Abstract
Purpose
Several studies have shown that estrogen receptor (ER) and progesterone receptor (PR) expression and human epidermal growth factor receptor 2 (HER2) expression may vary during tumoral progression. We aimed to describe and compare ER, PR, and HER2 expressions in primary breast tumors and synchronic axillary nodal metastases, and evaluate phenotypic correlations between them.
Methods
Patients were identified prospectively through surgical procedures between September 2013 and July 2016. The status of ER, PR, HER2, and Ki-67 were pathologically analyzed in breast cancers and axillary nodal metastases; these patients were classified based on the breast cancer phenotypes into five subgroups.
Results
Synchronic axillary nodal metastases were observed in 127 patients. In breast cancers and nodal metastases, correlation analyses of ER, PR, and Ki-67 expression showed a statistical dependence and concordance between these samples was unambiguously demonstrated through Bland-Altman plots for each determination. Primary breast tumors were classified as follows: luminal A, 41.6%; luminal B, 40.0%; luminal B/HER2, 9.6%; HER2, 2.4%; triple negative, 6.4%. Alterations in phenotype were observed in 28% of patients. The most frequent phenotypic alteration was from luminal B to A (36.4%). Ten cases (30.3%) showed alterations with therapeutic implications; six gained HER2 overexpression, and four, hormonal receptor (HR) expression. A moderate strength of agreement (Cohen's κ coefficient, 0.59; 95% confidence interval, 0.48–0.71) was observed. In multivariate analyses, high histologic grade (odds ratio [OR], 2.79; p<0.047) and high Ki-67 expression (OR, 1.05; p<0.037) were independent factors predictive of phenotypic alterations.
Conclusion
Strong correlations were observed in HR and Ki-67 expressions between primary breast tumors and axillary nodal metastases, and a moderate concordance was observed in their phenotypical characteristics. Nevertheless, alterations did exist, and one-third of these changes may have therapeutic implications. The nodal metastases of tumors with high grade and high Ki-67 expression may need to be analyzed, to obtain complete therapeutic information.
Keywords: Breast neoplasms, Ki-67 antigen, Lymphatic metastasis, Phenotype
INTRODUCTION
In Argentina, breast cancers have the highest incidence and mortality in women [1]. Seventy percent of these tumors are hormone-dependent, and the expressions of estrogen receptor (ER) and progesterone receptor (PR) are considered as prognostic and predictive factors. The overexpression of the human epidermal growth factor receptor 2 (HER2) occurs in 20% of these tumors, and its positive expression has important therapeutic implications. Treatment decisions are based on the clinical, histological, and immunohistochemical information obtained from the analysis of these tumors [2]. Nodal status is considered an indicator of tumor chronology; patients with node-negative cancers are believed to demonstrate better prognoses, although some authors may describe this as “lead time bias” [3]. However, nodal status is considered a marker of tumor phenotypes because this factor retains its prognostic importance after patients undergo relapses.
Several studies have shown that the expressions of the ER, PR, and HER2 may vary during tumoral progression and development of metastases [4,5]. Consequently, in several clinical scenarios, conducting biopsies of metastases is recommended, to adequately define their phenotypes and subsequent treatments [6]. Similarly, differences in the expressions of these receptors may be expected between primary breast tumors and synchronic nodal metastases [7].
In this study, we aimed to describe and compare the expressions of ER, PR, and HER2 in primary breast tumors and synchronic axillary nodal metastases, and evaluate the phenotypic alterations between these neoplastic tissues.
METHODS
Patient population
Patients were identified prospectively from the surgical procedures for breast cancers at the Alexander Fleming Institute between September 2013 and July 2016. Inclusion criteria included patients who (1) were older than 18 years, (2) signed informed consents, and (3) presented with synchronic axillary nodal metastases after undergoing therapeutic breast surgeries and axillary procedures. This study was approved by the Institutional Review Board (No. 725). They received treatments based on their pathologies, following international and institutional standards. Our analysis did not affect any diagnostic and therapeutic decisions.
Pathological analysis
During gross examinations, the sentinel lymph nodes >4 mm in diameter were bisected. Frozen sections were obtained, and the nodes were processed in individual blocks for the preparation of permanent sections and histopathologic evaluations using hematoxylin and eosin (H&E) staining. If the tumor could not be identified using H&E staining, then cytokeratin (Clone AE1-AE3; Dako, Glostrup, Denmark) immunohistochemistry (IHC) was performed, at one level (in a total of two faces in a bisected node) along with routine histologic examination of the sentinel lymph nodes using H&E. Thus, a sentinel node was examined in three sections (six faces): (1) frozen, (2) H&E stained, and (3) IHC stained. Cytokeratin immunostaining was considered positive, if the lymph node contained immune-reactive cells that appeared malignant or if there were cytologically atypical individual cells with strong cytokeratin reactivity in subcapsular sinuses. The cytokeratin IHC staining was reviewed independently by two pathologists [8].
We selected slides with greater amounts of tumor tissue, after a lymphatic mapping, as described above, for the analysis of ER (Clone 6F11; Leica Biosystems, Nussloch, Germany), PR (Clone 16; Leica Biosystems), and HER2 (Clone SP3; Cell Marque, Rocklin, USA). HER2 staining was interpreted as follows: membrane staining in 10% of invasive tumor cells was considered positive, when scoring for HER2. The IHC scoring method for HER2 was a semiquantitative method based on the intensities of the reaction products and the percentages of membrane-positive cells, yielding a score range of 0 to 3+. A score of 3+ was regarded as unequivocally positive, and scores of 0 or 1+, as negative. Borderline scores (2+) were regarded as equivocal, and fluorescence in situ hybridization was performed for those cases [9].
Classification
Hormonal receptors (HRs) were analyzed in terms of their percentages of expression; expressions >1% were considered positive. Overexpression of HER2 was considered positive, if more than 10% of tumor cells showed the presence of homogeneous and dark circumferential (chicken-wire) patterns.
Phenotypically, tumors and metastases were classified as follows: luminal A, if ER+, PR ≥20%, or Ki-67 ≤20%; luminal B, if ER+, PR <20%, or Ki-67 >20%; luminal B/HER2, if HR+ and HER2+; HER2, if HR− and HER2+; and triple negative, if HR− and HER2−
Statistical analysis
Categorical variables were expressed as absolute numbers and percentages. Continuous variables were described in terms of means and standard deviations if normally distributed, or medians and interquartile ranges otherwise. Comparison between groups were conducted using the Student t-test and the Wilcoxon rank sum test for continuous variables, and the chisquare test and Fisher exact test for categorical variables. Interrelationships between two continuous variables were examined using the Spearman's correlation. Bland-Altman plots were obtained for concordance analysis of continuous variables and Cohen's κ coefficient, for that of categorical variables. The relationships between each of the variables and the phenotype alterations were assessed individually using univariate analysis. The variables that were assessed as statistically significant using univariate analysis were included in multivariate analysis. Multivariate logistic regression was used to determine factors predictive of phenotype alterations. A p-value < 0.05 was considered statistically significant. Statistical analysis was carried out with Statistix 8.0 (Analytical Software, Tallahassee, USA).
RESULTS
During the period of study, 127 patients presented with synchronic axillary nodal metastases. The complete analysis of HR and HER2 in primary tumors and nodal samples was carried out in 118 patients. A summary of baseline characteristics is shown in Table 1.
Table 1. Baseline characteristics.
| Characteristic | No. (%) |
|---|---|
| No. of patients | 127 |
| Age (yr)* | 51.6±12.9 |
| Menopausal status | |
| Premenopause | 67 (52.7) |
| Postmenopause | 60 (47.2) |
| Breast tumor size (cm)† | 3.0 (2.0–4.5) |
| Stage at diagnosis | |
| I | 1 (0.8) |
| II | 82 (64.6) |
| III | 42 (33.0) |
| IV | 2 (1.6) |
| Type of surgery | |
| BCS | 83 (65.3) |
| Mastectomy | 44 (34.7) |
| Histologic subtypes | |
| Ductal | 103 (81.1) |
| Lobular | 16 (12.6) |
| Ductolobular | 3 (2.4) |
| Other | 5 (3.9) |
| Histologic grade | |
| 1 | 3 (2.5) |
| 2 | 77 (64.2) |
| 3 | 40 (33.3) |
| Estrogen receptor | |
| Positive | 110 (86.6) |
| Negative | 17 (13.4) |
| Progesterone receptor | |
| Positive | 97 (76.4) |
| Negative | 30 (23.6) |
| HER2 | |
| Positive | 15 (11.8) |
| Negative | 112 (88.2) |
| No. of positive nodes† | 2 (1–4) |
BCS=breast-conserving surgery; HER2=human epidermal growth factor receptor 2.
*Mean±SD; †Median (interquartile range).
Correlation between hormonal receptors and Ki-67 in breast tumors and nodal metastases
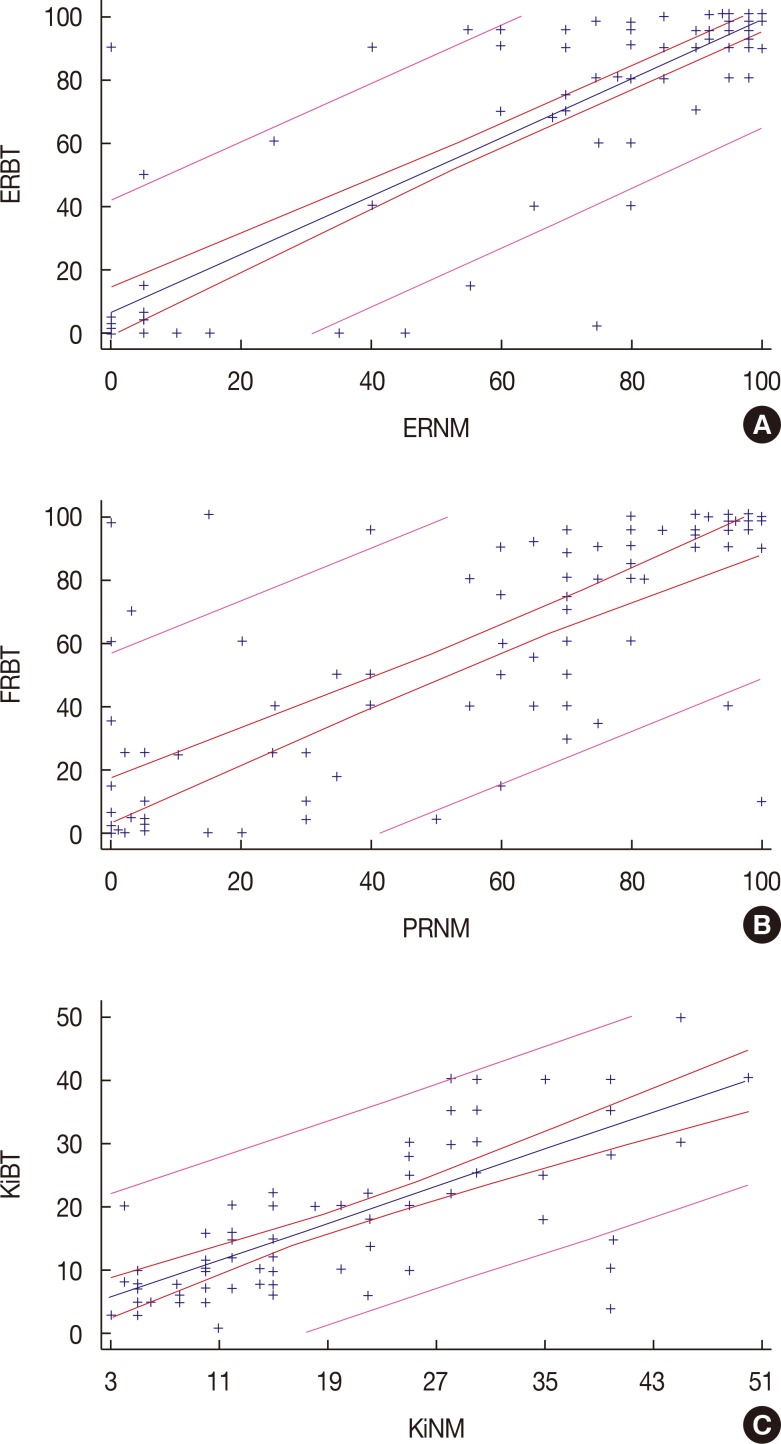
Correlation analysis between HRs and Ki-67 showed statistical dependence between these variables. Patients with high expressions of ER in primary breast tumors presented with high expressions of ER in nodal metastases (Spearman's rho 0.77, p<0.001). Similar dependence was observed between PR (Spearman's rho 0.81, p<0.001) and Ki-67 (Spearman's rho 0.72, p<0.001). The scatter plots shown in Figure 1 illustrate these relationships.
Figure 1. Correlation between estrogen receptor (ER), progesterone receptor (PR), and Ki-67 expression in primary breast tumors and synchronous axillary lymph node metastases. Scatter plot shows breast tumor expression (X-axis) and nodal metastases expression (Y-axis) of ER (A), PR (B) and Ki-67 (C). Strong correlation was observed through Spearman correlation in hormonal receptor and Ki-67 between primary breast tumor and axillary nodal metastases (ER Spearman rho 0.77, p<0.001; PR rho 0.81, p<0.001; and Ki-67 rho 0.72, p<0.001).
ERBT=ER percentage in breast tumor; ERNM=ER percentage in nodal metastases; PRBT=PR percentage in breast tumor; PRNM=PR percentage in nodal metastases; KiBT=Ki-67 percentage in breast tumor; KiNM=Ki-67 percentage in nodal metastases.
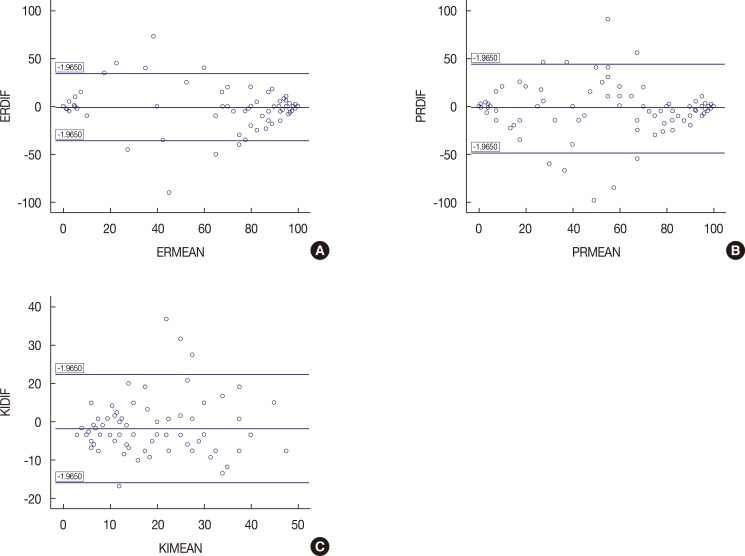
To adequately analyze the concordance between HRs and Ki-67 in both sets of samples, Bland-Altman plots were obtained for each determination (Figure 2).
Figure 2. Bland-Altman plots for concordance analysis. Agreement in primary breast tumors and synchronous axillary lymph node metastases for (A) estrogen receptor (ER) expression, (B) progesterone receptor (PR) expression and (C) Ki-67 expression. Y-axis shows the difference between the two paired measurements (measurement in primary breast tumors–measurement in nodal metastases) and the X-axis represents the average of these measures ([measurement in primary breast tumors+measurement in nodal metastases]/2). The difference of the two paired measurements is plotted against the mean of the two measurements. Ninety-five percent of the data points lie within±2 SD of the mean difference showing good correlation.
ERDIF=ER percentage difference between breast tumor and nodal metastases; ERMEAN=ER percentage mean between breast tumor and nodal metastases; PRDIF=PR percentage difference between breast tumor and nodal metastases; PRMEAN=PR percentage mean between breast tumor and nodal metastases; KIDIF=Ki-67 percentage difference between breast tumor and nodal metastases; KIMEAN=Ki-67 percentage mean between breast tumor and nodal metastases.
Phenotypic alterations between breast tumors and nodal metastases
Primary breast tumors were classified into five subgroups according to their immunohistochemical analysis: luminal A constituted 41.6% of the tumors; luminal B, 40.0%; luminal B/HER2, 9.6%; HER2, 2.4%; and triple negative, 6.4%. Nodal metastases were classified as follows: luminal A, 50.8%; luminal B, 30%; luminal B/HER2, 7.5%; HER2, 6.7%; and triple negative, 5%. The phenotypic correlation between these subgroups is shown in Table 2. Alterations of phenotypes between primary breast tumors and nodal metastases were observed in 28% of the patients. The most frequently observed alteration was from a luminal B subtype to a luminal A subtype (36.4%, n=12). Alterations with therapeutic implications occurred in 10 cases (30.3%); six showed overexpression of HER2 in nodal metastases that was not detected in the primary breast tumors, and four gained HR expression.
Table 2. Phenotype correlation.
| Breast phenotype | No. of lymph node phenotype | ||||
|---|---|---|---|---|---|
| Luminal A | Luminal B | Luminal/HER2 | HER2 | TN | |
| Luminal A | 44 | 3 | 0 | 1 | 0 |
| Luminal B | 12 | 30 | 0 | 2 | 3 |
| Luminal/HER2 | 2 | 1 | 8 | 1 | 0 |
| HER2 | 1 | 0 | 1 | 1 | 0 |
| Triple negative | 0 | 2 | 0 | 3 | 3 |
HER2=human epidermal growth factor receptor 2.
The number of observed agreements was 86 (72.9% of the observations). Nevertheless, 33.8% of these agreements could have occurred by chance. The Cohen's κ coefficient indicated a moderate strength of agreement (κ, 0.59; 95% confidence interval, 0.48–0.71).
Analysis of factors associated with phenotypic alterations
Clinical and pathological factors were analyzed, to describe their associations with the phenotypic alterations in nodal metastases. In univariate analysis, high histological grade and high Ki-67 expression showed associations with variations in the classification of phenotypes. In multivariate analysis, high histologic grade (odds ratio [OR], 2.79; p<0.047) and high Ki-67 expression (OR, 1.05; p<0.037) remained independent factors that were predictive of phenotypic alterations. The results of the analysis of all factors are shown in Table 3.
Table 3. Factors associated with phenotype change.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Univariate analysis | |||
| High histological grade (grade 3 vs. 1–2) | 4.45 | 1.77–11.20 | < 0.002 |
| Ki-67 expression (continuous, 1% change) | 1.07 | 1.03–1.10 | < 0.001 |
| Age (continuous) | 1.01 | 0.98–1.05 | 0.464 |
| Menopausal status (post vs. pre) | 1.81 | 0.76–4.30 | 0.179 |
| Tumor size (continuous) | 1.05 | 0.92–1.21 | 0.437 |
| Ductal histology (ductal vs. other) | 1.06 | 0.35–3.18 | 0.922 |
| Multivariate analysis | |||
| High histological grade | 2.79 | 1.02–7.66 | < 0.047 |
| Ki-67 expression | 1.05 | 1.00–1.09 | < 0.037 |
OR=odds ratio; CI=confidence interval.
DISCUSSION
Intratumoral heterogeneity is a recognized characteristic of breast neoplasms, in genetic, proteomic and macroscopic levels. Furthermore, heterogeneity exists between primary tumors and the corresponding metastases [10]. It is known that tumors may differentiate as they metastasize, changing their biological characteristics. The metastatic outcomes are dependent on a selection process that favors the survival and growth of a distinct special subpopulation of cells. The main purpose of investigating this heterogeneity is to evaluate its effects on prognosis and the efficacy of therapy.
Axillary nodes are the first sites of metastasis in breast cancers. Cells from these sites may represent the ones with greater invasive and proliferative capacities, and may be responsible for the occurrence of distant metastases. Between primary breast cancer and metastases, published literature reflects a discordance of 15% to 54% in HRs [6,11,12], and up to 34% in HER2 [6,12,13]. When the data is restricted exclusively to alterations in axillary nodal metastases, this discordance varies from 10% to 30% for HRs, and 10% to 20% for HER2 [14,15,16]. Comprehensive comparisons of biomarker expressions between primary breast carcinomas and the corresponding metastatic carcinomas in patients, as well as that between different metastatic sites from the same patient showed heterogeneous expressions of these biomarkers. In this report, the therapeutic targets identified in the primary breast carcinomas, or even in some metastatic breast carcinomas might not reflect the targets present universally in all metastatic sites [17].
In our study, a significant correlation was observed in the expressions of HRs and Ki-67 between primary breast tumors and axillary nodal metastases, and moderate concordance was observed in the phenotypic characteristics of these two different neoplastic tissues. This may support the argument for avoiding the use of immunohistochemical analysis, in this scenario. Nevertheless, as described earlier, phenotypic changes did exist between the two samples (28% in the patients in our study). One-third of these changes have therapeutic implications implications, thus adding more effective tools to the therapeutic arsenal. In our multivariate model, two pathological characteristics of the breast tumors were associated with phenotypic alterations: high histological grade and high Ki-67 expression.
This data strengthens the need to obtain corresponding information from patients in whom tumoral characteristics were associated with phenotypic alterations. These variables, namely, histologic grade 3 and high Ki-67 expression, which describe the capacities for differentiation and proliferation, may suggest the need to observe the pathological characteristics of nodal metastases, to effectively guide and optimize therapeutic strategies. The explanation for why these two pathological factors may influence the possibility of phenotypic alteration is based on the fact that undifferentiated tumors may show more intratumoral heterogeneity, wherein the population of cells that gain the characteristics of epithelial-tomesenchymal transition may differ from the principal types of cells observed in the originally analyzed samples. Therefore, a group of cells that are under-represented in the primary tumor may establish themselves by displaying metastatic capacity. The data obtained in this study is insufficient to confirm this theory; moreover, technical details of the experimental approaches may also contribute significantly to the resulting data.
In conclusion, the explanations for our data may range from the presence of technical issues or false negatives, to intratumoral heterogeneities, or to an actual alteration in the characteristics of the metastases observed in this study. Nevertheless, taken pragmatically, the selection of tumors with the abovementioned characteristics underscores the importance of the evaluation of the expressions of HRs and HER2 in different tissues, to appropriately tailor the individual therapeutic strategies.
Despite the fact that we do not know yet if this may offer further prognostic or predictive information [18], longer follow-ups and larger sample sizes may allow us to determine if the prognoses of patients may change as immunohistochemical information shift in metastatic development. Such a study will be presented in the future, when the required results are available.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Cancer situation analysis in Argentina. Ministerio de Salud de la Nación. [Accessed August 25th, 2016]. http://www.msal.gov.ar/inc/category/acerca-del-cancer/estadisticas.
- 2.Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:753–760. doi: 10.6004/jnccn.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittra I. Axillary lymph node metastasis in breast cancer: prognostic indicator or lead-time bias? Eur J Cancer. 1993;29A:300–302. doi: 10.1016/0959-8049(93)90371-l. [DOI] [PubMed] [Google Scholar]
- 4.Lindström LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 5.Gancberg D, Di Leo A, Cardoso F, Rouas G, Pedrocchi M, Paesmans M, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 6.Guarneri V, Giovannelli S, Ficarra G, Bettelli S, Maiorana A, Piacentini F, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist. 2008;13:838–844. doi: 10.1634/theoncologist.2008-0048. [DOI] [PubMed] [Google Scholar]
- 7.Falck AK, Fernö M, Bendahl PO, Rydén L. St Gallen molecular subtypes in primary breast cancer and matched lymph node metastases-aspects on distribution and prognosis for patients with luminal A tumours: results from a prospective randomised trial. BMC Cancer. 2013;13:558. doi: 10.1186/1471-2407-13-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997;226:271–276. doi: 10.1097/00000658-199709000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68:93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunelli M, Manfrin E, Martignoni G, Miller K, Remo A, Reghellin D, et al. Genotypic intratumoral heterogeneity in breast carcinoma with HER2/neu amplification: evaluation according to ASCO/CAP criteria. Am J Clin Pathol. 2009;131:678–682. doi: 10.1309/AJCP09VUTZWZXBMJ. [DOI] [PubMed] [Google Scholar]
- 11.Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, et al. Changes in estrogen receptor, progesterone receptor and HER-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–1562. [PubMed] [Google Scholar]
- 12.Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lower EE, Glass E, Blau R, Harman S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009;113:301–306. doi: 10.1007/s10549-008-9931-6. [DOI] [PubMed] [Google Scholar]
- 14.Nedergaard L, Haerslev T, Jacobsen GK. Immunohistochemical study of estrogen receptors in primary breast carcinomas and their lymph node metastases including comparison of two monoclonal antibodies. APMIS. 1995;103:20–24. doi: 10.1111/j.1699-0463.1995.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 15.Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2010;21:1254–1261. doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 16.Yao ZX, Lu LJ, Wang RJ, Jin LB, Liu SC, Li HY, et al. Discordance and clinical significance of ER, PR, and HER2 status between primary breast cancer and synchronous axillary lymph node metastasis. Med Oncol. 2014;31:798. doi: 10.1007/s12032-013-0798-y. [DOI] [PubMed] [Google Scholar]
- 17.Wu JM, Fackler MJ, Halushka MK, Molavi DW, Taylor ME, Teo WW, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falck AK, Fernö M, Bendahl PO, Rydén L. Does analysis of biomarkers in tumor cells in lymph node metastases give additional prognostic information in primary breast cancer? World J Surg. 2010;34:1434–1441. doi: 10.1007/s00268-010-0499-z. [DOI] [PubMed] [Google Scholar]