | (5.9–7.0) | 3.3 | (3.0–3.6) c | 5.7 | (5.2–6.1) | 4.0 × 10−25 |
| DHBMA | 308.8 | (292.8–325.8) | 362.0 | (332.5–394.1) c | 292.7 | (270.1–317.3) | 294.6 | (274.1–316.7) | 3.3 × 10−4 |
| MHBMA / (MHBMA + DHBMA) | 0.017 | (0.016–0.018) | 0.018 | (0.016–0.019) | 0.012 | (0.011–0.012) c | 0.020 | (0.018–0.021) | 1.7 × 10−14 |
|
| |||||||||
| Males | |||||||||
| N | 444 | 94 | 181 | 169 | |||||
|
|
|||||||||
| MHBMA | 5.3 | (4.9–5.7) | 7.6 | (6.5–8.9) | 3.9 | (3.4–4.3) c | 6.4 | (5.7–7.2) | 7.5 × 10−11 |
| DHBMA | 345.3 | (322.3–370) | 411.5 | (355.8–475.8) c | 335.0 | (300.5–373.4) | 341.5 | (306.7–380.3) | 0.07 |
| MHBMA / (MHBMA + DHBMA) | 0.016 | (0.015–0.018) | 0.019 | (0.016–0.023) | 0.012 | (0.011–0.014) c | 0.020 | (0.017–0.022) | 2.2 × 10−6 |
|
| |||||||||
| Females | |||||||||
| N | 628 | 233 | 168 | 227 | |||||
|
|
|||||||||
| MHBMA | 4.6 | (4.3–4.8) | 5.6 | (5.0–6.2) | 2.9 | (2.5–3.2) c | 5.1 | (4.6–5.7) | 1.7 × 10−15 |
| DHBMA | 287.2 | (271.2–304.1) | 322.6 | (291–357) c | 258.2 | (299.1–291.1) | 257.7 | (233.7–284.1) | 4.1 × 10−3 |
| MHBMA / (MHBMA + DHBMA) | 0.017 | (0.016–0.018) | 0.018 | (0.016–0.021) | 0.012 | (0.010–0.014) c | 0.021 | (0.019–0.023) | 2.8 × 10−9 |
P-values and geometric least square means have been adjusted for BMI, age, batch, TNE (and sex where appropriate).
P-values are comparing overall differences across ethnic groups.
P-values across ethnic groups (with Whites as the reference) were indicated where significant with p < 0.05.
GST catalyzed glutathione conjugation is the major metabolic pathway leading to MHBMA and DHBMA (Scheme 1). Therefore, MHBMA and DHBMA concentrations across ethnic groups were stratified by GSTT1 copy number (Table 3). Of the three ethnic groups, Japanese Americans had the highest frequency of the null genotype (48%), followed by African Americans (23%) and Whites (19%) (Table 3). For all ethnic groups, both MHBMA levels and metabolic ratios were strongly associated with GSTT1 deletion copy number genotype (p < 0.0001). Individuals with two copies of the gene (1/1) excreted the highest amount of MHBMA (6.9 ng/mL urine), followed by those with one copy of GSTT1 (1/0, 5.4 ng/mL urine) and those with null deletion genotype (0/0) (3.1 ng/mL urine) (Table 3). Among individuals null for GSTT1, Japanese American smokers had significantly lower urinary MHBMA levels than Whites (p < 0.05). No significant association between GSTT1 deletion and excretion of DHBMA was seen in any of the ethnic groups (Table 3).
Table 3.
Geometric means (95% CI) of BD metabolites stratified by GSTT1 and GSTM1 CNV and race/ethnicity.
| GSTT1 copy number genotype* | All | Whites | African Americans | Japanese Americans | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | Geometric means | N | Geometric means | N | Geometric means | N | Geometric means | ||||||
|
|
|
|
|
||||||||||
| (95% CL) a | (95% CL) a | (95% CL) a | (95% CL) a | ||||||||||
| MHBMA (ng/mL) | |||||||||||||
| 1/1 | 232 | 6.9 | (6.3–7.6) | 126 | 7.6 | (6.8–8.6) | 78 | 9.7 | (8.4–11.2) b | 28 | 4.1 | (3.0–5.6) b | 1.6 × 10−3 |
| 1/0 | 482 | 5.4 | (5.1–5.8) | 187 | 5.7 | (5.1–6.2) | 155 | 8.2 | (7.4–9.1) b | 140 | 3.6 | (3.1–4.1) b | 3.2 × 10−6 |
| 0/0 | 295 | 3.1 | (2.9–3.4) | 72 | 3.0 | (2.6–3.5) | 71 | 5.1 | (4.4–5.9) | 152 | 2.0 | (1.8–2.3) b | 2.8 × 10−6 |
| p-value | <.0001 | <.0001 | <.0001 | <.0001 | |||||||||
|
| |||||||||||||
| DHBMA (ng/mL) | |||||||||||||
| 1/1 | 232 | 293.9 | (266.7–323.8) | 126 | 284.8 | (250.2–324.2) | 78 | 406.2 | (341.6–483.0) | 28 | 222.3 | (172.6–286.2) | 0.28 |
| 1/0 | 482 | 322.4 | (302.1–344.1) | 187 | 302.3 | (272.0–335.9) | 155 | 455.1 | (402.3–514.7) b | 140 | 241.1 | (215.3–270.0) | 1.7 × 10−3 |
| 0/0 | 295 | 325.5 | (290.0–354.4) | 72 | 337.7 | (284.6–400.7) | 71 | 425.4 | (355.5–509.1) | 152 | 244.6 | (219.5–272.6) | 0.19 |
| p-value | 0.226 | 0.293 | 0.536 | 0.790 | |||||||||
|
| |||||||||||||
| MHBMA / (MHBMA + DHBMA) ratio | |||||||||||||
| 1/1 | 232 | 0.025 | (0.022–0.027) | 126 | 0.028 | (0.024–0.032) | 78 | 0.025 | (0.021–0.030) | 28 | 0.020 | (0.014–0.027) b | 9.0 × 10−3 |
| 1/0 | 482 | 0.018 | (0.017–0.019) | 187 | 0.020 | (0.018–0.022) | 155 | 0.019 | (0.017–0.022) | 140 | 0.016 | (0.014–0.018) b | 0.04 |
| 0/0 | 295 | 0.010 | (0.009–0.011) | 72 | 0.010 | (0.008–0.011) | 71 | 0.013 | (0.010–0.015) | 152 | 0.009 | (0.008–0.010) | 4.7 × 10−3 |
| p-value | <.0001 | <.0001 | <.0001 | <.0001 | |||||||||
|
| |||||||||||||
| GSTM1 copy number genotype | All | Whites | African Americans | Japanese Americans | p-value | ||||||||
|
| |||||||||||||
| N | Geometric means | N | Geometric means | N | Geometric means | N | Geometric means | ||||||
|
|
|
|
|
||||||||||
| (95% CL) a | (95% CL) a | (95% CL) a | (95% CL) a | ||||||||||
|
| |||||||||||||
| MHBMA (ng/mL) | |||||||||||||
| 1/1 | 137 | 5.5 | (4.8–6.2) | 207 | 7.4 | (5.8–9.3) | 75 | 7.7 | (6.6–9.0) | 27 | 3.0 | (2.1–4.1) b | 4.2 × 10−3 |
| 1/0 | 452 | 5.3 | (5.0–5.7) | 153 | 6.4 | (5.7–7.2) | 157 | 7.6 | (6.8–8.5) | 142 | 3.2 | (2.7–3.6) b | 1.3 × 10−11 |
| 0/0 | 479 | 4.4 | (4.1–4.8) | 35 | 4.9 | (4.4–5.4) | 94 | 7.6 | (6.6–8.7) b | 178 | 2.6 | (2.3–3.0) b | 1.6 × 10−12 |
| p-value | <.0001 | <.0001 | 0.986 | 0.134 | |||||||||
|
| |||||||||||||
| DHBMA (ng/mL) | |||||||||||||
| 1/1 | 137 | 310.7 | (274.8–351.3) | 207 | 294.0 | (230.1–375.5) | 75 | 412.5 | (347.4–489.9) | 27 | 245.9 | (190.1–318.1) | 0.39 |
| 1/0 | 452 | 318.3 | (297.7–340.4) | 153 | 302.1 | (268.6–339.7) | 157 | 434.1 | (383.8–490.1) b | 142 | 245.7 | (219.7–274.7) | 0.01 |
| 0/0 | 479 | 315.4 | (295–337.3) | 35 | 300.1 | (271.2–332.1) | 94 | 436.1 | (370.9–512.6) b | 178 | 244.5 | (221.3–270.1) | 0.05 |
| p-value | 0.94 | 0.098 | 0.868 | 0.997 | |||||||||
|
| |||||||||||||
| MHBMA / (MHBMA + DHBMA) ratio | |||||||||||||
| 1/1 | 137 | 0.019 | (0.014–0.016) | 207 | 0.026 | (0.020–0.035) | 75 | 0.020 | (0.016–0.024) | 27 | 0.013 | (0.009–0.018) b | 8.8 × 10−4 |
| 1/0 | 452 | 0.018 | (0.016–0.019) | 153 | 0.022 | (0.019–0.025) | 157 | 0.018 | (0.016–0.021) b | 142 | 0.014 | (0.012–0.016) b | 5.8 × 10−8 |
| 0/0 | 479 | 0.015 | (0.014–0.016) | 35 | 0.017 | (0.015–0.019) | 94 | 0.018 | (0.015–0.022) | 178 | 0.011 | (0.010–0.013) b | 1.1 × 10−6 |
| p-value | <.0001 | <.0001 | 0.808 | 0.157 | |||||||||
(0/0) is equivalent to the gene deletion
Adjusted for age, sex, BMI, batch and TNE
P-value <0.05 when compared to Whites
To confirm that GSTT proteins can catalyze conjugation of EB glutathione, EB was incubated with glutathione in the presence of increasing amounts of recombinant human GSTT1 and GSTT2 enzymes, and the conjugation products were analyzed by HPLC-ESI-MS/MS (Figure S-1). We found that both enzymes can catalyze this reaction, with GSTT1 exhibiting a faster rate, confirming the mechanistic involvement of these genes in MHBMA/DHBMA formation. In addition, MHBMA values were associated with GSTM1 genotype, suggesting that another isoform of glutathione S-transferase may be involved in metabolism of butadiene (Table 3).
Additional analyses were conducted to identify factors responsible for the variability noted in BD metabolite excretion. We examined the associations of urinary MHBMA and DHBMA concentrations in relation to smokers’ sex, age, BMI, batch, TNE and CPD (Table 4). We found that all together, these factors explained 44.23% of the variability in MHBMA and 32.12% of the variability in DHBMA concentrations. For both MHMBM and DHBMA models, significant differences in adjusted means were observed for Japanese Americans (p < 0.0001) when compared to Whites. The same factors explained 11.05% of the variance in the metabolic ratio and were also significant for Japanese Americans as compared to the Whites (p < 0.0001).
Table 4.
Percent variation explained.
| MHBMA (ng/mL) N = 1009 |
Overall Percent Variation Explained | Whites | African Americans | Japanese Americans | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | Meana | n | Meana | n | Meana | |||
| Sex + Age + BMI + Batch + TNE + CPD | 44.23% | 385 | 5.73 | 304 | 6.15 | 320 | 3.29 | |
| p-values | – | 0.27 | <0.0001 | |||||
| + GSTT1_deletion | 7.30% | 385 | 5.30 | 304 | 5.95 | 320 | 3.76 | |
| p-values | – | 0.05 | <0.0001 | |||||
| + GSTM1_deletion | 0.88% | 385 | 5.56 | 304 | 5.92 | 320 | 3.91 | |
| p-values | – | 0.29 | <0.0001 | |||||
|
| ||||||||
|
DHBMA (ng/mL) N = 1009 |
||||||||
|
|
||||||||
| Sex + Age + BMI + Batch + TNE + CPD | 32.12% | 385 | 306.1 | 304 | 352.2 | 320 | 294.7 | |
| p-values | – | 0.02 | 0.506 | |||||
| + GSTT1_deletion | 0.16% | 385 | 306.7 | 304 | 350.3 | 320 | 288.3 | |
| p-values | – | 0.03 | 0.31 | |||||
| + GSTM1_deletion | 0.00% | 385 | 305.3 | 304 | 349.2 | 320 | 286.8 | |
| p-values | – | 0.03 | 0.29 | |||||
|
| ||||||||
|
MHBMA / (MHBMA + DHBMA) N = 1009 |
||||||||
|
|
||||||||
| Sex + Age + BMI + Batch + TNE + CPD | 11.05% | 385 | 0.020 | 304 | 0.018 | 320 | 0.012 | |
| p-values | – | 0.32 | <0.0001 | |||||
| + GSTT1_deletion | 11.40% | 385 | 0.018 | 304 | 0.018 | 320 | 0.014 | |
| p-values | – | 0.77 | <0.0001 | |||||
| + GSTM1_deletion | 1.12% | 385 | 0.019 | 304 | 0.018 | 320 | 0.015 | |
| p-values | – | 0.28 | <0.0001 | |||||
All Means are expressed as geometric least squares means adjusted for age, gender, BMI, batch, TNE (CPD, GSTT1, and GSTM1 where appropriate).
After adjusting for age, gender, batch, BMI, and race, MHBMA and DHBMA showed a strong correlation with TNE (r = 0.55 and 0.47, respectively; Table 5), while the metabolic ratio showed a moderate association (r = 0.14, Table 5). Upon adjustment for the same variables and TNE, CPD accounted for only 0.48%, 1.27%, and 0.09% of the variability in MHBMA concentration, DHBMA concentration, and the metabolic ratio, respectively (Table 5). This suggests that measured TNE is a better predictor of urinary BD metabolites than self-reported CPD. These correlations did not differ greatly when categorized by ethnic group or by GSTT1 copy number (Table 5).
Table 5.
Partial correlations.
| N | MHBMA & TNEa | N | DHBMA & TNEa | N | MHBMA / (MHBMA + DHBMA) & TNEa | |
|---|---|---|---|---|---|---|
| Overall | 1072 | 0.55 | 1072 | 0.47 | 1072 | 0.14 |
| African Americans | 327 | 0.60 | 327 | 0.54 | 327 | 0.03 |
| Whites | 396 | 0.59 | 396 | 0.41 | 396 | 0.23 |
| Japanese Americans | 349 | 0.48 | 349 | 0.46 | 349 | 0.12 |
| GSTT1 = 1/1 | 232 | 0.70 | 232 | 0.51 | 232 | 0.25 |
| GSTT1 = 1/0 | 482 | 0.56 | 482 | 0.44 | 482 | 0.15 |
| GSTT1 = 0/0 | 295 | 0.54 | 295 | 0.54 | 295 | 0.07 |
All partial correlations have been adjusted for age, gender, batch, BMI (and race where appropriate)
Aside from the GSTT1 deletion (which explains 7.3% of the variability in MHBMA levels) we also investigated the association between the metabolites and the GSTM1 deletion, which explains 0.88% of the variability in MHBMA and 1.1% of the variability in the metabolic ratio (p < 0.0001, Table 4). The GSTT1 deletion explained 0.16% of variability in DHBMA and the GSTM1 explained close to zero percent of the variability in DHBMA (0.01%). The GSTT1 deletion explains 11.4% of the variability in metabolic ratio values, with an additional 1.12% of the variability explained by the GSTM1 deletion.
Table 4 also provides tests for ethnic differences in metabolite levels before and after adjustment for the two deletion genotypes. For MHBMA, tests for heterogeneity gave a t-statistic value of 1.15 for African Americans before adjustment for the two deletion genotypes, and 1.05 after adjustment, a 9% variability attributed to racial groups provided by the deletion genotypes. Importantly, strong differences in MHBMA ecretion between Japanese and White smokers remained even after adjustment for GSTT1 deletion (p < 0.0001), suggesting that additional factors contribute to ethnic differences in BD metabolism.
Besides GSTT1 and GSTM1 deletions, GWAS analyses were conducted to identify any other genomic determinants of BD metabolism (Figures S2–S6). For the GWAS of MHBMA (Figures S2–S7), we detected associations at p < 5 ×10−8 with 136 variants. However, all of them were located between 24.2—24.4 Mb near the GSTT1 gene on chromosome 22q11. Although there was one other rare association on 2p22.3 that was globally significant, this was a potentially unreliable rare variant that was not further considered. The significant associations at 22q11 were fully explained by the GSTT1 deletion, as no secondary signal was detected after conditioning on the GSTT1 deletion genotype (Figure S3). The deletion allele was significantly associated with lower MHBMA levels, found to be lowest among Japanese Americans (Table 3).
In ethnic-specific analyses, 108 globally significant associations were observed in Whites near GSTT1 (Figure S6). As in the overall analyses, the significance of the SNP associations was greatly diminished when the analyses were conditioned on the GSTT1 deletion genotype. The strongest remaining ethnic-specific association after conditioning on the GSTT1 deletion was in Whites for rs62241865 (p=6.6×10−5). The neighboring gene for this SNP is SYN3. The minor allele frequency of this SNP was 6 percent in Whites and 1 percent or less in the other ethnic groups. A single association in Japanese Americans in rs6004031 (our top-most significant SNP in overall analysis) near GSTT1 was noted to be globally significant at p = 5.13 ×10−9 (Figure S5), and four globally significant associations were observed for African Americans (Figure S4). None of these associations remained strongly significant after conditioning on GSTT1.
For DHBMA levels (Figure S8), no genome-wide significant associations were observed in either overall or ethnic specific analyses for any of the genotyped GWAS variants, or for SNPs and other variants that were imputed based on the GWAS.
For the MHBMA / (MHBMA + DHBMA) metabolic ratio, there were 144 associations at p < 5 × 10−8 located between 24.2–24.4 Mb near GSTT1 gene on chromosome 22q11 (Figure S9). As was the case for MHBMA, these associations were explained by the GSTT1 deletion, and no secondary signal was noted after conditioning on the deletion in any ethnic group. In ethnic-specific analyses, 54 globally significant associations in Whites were observed near GSTT1. A single association in Japanese Americans in rs6004031 (our top-most significant SNP in overall analysis) near GSTT1 was noted to be globally significant at p = 1.37 × 10−9. No globally significant associations were observed for African Americans. Again, the associations observed near GSTT1 were explained by the GSTT1 deletion genotype.
Discussion
Of over 60 known carcinogens present in tobacco smoke, 1,3-butadiene (BD) has the second highest cancer risk index.(14,58) BD inhalation induces tumors in laboratory mice and rats (15–18), and there is a strong association between occupational exposure to BD and the development of lymphoma and leukemia in humans (19,21–24,26). However, epidemiological studies have reported a weak association between lung cancer cases in women and occupational exposure to BD (59,60), and the potential role of BD in smoking induced lung cancer has yet to be fully understood.
We quantified urinary BD-mercapturic acids MHBMA and DHBMA (Scheme 1) as biomarkers of BD exposure and metabolic activation in African American, White, and Japanese American smokers (42,43). Our results show significant ethnic differences in the excretion of MHBMA, with African American smokers excreting the highest levels, followed by Whites and Japanese Americans (Table 2). These results correlate with the high lung cancer risk of African Americans and low lung cancer risk of Japanese Americans as compared to Whites, suggesting that BD could play a role in the differences in lung cancer etiology seen between these groups (3). Interestingly, a similar trend was recently reported for mercapturic acids derived from acrolein, crotonaldehyde, and benzene (51,61). Furthermore, levels of the mercapturic acid formed from benzene were strongly influenced by GSTT1 deletion, highlighting the important role of this GST gene in the detoxification of structurally distinct carcinogens (61).
In the present study, individuals with the GSTT1 deletion excreted the lowest levels of MHBMA, followed by individuals with one copy of the gene and those with two copies (Table 3). This is consistent with our recent small study of White, Native Hawaiian, and Japanese smokers, which also reported the lowest MHBMA levels from individuals with the GSTT1 deletion, followed by those with one and two copies of the gene (9). Adjusting for GSTT1 deletion explained the difference in urinary MHBMA between Japanese Americans and Whites; however, the difference between Whites and African Americans remained (Table 4).
The effect of GSTM1 deletion on urinary MHBMA concentrations was also investigated, and among null individuals, the same trend was seen, with African Americans excreting the most MHBMA, followed by Whites and Japanese Americans (Table 3). Other glutathione S-transferases such as GSTT2 could contribute to MHBMA formation (Figure S1) and account for the differences in MHBMA excretion between African Americans and Whites. However, we did not see evidence supporting a role of other GST genes in our GWAS, which found no additional signal after conditioning on GSTT1 deletion genotype.
To the best of our knowledge, this study is the first to employ a GWAS to identify single nucleotide polymorphisms (SNPs) or other genetic variants associated with MHBMA and DHBMA excretion. With regard to MHBMA, the GWAS showed significant associations of 136 SNPs located near the GSTT1 gene. However, when the GWAS was conditioned on GSTT1 deletion, these SNPs were no longer detected in any ethnic group. Similar results were seen in the GWAS for the metabolic ratio, which was significantly associated with 144 SNPs near the GSTT1 gene; these SNPs were also explained by the GSTT1 deletion. Experiments with recombinant GSTT1 and GSTT2 have confirmed the ability of these enzymes to catalyze glutathione conjugation with EB (Figure S1).
The strong relationship between the GSTT1 deletion and MHBMA levels may potentially complicate the use of MHBMA as a biomarker of BD exposure since this protein is required for MHBMA formation. However, in our study, the ethnic differences in MHBMA excretion remained regardless of GSTT1 genotype (Table 3). For studies where genotyping is not available, biomarkers directly reflecting BD-induced cellular damage, such as a BD-DNA adducts, might be a better choice to evaluate cancer risk specifically caused by BD.
Urinary concentrations of DHBMA also differed by ethnic group, with African Americans excreting the highest amounts, followed by Whites and Japanese Americans (Table 2). With respect to GSTT1, individuals with the deletion excreted the highest amounts of DHBMA, followed by individuals with one and two copies of GSTT1 (Table 3), but these differences were not significant (p = 0.226). These findings are analogous to those reported by Fustinoni et al., who did not see a difference in urinary DHBMA levels between occupationally exposed workers with the GSTT1 or GSTM1 deletion genotype and workers containing one or more copies of either gene (40).
Overall, this study is the first of its kind to use a GWAS to identify potential SNPs associated with urinary MHBMA and DHBMA levels, clearly showing an association between GSTT1 genotype and MHBMA levels in smokers from three different ethnic groups. Furthermore, our results reveal that MHBMA levels, expressed in ng/mL urine, are highest in African Americans and lowest in Japanese Americans as compared to Whites, which is consistent with their respective lung cancer risks.
Supplementary Material
Acknowledgments
Financial support: This study was supported by NIH grants 5P01CA138338 and R01 CA85997. This work is also funded in part by NIH grants P-30 CA014089 to the USC Norris Comprehensive Cancer Center and P-30 CA071789 to the UH Cancer Center Genomics Shared Resource. The MEC study is supported by U01 CA164973. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, supported in part by National Cancer Institute Cancer Center Support grant CA-77598.
The authors thank Robert Carlson for preparing figures for the manuscript and his editorial assistance.
Abbreviations
- MHBMA
2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene and 1-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene)
- DHBMA
N-acetyl-S-(3,4-dihydroxybutyl)-L-cysteine
- BD
1,3-butadiene
- EB
3,4-epoxybut-1-ene
- DEB
1,2,3,4-diepoxybutane
- EBD
3,4-epoxy-1,2-butanediol
- HMVK
hydroxymethylvinyl ketone
- EH
epoxide hydrolase
- GSTT1
glutathione S-transferase theta 1
- GSTT2
glutathione S-transferase theta 2
- GSTM1
glutathione S-transferase mu 1
- GSH
glutathione
- EB-GSH
1,2-epoxybutene glutathione conjugate
- CYP
cytochrome P450 monoxygenase
- SNP
single nucleotide polymorphism
- GWAS
genome wide association study
Footnotes
Conflicts of interest: We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.U.S.Department of Health and Human Services. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking--50 Years of Progress. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: 2014. pp. 43–48. [Google Scholar]
- 3.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, Shields PG, Caporaso NE, Sugimura H, Trivers GE, Tucker MA, et al. Analysis of cytochrome P450 2E1 genetic polymorphisms in relation to human lung cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:515–18. [PubMed] [Google Scholar]
- 5.Kihara M, Kihara M, Noda K. Risk of smoking for squamous and small cell carcinomas of the lung modulated by combinations of CYP1A1 and GSTM1 gene polymorphisms in a Japanese population. Carcinogenesis. 1995;16:2331–36. doi: 10.1093/carcin/16.10.2331. [DOI] [PubMed] [Google Scholar]
- 6.Le Marchand L, Sivaraman L, Pierce L, Seifried A, Lum A, Wilkens LR, et al. Associations of CYP1A1, GSTM1, and CYP2E1 polymorphisms with lung cancer suggest cell type specificities to tobacco carcinogens. Cancer Res. 1998;58:4858–63. [PubMed] [Google Scholar]
- 7.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–33. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JY, Chen L, Elahi A, Lazarus P, Tockman MS. Genetic analysis of microsomal epoxide hydrolase gene and its association with lung cancer risk. Eur J Cancer Prev. 2005;14:223–30. doi: 10.1097/00008469-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SL, Kotapati S, Wilkens LR, Tiirikainen M, Murphy SE, Tretyakova N, et al. 1,3-Butadiene exposure and metabolism among Japanese American, Native Hawaiian, and White smokers. Cancer Epidemiol Biomarkers Prev. 2014;23:2240–49. doi: 10.1158/1055-9965.EPI-14-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider J, Bernges U, Philipp M, Woitowitz HJ. GSTM1, GSTT1, and GSTP1 polymorphism and lung cancer risk in relation to tobacco smoking. Cancer Lett. 2004;208:65–74. doi: 10.1016/j.canlet.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Stephens EA, Taylor JA, Kaplan N, Yang CH, Hsieh LL, Lucier GW, et al. Ethnic variation in the CYP2E1 gene: polymorphism analysis of 695 African-Americans, European-Americans and Taiwanese. Pharmacogenetics. 1994;4:185–92. doi: 10.1097/00008571-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 13.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis. 1990;11:1863–68. doi: 10.1093/carcin/11.10.1863. [DOI] [PubMed] [Google Scholar]
- 14.International Agency for the Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. 83. Lyon, France: 2004. Tobacco Smoke and Involuntary Smoking; pp. 59–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Huff JE, Melnick RL, Solleveld HA, Haseman JK, Powers M, Miller RA. Multiple organ carcinogenicity of 1,3-butadiene in B6C3F1 mice after 60 weeks of inhalation exposure. Science. 1985;227:548–49. doi: 10.1126/science.3966163. [DOI] [PubMed] [Google Scholar]
- 16.Melnick RL, Huff J, Chou BJ, Miller RA. Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res. 1990;50:6592–99. [PubMed] [Google Scholar]
- 17.Owen PE, Glaister JR, Gaunt IF, Pullinger DH. Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. Am Ind Hyg Assoc J. 1987;48:407–13. doi: 10.1080/15298668791384959. [DOI] [PubMed] [Google Scholar]
- 18.Owen PE, Glaister JR. Inhalation toxicity and carcinogenicity of 1,3-butadiene in Sprague-Dawley rats. Environ Health Perspect. 1990;86:19–25. doi: 10.1289/ehp.908619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delzell E, Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R. An updated study of mortality among North American synthetic rubber industry workers. Res Rep Health Eff Inst. 2006:1–63. [PubMed] [Google Scholar]
- 20.International Agency for the Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. 97. Lyon, France: 2008. 1,3-Butadiene, Ethylene Oxide, and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide) pp. 45–184. [PMC free article] [PubMed] [Google Scholar]
- 21.Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, et al. Leukemia and cumulative exposure to butadiene, styrene and benzene among workers in the synthetic rubber industry. Toxicology. 1996;113:190–202. doi: 10.1016/0300-483x(96)03444-0. [DOI] [PubMed] [Google Scholar]
- 22.Matanoski G, Francis M, Correa-Villasenor A, Elliott E, Santos-Burgoa C, Schwartz L. Cancer epidemiology among styrene-butadiene rubber workers. IARC Sci Publ. 1993:363–74. [PubMed] [Google Scholar]
- 23.Matanoski G, Elliott E, Tao X, Francis M, Correa-Villasenor A, Santos-Burgoa C. Lymphohematopoietic cancers and butadiene and styrene exposure in synthetic rubber manufacture. Ann N Y Acad Sci. 1997;837:157–69. doi: 10.1111/j.1749-6632.1997.tb56872.x. [DOI] [PubMed] [Google Scholar]
- 24.Meinhardt TJ, Lemen RA, Crandall MS, Young RJ. Environmental epidemiologic investigation of the styrene-butadiene rubber industry. Mortality patterns with discussion of the hematopoietic and lymphatic malignancies. Scand J Work Environ Health. 1982;8:250–59. doi: 10.5271/sjweh.2469. [DOI] [PubMed] [Google Scholar]
- 25.National Toxicology Program (NTP) Report on Carcinogens. 13. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; 2014. [Google Scholar]
- 26.Sathiakumar N, Delzell E, Hovinga M, Macaluso M, Julian JA, Larson R, et al. Mortality from cancer and other causes of death among synthetic rubber workers. Occup Environ Med. 1998;55:230–35. doi: 10.1136/oem.55.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csanady GA, Guengerich FP, Bond JA. Comparison of the biotransformation of 1,3-butadiene and its metabolite, butadiene monoepoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis. 1992;13:1143–53. doi: 10.1093/carcin/13.7.1143. [DOI] [PubMed] [Google Scholar]
- 28.Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (+/−)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch Biochem Biophys. 1997;337:176–84. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 29.Sprague CL, Elfarra AA. Mercapturic acid urinary metabolites of 3-butene-1,2-diol as in vivo evidence for the formation of hydroxymethylvinyl ketone in mice and rats. Chem Res Toxicol. 2004;17:819–26. doi: 10.1021/tx049949f. [DOI] [PubMed] [Google Scholar]
- 30.Duescher RJ, Elfarra AA. Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys. 1994;311:342–49. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 31.Krause RJ, Sharer JE, Elfarra AA. Epoxide hydrolase-dependent metabolism of butadiene monoxide to 3-butene-1,2-diol in mouse, rat, and human liver. Drug Metab Dispos. 1997;25:1013–15. [PubMed] [Google Scholar]
- 32.Adler ID, Cochrane J, Osterman-Golkar S, Skopek TR, Sorsa M, Vogel E. 1,3-Butadiene working group report. Mutat Res. 1995;330:101–14. doi: 10.1016/0027-5107(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 33.Boogaard PJ, Bond JA. The role of hydrolysis in the detoxification of 1,2:3,4-diepoxybutane by human, rat, and mouse liver and lung in vitro. Toxicol Appl Pharmacol. 1996;141:617–27. doi: 10.1006/taap.1996.0328. [DOI] [PubMed] [Google Scholar]
- 34.Bechtold WE, Strunk MR, Chang IY, Ward JB, Jr, Henderson RF. Species differences in urinary butadiene metabolites: comparisons of metabolite ratios between mice, rats, and humans. Toxicol Appl Pharmacol. 1994;127:44–49. doi: 10.1006/taap.1994.1137. [DOI] [PubMed] [Google Scholar]
- 35.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotapati S, Matter BA, Grant AL, Tretyakova NY. Quantitative analysis of trihydroxybutyl mercapturic acid, a urinary metabolite of 1,3-butadiene, in humans. Chem Res Toxicol. 2011;24:1516–26. doi: 10.1021/tx2001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotapati S, Sangaraju D, Esades A, Hallberg L, Walker VE, Swenberg JA, et al. Bis-butanediol-mercapturic acid (bis-BDMA) as a urinary biomarker of metabolic activation of butadiene to its ultimate carcinogenic species. Carcinogenesis. 2014;35:1371–78. doi: 10.1093/carcin/bgu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabourin PJ, Burka LT, Bechtold WE, Dahl AR, Hoover MD, Chang IY, et al. Species differences in urinary butadiene metabolites; identification of 1,2-dihydroxy-4-(N-acetylcysteinyl)butane, a novel metabolite of butadiene. Carcinogenesis. 1992;13:1633–38. doi: 10.1093/carcin/13.9.1633. [DOI] [PubMed] [Google Scholar]
- 39.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol Sci. 2000;56:189–202. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 40.Fustinoni S, Soleo L, Warholm M, Begemann P, Rannug A, Neumann HG, et al. Influence of metabolic genotypes on biomarkers of exposure to 1,3-butadiene in humans. Cancer Epidemiol Biomarkers Prev. 2002;11:1082–90. [PubMed] [Google Scholar]
- 41.Wiencke JK, Pemble S, Ketterer B, Kelsey KT. Gene deletion of glutathione S-transferase theta: correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol Biomarkers Prev. 1995;4:253–59. [PubMed] [Google Scholar]
- 42.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009;11:1216–25. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 43.Urban M, Gilch G, Schepers G, van Miert E, Scherer G. Determination of the major mercapturic acids of 1,3-butadiene in human and rat urine using liquid chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:131–40. doi: 10.1016/j.jchromb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Henderson RF, Thornton-Manning JR, Bechtold WE, Dahl AR. Metabolism of 1,3-butadiene: species differences. Toxicology. 1996;113:17–22. doi: 10.1016/0300-483x(96)03422-1. [DOI] [PubMed] [Google Scholar]
- 45.Thornton-Manning JR, Dahl AR, Bechtold WE, Griffith WC, Jr, Henderson RF. Comparison of the disposition of butadiene epoxides in Sprague-Dawley rats and B6C3F1 mice following a single and repeated exposures to 1,3-butadiene via inhalation. Toxicology. 1997;123:125–34. doi: 10.1016/s0300-483x(97)00112-1. [DOI] [PubMed] [Google Scholar]
- 46.Thornton-Manning JR, Dahl AR, Bechtold WE, Henderson RF. Gender and species differences in the metabolism of 1,3-butadiene to butadiene monoepoxide and butadiene diepoxide in rodents following low-level inhalation exposures. Toxicology. 1996;113:322–25. doi: 10.1016/0300-483x(96)03466-x. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Salguero P, Hoffman SM, Cholerton S, Mohrenweiser H, Raunio H, Rautio A, et al. A genetic polymorphism in coumarin 7-hydroxylation: sequence of the human CYP2A genes and identification of variant CYP2A6 alleles. Am J Hum Genet. 1995;57:651–60. [PMC free article] [PubMed] [Google Scholar]
- 48.Gsur A, Zidek T, Schnattinger K, Feik E, Haidinger G, Hollaus P, et al. Association of microsomal epoxide hydrolase polymorphisms and lung cancer risk. Br J Cancer. 2003;89:702–06. doi: 10.1038/sj.bjc.6601142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–97. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa M, Hiyama K, Ishioka S, Maeda H, Maeda A, Yamakido M. Microsomal epoxide hydrolase genotypes and chronic obstructive pulmonary disease in Japanese. Int J Mol Med. 2000;5:49–53. doi: 10.3892/ijmm.5.1.49. [DOI] [PubMed] [Google Scholar]
- 51.Park SL, Carmella SG, Chen M, Patel Y, Stram DO, Haiman CA, et al. Mercapturic acids derived from the toxicants acrolein and crotonaldehyde in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PLoS One. 2015;10:e0124841. doi: 10.1371/journal.pone.0124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Liang Q, Mendes P, Sarkar M. Is 24h nicotine equivalents a surrogate for smoke exposure based on its relationship with other biomarkers of exposure? Biomarkers. 2011;16:144–54. doi: 10.3109/1354750X.2010.536257. [DOI] [PubMed] [Google Scholar]
- 53.Kotapati S, Esades A, Matter B, Le C, Tretyakova N. High throughput HPLC-ESI-MS/MS methodology for mercapturic acid metabolites of 1,3-butadiene: Biomarkers of exposure and bioactivation. Chem Biol Interact. 2015;241:23–31. doi: 10.1016/j.cbi.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel YM, Stram DO, Wilkens LR, Park SS, Henderson BE, Le Marchand L, et al. The contribution of common genetic variation to nicotine and cotinine glucuronidation in multiple ethnic/racial populations. Cancer Epidemiol Biomarkers Prev. 2015;24:119–27. doi: 10.1158/1055-9965.EPI-14-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9:179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 56.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–30. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sathiakumar N, Delzell E. A follow-up study of mortality among women in the North American synthetic rubber industry. J Occup Environ Med. 2009;51:1314–25. doi: 10.1097/JOM.0b013e3181bd8972. [DOI] [PubMed] [Google Scholar]
- 60.Sathiakumar N, Brill I, Delzell E. 1,3-butadiene, styrene and lung cancer among synthetic rubber industry workers. J Occup Environ Med. 2009;51:1326–32. doi: 10.1097/JOM.0b013e3181c3c663. [DOI] [PubMed] [Google Scholar]
- 61.Haiman CA, Patel YM, Stram DO, Carmella SG, Chen M, Wilkens LR, et al. Benzene uptake and glutathione S-transferase T1 status as determinants of S-phenylmercapturic acid in cigarette smokers in the multiethnic cohort. PLoS One. 2016;11:e0150641. doi: 10.1371/journal.pone.0150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.