Abstract
Estimates of those living in rural counties vary from 46.2–59 million, or 14–19% of the U.S. population. Rural communities face disadvantages compared to urban areas, including higher poverty, lower educational attainment, and lack of access to health services. We aimed to demonstrate rural-urban disparities in cancer and to examine NCI-funded cancer control grants focused on rural populations. Estimates of five-year cancer incidence and mortality from 2009–2013 were generated for counties at each level of the rural-urban continuum and for metropolitan versus non-metropolitan counties, for all cancers combined and several individual cancer types. We also examined the number and foci of rural cancer control grants funded by NCI from 2011–2016. Cancer incidence was 447 cases per 100,000 in metropolitan counties and 460 per 100,000 in non-metropolitan counties (p<0.001). Cancer mortality rates were 166 per 100,000 in metropolitan counties and 182 per 100,000 in non-metropolitan counties (p<0.001). Higher incidence and mortality in rural areas were observed for cervical, colorectal, kidney, lung, melanoma, and oropharyngeal cancers. There were 48 R- and 3 P-mechanism rural-focused grants funded from 2011–2016 (3% of 1655). Further investment is needed to disentangle the effects of individual-level SES and area-level factors to understand observed effects of rurality on cancer.
Keywords: rural, rural-urban, RUCC, cancer, funding
Introduction
Estimates of the total population living in non-metropolitan (rural) counties in the United States vary from 46.2 million (1) to 59 million (2) people, compared to more than 250 million people living in urban areas. This represents 14 to 19 percent of the U.S. population (1,2). Rural communities face notable disadvantages compared to urban areas, including higher poverty rates, lower educational attainment, a higher proportion of elderly individuals, lack of access to health services, and a lack of resources needed to support the public health infrastructure (3). As a result of these and other factors, rural communities face elevated rates of morbidity and mortality, as well as greater percentages of potentially excess deaths from the five leading causes of death, including cancer (4). Individuals in rural counties not only have an 8 percent higher overall cancer mortality than those in urban areas, but a rural-urban disparity in mortality has also been observed for lung, colorectal, prostate, and cervical cancers, though, in several cases, adjusting for socioeconomic status attenuates or completely explains the relationship between rurality and higher cancer mortality (5).
Additional rural-urban disparities across the cancer control continuum have been documented, though the existing literature is nascent and methodologically inconsistent compared with other research identifying race-, economic-, and age-based disparities in diagnosis, treatment, and survival of cancer (6). At least two studies have demonstrated that cervical cancer incidence is higher in rural areas (7,8). There is also some evidence that rural residents are less likely to get screened for cancer (6): for example, an analysis of 2008 Behavioral Risk Factor Surveillance System (BRFSS) data showed that rural women were less likely to meet recommendations for mammography than urban women; that the proportion of women reporting appropriate cervical cancer screening decreased as rurality increased; and that individuals from rural areas were less likely to report colorectal cancer screening than individuals from urban areas (9). Furthermore, rural individuals may be less likely to receive follow-up testing after receiving abnormal screening results (10), and although findings are not consistent with regard to rural-urban differences in stage at diagnosis, some research suggests that women from rural areas are more likely to be diagnosed with more advanced breast cancer compared to their urban counterparts (11).
Evidence also suggests that there are rural-urban differences in cancer treatment. For example, rural women are more likely to receive mastectomies than breast conserving surgery, and rural patients with either endometrial cancer or prostate cancer are less likely to receive radiation therapy than non-rural patients (6). Another marked disparity in treatment between rural and urban cancer patients concerns recruitment into clinical trials. A study evaluating treatment trials in North Carolina found that the rate of enrollment was higher among urban populations than among the most rural populations (2.4% vs. 1.5%) (12). The authors suggested that despite initiatives like the Community Clinical Oncology Program (CCOP) and the National Community Cancer Centers Program (NCCCP) to enhance access to cancer trials and increase enrollment of diverse patients, rural residents still face access barriers to trial participation (12).
Although most research does not indicate that residing in a rural area is independently associated with substantially worse cancer survivorship, there is evidence suggesting that individuals treated at smaller hospitals experience worse survival than those treated in larger facilities, and that rural cancer patients are more likely to receive treatment in smaller hospitals (13, 16). There is also evidence to suggest that rural cancer survivors experience worse quality of life and mental health outcomes than survivors from urban areas (14, 15), and they may be less likely to use hospice care at the end of life (13).
Despite these and other rural-urban disparities in cancer, few national level priority-setting reports or initiatives have encouraged research on cancer in rural populations. In the 2003 report entitled “Unequal Treatment; Confronting Racial and Ethnic Disparities in Healthcare,” the Institute of Medicine identified rural residence as a potential risk factor for patient health disparities, stating that “for all patients, processes of care… were of lower quality in rural hospitals and best in urban teaching hospitals,” (16) but the disparities identified in the report were focused on cardiac care and few were cancer-specific. Similarly, while Healthy People 2020 (17) does not have specific objectives or targets related to rural health or rural cancer control, it does designate rurality as one of fourteen disparities contributing to poor health. The Health Resources and Services Administration’s Federal Office of Rural Health Policy(18) funds grants and community programs related to rural health, but few are cancer-specific.
The goals of this commentary were to demonstrate rural-urban disparities in cancer incidence and mortality from 2009–2013, and to examine five-year funding trends for rural-focused, NCI-funded cancer control grants awarded from 2011–2016.
Materials and Methods
Our aims and approaches were two-fold. First, to examine rural-urban disparities in cancer incidence and mortality, we generated estimates of five-year cancer incidence and mortality rates for counties at each level of the rural-urban continuum and for metropolitan versus non-metropolitan (rural) counties, for all cancers combined as well as several individual cancer types. Second, to determine the number and foci of funded studies examining cancer control in rural populations, we conducted a portfolio analysis of R- and P-mechanism grants funded between 2011 and 2016 by the National Cancer Institute’s Division of Cancer Control and Population Sciences (DCCPS), which funds the majority of cancer control grants at NCI.
Rural-Urban Disparities in Cancer Incidence and Mortality
Cancer incidence rates are based on data from the Surveillance, Epidemiology, and End Results (SEER) program. SEER includes 18 cancer registries across the United States, each of which captures nearly 100% of incident cases in their catchment area. Together, the registries cover about 30% of the U.S. population. Mortality data are provided to SEER by the National Center for Health Statistics; thus, estimates of cancer mortality cover the entire U.S. population. Information on county rurality came from the United States Department of Agriculture, Economic Research Service’s 2013 rural-urban continuum codes (RUCC). These codes are calculated based on counties’ population size, degree of urbanization, and proximity to a metropolitan area, drawing on data from the 2010 Census and the 2006–2010 American Community Survey. The codes range from 1 to 9, with codes 1 to 3 designating metropolitan counties and codes 4 to 9 designating non-metropolitan (rural) counties (Table 1). We generated estimates of five-year (2009–2013) overall cancer incidence and mortality rates for counties at each level of the rural-urban continuum, as well as for metropolitan versus rural counties. In addition, we estimated incidence and mortality for metropolitan versus rural counties for the following individual cancer sites: cervical, colorectal, kidney, lung and bronchus, melanoma, oropharyngeal, breast, liver, and thyroid. We created a line chart depicting overall cancer incidence and mortality at each RUCC. Finally, we conducted t-tests to statistically compare cancer rates for metropolitan versus rural counties. Cancer rates were generated using SEER*Stat version 8.3.2, and analyses were conducted using SAS version 9.3. Statistical tests used a two-sided p value of .05.
Table 1.
Definition of rural-urban continuum codes (RUCC) and population sizes for counties in the United States.
| RUCC | Definition | k counties | Population in SEER1 | Population in U.S.1 | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 1 | Counties in metro areas of 1 million population or more | 472 | 54,360,203 | 62.7% | 170,707,445 | 54.8% |
| 2 | Counties in metro areas of 250,000 to 1 million population | 395 | 17,963,604 | 20.7% | 66,226,176 | 21.2% |
| 3 | Counties in metro areas of fewer than 250,000 population | 369 | 6,104,298 | 7.0% | 28,501,828 | 9.1% |
| 4 | Urban population of 20,000 or more, adjacent to a metro area | 217 | 1,845,954 | 2.1% | 13,544,583 | 4.3% |
| 5 | Urban population of 20,000 or more, not adjacent to a metro area | 92 | 1,374,217 | 1.6% | 4,976,874 | 1.6% |
| 6 | Urban population of 2,500 to 19,999, adjacent to a metro area | 597 | 2,427,381 | 2.8% | 14,747,534 | 4.7% |
| 7 | Urban population of 2,500 to 19,999, not adjacent to a metro area | 434 | 1,736,695 | 2.0% | 8,243,858 | 2.6% |
| 8 | Completely rural or less than 2,500 urban population, adjacent to a metro area | 220 | 415,639 | 0.5% | 2,146,218 | 0.7% |
| 9 | Completely rural or less than 2,500 urban population, not adjacent to a metro area | 425 | 492,659 | 0.6% | 2,595,449 | 0.8% |
| 1–3 | Metropolitan | 1,236 | 78,428,104 | 90.4% | 265,435,449 | 85.2% |
| 4–9 | Non-metropolitan | 1,985 | 8,292,544 | 9.6% | 46,254,517 | 14.8% |
Average population across 2009–2013.
Five-Year Funding Trends
Using NCI’s Portfolio Management Application software package version 16.1, we conducted a portfolio analysis of R-mechanism (hypothesis-driven, investigator initiated) and P-mechanism (program project) grants funded by NCI’s Division of Cancer Control and Population Sciences (DCCPS) between 2011 and 2016. DCCPS is the primary funder of cancer control research at NCI. Because the analysis was intended to examine the current cancer control research portfolio and to highlight gaps in the science, our analysis was restricted to grants that focused largely on cancer control, which falls within the mission of DCCPS, to support research that reduces the risk, incidence, and deaths from cancer as well as enhance the quality of life for cancer survivors. The Division conducts and supports genetic, epidemiologic, behavioral, social, and surveillance cancer research. We did not include grants funded by other NCI divisions, such as the Division of Cancer Prevention, whose goal is to support clinical and laboratory research with community and multidisciplinary teams, and collaborative scientific networks.
Inclusion criteria included those funded grants for which the keywords “rural,” “Appalachia,” “frontier,” “reservation,” or “tribal lands” appeared in the title, abstract, or specific aims. Manual exclusions were applied to irrelevant grants upon review by the research team. Grants that used any of the rural keywords from the above taxonomy only as descriptors of the catchment area of the study but without an explicit focus on rural populations or rural disparities were excluded from further analysis.
Results
Rural-Urban Disparities in Cancer Incidence and Mortality
Out of the 3,221 counties in the U.S., most were rural (k=1,985, or 62%) (Table 1). However, averaged across 2009–2013, the majority of individuals in the population lived in metropolitan counties (265,435,449 of 311,689,517 people, or 85%, compared to 46,245,517, or 14%, in rural counties).
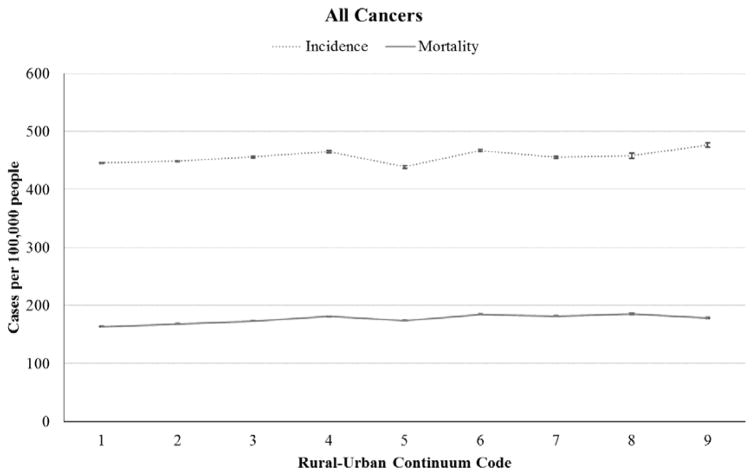
Figure 1 depicts 2009–2013 overall cancer incidence rates (in SEER areas) and mortality rates (in the entire U.S.) across the rural-urban continuum. In metropolitan counties, cancer incidence was 447 cases per 100,000 people (range across RUCCs: 446–456), while in rural counties, incidence was 460 per 100,000 people (range: 439–477). Mortality rates were 166 per 100,000 people in metropolitan counties (range: 164–173) and 182 per 100,000 people in rural counties (range: 174–185). Thus, cancer rates were higher in rural than metropolitan counties: +12.3 cases per 100,000 people or 2.7% for incidence and +15.9 cases or 9.6% for mortality (both p<.001) (Table 2).
Figure 1.
Incidence and mortality rates per 100,000 people across rural-urban continuum codes for all cancers, 2009–2013. Codes 1–3 designate metropolitan counties. Codes 4–9 designate non-metropolitan (rural) counties.
Table 2.
Cancer incidence and mortality rates in metropolitan versus non-metropolitan (rural) counties per 100,000 people per year, 2009–2013.
| Cancer type | Incidence | Mortality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metro | Rural | Diff. | % diff. | p | Metro | Rural | Diff. | % diff. | p | |||||
| Rate | SE | Rate | SE | Rate | SE | Rate | SE | |||||||
| All cancers | 447.3 | 0.3 | 459.6 | 1.0 | 12.3 | 2.7% | <.001 | 165.8 | 0.1 | 181.7 | 0.3 | 15.9 | 9.6% | <.001 |
| Individual cancers | ||||||||||||||
| Cervical1 | 7.4 | 0.1 | 8.5 | 0.2 | 1.1 | 14.9% | <.001 | 2.3 | 0.0 | 2.6 | 0.0 | 0.3 | 13.0% | <.001 |
| Colorectal | 40.4 | 0.1 | 45.8 | 0.3 | 5.4 | 13.4% | <.001 | 14.7 | 0.0 | 17.0 | 0.1 | 2.3 | 15.6% | <.001 |
| Kidney | 15.4 | 0.1 | 17.2 | 0.2 | 1.8 | 11.7% | <.001 | 3.8 | 0.0 | 4.4 | 0.0 | 0.6 | 15.8% | <.001 |
| Lung and bronchus | 55.4 | 0.1 | 71.9 | 0.4 | 16.5 | 29.8% | <.001 | 44.4 | 0.1 | 53.4 | 0.1 | 9.0 | 20.3% | <.001 |
| Melanoma | 21.8 | 0.1 | 22.4 | 0.2 | 0.6 | 2.8% | 0.02 | 2.7 | 0.0 | 3.0 | 0.0 | 0.3 | 11.1% | <.001 |
| Oropharyngeal | 10.9 | 0.1 | 12.7 | 0.2 | 1.8 | 16.5% | <.001 | 2.4 | 0.0 | 2.8 | 0.0 | 0.4 | 16.7% | <.001 |
| Breast1 | 126.1 | 0.2 | 114.8 | 0.7 | −11.3 | −9.0% | <.001 | 21.5 | 0.1 | 21.5 | 0.1 | 0.0 | 0.0% | 0.99 |
| Liver | 8.6 | 0.0 | 6.7 | 0.1 | −1.9 | −22.1% | <.001 | 6.3 | 0.0 | 5.6 | 0.0 | −0.7 | −11.1% | <.001 |
| Thyroid | 14.10 | 0.10 | 12.30 | 0.20 | −1.8 | −12.8% | <.001 | 0.51 | 0.01 | 0.48 | 0.01 | 0.0 | −5.9% | 0.45 |
Measured among women only.
Note. Metro=metropolitan counties; Rural=non-metropolitan counties; SE=standard error; Diff.=absolute difference between rates (non-metropolitan minus metropolitan); % diff.=relative difference between rates (absolute difference divided by rate in metropolitan counties).
Similar patterns emerged for cervical cancer (measured among women only) as well as colorectal, kidney, lung and bronchus, melanoma, and oropharyngeal cancers (Table 2). For example, lung cancer incidence was 29.8% higher and mortality was 20.3% higher in rural compared to metropolitan counties (both p<.001) (Table 2). In contrast, cancer rates were higher in metropolitan versus rural counties for three cancer types: breast (incidence only), liver, and thyroid (incidence only) (Table 2). For example, liver cancer incidence was 22.1% lower and mortality was 11.1% lower in rural compared to metropolitan areas (both p<.001) (Table 2).
Five-Year Funding Trends
There were 48 R-mechanism grants (3 percent of 1589 total) that focused on cancer control in rural populations funded by NCI’s Division of Cancer Control and Population Sciences (DCCPS) from 2011–2016. Mechanisms included: R03 (12 grants); R21 (17 grants); R15 (2 grants); and R01 (17 grants). Most of the R01s (n=16) focused on intervention research. Of the 48 grants, 29 (60%) focused on rural populations alone; the 19 others (40%) focused on rural-urban comparisons or were part of a larger study that included rural areas/populations.
For those 29 funded grants focused solely on rural areas, 9 were descriptive and 20 were intervention studies. Among the descriptive studies, 4 sought to identify survivorship needs for health care follow-up, and the remaining 5 studies focused on patient navigation, adjuvant therapy, tobacco use in youth, patterns of patient care, and risk factors for childhood leukemia. Among the intervention studies, 8 were on screening and patient-provider communication; 5 on diet and physical activity; 3 on tobacco use; 3 on health care planning for cancer survivors; and 1 on tanning salons.
For those 19 grants that focused partially on rural populations or conducted rural-urban comparisons, 13 were descriptive and 6 were intervention studies. Of the 13 descriptive studies, 3 focused on diet and physical activity; 2 on breast cancer treatment differences; 2 on attitudes toward cancer trials accrual and biospecimen collection; and 1 each on cost of cancer care, HPV vaccination, tobacco use, risk factors for testicular cancer, Medicare claims and cancer relapse, and risk factors for cancer in the Southern Community Cohort. Among the intervention studies, 2 focused on colorectal cancer screening; 1 on tobacco cessation; 2 on long term survivorship and preventive care planning; and 1 on provider training to reduce racism in the patient-provider encounter.
DCCPS funded 3 P-mechanism grants (4 percent of 66 total) that focused on rural populations between 2011–2016. All were P50 (specialized center) awards. Of these, 2 focused solely on rural populations (one on cervical cancer and one on tobacco), and one had a partial focus on rural populations (related to tobacco use and breast cancer screening).
Discussion
In addition to summarizing what is known from the literature about cancer-related rural-urban disparities, the goals of this commentary were two-fold. We examined rural-urban disparities in cancer incidence and mortality from 2009–2013, and examined five-year funding trends for NCI-funded rural cancer control grants awarded from 2011–2016. Our study revealed significant rural-urban differences in cancer incidence and mortality rates in the U.S. for all cancers combined and for several individual cancer types. It also demonstrated that research funding focused on cancer control in rural populations has been limited in the last 5 years.
Some of the rural-urban disparities in cancer incidence and mortality, as identified in our study and others cited within, likely can be attributed to barriers to accessing health services in rural areas. Charlton and colleagues have pointed out that financial barriers, such as being uninsured, and a general shortage of physicians may limit access to preventive and screening services for rural residents (13). In addition, the absence of specialists in rural areas is especially striking: while there are 134 specialists per 10,000 people in urban areas, there are only around 40 specialists per 10,000 people in rural areas, and this has an impact on the availability of cancer screening and treatment (13). In fact, there are many rural counties in the United States that don’t have a single medical or radiation oncologist, which results in patients having to travel greater distances to obtain care (13). Further, the large distance between many rural patients and oncology services may result in patients without personal vehicles or access to public transportation being unable to access care at all (13).
Although we have highlighted research examining rural-urban disparities in cancer screening, incidence, treatment, and mortality, less attention has been paid to disparities in social and behavioral risk factors for cancer, even though there is evidence to suggest that rural and urban populations engage in certain health behaviors at different rates. Studies examining rural-urban differences in tobacco use (19–26); obesity (27–29), diet(30–32), weight and physical activity(33); sun safety(34–36); alcohol consumption(25,37,38); and HPV vaccination(39) deserve more attention.
Beyond access to care and behavioral risk factors for cancer, the next generation of studies designed to explain rural-urban differences in cancer should include a more extensive list of variables in order to attribute variance appropriately and avoid misinterpretation of findings. For example, the development of effective interventions to reduce rural-urban health disparities will depend on a more sophisticated analysis of differences that should address broader social determinants of health, including cultural differences that influence trust in and attitudes toward institutions, medical providers, and government-sponsored programs. Moreover, one of the greatest challenges in addressing rural-urban health disparities will be the complex relationship between comorbidities not traditionally associated with cancer prevention and control (e.g., opiate drug abuse) and established cancer risk factors. Collaboration with organizations and programs with experience or infrastructure (e.g., telemedicine, behavioral health services) initially designed to address other health or social problems in rural populations could afford substantial opportunities to cancer prevention and control investigators.
The specific nature of rural-urban differences in social and physical context in one region of the country might not generalize to another (e.g., in Alaska versus Mississippi, or frontier areas versus Appalachia), and will need to be considered in future research. Similarly, the specific contextual and cultural factors that affect rural-urban differences in one domain of cancer control (e.g., differences in HPV vaccination rates and associated cervical cancer incidence) might not generalize to another (e.g., low uptake of colorectal cancer screening and associated colorectal cancer incidence). The breadth and complexity of unanswered research questions concerning rural cancer control and the need for greater integration of economic, health, and educational programs for rural populations poses a substantial challenge for the development of effective and sustainable cancer control interventions. Nevertheless, we believe that new efforts are warranted to address significant disparities that might otherwise worsen in the coming decade.
In reviewing the literature for this commentary, it became increasingly evident that the various definitions of “rural” (at least 15 federal definitions, and 11 within the USDA alone) can complicate the interpretation of studies examining rural-urban differences in cancer. Meilleur and colleagues’ 2013 article (6) is a great start toward developing conventions for how rural residence should be defined, measured, and analyzed in cancer-related research, but more work is necessary to be sure that derived rural-urban variables are available in public-use datasets and that their analytic utility is maximized. Further, more work is necessary to disentangle the effects of individual-level SES and area-level factors (e.g., census tract poverty) in multivariable, multi-level models, to understand the independent association of rurality on outcomes relevant to cancer prevention and control. Many of the published studies, including our own, present only unadjusted estimates for rural-urban disparities in cancer. Finally, with just 3 percent of the portfolio of cancer control grants being focused on rural populations in the past five years (48 R-mechanism and 3 P-mechanism grants of 1655 total), further investment is needed to address these and other questions, and to make a meaningful difference in reducing the burden of cancer in rural populations.
Acknowledgments
The authors gratefully acknowledge Fekade Shewakena for his assistance with the grant portfolio analysis conducted for this commentary.
Footnotes
COI Statement: The authors declare no potential conflicts of interest.
References
- 1.Kusmin L. Rural America at a glance, 2016 edition. Washington, DC: United States Department of Agriculture; 2016. [Google Scholar]
- 2.2010 Census Urban and Rural Classification and Urban Area Criteria [Internet] United States Census Bureau; Available from: < https://www.census.gov/geo/reference/ua/urban-rural-2010.html>. [Google Scholar]
- 3.Bolin JN, Bellamy GR, Ferdinand AO, Vuong AM, Kash BA, Schulze A, et al. Rural Healthy People 2020: New Decade, Same Challenges. J Rural Health. 2015;31(3):326–33. doi: 10.1111/jrh.12116. [DOI] [PubMed] [Google Scholar]
- 4.Moy E, Garcia MC, Bastian B, Rossen LM, Ingram DD, Faul M, et al. Leading Causes of Death in Nonmetropolitan and Metropolitan Areas — United States, 1999–2014. Centers for Disease Control and Prevention; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1657–67. doi: 10.1158/1055-9965.epi-13-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benard VB, Coughlin SS, Thompson T, Richardson LC. Cervical cancer incidence in the United States by area of residence, 1998 2001. Obstet Gynecol. 2007;110(3):681–6. doi: 10.1097/01.aog.0000279449.74780.81. [DOI] [PubMed] [Google Scholar]
- 8.Singh GK. Rural-urban trends and patterns in cervical cancer mortality, incidence, stage, and survival in the United States, 1950–2008. J Community Health. 2012;37(1):217–23. doi: 10.1007/s10900-011-9439-6. [DOI] [PubMed] [Google Scholar]
- 9.Bennett KJ, Probst JC, Bellinger JD. Receipt of cancer screening services: surprising results for some rural minorities. J Rural Health. 2012;28(1):63–72. doi: 10.1111/j.1748-0361.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 10.Paskett ED. Breast Cancer Among Special Populations: Disparities in Care Across the Cancer Control Continuum. Adv Exp Med Biol. 2015;862:39–52. doi: 10.1007/978-3-319-16366-6_4. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228–35. doi: 10.1016/j.annepidem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Zullig LL, Fortune-Britt AG, Rao S, Tyree SD, Godley PA, Carpenter WR. Enrollment and Racial Disparities in Cancer Treatment Clinical Trials in North Carolina. N C Med J. 2016;77(1):52–8. doi: 10.18043/ncm.77.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of Rural Cancer Care in the United States. Oncology (Williston Park, NY) 2015;29(9):633–40. [PubMed] [Google Scholar]
- 14.Reid-Arndt SA, Cox CR. Does rurality affect quality of life following treatment for breast cancer? J Rural Health. 2010;26(4):402–5. doi: 10.1111/j.1748-0361.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrykowski MA, Steffens RF, Bush HM, Tucker TC. Disparities in mental health outcomes among lung cancer survivors associated with ruralness of residence. Psycho-oncology. 2014;23(4):428–36. doi: 10.1002/pon.3440. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academy of Sciences; 2002. [Google Scholar]
- 17.Healthy People 2020 [Internet] U.S. Department of Health and Human Services; Available from: < https://www.healthypeople.gov/>. [Google Scholar]
- 18.Rural Health Funding Opportunities [Internet] Federal Office of Rural Health Policy, Health Resources & Services Administration, U.S. Department of Health and Human Services; Available from: < https://www.hrsa.gov/ruralhealth/programopportunities/fundingopportunities/default.aspx>. [Google Scholar]
- 19.Doescher MP, Jackson JE, Jerant A, Gary Hart L. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22(2):112–8. doi: 10.1111/j.1748-0361.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh GK, Siahpush M, Williams SD. Changing urbanization patterns in US lung cancer mortality, 1950–2007. J Community Health. 2012;37(2):412–20. doi: 10.1007/s10900-011-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard-Pitney B, Winkleby MA. Chewing tobacco: who uses and who quits? Findings from NHANES III, 1988–1994. National Health and Nutrition Examination Survey III. Am J Public Health. 2002;92(2):250–6. doi: 10.2105/ajph.92.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell RA, Spangler JG, Quandt SA. Smokeless tobacco use among adults in the Southeast. South Med J. 2000;93(5):456–62. [PubMed] [Google Scholar]
- 23.Rodu B, Cole P. Smokeless tobacco use among men in the United States, 2000 and 2005. J Oral Pathol Med. 2009;38(7):545–50. doi: 10.1111/j.1600-0714.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 24.Atav S, Spencer GA. Health risk behaviors among adolescents attending rural, suburban, and urban schools: a comparative study. Fam Community Health. 2002;25(2):53–64. doi: 10.1097/00003727-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Gfroerer JC, Larson SL, Colliver JD. Drug use patterns and trends in rural communities. J Rural Health. 2007;23(Suppl):10–5. doi: 10.1111/j.1748-0361.2007.00118.x. [DOI] [PubMed] [Google Scholar]
- 26.Weaver KE, Palmer N, Lu L, Case LD, Geiger AM. Rural-urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control. 2013;24(8):1481–90. doi: 10.1007/s10552-013-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005–2008) J Rural Health. 2012;28(4):392–7. doi: 10.1111/j.1748-0361.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis AM, Bennett KJ, Befort C, Nollen N. Obesity and related health behaviors among urban and rural children in the United States: data from the National Health And Nutrition Examination Survey 2003–2004 and 2005–2006. J Pediatr Psychol. 2011;36(6):669–76. doi: 10.1093/jpepsy/jsq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JA, 3rd, Johnson AM. Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes (Print) 2015;11(3):233–41. doi: 10.1089/chi.2014.0085. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi T, Liu J, Probst J, Merchant A, Jhones S, Martin AB. Obesity and obesity-related behaviors among rural and urban adults in the USA. Rural Remote Health. 2015;15(4):3267. [PubMed] [Google Scholar]
- 31.McCormack LA, Meendering J. Diet and Physical Activity in Rural vs Urban Children and Adolescents in the United States: A Narrative Review. J Acad Nutr Diet. 2016;116(3):467–80. doi: 10.1016/j.jand.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Liu JH, Jones SJ, Sun H, Probst JC, Merchant AT, Cavicchia P. Diet, physical activity, and sedentary behaviors as risk factors for childhood obesity: an urban and rural comparison. Child Obes (Print) 2012;8(5):440–8. doi: 10.1089/chi.2012.0090. [DOI] [PubMed] [Google Scholar]
- 33.Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151–9. doi: 10.1111/j.1748-0361.2004.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 34.Zahnd WE, Goldfarb J, Scaife SL, Francis ML. Rural-urban differences in behaviors to prevent skin cancer: an analysis of the Health Information National Trends Survey. J Am Acad Dermatol. 2010;62(6):950–6. doi: 10.1016/j.jaad.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 35.Quinn M, Alamian A, Hillhouse J, Scott C, Turrisi R, Baker K. Prevalence and Correlates of Indoor Tanning and Sunless Tanning Product Use among Female Teens in the United States. Prev Med Rep. 2015;2:40–3. doi: 10.1016/j.pmedr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the United States. Arch Pediatr Adolesc Med. 2003;157(9):854–60. doi: 10.1001/archpedi.157.9.854. [DOI] [PubMed] [Google Scholar]
- 37.Borders TF, Booth BM. Rural, suburban, and urban variations in alcohol consumption in the United States: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Rural Health. 2007;23(4):314–21. doi: 10.1111/j.1748-0361.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JE, Doescher MP, Hart LG. Problem drinking: rural and urban trends in America, 1995/1997 to 2003. Prev Med. 2006;43(2):122–4. doi: 10.1016/j.ypmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR. Uptake of free HPV vaccination among young women: a comparison of rural versus urban rates. J Rural Health. 2011;27(4):380–4. doi: 10.1111/j.1748-0361.2010.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]