Introduction
The cochlear implant is a neural prosthesis implanted in the periauricular soft tissue and inner ear that allows electric stimulation of remaining cochlear neurons in severely to profoundly deaf patients. Contemporary multi-channel implants consist of platinum or platinum/iridium electrodes embedded in a silicone carrier. These biomaterials are biocompatible, but they are not bio-inert. A foreign body response to implanted biomaterials is common1,2 and has been described in response to a cochlear implant.3–12
In a series of temporal bones from patients who had undergone cochlear implantation during life, a foreign body giant cell reaction was described in 96% and a lymphocytic infiltrate in 89% of specimens13 providing evidence of a cellular immune response in all or nearly all cases. The severity of the inflammatory response to a cochlear implant varies greatly from patient to patient.13 In some cases, the response may be limited to mild fibrosis or new bone formation, whereas in other cases a foreign body granuloma with necrosis has been described.14 The histologic correlate of the cellular immune response consists of an infiltrate of B and T lymphocytes, macrophages, and multinucleated foreign body giant cells.15 Particulate material found within the fibrous sheath surrounding the cochlear implant has been identified as platinum, presumably a degradation product derived from the implanted electrode and was seen in both an extracellar location and within macrophages.
A foreign body giant cell reaction has been described following implantation of silicone containing prostheses, including skin expanders,16 arthroplasty prosthetics,17 cardiac pacemakers,18 cerebral spinal fluid shunts,19 and breast implants,20 but to date there has been no histologic evidence of a role for silicone in the cellular immune response to cochlear implants.
The goal of the current investigation was to seek histologic evidence for a foreign body reaction to silicone following cochlear implantation.
Materials and Methods
Histologic preparation
Temporal bones were removed and fixed in 10% buffered formalin. The specimens were decalcified in ethylene/diamine tetra acetic acid. Following decalcification, the cochlear implant electrode was removed. The specimens were dehydrated in graded alcohols followed by embedment in celloidin. Specimens were serially sectioned in the axial (horizontal) plane with an average thickness of 20 micrometers. Every tenth section was stained with hematoxylin and eosin and mounted on glass slides for histologic examination and two-dimensional graphic reconstruction. All H & E stained sections containing the cochlea, cochleostomy site, and middle ear were surveyed for the occurrence of presumptive platinum and silicone particles.
Immunohistochemical methods
Intervening unstained sections were prepared for immunostaining. Selected intervening celloidin sections were mounted on a gelatin subbed glass slide. Celloidin was removed with changes of a solution of sodium hydroxide in methanol followed by 100% methanol rinses. The sections were rehydrated from 100% methanol to 70% methanol and then into distilled water. The sections were rinsed in phosphate buffered saline (PBS) and incubated in 5% normal horse serum followed by incubation in primary antibodies overnight in a humid chamber at room temperature.
After primary incubation, sections were rinsed in three washes of PBS, secondary antibodies diluted 1:200 and appropriate for the host species of primaries were applied for one hour, and sections were then rinsed three times with PBS. Avidin-Biotin horseradish peroxidase (standard ABC kit, Vector Labs, Burlingame, CA) was applied for one hour and rinsed in PBS. Colorization was accomplished with 0.01% diaminobenzidine and 0.01% hydrogen peroxide (H2 02) for five to ten minutes. The sections were then rinsed in PBS, dehydrated in graded alcohols, and cover slips were applied.21,22
Scanning electron microscopy (SEM)
In each temporal bone specimen the electrode array was removed following decalcification. The arrays were then post-fixed in ½ strength Karnovsky fixative (2% paraformaldehyde, 2.5% glutaraldahyde in phosphate buffer at a ph of 7.4). After fixation the electrodes were rinsed 3 times in phosphate buffer, dehydrated in graded series of ethanol, critical point dried in a Tousimis semiautomatic Samdri-795 critical point dryer, and coated with a 150 Å chromium layer in a Gatan 610 ion beam coater.
Control electrode arrays (not previously implanted) were not fixed, but were coated using the same protocol as for implanted implant arrays. Electrodes were mounted on 12mm diameter aluminum mounting stubs before the coating was applied. They were then examined in a JEOL 7401F field emission scanning electron microscope.
The following specimens were submitted for SEM analysis: (1) Advanced Bionics High Res 90 Helix array, implanted in case #27; (2) Nucleus modiolar hugging array, implanted in case #19; (3) an unimplanted Advanced Bionics High Res 90 Helix implant array; (4) an unimplanted Nucleus 24 implant array.
Energy dispersive spectroscopy by scanning electron microscopy (EDS-SEM)
Tissue sections adjacent to those used for H&E staining or immunostaining were prepared by a similar method as described for immunostaining on glass slides (or plastic sides when silicon was the elemental target) and air dried.
The mounted samples were then examined using a JEOL (Japan Electron Optics Laboratory) Model 6610LV scanning electron microscope (SEM) in low vacuum mode with a pressure of approximately 35Pa. Images were generated using the Back Scatter Electron Detector. Back Scatter imaging provides composition information whereby high atomic number materials appear brighter in the image. These images were useful as a guide as the investigation focused on platinum (atomic weight 78), or silicon (atomic weight 14), as compared to the surrounding organic material. The SEM was equipped with an IXRF energy dispersive spectroscopy system (EDS), which provides identification of elements based on X-ray radiation caused by the electron beam interacting with the sample. The IXRF system was equipped with a silicon drift detector (Model 550I pulse processor and iridium analysis software).
Results
Prevalence of presumed platinum and/or silicone foreign body particles
A total of 44 temporal bone specimens derived from 36 cases (Table) were reviewed for light microscopic evidence of presumed presence of platinum and/or silicone. Platinum was presumed to be present when small black particles were seen in an extracellular or intracellular location. Silicone was presumed to be present when birefringent material was seen in either an extracellular or intracellular location.
Table 1.
Platinum and Silicon in Celloidin Embedded Cochlear Implant Cases
| Case # | Age at Death | Sex | PM (Hours) | Type CI -Ear | Years with CI | Last Recorded Post-op WRS |
|---|---|---|---|---|---|---|
| 1 | 67 | M | 23 | Nucleus 22-L | 10 | 0% (N/A) |
| 2 | 83 | M | 19.5 | Nucleus 22-R | 7 | 30% (NU6) |
| 3 | 81 | M | 27 | Nucleus 22-L | 6 | 44% (NU6) |
| 4 | 70 | M | 15 | Nucleus 22-R | 11 | 14% (NU6) |
| 5 | 74 | M | 31 | Nucleus 22-R | 12 | 16% (NU6) |
| 6 | 91 | M | 17 | Nucleus 22-L | 11 | 12% (NU6) |
| 7 | 59 | M | 96 | Nucleus 22-R | 15 | 6% (NU6) |
| 8 | 72 | F | 22 | Nucleus 22-R Nucleus-L |
12 4 |
24% (NU6) (N/A) |
| 9 | 84 | F | 2 | Nucleus 22-R | 12 | 34% (NU6) |
| 10 | 71 | M | 58.5 | Nucleus-R Nucleus-L |
13 12 |
0% (N/A) 0% (N/A) |
| 11 | 76 | M | 28 | Nucleus-L | 17.5 | 0% (NU6) |
| 12 | 57 | M | 3 | Nucleus 24-R | 7 | 28% (CNC) |
| 13 | 89 | M | 24 | Ineraid-R Nucleus-R |
5 18 |
(N/A) 0% (N/A) |
| 14 | 66 | M | 4 | Nucleus 22-L | 18.5 | 26% (CNC) |
| 15 | 74 | F | 32 | CII Hi-focus with positioner R CII Hi-focus with positioner L |
8 8 |
66% (CNC) 66% (CNC) |
| 16 | 78 | M | ? | Nucleus Modiolar hugging-L | 7 | (N/A) |
| 17 | 83 | M | 12 | Nucleus-L | 23 | 36% (CNC) |
| 18 | 82 | M | ? | Clarion CII HF-L | 9 | 15% (CNC) |
| 19 | 68 | M | 27.5 | Nucleus 22-R Nucleus Modiolar hugging-L |
20 3 |
0% (N/A) 0% (N/A) |
| 20 | 89 | F | 54 | Clarion w/o positioner-L | 9 | 10% (CNC) |
| 21 | 71 | F | 19.5 | Nucleus 24-R Nucleus 24-L |
11.5 11.5 |
56% (CNC) 60% (CNC) |
| 22 | 89 | F | 48 | Nucleus 24 Modiolar hugging-L | 10.5 | 50% (CNC) |
| 23 | 88 | M | 48 | Contour advance MH-R Nucleus 22-L |
6 17 |
60% (CNC) 0% (N/A) |
| 24 | 82 | F | 39.5 | Clarion-L | 8 | 44% (CNC) |
| 25 | 89 | F | 48 | Clarion-L | 19 | 0% (CNC) |
| 26 | 96 | M | 96 | Nucleus FreedomModiolar hugging-R | 5 | 20% (CNC) |
| 27 | 89 | M | 20 | Advanced Bionics High Res Helix MH-R | 2 | 74% (CNC) |
| 28 | 81 | F | 29.5 | Nucleus 22-R | 20.5 | 54% (CNC) |
| 29 | 83 | M | 12 | Nucleus 24-R Nucleus 24-L |
12 12 |
57% (CNC) 58% (CNC) |
| 30 | 70 | M | 35 | 6 electrode Nucleus hybrid S8-L | 6 | 28% (CNC) |
| 31 | 80 | M | 8 | Nucleus C122M-R | 23 | 46% (N/A) |
| 32 | 92 | F | 4 | Clarion Advanced Bionics-L | 8 | (N/A) |
| 33 | 81 | M | 24 | Advanced Bionics-R | 1 | 41%(AzBio) |
| 34 | 64 | M | 22.5 | Advanced Bionics Helix-R Advanced Bionics Helix-L |
2 2 |
60% (CNC) 50% (CNC) |
| 35 | 94 | F | 17 | CII Hi-Focus with positioner-R | 12 | (N/A) |
| 36 | 70 | F | 10 | Nucleus 24 Modiolar hugging-R | 7 | (N/A) |
M: male
F: female
WRS: word recognition score
NU6: Northwestern University Auditory Test Number 6
AzBio: Arizona State University/Biomedical Institute Sentence Test
CI: cochlear implant
Evidence for both platinum and silicone was found in all 44 specimens. Platinum was found in both the cochlea and in the middle ear in 39 of 44 specimens, in the cochlea only in five specimens, and in the middle ear only in 0 specimens. The presence of presumptive silicone was found both in the cochlea and in the middle ear in 36 of 44 temporal bone specimens, in the cochlea only in 6, and in the middle ear only in 2.
Presumptive platinum was found in an intracellular or extracellular location in all 44 specimens. It was located both in an intracellular location and also in an extracellular location in 43 of 44 specimens; intracellular only in 1, and extracellular only in 0 cases. Presumptive silicone was found in an intracellular location and also an extracellular location in 43 of 44 specimens, in an intracellular location only in 1, and in extracellular location only in 0 specimens. It should be noted that not every section through all cochleae were reviewed and the middle ears of two specimens could not be evaluated due to removal artifact.
Immunostaining
The presence of macrophages containing phagocytized presumed platinum and silicone were demonstrated in the fibrous tissue sheath surrounding the cochlear implant track in three cases, #17, #19; #28; using anti-CD163 immunostaining, a marker for cells of the monocyte/macrophage lineage (Figs. 1,2,3).
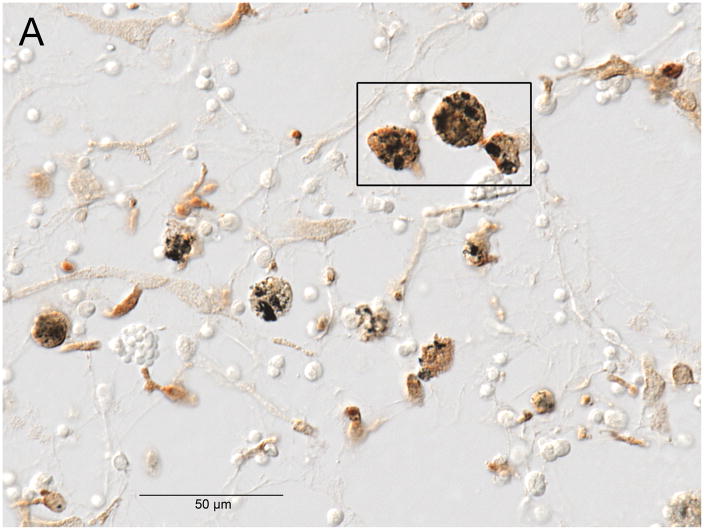
Fig. 1.
Fig. 1A. Anti CD-163 immunostaining (a marker of cells of monocyte/macrophage lineage) demonstrated macrophages with phagocytized black particulate material (presumed platinum foreign body) and also birefringent material (presumed silicone foreign body) in Case #19. The boxed area is shown at higher magnification in Figure 1B.
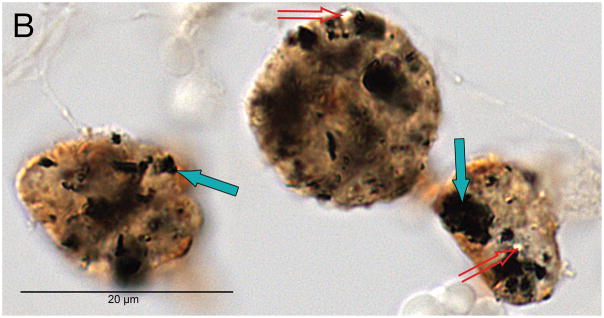
Fig. 1B. Black particulate material consistent with platinum as a phagocytized foreign body (solid arrows), and birefringent material (hollow arrows) consistent with silicone as a phagocytized foreign body.
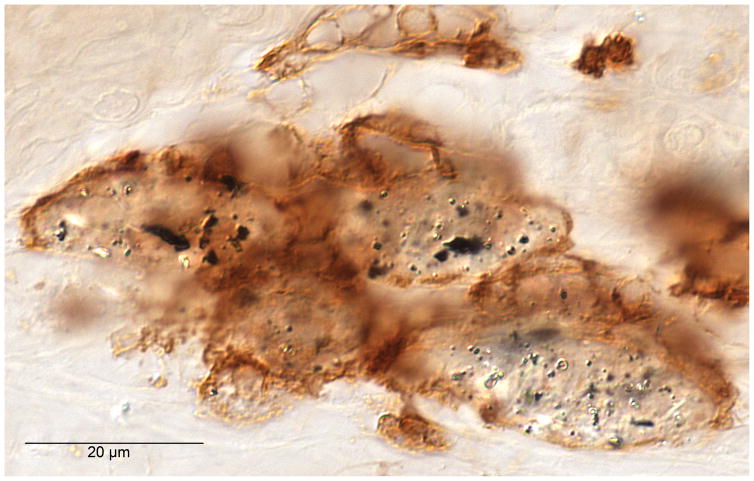
Fig. 2.
Anti CD-163 immunostaining of macrophages located in the fibrous tissue layers surrounding a cochlear implant in case #28. The black particulate material (solid arrow) is consistent with phagocytized platinum foreign body, whereas the birefringent material (hollow arrow) is consistent with phagocytized silicone.
Fig. 3.
Anti CD-163 immunostaining of a macrophage located in fibrous tissue surrounding a cochlear implant in case #17. The black particulate material is consistent with phagocytized platinum, and the birefringent material is consistent with phagocytized silicone.
Energy dispersive X-ray spectroscopy (EDS-SEM)
The EDS system provides a spectrum showing the elements presented in each identified area, but also reports elemental concentration and weight percent. The reported concentration is not directly applicable, but provides a guide to the amount of a given element (platinum or silicon), and the confidence interval in the measurement of that element. The degree of confidence is provided as an error indication and a minimum detection limit (MDL).
Opaque particles consistent with foreign body material were identified as platinum in a previous study (Nadol, O’Malley, Burgess, Galler 2014). This was usually found in areas of lymphocytic infiltration, either in an extracellular location or within macrophages.
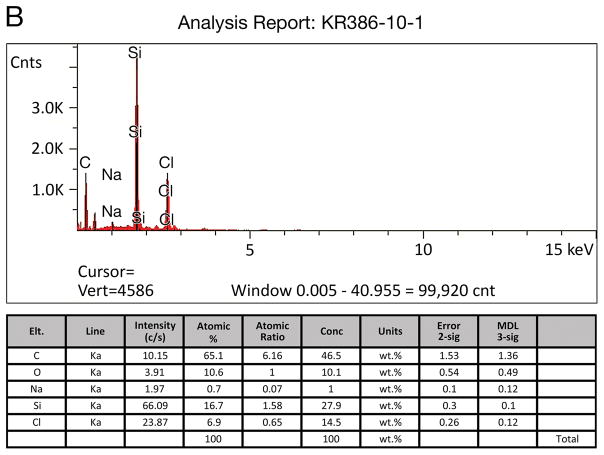
Five specimens in the current study (#11; #21; #19; #28; #34) were evaluated by EDS/SEM for the presence of silicon. In case #21, EDS/SEM analysis applied to intracellular collections of birefringent material (Fig. 4) demonstrated a silicon concentration of 27.9% (Fig. 5). The specimen was mounted on plastic (polyvinyl chloride) slides containing no silicon. A positive control specimen consisted of a small piece of silicone carrier which showed a concentration of silicon of 37%, whereas a negative control in an adjacent area without birefringent material showed a silicon concentration of 1.5 weight %.
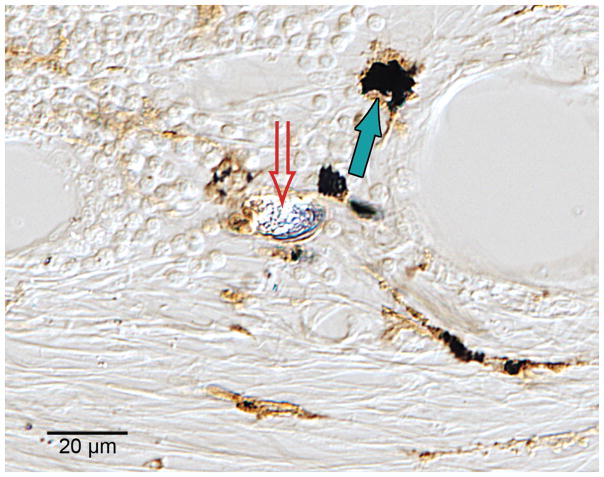
Fig. 4.
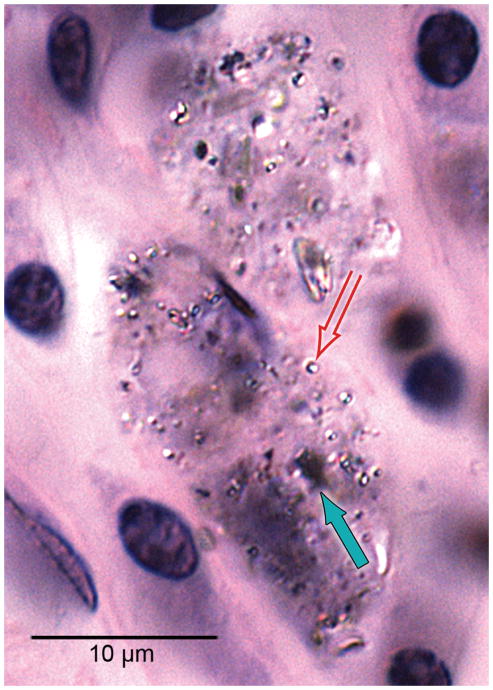
Macrophages containing both black particulate material (solid arrow) and birefringent material (hollow arrow) consistent with phagocytized platinum and silicone respectively were commonly found in the fibrous tissue sheath surrounding the cochlear implant electrode, here in case #21 (H&E stain).
Fig. 5.
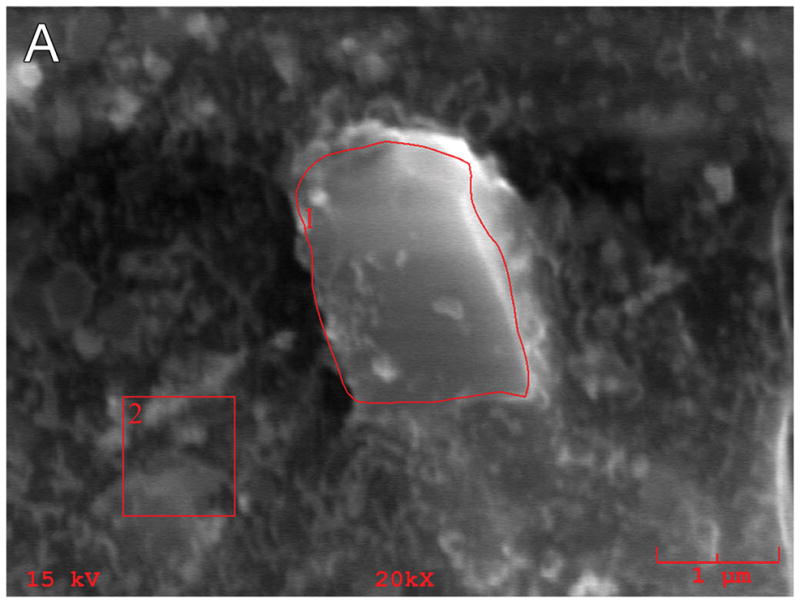
Energy dispersive X-ray spectroscopy (EDS-SEM) of particulate material in a microscopic field (Fig. 5A) adjacent to that seen containing birefringent material in Figure 4 in case #21. The EDS analysis (Fig. 5B) is consistent with silicon as a foreign body with a concentration of 27.9 weight percent.
SEM
Scanning electron microscopy of the electrodes removed from temporal bones during the process of histologic preparation demonstrated cracks or fragmentation of the silicone carrier (Fig 6A) as compared to control electrodes which had never been implanted (Fig. 6B), and the creation of apparent small circular defects in the silicone carrier (Fig. 7).
Fig. 6.
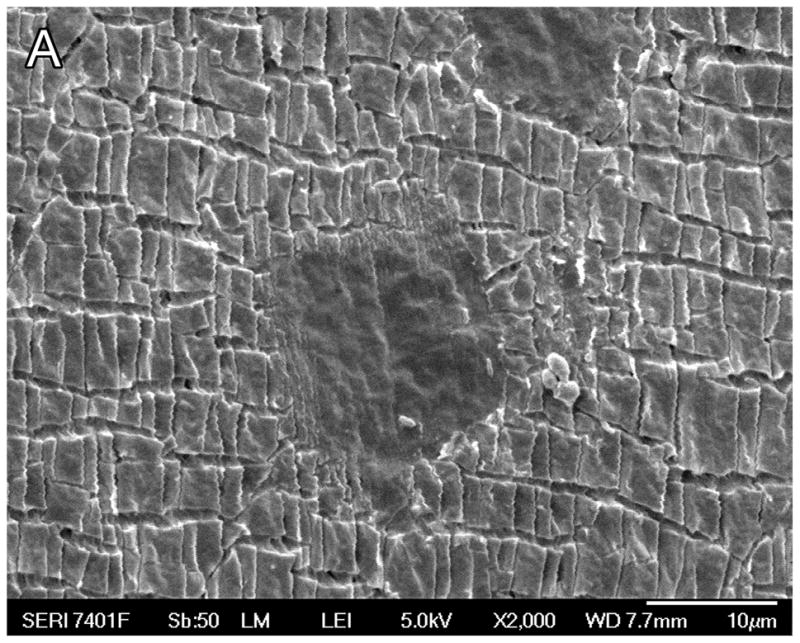

Fig. 6A. Scanning electron microscopy (SEM) of case #27, an individual who had a cochlear implant (Advanced Bionics High Res Helix) implanted two years previously in the right ear. Micro-fragmentation of the silicone carrier was seen.
Fig. 6B. SEM of a previously unimplanted control (Advanced Bionics High Res 90 Helix cochlear implant). The silastic carrier did not demonstrate the micro-fragmentation seen in Figure 6A.
Fig. 7.

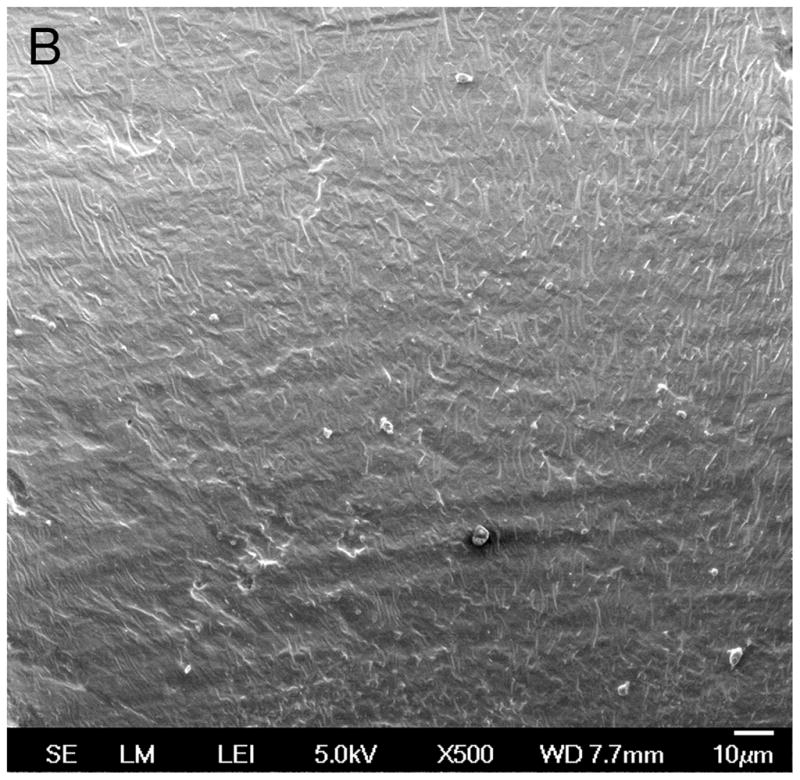
Fig. 7A. Scanning electron microscopy (SEM) of the silastic carrier implanted in case #19 (Nucleus modiolar-hugging cochlear implant) implanted three years prior to death, demonstrated small circular defects (arrows) consistent with loss of material from the silicone carrier.
Fig. 7B. Control Nucleus 24 electrode array not previously implanted. The silicone carrier showed no circular defects, as were shown in Figure 7A.
Discussion
In a previous report,15 particulate matter consistent with foreign body, both in an extracellular location and in an intracellar location as phagocytized by macrophages, were identified as platinum using EDS/SEM. These findings support the previous observation of particulate material consistent with platinum as described by Clark et al23,24 in the inner ear of an implanted patient.
In the current report, evidence of additional foreign material was identified by its birefringent nature as seen histopathologically. It was co-located with black particulate matter previously identified as platinum in areas of lymphocytic infiltration and was identified as silicon by EDS-SEM.
The foreign body reaction to the presence of implanted biomaterials includes absorption of plasma protein onto the surface of the material, followed by monocyte or macrophage adhesion and fusion of macrophages to form foreign body giant cells.2 The accumulation of monocytes and lymphocytes is consistent with a chronic inflammation. The recruitment of monocytes and macrophages is hypothesized to be in response to chemokines.2 Henson25,26 has reported the release of mediators of degradation from macrophages and foreign body giant cells which include reactive oxygen intermediates and degradative enzymes and the creation of an acid environment at the surface of the implanted biomaterial, thus exposing the implanted biomaterial to multiple degradative agents. Degradation of biomaterials of the implanted device includes “stress cracking” of elastomers and metal ion oxidation of an implanted electrode array.2,27
Macrophages that have become activated by the presence of a foreign body produce factors enhancing fibrogenesis by stimulating fibroblast activity. In addition, following adhesion of plasma proteins to implanted biomaterial surfaces, a state of reduced bactericidal capability is produced.28
An active foreign body response to the presence of a cochlear implant may be anticipated to result in device failure in some cases as has been reported.3,6,14,29,30,31 Benatti et al29 presented a meta-analysis of cochlear implant cases demonstrating that severe tissue response to the implant may result in extrusion. The severity of the cellular immune response has been demonstrated to be negatively correlated with performance32 and with the preservation of acoustic hearing following implantation.12
Reduction of the foreign body response to implanted electrodes may be achieved by alterations in surface chemistry of implanted biomaterials,33,34,35,36 or by changing the surface topography of the implanted electrode array,37 or the use of biomimetic silicone surfaces.38 It also remains to be seen what kind of response is initiated with implantation through the RW membrane versus a drilled cochleostomy site. It is reasonable to expect less reaction with less coating of blood (plasma proteins) on the carrier as it is inserted, and less overall trauma using a round window route.
Acknowledgments
This study was supported by grant #R01 DC000152 and #U 24DC013983 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders
We would like to thank Ann Tisdale from the Schepens Eye Research Institute of Boston for her expertise in processing, scanning, and photography of the specimens for scanning electron microscopy.
BIBLIOGRAPHY
- 1.Peppas NA, Langer R. New Challenges in biomaterials. Science. 1994;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertuleit H, Groden C, Schafer HJ, Leuwer R. Removal of a cochlear implant with chronic granulation labyrinthitis and foreign body reaction. Laryngo-rhinio-otologie. 1999;78:304–306. doi: 10.1055/s-2007-996876. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg J, Wolf M, Migirov L, Shapira Y, Aviel –Ronen S, Hildesheimer M. Foreign body reaction to cochlear implant. Oto-rhino-laryngol Nova. 2001;11:207–209. [Google Scholar]
- 5.Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol. 2004;25:257–262. doi: 10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB., Jr Silicone allergy: a new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27:1078–1082. doi: 10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- 7.Huang CQ, Tkyocinski M, Stathopoulos D, Cowan R. Effects of steroids and lubricants on electrical impedance and tissue response following cochlear implantation. Coch Implants Int. 2007 Sep;8(3):123–147. doi: 10.1179/cim.2007.8.3.123. [DOI] [PubMed] [Google Scholar]
- 8.Ho EC, Proops D, Andrews P, Graham J. Unexpected exit of a cochlear implant electrode to the wall of the basal turn of the cochlea – a report on two patients. Coch Implants Int. 2007;8:162–161. doi: 10.1179/cim.2007.8.3.162. [DOI] [PubMed] [Google Scholar]
- 9.Migirov L, Taitelaum-Swead R, Hildesheimer M, Kronenberg J. Revision surgeries in cochlear implant patients: a review of 45 cases. Eur Arch Oto-rhino-laryngol. 2007;264:3–7. doi: 10.1007/s00405-006-0144-5. [DOI] [PubMed] [Google Scholar]
- 10.Migirov L, Kronenberg J, Volkov A. Local tissue response to cochlear implant device housings. Otol Neurotol. 2011 Jan;32(1):55–57. doi: 10.1097/MAO.0b013e3182009d5f. [DOI] [PubMed] [Google Scholar]
- 11.Farhadi M, Jalessi M, Salehian P, Ghavi FF, Emamjomeh H, Mirzadeh H, Imani M, Jolly C. Dexamethasone eluting cochlear implant: Histological study in animal model. Cochlear Implants Int. 2013 Jan;14(1):45–50. doi: 10.1179/1754762811Y.0000000024. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O’Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in the human. Otol Neurotol. 2014;35:1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadol JB, Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol Neurotol. 2008;29:1076–1084. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadol JB, Jr, O’Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. 2014;318:11–17. doi: 10.1016/j.heares.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marturri L, Azzolini A, Campiglio GL, Tardito E. Are synthetic prostheses really inert: preliminary results of study on the biocompatibility of Dacron vascular prostheses and Silicone skin expanders. Int Surg. 1991;76:115–118. [PubMed] [Google Scholar]
- 17.Kistler U, Weiss AP, Simmen BR, Herren DB. Long term results of silicone wrist arthroplasty in patients with rheumatoid arthritis. J Hand Surg (Am) 2005;30:1282–1287. doi: 10.1016/j.jhsa.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Maushagen E, Reischle B, Simon H. Circumscribed erythema after cardiac pacemaker implantation. Z Kardiol. 1994;83:340–342. [PubMed] [Google Scholar]
- 19.Snow RB, Kossovosky N. Hypersensitivity reactions associated with sterile ventriculo-peritoneal shunt malfunction. Surg Neurol. 1989;31:209–214. doi: 10.1016/0090-3019(89)90119-5. [DOI] [PubMed] [Google Scholar]
- 20.Meyer DR, Bui HX, Carlson JA, et al. Silicone granulomas and dermatomyositis-like changes associated with chronic eyelid edema after silicone breast implant. Ophthal Plast Reconstr Surg. 1998;14:182–188. doi: 10.1097/00002341-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 21.O’Malley JT, Burgess BJ, Jones DD, Adams JC, Merchant SN. Techniques of celloidin removal from temporal bone sections. Ann Otol Rhinol Laryngol. 2009a;118(6):435–441. doi: 10.1177/000348940911800606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Malley JT, Merchant SN, Burgess BJ, Jones DD, Adams JC. Effects of fixative in embedding medium on morphology and immunostaining of the cochlea. Audiol Neuro Otol. 2009b;14(2):78–87. doi: 10.1159/000158536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark GM, Clark JC, Furness JB. The evolving science of cochlear implants (viewpoint) JAMA. 2013;310:1225–1226. doi: 10.1001/jama.2013.278142. [DOI] [PubMed] [Google Scholar]
- 24.Clark GM, Clark J, Cardamone T, Clarke M, Nielsen P, Jones R, Arhatari B, Birbilis N, et al. Biomedical studies on temporal bones of the first multi-channel cochlear implant patient at the University of Melbourne. Coch Implants Int. 2014;(Suppl 2):S1–15. doi: 10.1179/1754762814Y.0000000087. [DOI] [PubMed] [Google Scholar]
- 25.Henson PM. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971a;107(6):1535–1546. [PubMed] [Google Scholar]
- 26.Henson PM. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971b;107(6):1547–1547. [PubMed] [Google Scholar]
- 27.Barrese JC, Aceros J, Donoghue J. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J Neural Eng. 2016;13:026003, 27. doi: 10.1088/1741-2560/13/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodbeck WG, Shive MS, Colton E, Nakayama Y, Matsuda T, Anderson JM. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J Biomed Mater Res. 2001;55(4):661–668. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Benatti A, Castiglione A, Trevisi P, et al. Endocochlear inflammation in cochlear implant users: case report and literature review. Int J Pedi Otorhinolaryngol. 2013;77:885–893. doi: 10.1016/j.ijporl.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005;115:1760–1762. doi: 10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- 31.Neilan RE, Pawlowski K, Isaacson B, et al. Cochlear implant device failure secondary to cholesterol granuloma-mediated cochlear erosion. Otol Neurotol. 2012;33:733–735. doi: 10.1097/MAO.0b013e3182544fff. [DOI] [PubMed] [Google Scholar]
- 32.Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hetrick EM, Prichard HL, Klitzman B, Schoenfisch MH. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2007 Nov;28(31):4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeplin PH, Larena-Avellaneda A, Jordan M, Laske M, Schmidt K. Phosphorylcholine-coated silicone implants: Effect on inflammatory response and fibrous capsule formation. Ann Plast Surg. 2010 Dec;65(6):560–564. doi: 10.1097/SAP.0b013e3181d6e326. [DOI] [PubMed] [Google Scholar]
- 35.Joseph J, Mohanty M. Influence of curing agent on fibrosis around silicone implants. J Biomater Sci Polym Ed. 2013;24(9):1140–1151. doi: 10.1080/09205063.2012.743060. [DOI] [PubMed] [Google Scholar]
- 36.Moore LB, Sawyer AJ, Charokopos A, Skokos EA, Kyriakides TR. Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFkB activation in the foreign body response. Acta Biomater. 2015 Jan;11:37–47. doi: 10.1016/j.actbio.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier TO, Anderson JM, Brodbeck WG, Barber T, Healy KE. Inhibition of macrophage development and foreign body giant cell formation by hydrophilic interpenetrating polymer network. J Biomed Mater Res A. 2004 Jun 15;69(4):644–650. doi: 10.1002/jbm.a.30030. [DOI] [PubMed] [Google Scholar]
- 38.Kyle DJ, Oikonomou A, Hill E, Bayat A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials. 2015 Jun;52:88–102. doi: 10.1016/j.biomaterials.2015.02.003. [DOI] [PubMed] [Google Scholar]