Abstract
Background
Lung cancer treatment has become increasingly dependent upon invasive biopsies to profile tumors for personalized therapy. Recently, tumor expression of PD-L1 has gained interest as a potential predictor of response to immunotherapy. Circulating biomarkers present an opportunity for tumor profiling without the risks of invasive procedures. We characterized PD-L1 expression within populations of nucleated cells in the peripheral blood of lung cancer patients in hopes of expanding the role of liquid biopsy in this setting.
Methods
Peripheral blood samples from a multi-institutional prospective study of patients with clinical diagnosis of lung cancer were subjected to cytomorphometric and immunohistochemical evaluation using single-cell, automated slide-based, digital pathology. PD-L1 expression was determined by immunofluorescence.
Results
PD-L1 expression was detected within peripheral circulating cells associated with malignancy (CCAM) in 26/112(23%) non-small cell lung cancer patients. Two distinct populations of nucleated, non-hematolymphoid, PD-L1 expressing cells were identified; cytokeratin positive (CK+, PD-L1+, CD45−) and cytokeratin negative (CK−, PD-L1+, CD45−) cells, both with cytomorphometric features (size, nuclear to cytoplasm ratio) consistent with tumor cells. Patients with >1.1 PD-L1(+) cell/mL (n=14/112) experienced worse overall survival than patients with ≤1.1 PD-L1(+) cell/ml (2-yearOS:31.2% vs 78.8%, p=0.00159). In a Cox model adjusting for stage, high PD-L1(+) cell burden remained a significant predictor of mortality (HR=3.85, 95%CI:1.64–9.09, p=0.002).
Conclusions
PD-L1 expression is detectable in two distinct cell populations in the peripheral blood of lung cancer patients and is associated with worse survival.
Keywords: Lung cancer, PD-L1, biomarkers, liquid biopsy, digital pathology
Introduction
Expression of programmed death-ligand 1 (PD-L1) on tumor cells allows them to evade immune effector mechanisms. Modulation of the PD-1 axis has rapidly emerged as a promising therapeutic approach in heavily pre-treated cancer patients across multiple tumor types (1–4). Recently, anti PD-1 axis agents pembrolizumab, nivolumab, and atezolizumab have gained approval as single agents in recurrent lung cancer (5–9). Thus far these agents appear to have superior toxicity profiles, sustained progression-free responses, and improved overall survival compared to cytotoxic chemotherapy.
Unfortunately, not all patients respond to anti PD-1 axis therapeutics. Therefore, paralleling PD-1 axis clinical development is the need for biomarkers to predict response and toxicity. For example, in recent clinical trials of the checkpoint inhibitors nivolumab and pembrolizumab, the mortality risk for patients treated with either agent was lower among patients in whom PD-L1 expression was identified in biopsy specimens (5–8). As a result, profiling for PD-L1 in solid tumor tissue biopsies has become increasingly incorporated into the treatment paradigm for metastatic lung cancer.
Currently lung cancer profiling is dependent upon invasive biopsies to obtain tumor tissue. Not only do invasive procedures expose patients to risks for complications (e.g. pneumothorax, bleeding)(10), but the scheduling of biopsies can impose significant treatment delays and logistical challenges for patients. Furthermore, heterogeneity among tumor foci may result in discordant responses to systemic therapy. Clinicians often repeat biopsies to optimize their approach to resistant disease. As a result, there is increasing interest in tumor profiling through peripheral blood analysis to avoid the hazards and inconvenience of invasive (potentially multiple) biopsy procedures.
Circulating tumor cells (CTC) have been studied using a diverse array of platforms with distinct strategies to enrich and evaluate the populations of interest. Most commonly, CTC assays impose a positive or negative selection step for enrichment that narrows the populations of circulating cell species that are able to be studied. Enrichment-free technologies, such as automated digital microscopy and computational pathology, image and categorize all nucleated cells and allow a broad array of circulating cell types to be analyzed. Because immunohistochemistry studies of tumor biopsies have identified PD-L1 expression within a variety of tumor and tumor-associated cell types, (11) the non-selection based digital microscopy approach represents an ideal platform to study PD-L1 expression among the broad array of cell types in the peripheral blood.
Materials and Methods
Patient Selection
The patients in the current study represent a subset of a prospective multi-institutional study to evaluate a novel, non-enrichment rare blood cell detection platform (EPIC Sciences) in a lung cancer population (NCT01830426). The parent study enrolled patients who were suspected to have clinical stage I-IV primary lung cancer by imaging, prior to undergoing a procedure for tissue confirmation from 3 institutions (Yale New Haven Hospital, Billings Clinic, and University of California, San Diego). Patients with a prior history of malignancy were excluded. All patients were consented according to an Institutional Review Board protocol approved at the three respective institutions and in accordance with the Declaration of Helsinki.
This current analyses represent secondary outcome measures of the original study. In order to maximize the potential utility of PD-L1 as a biomarker, the study was limited to a prognostic subset. More specifically, the current lung cancer subset was restricted to only those patients with: 1) confirmed primary non-small cell lung cancer (NSCLC), because NSCLC represented the majority population in our dataset (95%) and the bulk of the clinical experience with anti-PD-L1 therapy has been with NSCLC patients, 2) complete staging data, and 3) longitudinal follow-up. As an exploratory analysis, 6 patients with small cell carcinoma were evaluated. PD-L1 positive CCAMs were identified in 3 of the patients.
Two populations were used for controls. First a cohort of “healthy controls” was evaluated which included volunteers with no active medical conditions. Recognizing that PD-L1 expression may be influenced by a multitude of factors unrelated to cancer, we developed a second control population comprised of “unhealthy controls.” The population of “unhealthy controls” consisted of patients who were included in the parent trial (NCT01830426 described above) under the suspicion of having lung cancer, but were ultimately deemed not to have lung cancer. More specifically, these patients were enrolled based on an abnormality on thoracic imaging that raised suspicion for primary lung cancer yet after biopsy or additional observation, the clinical team determined these patients did not have lung cancer.
Rare Cell Collection
A “baseline” blood sample was drawn from a peripheral venipuncture (5cc waste) prior to the patient undergoing any invasive procedure for diagnostic, staging, or therapeutic purposes (to avoid contamination from noncancerous epithelial cells resulting from tissue trauma). Blood (7.5 mL) from each subject was collected in Streck (La Vista, Nebraska) tubes and shipped to Epic Sciences within 48 hours and processed immediately on arrival. Erythrocytes were lysed, and approximately 3 million nucleated blood cells were dispensed onto each of 10–16 glass microscope slides and placed at −80°C for long term storage according to methods previously described (12, 13). Sample processing and testing was conducted in laboratories certified under the Good Laboratory Practices (GLP).
PD-L1 Assay Development
Anti-PD-L1 rabbit monoclonal antibody from Cell Signaling (clone E1L3N, #13684)(14–16) was titered on PD-L1 negative (Colo205, H23), low (SU-DHL1), medium (H441) and high (H820) expressing cell line cells that had been spiked into healthy donor blood and run on the automated digital microscopy platform to evaluate the analytical performance of the antibody. PD-L1 expression levels demonstrated excellent antibody sensitivity and specificity for PD-L1 protein. Little-to-no cross-reactivity was observed in negative control cell lines and leukocytes from healthy donors (Supplemental Figure S1). PD-L1 antibody was visualized through secondary staining with Alexa Fluor-labeled secondary antibody. Optimal antibody and assay concentrations allowing for the highest signal-to-background detection of the various PD-L1 expression levels were selected for assay qualification and subsequent patient staining.
Rare Cell PD-L1 Immunofluorescent Staining and Analysis
Rare cell identification and characterization took place as previously described (13, 17, 18). In brief, prepared slides were subjected to automated immunofluorescent staining for cytokeratin (CK), DAPI (DNA marker), CD45 (blood lineage marker) and PD-L1. Two slides were stained and evaluated per patient sample with the PD-L1 assay, and processed in tandem with aforementioned high and low PD-L1 expressing cell line control slides.
Automated scanning identified “candidate” cells of interest among nucleated cell populations based on size/morphology of cell, nuclear features, cytokeratin expression, and PD-L1 expression in the absence of blood-lineage CD45 expression. Candidate cells were then reviewed by California-licensed Clinical Laboratory Scientists to confirm immunohistochemistry staining profile, as well as to assess the cytomorphometric features of the cell (size, shape, nucleus to cytoplasm ratio, etc. as they relate to the features associated with circulating tumor cells). Candidate cells were given histological classification of: single cells, clusters (more than one sharing cytoplasmic boundaries) or apoptotic cells (nuclear features consistent with apoptosis).
The analytical threshold for single-cell PD-L1 positivity of the assay was a signal to noise ratio set at the 95th percentile of intensity observed in the Colo205 negative control cell line cells spiked into whole blood and processed as process controls for patient sample staining. More than 95% of both high endogenous expressing (H820, H441) and induced PD-L1 expressing (SU-DHL1) cell line cells were above this analytical threshold. Apoptotic cells were not considered PD-L1(+) due to yet-to-be-explored effects of epitope availability during apoptotic enzymatic cascade, and were excluded from analyses. For analyses, cell counts per slide were converted to counts per mL of blood via the amount of blood utilized to create patient slides.
Circulating Cells Associated with Malignancy (CCAM)
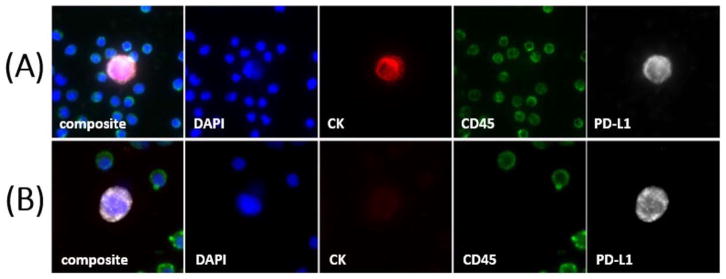
Many of the identified circulating cells met the field consensus criteria for circulating tumor cells (CTCs): epithelial protein (cytokeratin) expression, absence of blood lineage CD45 expression, and an intact nucleus (19). On the other hand, many PD-L1 positive, CD45 negative cells in patient samples both contained a nucleus morphologically distinct from surrounding white blood cells, and had cytokeratin expression below the analytical threshold of the assay (Figure 1). While these cells were not observed in “healthy control” donor samples, these cells have not been genetically confirmed to be of malignant origin, and we refrained from labeling them as “CTCs”. Therefore, throughout this report, we have adopted the nomenclature Circulating Cells Associated with Malignancy (CCAM) to refer to all cells that are 1) nonapoptotic, 2) have a nucleus, 3) are CD45(−), and 4) have cytomorphometric features consistent with circulating tumor cells (size, shape, nuclear to cytoplasm ratio, etc). In other words, the CCAM category includes classic CTCs, as well as cells that meet all other criteria to be a CTC, but don’t express cytokeratin.
Figure 1. Circulating Cells Associated with Malignancy (CCAM).
Panels include DNA (blue), Pan-Cytokeratin (red), CD45 (green), and PD-L1 (white). Example CK(+)/PD-L1(+) CCAM (A) and CK(−)/PD-L1(+) CCAM (B) are shown. DAPI = 4′,6-diamidino-2-phenylindole. CK = Cytokeratin. PD-L1 = Programmed death-ligand 1.
Statistical Analyses
All statistical analyses were performed with R v3.2.0 packages ‘stats’, ‘survival’, ‘survminer’, ‘ggplot’, and ‘maxstat’. Fisher’s Exact and ANOVA tests were used to compare groups for categorical and continuous characteristics, respectively. The optimal cutoff for dichotomizing PD-L1(+) CCAMs for overall survival was determined by a 10-fold cross-validation approach using maximally selected log-rank statistics in the maxstat package in R.(20) Overall survival was calculated in months from the time of blood draw to death from any cause. Patients still alive at time of last follow-up were right censored. Differences in survival between defined patient groups were evaluated using the log-rank test. Mortality hazard was estimated from univariable and multivariable Cox proportional hazards (PH) regression models. The covariates considered in the multivariable Cox PH models included: American Joint Committee on Cancer (AJCC) Staging (IV vs. I–III), age, and PD-L1(+) CCAMs. Log-log plot comparisons and Schoenfeld residuals were evaluated for violations of the proportional hazards assumption. The models were refined using a stepwise selection method in which individual covariates had to be significantly associated (p < 0.05) with overall survival in order to be kept in the model. Age did not meet this criterion. All statistical tests were two-sided and a p-value < 0.05 was considered statistically significant.
Results
Distinct Subpopulations of PD-L1(+) Circulating Cells Associated with Malignancy (CCAM)
Of the 112 NSCLC patients studied, PD-L1 (+) CCAMs (see Methods) were detected in the peripheral blood of 26 (23%). No PD-L1(+) CCAMs were detected in “healthy controls”, while PD-L1 (+) CCAMs were detected in 4 of 20 “unhealthy controls” (Methods). Within the PD-L1(+) CCAM population (47 cells from 26 lung cancer patients), two distinct subpopulations were noted based on the differential expression of cytokeratin (CK) (Figure 1). More specifically, 19 cells were positive for cytokeratin (PD-L1(+) CK(+)), while 28 (60%) were negative for cytokeratin (PD-L1(+)CK(−)). Table 1 shows the Profile of CCAMs detected in lung cancer patients.
Table 1.
Profile of (+) Circulating Cells Associated with Malignancy (CCAM) Detected in Lung Cancer Patients.
| # of Cells | PD-L1 | Cytokeratin | Malignant Cytomorphometrics |
|---|---|---|---|
| 19 | + | + | + |
| 28 | + | − | + |
| 942 | − | + | + |
| 232 | − | − | + |
PD-L1 (+) CCAM Enumeration by Histology and AJCC Stage
The distribution of PD-L1 (+) CCAMs was evaluated across tumor subtypes and AJCC stage (Supplemental Figure S2). In general, the PD-L1 (+) CCAMs appeared to be more common in patients with stage IV cancer, yet the differences did not reach statistical significance. Table 2 summarizes the 132 blood samples including 112 from cancer patients and 20 from “unhealthy control” patients.
Table 2.
Patient Cohort Summary by PD-L1(+) Circulating Cells Associated with Malignancy (CCAM) Incidence.
| Characteristic | No detected PD-L1(+) CCAMs | >0–1.1 PD-L1(+) CCAMs/mL | >1.1 PD-L1(+) CCAMs/mL | p-value |
|---|---|---|---|---|
| Lung Cancer Cohort | 86 | 12 | 14 | |
|
| ||||
| Age, median (IQR), years | 67 (60–74) | 68.5 (61.75–75.75) | 70 (59–76.5) | 0.851 |
|
| ||||
| Tumor AJCC Stage | ||||
| I | 42 | 7 | 2 | 0.0265 |
| II | 12 | 2 | 2 | |
| III | 22 | 0 | 4 | |
| IV | 10 | 3 | 6 | |
|
| ||||
| Tumor Histological Type | ||||
| Adenocarcinoma | 62 | 7 | 11 | 0.918 |
| Squamous Cell Carcinoma | 14 | 3 | 2 | |
| Other | 10 | 2 | 1 | |
|
| ||||
| Clinical Site | ||||
| Yale | 61 | 8 | 3 | 0.00743 |
| Billings | 19 | 2 | 8 | |
| UCSD | 6 | 2 | 3 | |
|
| ||||
| Unhealthy Controls | 16 | 4 | 0 | |
CCAM = Circulating Cells Associated with Malignancy
IQR = Interquartile range
AJCC = American Joint Committee on Cancer
PD-L1(+) CCAMs Associated with Worse Survival
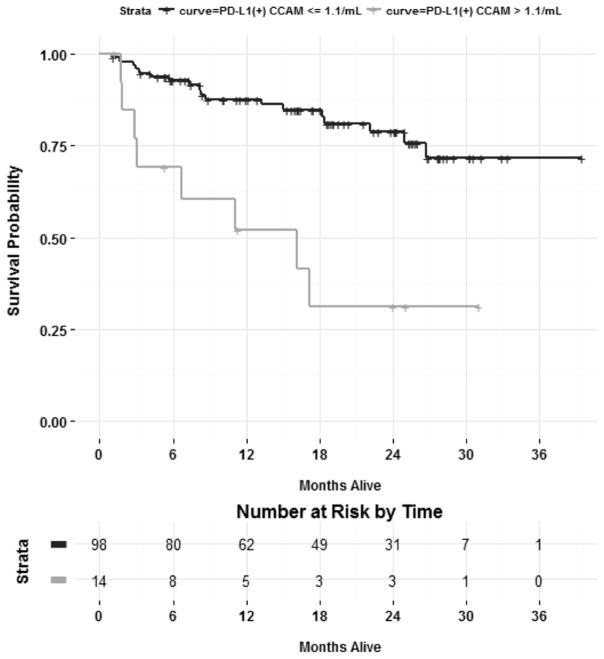
In an effort to better understand the prognostic relevance of the PD-L1(+) CCAM population, the relationship between PD-L1(+) CCAMs and long-term survival was evaluated. The population was stratified using an optimal cutoff (see Methods) of >1.1 PD-L1 (+) CCAM per milliliter as the threshold, (all patients with >1.1 PD-L1(+) CCAM/mL had lung cancer). Kaplan Meier survival curves showed that lung cancer patients with >1.1 PD-L1(+) CCAM/mL (n=14) experienced a worse median survival (16.1 months versus not reached) and worse 2-year survival than those with ≤ 1.1 PD-L1 (+) CCAM/mL (31.2% vs. 78.8%, p = 0.00159) (Figure 2). In a multivariable Cox PH model adjusting for AJCC staging, expression of >1.1 PD-L1(+) CCAM/mL was an independent predictor of mortality risk (HR = 3.85, 95% CI: 1.64 to 9.09, p = 0.002) (Supplemental Table S1).
Figure 2. PD-L1(+) CCAMs are Prognostic for Overall Survival in Lung Cancer Patients.
Kaplan-Meier estimates of overall survival of patients stratified by those with > 1.1 PD-L1(+) CCAM (gold line) or ≤ 1.1 PD-L1(+) CCAM (blue line).
To further characterize the prognostic implications of PD-L1(+) CCAMs, separate Cox PH models were created using progressively higher thresholds of PD-L1(+) CCAMs concentrations (Table 3). A dose-response relationship was observed, where the mortality risk appeared to increase as the threshold increased (indicating greater numbers of PD-L1(+) CCAMs is associated with worse prognosis), yet the confidence intervals widened as the number of high risk patients declined.
Table 3.
Impact of “threshold” PD-L1(+) Circulating Cells Associated with Malignancy concentration on prognostic ability.
| Threshold | Univariate Hazard Ratio (95% CI) | p-Value | Patients Positive | % Patients Positive |
|---|---|---|---|---|
| >3/mL | 4.54 (1.35 – 15.2) | 0.0417 | 4 | 3.6 |
| >2/mL | 7.04 (2.77 – 18.0) | < 0.0001 | 8 | 7.2 |
| >1/mL | 3.06 (1.32 – 7.04) | 0.0159 | 18 | 16 |
| >0/mL | 2.32 (1.05 – 5.12) | 0.0458 | 26 | 23.2 |
CI = Confidence Interval
Recognizing the PD-L1(+) CCAMs that are negative for cytokeratin (PD-L1+/CK− CCAMs) represent a previously undescribed circulating species, a subsequent analysis was performed by stratifying patients exclusively on the presence or absence of this population of cells. The presence of PD-L1+/CK− CCAMs was associated with a worse prognosis, indicating that this represents a clinically relevant cellular population (Supplemental Figure S3 and Table 4). As a supplementary analysis, patients were stratified by the presence or absence of PD-L1 negative cells that met our most stringent criteria of circulating tumor cells (cytokeratin positive CCAMs that were PD-L1 negative), however this population of cells was not found to be prognostic (Table 4).
Table 4.
Cox Models for 2-year Mortality Stratified by CCAM Expression of Cytokeratin and PD-L1
| CCAM Subset | Na | HR | LCL | UCL | p-Value |
|---|---|---|---|---|---|
| CK(−)PD-L1(+) | 10 | 4.570 | 1.790 | 11.60 | 0.00147 |
| CK(−)PD-L1(−) | 30 | 2.060 | 0.936 | 4.530 | 0.0722 |
| CK(+)PD-L1(+) | 6 | 2.560 | 0.582 | 11.200 | 0.214 |
| CK(+)PD-L1(−) | 59 | 0.806 | 0.369 | 1.760 | 0.586 |
N = number of patients with >1.1 CCAM/ml
CCAM = Circulating Cells Associated with Malignancy
CK = Cytokeratin
HR = Hazard ratio
LCL = Lower confidence limit
UCL = Upper confidence limit
Discussion
PD-L1(+) CCAMs were detected in the peripheral blood of 23% of treatment-naïve primary NSCLC patients. The tendency for the prevalence of PD-L1(+) CCAMs to increase among tumors at highest risk for systemic progression (advanced-stage tumors) is not surprising, and is consistent with the results of circulating tumor cell studies that trend towards higher prevalence in later stages patients (21). The prevalence in the peripheral blood is roughly half of what has been reported for tissue biopsies. More specifically, previous studies investigating the incidence of PD-L1 expression in lung cancer tissue samples (using a variety of antibodies and positivity thresholds), estimate that around 50% of lung cancers contain PD-L1 positive cells (11). The most obvious explanation for the lower frequency of PD-L1 detection in blood compared to tumor samples is disease state, as tumor cells (or CCAMs) would be far less likely to be found in the circulation in patients with completely localized cancer (although they may be). Among Stage IV patients, 9 of 19 (47%) had at least one PD-L1(+) CCAM. Most of the trial data that has defined the prevalence of PD-L1 positive cells in the tumor specimens of lung cancer has been in stage IV or recurrent patients (11). The difference may also relate to the sensitivity of the assay. While up to half of the cells in a solid tumor specimen may be PD-L1 positive, circulating tumor cells or CCAMs are rare populations in the blood compartment.
In the current study, we report a population of PD-L1(+) cells previously undescribed in lung cancer that share many characteristics with circulating tumor cells (i.e. nucleus present, CD45(−), non-apoptotic, size, shape, nucleus to cytoplasm ratio of CTCs), but do not express cytokeratin. A recent publication has reported similar circulating cells in bladder cancer patients (22). The study was too small for prognostic interpretation, but single-cell sequencing of these CD45(−), PD-L1(+), cytokeratin-negative cells revealed copy number variations consistent with malignant origin. In both studies, this population was only able to be imaged because the automated digital microscopy platform used to evaluate the rare cell populations (Epic Sciences CTC Detection Platform) did not include a positive or negative selection step. This turned out to be a critically important aspect of the current study, as just over half of the PD-L1(+) cells did not express cytokeratin. We have employed the phrasing “circulating cells associated with malignancy”, or CCAMs to describe a population of cells that have features consistent with circulating tumors cells, yet recognize that 20% of the unhealthy control patients had PD-L1 (+) CCAMs. It is not unusual to identify low levels of circulating tumor cells in patients without cancer,(23) and for this reason most assays ultimately impose a threshold of positivity, as we have done in prospective analyses (>1.1 PD-L1(+) CCAM/mL). The potential explanation for these cells include clinically occult cancer, particularly as not all of the unhealthy controls had the lesions removed (and may still have a lung cancer that has not grown).
There is also the possibility that these cells represent a transition in cancer cell phenotype, such as epithelial-mesenchymal transition (EMT). In a case study, utilizing a filtration-enrichment strategy (ISET), Chinen et al reported lung cancer associated cells positive for N-cadherin and negative for CK7 and CK8 (24). The authors proposed an EMT related mechanism (25, 26) to explain the observed phenomenon. PD-L1 expression and EMT were found to be co-regulated by microRNA-200 in a preclinical model (27). In breast cancer tissue samples, EMT-like signatures were found to be highly associated with higher PD-L1 expression (28). A recent study reported detection of CD45(−)/PD-L1(+)/vimentin(+) cells in the peripheral blood of colorectal carcinoma and prostate cancer patients, presumably CTCs that had undergone EMT (29). Ultimately we refrained from referring to these populations as circulating tumor cells because we recognize the possibility that they are related to malignancy but might not actually be tumor derived.
The presence of PD-L1(+) CCAMs was significantly associated with increased 2-year mortality risk. This is consistent with prior reports that have demonstrated a poorer prognosis in patients whose tumors express PD-L1 (30). Furthermore, high concentrations of non-cell bound (soluble) circulating PD-L1 protein assessed via ELISA assay in 109 cancer patients was previously associated with shorter median survival (18.7 vs. 26.8 months) (31). We recognize that the prognostic ability of PD-L1(+) CCAM status in the peripheral blood, while significant, is unlikely to change patient care. Nonetheless, we feel this clinical association provides strong evidence that the PD-L1(+) CCAM population is clinically relevant to the patients, potentially representing PD-L1 expression at some level of the host-tumor interface. Because these samples were collected as a part of a prospective trial, we are not able to compare the PD-L1 expression in the peripheral blood to that of the primary tumors (primary tumors not currently available for profiling). However, we propose that the peripheral blood offers an important perspective of PD-L1 status that is independent of the status of the primary tumor (i.e. if the primary tumor was negative, it is possible that the patient may still benefit from checkpoint inhibitors).
In conclusion, an enrichment-free, whole plasma scanning, rare cell detection platform has enabled the identification of two species of PD-L1 expressing cells in lung cancer patients that appear to be clinically relevant. Further study is warranted to evaluate the relationship between the cellular expression of PD-L1 in the peripheral blood and the efficacy of immunotherapy affecting the PD-1 axis.
Supplementary Material
Impact.
These findings could represent a step forward in the development of minimally invasive liquid biopsies for the profiling of tumors.
Acknowledgments
This work was supported by National Institute of Health grant SBIR HHSN261201200049C awarded to R. P. Graf.
We would like to thank the patients and their families that took part in this study, and the clinical and laboratory staff at Yale New Haven Hospital, the Billings Clinic, University of California San Diego, and Epic Sciences. We would additionally like to thank Jordan Warburg for laboratory support in PD-L1 assay validation. This work was supported by National Institute of Health grant SBIR HHSN261201200049C.
References
- 1.Razzak M. From ASCO-targeted therapies: Anti-PD-1 approaches--important steps forward in metastatic melanoma. Nature reviews Clinical oncology. 2013;10:365. doi: 10.1038/nrclinonc.2013.98. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27C:89–97. doi: 10.1016/j.coi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014 doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Opin Biol Ther. 2016 doi: 10.1517/14712598.2016.1145652. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haramati LB, Austin JH. Complications after CT-guided needle biopsy through aerated versus nonaerated lung. Radiology. 1991;181:778. doi: 10.1148/radiology.181.3.1947096. [DOI] [PubMed] [Google Scholar]
- 11.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics. 2015 doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 12.Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, Louw J, et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner SL, Graf RP, Landers ML, Valenta DT, Schroeder M, Greene SB, et al. Analytical Validation and Capabilities of the Epic CTC Platform: Enrichment-Free Circulating Tumour Cell Detection and Characterization. Journal of Circulating Biomarkers. 2015:4. doi: 10.5772/60725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017:8. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulsen E-E, Kilvaer TK, Khanehkenari MR, Al-Saad S, Hald SM, Andersen S, et al. Assessing PDL-1 and PD-1 in Non–Small Cell Lung Cancer: A Novel Immunoscore Approach. Clinical Lung Cancer. 2017;18:220–33.e8. doi: 10.1016/j.cllc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for pd-l1 expression in non–small cell lung cancer. JAMA Oncology. 2017 doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Physical biology. 2012;9:016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Physical biology. 2012;9:016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attard G, Crespo M, Lim AC, Pope L, Zivi A, de Bono JS. Reporting the capture efficiency of a filter-based microdevice: a CTC is not a CTC unless it is CD45 negative--letter. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:3048–9. doi: 10.1158/1078-0432.CCR-10-3234. author reply 50. [DOI] [PubMed] [Google Scholar]
- 20.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Computational Statistics & Data Analysis. 2003;43:121–37. [Google Scholar]
- 21.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1556–63. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 22.Anantharaman A, Friedlander TW, Lu D, Krupa R, Premasekharan G, Hough J, et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) and white blood cells (WBCs) in muscle invasive and metastatic bladder cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(suppl 2S) doi: 10.1186/s12885-016-2758-3. abstr 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantel K, Deneve E, Nocca D, Coffy A, Vendrell JP, Maudelonde T, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–40. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 24.Chinen LT, de Carvalho FM, Rocha BM, Aguiar CM, Abdallah EA, Campanha D, et al. Cytokeratin-based CTC counting unrelated to clinical follow up. J Thorac Dis. 2013;5:593–9. doi: 10.3978/j.issn.2072-1439.2013.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Seminars in cancer biology. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nature medicine. 2013;19:1438–49. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nature communications. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Molecular cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, et al. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep. 2016;6:28910. doi: 10.1038/srep28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PloS one. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Gao J, Li Y, Nie J, Dai L, Hu W, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thoracic cancer. 2015;6:534–8. doi: 10.1111/1759-7714.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.