Abstract
Ultraviolet (UV) exposure has an array of damaging effects and is the main cause of skin cancer in humans. Non-melanoma skin cancer (NMSC), including basal cell carcinoma and squamous cell carcinoma, is the most common type of cancer. Incidence of NMSC has increased due to greater UV radiation, increased life expectancy, and other changes in lifestyle; the annual cost of skin cancer treatment in the United States has increased concurrently to around eight billion dollars. Because of these trends, novel approaches to skin cancer prevention have become an important area of research to decrease skin cancer morbidity and defray the costs associated with treatment. Chemoprevention aims to prevent or delay the development of skin cancer through the use of phytochemicals. Use of phytochemicals as chemopreventive agents has gained attention due to their low toxicity and anti-carcinogenic properties. Phytochemicals also exhibit antioxidant, anti-inflammatory, and anti-proliferative effects which support their use as chemopreventive agents, particularly for skin cancer. Preclinical and human studies have shown that phytochemicals decrease UV-induced skin damage and photocarcinogenesis. In this review article, we discuss the selected phytochemicals that may prevent or delay UV-induced carcinogenesis and highlights their potential use for skin protection.
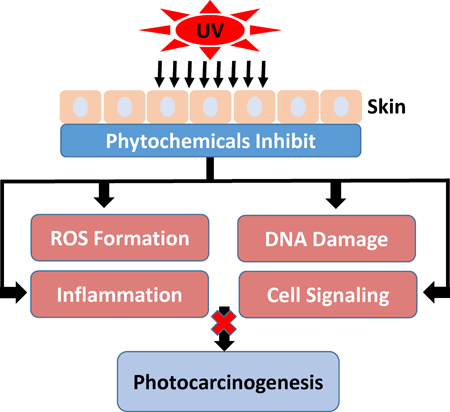
Graphical Abstract
Ultraviolet (UV) exposure is the main cause of human skin cancer due to its damaging effects on skin cells. Chemoprevention prevents or delays the development of skin cancer through the use of phytochemicals. Phytochemicals impede the damaging effects of UV radiation and may lead to inhibition of photocarcinogenesis.
INTRODUCTION
Skin is the largest organ in the human body and acts as an external barrier to the environment. Thus, skin is accessible to ultraviolet (UV) exposure through solar radiation or through UV-emitting tanning devices. The three main subtypes of UV radiation are UVA (320–400 nm), UVB (280–320 nm), and UVC (200–280 nm) (1). While UV radiation yields positive effects such as vitamin D production for proper bone health, UV exposure is the main cause of skin cancer development in humans due to its damaging effects (1,2). The skin is most susceptible to UV irradiation because macromolecules in the skin easily absorb UVB (3,4). UV irradiation produces damaging effects through DNA damage, reactive oxygen species (ROS) production, inflammation and activation of signal transduction pathways, and these effects comprise driving factors for skin cancer development. Because UVC is absorbed by the stratospheric ozone, it plays an insignificant role in human skin carcinogenesis. Although UVB only accounts for 5% of all UV radiation, it causes carcinogenesis to a higher magnitude than UVA. UVA accounts for 95% of all solar UV radiation but is less intense than UVB and does not induce carcinogenesis as effectively as UVB (1,5). Nonetheless, both UVA and UVB have been suggested as contributing factors to skin cancer development (6).
Non-melanoma skin cancer (NMSC) is the most common type of cancer, and its incidence exceeds breast, colon, lung, and prostate combined (7). However, NMSCs have a relatively good prognoses and low mortality rate (8,9). There are two primary types of non-melanoma skin cancer: squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), and they are both derived from the stratum basale of the epidermis. NMSC is commonly found in populations with fair skin (8). In the United States, NMSC incidence has increased with more than five million new cases each year (10). Further, those with a history of skin cancer are at an increased risk of developing other lethal types of cancer (11,12). Basal cell carcinoma accounts for 80–85% of skin cancer and is caused by intermittent UV exposure, particularly during youth (13,14). BCC rarely invades other tissues and grows slowly. Squamous cell carcinoma results from cumulative sun exposure and makes up around 15 to 20% of all skin cancers (13). SCC is common among those who work outdoors and are exposed to substantial UV radiation (15). SCC most frequently occurs on areas of the body which are most exposed to sunlight such as the face and head, and these cancers are capable of metastasizing (13,16,17). Sunscreen and protective clothing can prevent skin cancer with limited efficacy, and patients are often noncompliant with sunscreen use (18). Increased outdoor exposure to UV radiation, changes in lifestyle, and an increased life expectancy may contribute to the increased risk of developing skin cancer [www.cancer.org/statistics]. In the United States, the average annual cost of skin cancer treatment is more than eight billion dollars [cdc.gov/cancer/skin]. Therefore, preventive approaches must be developed in order to protect from skin cancer or its precursor conditions. Various phytochemicals are known to target UV-induced cellular events involved in skin tumorigenesis. The purpose of this review is to discuss the effects of UV radiation on skin carcinogenesis and the use of phytochemicals to prevent or delay the development of skin cancer.
UV-INDUCED DNA DAMAGE
The primary intracellular target for UV radiation is DNA, and UV light absorbed by DNA forms photoproducts (8). Cyclobutane pyrimidine dimers (CPDs) are the most common type of DNA damage and are formed in mammalian cells when DNA absorbs photons from UV radiation, particularly UVB (19,20). The skin is capable of naturally repairing dimers through photoreactivation via photolyases or through nucleotide excision repair (NER), which removes dimers and seals nicks in DNA (21). If mutations are present, p53 acts as a guardian of the genome by causing cell cycle arrest and regulating NER (22). NER is a critical protective mechanism, and its significance was revealed in humans with an autosomal recessive disorder of defective NER known as xeroderma pigmentosum (XP). These individuals are unable to repair UV-induced DNA damages and are much more predisposed to skin photocarcinogenesis. If the cell is incapable of repairing DNA damage, the mutagenic dimers can lead to proliferation and ultimately carcinogenesis. Such mutations are commonly found in tumor suppressor genes and oncogenes (23). p53 is a common mutated tumor suppressor gene found in about ninety percent of individuals with non-melanoma skin cancer (24). When p53 is mutated, cells enter the cell cycle without undergoing DNA repair and become resistant to apoptosis (25).
UV-INDUCED ROS FORMATION
UV radiation also generates ROS including superoxide anion, hydrogen peroxide (H2O2), and singlet oxygen, which further cause oxidative damage to DNA, proteins and lipids (7,26). ROS damage the deoxyribosyl backbone of DNA and produce pyrmidine dimers, including CPDs and 6,4-pyrimidine-pyrimidones, as well as DNA cross-links or strand breaks. Free radical oxidation of proteins causes a change in protein structure and function, which may alter or activate various signaling pathways and lead to inflammation, cell proliferation, angiogenesis and tumorigenesis (27). Free radicals may also extract electrons from lipids in the process of lipid peroxidation. This may lead to membrane damage and programmed cell death (28). The skin contains endogenous antioxidant systems (enzymatic and non-enzymatic), which function to neutralize free radicals and prevent cellular damages induced by oxidative damage (29,30). However, UV exposure overwhelms the skin’s endogenous ability to neutralize ROS, leading to a state of oxidative stress in the skin.
UV-INDUCED INFLAMMATION
UV exposure activates pro-inflammatory cytokines from keratinocytes, fibroblasts, and tumor cells. Tumor initiation, promotion, and progression are all influenced by UV-induced inflammation (31). Visible signs of skin inflammation include erythema (sunburn), edema and increased skin thickness, which result from dermal vasodilation and increased vasculature permeability (31,32).
UVB induces activation of the nuclear factor kappa B (NFκB) and mitogen-activated protein kinase (MAPK) pathways which enables transcription of lipid mediators and inflammatory genes such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) (33,34). UV-induced iNOS activation leads to the production of nitric oxide (NO) and subsequent inflammation and photocarcinogenesis. NO may interact with superoxide to generate ROS and further upregulate the expression of COX-2 (34). UV-induced COX-2 expression is also activated by AKT, AMPK and SIRT6 (13,35–37). COX-2 is the rate-limiting enzyme involved in the synthesis of prostaglandins which facilitate inflammatory processes (38). UV-induced expression of COX-2 has been implicated in inflammation and cancer including SCC and BCC (39,40). Prostaglandins, particularly PGE2, mediate skin inflammation in humans (41). One endogenous protective mechanism against inflammation is regulated by Nrf2, and activation of Nrf2 has been shown to protect against UV-induced inflammation in mice and humans (42).
UV exposure causes infiltration of activated macrophages and neutrophils (CD11b+ cells) which express iNOS and produce NO, a hallmark of inflammation (43). These oxidative products produced by inflammatory cells may induce tumor development at sites of chronic inflammation (44,45). Inflammatory leukocytes also produce myeloperoxidase (MPO) which is used as a marker of infiltration of inflammatory cells (46). MPO synthesis and secretion induces vasodilation during the inflammatory reaction (47). UV exposure of the skin also increases the expression of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α which aid in tumor promotion and carcinogenesis. TNF-α induces ROS formation and may lead tumor growth (48). IL-12 expression protects against tumorigenesis, and deficiency of IL-12 leads to increased COX-2 and PGE2 levels as well as increased levels of IL-1β, IL-6 and TNF-α (49,50).
UV-INDUCED CELL SIGNALING PATHWAYS
The MAPK family of serine/threonine kinases consists of ERKs, JNK, and p38 proteins (51). UV exposure leads to direct phosphorylation and activation of the MAPK pathway, or UV-induced ROS formation may indirectly activate the MAPK pathway (52). Once activated, p38, JNK, and ERK phosphorylate target proteins such as cyclic-AMP-response-element-binding protein (CREB). This results in the activation of various transcription factors including activator protein-1 (AP-1). The AP-1 complex is a dimer of c-Fos and c-Jun which mediates inflammation, cell proliferation, tumorigenesis, and extracellular matrix damage (13). AP-1 mediates cell proliferation by controlling the expression of cell cycle regulatory proteins such as cyclins, p53 and p21 (53).
UV irradiation activates the epidermal growth factor receptor (EGFR) which subsequently phosphorylates AKT in a PI3K-dependent mechanism. Activation of the PI3K pathway regulates cell proliferation, apoptosis, and inflammation. (54–57). UV exposure also activates NFκB which translocate to the nucleus to activate genes involved in stress, inflammation, apoptosis and growth. Epidermal proliferative cells exposed to continuous UV radiation rely on NFκB for defense and survival (58,59). The Keap1-Nrf2-ARE pathway controls the oxidative stress response, and oxidative stress causes the transcription factor Nrf2 to dissociate from Keap1, translocate to the nucleus, and bind with the ARE (60). Activation of the Nrf2 signal pathway results in enhanced expression of antioxidant enzymes such as heme oxygenase-1 (HO-1) and glutathione reductase (GR) (61). UV irradiation significantly decreases Nrf2 mRNA expression in skin, and inhibition or deficiency of Nrf2 leads to UVB-induced skin damage and inflammation. Targeting various signal transduction pathways may reduce damage caused by UV radiation.
Studies have found that UVA and UVB exposure activates signal transducer and activator of transcription 3 (STAT3) that induces cell proliferation and inhibits apoptosis (62,63). In fact, constitutive activation of STAT3 has been found in various human cancers, and STAT3 activation is necessary for carcinogenesis in SCC (62,64,65). Studies have found that STAT3 phosphorylation at Tyr705 and subsequent activation are mediated by UV-induced ROS formation and DNA damage in human keratinocytes and fibroblasts (66,67).
As a tumor suppressor gene, p53 detects DNA damage and induces cell cycle arrest in order to allow cells to repair DNA before the cell cycle may again progress without damage or allow the cells to undergo apoptosis. In fact, the cause of most human cancers including NMSC may be due to defects in the p53 pathway involving the WAF1/p21 and Bax genes. When p53 is phosphorylated and activated by DNA damage, it upregulates gene expression of WAF1/p21 which participates in cell cycle arrest by inhibiting cyclin-CDK complexes (68). After UVB exposure, SKH-1 mouse skin express increased levels of WAF1/p21 and p53, causing cell cycle arrest and apoptosis (69).
Ornithine decarboxylase (ODC) is an enzyme involved in polyamine synthesis that regulates cell proliferation, cell transformation, and cell cycle regulation. UV irradiation activates ODC, and there is a direct relationship between increased levels of ODC and tumor growth (70). The MAPK signal pathway is involved in the activation of ODC (71). In order to verify the role of ODC in photocarcinogenesis, studies have shown that α-difluoromethylornithine, an irreversible inhibitor of ODC, blocked tumor formation (72). COX is an important enzyme in inflammation and prostaglandin synthesis. Although the isoform COX-1 is constitutively active, UV radiation induces COX-2 expression and subsequent prostaglandin formation. Increases in prostaglandin formation induce tumorigenesis through cell proliferation and angiogenesis, both of which are important events in SCC and BCC. COX-1 and COX-2 contribute to the development of BCC, while COX-2 overexpression has been found in UV-induced SCC (73). Various signaling pathways are involved in UV-induced COX-2 expression. Activation of AKT, p38, and SIRT6 promotes COX-2 expression while inhibition of AMPK yields similar effects (13,36).
PHOTOCARCINOGENESIS AND CHEMOPREVENTION
Photocarcinogenesis is a three-step process involving initiation, promotion, and progression. As a complete carcinogen, UV radiation is capable of inducing each step of carcinogenesis (74,75). During initiation, UV radiation induces DNA damage in the form of CPDs, 6-4 pyrimidone photoproducts, DNA strand breaks, and cross-links. DNA mutations, if unrepaired, will replicate and result in signature mutations in various genes such as tumor suppressor genes or proto-oncogenes in epidermal basal cells. As a result, these cells become “initiated” (76). Because tumor initiation is an irreversible stage and may occur at any time in a person’s life, it is a difficult stage to target with chemopreventive strategies (77). Promotion occurs when the mutated, tumor-initiated cells are repeatedly exposed to UV, causing the cells to proliferate and expand into a benign tumor. UV exposure activates various signal transduction pathways resulting in clonal expansion of the initiated cells (78). Tumor promotion occurs for a longer duration of time, may involve multiple exposures of the carcinogen, and is a reversible process (76). Therefore, chemoprevention approaches are more promising when tumor promotion is targeted. Progression occurs when the benign tumor transforms into a malignant neoplasm that is able to invade and metastasize. The process of progression may be induced by gene modifications and selection and gene instability (79,80). Because tumor progression is slow and rate-limiting, this stage may be a promising target for chemoprevention as well (13).
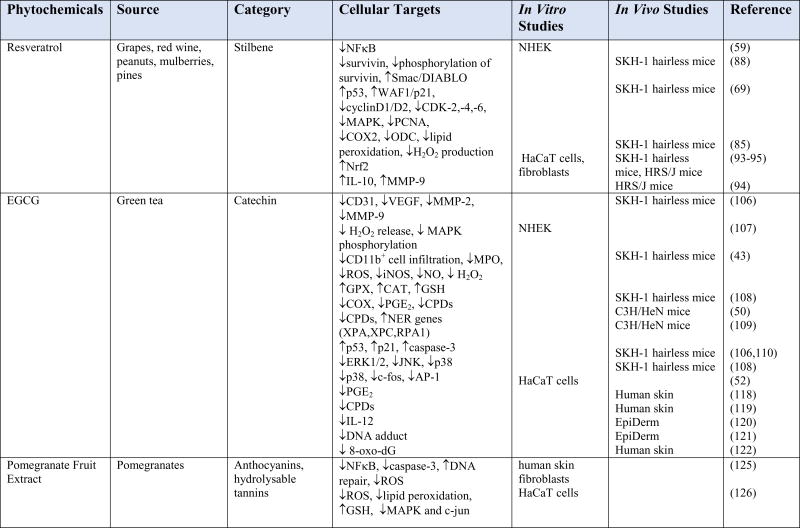
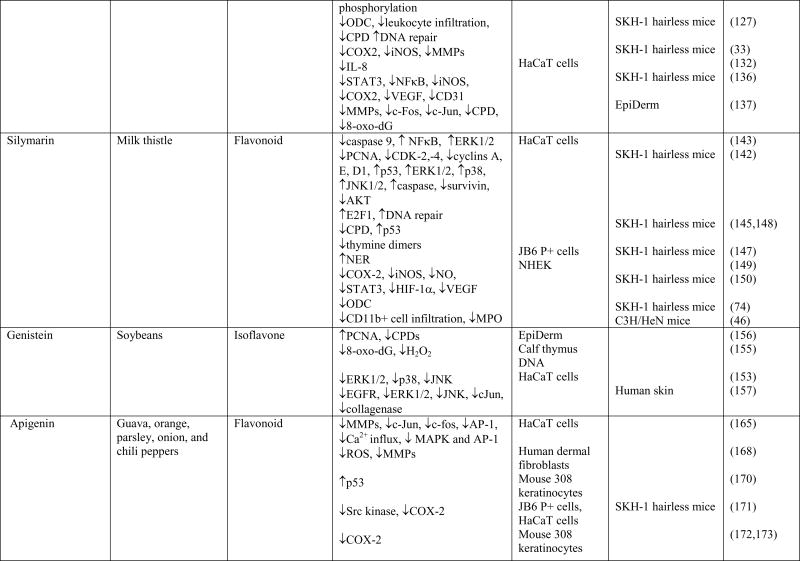
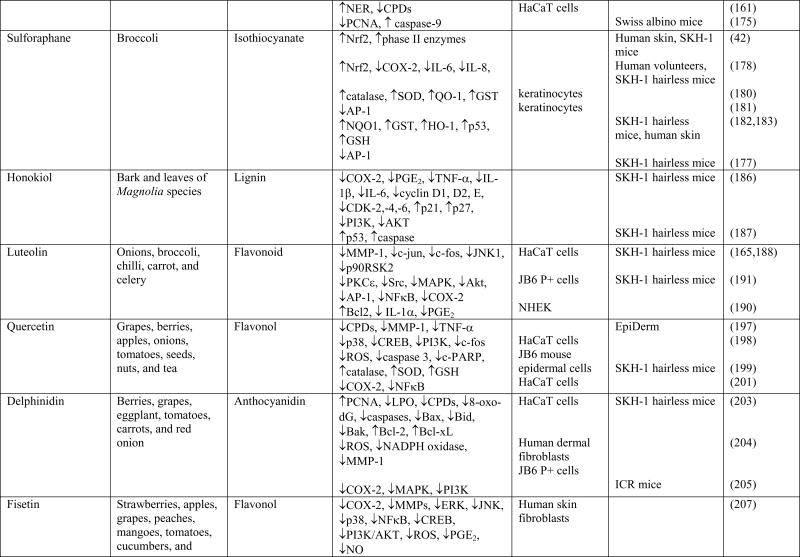
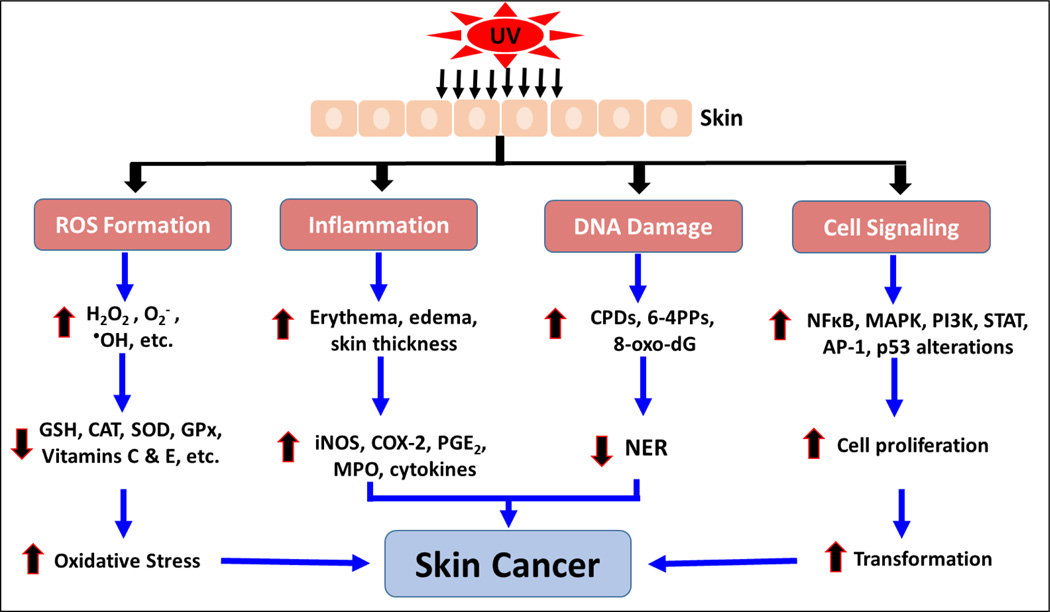
Chemoprevention is an increasingly studied approach to preventing or delaying the development of skin cancer, particularly tumor promotion and progression, through the use of phytochemicals. Phytochemicals have shown promise by modulating cell signaling pathways, inducing cell cycle arrest, stimulating DNA repair, acting as antioxidants, and inhibiting inflammation (13,31,81–84). Phytochemicals are active compounds found in plants and include flavonoids, carotenoids, stylbenes, lignans, and many others. The active compounds are extracted from tea, fruits, vegetables, beans, seeds, cocoa, and soy and are used topically or orally to prevent or protect from skin cancer. Many phytochemicals have polyphenol groups consisting of multiple hydrophilic hydroxyl groups that act as antioxidants to scavenge free radicals or ROS. By inhibiting the formation of ROS, polyphenols inhibit oxidative damage to DNA, proteins, and lipids. Some phytochemicals act as anti-inflammatory agents by inhibiting the production of inflammatory mediators and cytokines. In addition, phytochemicals exert anti-photocarcinogenic properties by modulating cell signaling pathways and regulating the cell cycle, cell proliferation, and angiogenesis (31,81,84). We have discussed selected phytochemicals (Table 1) which have significant protective effects against UV-induced skin cancer (Figure 1).
Table 1.
Cellular targets of phytochemicals against UVB-induced carcinogenesis
Fig. 1.
Schematic diagram depicting the adverse biological effects of UV radiation leading to skin cancer
RESVERATROL
Resveratrol, a naturally occurring polyphenolic stilbene found in red wine, grapes, peanuts, fruits, mulberries and pines, has antioxidant, anti-inflammatory, anti-proliferative, and anti-tumorigenic properties (84–86). Resveratrol is the active component found in the root of Polygonum cuspidatum and may be produced in response to stress or infection (87). Topical application of resveratrol both before and after UVB exposure imparts protection against UVB-induced skin carcinogenesis in SKH-1 hairless mouse model (88). Resveratrol increases UVB-induced apoptosis through modulations in NFκB, MAPK, survivin, WAF1/p21, SIRT1, p53 and cyclin-CDK (59,69,88,89). NFκB is a transcription factor that responds to ROS-induced changes in the redox status of cells, and it plays a vital role in the chemopreventive effects of resveratrol. Human keratinocytes exposed to UVB resulted in decreased levels of inhibitory IκBα in the cytosol and increased phosphorylation of IκBα at Ser32, which both indicate activation of the NFκB pathway. However, resveratrol treatment suppressed UVB-mediated activation of NFκB by increasing the expression of IκBα and reducing the phosphorylation of IκBα (59). Survivin, a protein that inhibits apoptosis, acts by binding caspases and inhibiting their activation. Survivin is expressed in most aggressive human cancers, and the onset and progression of BCC and SCC may be due to induction of survivin (90,91). Studies have found a positive relationship between NFκB levels and survivin expression, supporting the evidence that resveratrol may protect skin from UVB-induced damage by controlling the expression of survivin (58). In fact, resveratrol treatment before and after UVB exposure resulted in decreased mRNA and protein levels of survivin, and the down-regulation of survivin phosphorylation led to induced apoptosis. Smac/DIABLO directly interacts with survivin and is necessary for the antiapoptotic properties of survivin. UVB exposure both to normal human epidermal keratinocytes and to SKH-1 hairless mouse skin resulted in increased levels of survivin and decreased levels of Smac/DIABLO [88,89]. Resveratrol acts by inhibiting phosphorylation of survivin, decreasing survivin levels, and increasing Smac/DIABLO levels to induce cell death (88).
After UVB exposure, SKH-1 hairless mouse skin expressed increased levels of WAF1/p21 and p53, which both act to induce cell cycle arrest and apoptosis. Resveratrol further stimulated UVB-mediated increases in p53 and WAF1/p21, leading to cell cycle arrest in the G1 phase and eventually apoptosis (69). Resveratrol acts as an anti-proliferative agent by targeting various cell proliferation molecules including the CKI–cyclin–CDK network and PCNA. CDK–cyclin complexes regulate the cell cycle and lead to cell cycle progression when activated. UVB exposure resulted in increased expression of CDK-2, −4, and −6 as well as cyclins D1 and D2 which led to cell proliferation (69). CKIs are a class of proteins that inhibit cyclin-CDK complexes (69,92). Topical application of resveratrol resulted in down regulation of UVB-induced increase in cyclin D1, cyclin D2, CDK-2, CDK-4 and CDK-6. By decreasing the levels of cyclins and CDKs, resveratrol acts as an anti-proliferative agent. MAPK regulates the cell cycle by enhancing proliferation and may also play a role in increasing the expression of cyclin D1. MAPK phosphorylation is increased in response to UVB exposure, but this phosphorylation was inhibited in SKH-1 hairless mouse skin treated with resveratrol. PCNA also causes cell proliferation and is upregulated in response to UVB exposure. However, SKH-1 hairless mouse skin treated with resveratrol prior to UVB exposure resulted in downregulation of UVB-induced increase in PCNA levels (69). Thus, by modulating the CKI–cyclin–CDK network, the MAPK pathway, and PCNA, resveratrol plays an important role against UVB-induced skin carcinogenesis.
UVB-induced skin edema and hyperplasia were inhibited by topical application of resveratrol to SKH-1 hairless mice (69,85). UVB exposure also resulted in infiltration of leukocytes which may provide an additional oxidative burst. By inhibiting UVB-induced infiltration of leukocytes and subsequent ROS formation, resveratrol may contribute antioxidant activity (69). Topical application of resveratrol to SKH-1 hairless mice inhibited UVB-induced COX-2 protein expression and ODC enzyme activity (85). Thus resveratrol acts as a chemopreventive agent by inhibiting tumor promotion. UVB-exposed mouse skin contained increased levels of H2O2 and lipid peroxides. Resveratrol treatment acts as an antioxidant by inhibiting UVB-induced lipid peroxidation and H2O2 generation in SKH-1 hairless mouse skin (85). UVB irradiation significantly decreases Nrf2 mRNA expression in skin, and inhibition or deficiency of Nrf2 leads to UVB-induced skin damage, oxidative stress, and inflammation. Therefore, resveratrol acts as a chemopreventive agent that targets Nrf2 activation, increases expression of antioxidant enzymes, and protects against UVA-and UVB-induced apoptosis. (93–95). Ethanol extract of peanut sprout, which contains resveratrol, protected against UVB-induced oxidative stress in human dermal fibroblasts by activating Nrf2 and upregulating the expression detoxifying enzymes (60). Treatment of UV-exposed human keratinocytes with resveratrol resulted in decreased levels of ROS, IL-6 and COX-2, demonstrating the anti-inflammatory and antioxidant effects of resveratrol (96). Resveratrol also acts as an anti-inflammatory agent and promotes healing by upregulating IL-10 and MMP-9 production. IL-10 is one of the primary anti-inflammatory cytokines that limits the activity of enzymes involved in respiratory burst in tissues exposed to oxidative stress. Hence, resveratrol-mediated upregulation of IL-10 induces anti-inflammatory effects. Upregulation of MMP-9 activity may allow inflammatory cells to cross the extracellular matrix after UVB irradiation in order for skin repair to occur. Therefore, resveratrol-mediated increase in MMP-9 may induce skin healing (94). Pterostilbene, the analog of resveratrol, is more potent than resveratrol in its chemopreventive and anti-inflammatory effects in UVB-exposed mice (93).
Due to its poor solubility in aqueous solution, resveratrol has limited therapeutic effectiveness and low bioavailability in vivo when administered orally. Nanostructured delivery systems such as the liquid-crystalline system (LCS) have been developed for proper delivery of resveratrol. LCS shelters the active molecule and sustains drug release, making it an effective delivery system for molecules in topical preparations. Hairless mice treated with resveratrol-loaded LCS were protected from UVB-induced skin damage through inhibition of skin edema, leukocyte recruitment, lipid peroxidation, superoxide anion production, and oxidative stress. MMP-9, IL-10, Nrf2, and HO-1 were upregulated following resveratrol-loaded LCS treatment. Topical treatment with resveratrol-loaded LCS maintained catalase (CAT) activity, glutathione (GSH) levels, expression of glutathione peroxidase (GPx) and GR. Delivery systems such as LCS may allow for proper topical administration of chemopreventive agents (94). Resveratrate, a stable derivative of resveratrol, is more easily absorbed into skin tissue and becomes converted to resveratrol in the skin (97). Resveratrate was applied to human volunteers after UV exposure, and treatment resulted in decreased UV-induced erythema (98). A recent study found that pretreatment with resveratrol-containing microemulsion gel inhibited UVB-induced erythema in guinea pig skin (99). Therefore, resveratrate and various emulsions may be used for effective delivery and enhanced efficacy of resveratrol in preventing photocarcinogenesis.
EGCG
Green tea is a popular beverage derived from the Camellia sinensis plant by steaming and drying tea leaves (100). The active ingredients of green tea are polyphenolic compounds known as catechins or flavonols which exert anti-inflammatory, antioxidant, and anti-carcinogenic properties. The primary catechins in green tea are (–)-epicatechin, (–)- epigallocatechin, (–)-epicatechin-3-gallate and (–)-epigallocatechin-3-gallate (EGCG), and EGCG is the most photoprotective agent of these (84,101,102). Studies have shown that topical application of EGCG inhibits UV-induced carcinogenesis and thus may prevent NMSC development (13,84,102,103).
EGCG acts as an antioxidant and anti-proliferative agent to prevent skin tumorigenesis (84,102). EGCG prevents tumor-promoting ligands from binding epidermal growth factor receptor (EGFR). There is evidence that activation of the EGFR activates AP-1 and initiates p38 and PI3K pathways (104,105). Thus, EGCG acts to inhibit the p38 MAPK pathway and AP-1 activation and therefore inhibits tumorigenesis (13). In order for tumors to grow, invade, and metastasize, new blood vessels must form to allow for proper oxygen supply. CD31 and VEGF are essential endothelial markers, which are expressed in order to induce angiogenesis in tumors, including UVB-induced tumors. Oral administration of green tea polyphenols (GTPs) to UVB-irradiated SKH-1 hairless mice resulted in inhibition of CD31 and VEGF, suggesting that GTPs act by inhibiting angiogenesis in UVB-induced skin tumors. Increased expression of MMP-2 and MMP-9 plays a crucial role in tumor growth and angiogenesis, and administration of GTPs in UVB irradiated mice inhibited the expression of these MMPs. In addition, TIMP1 acts by inhibiting MMPs, and oral administration of GTPs increased TIMP1 in skin tumors of SKH-1 hairless mice. Administration of GTPs in UVB irradiated mice increased the numbers of cytotoxic T cells in skin tumors that cause an inhibition or regression of tumors in SKH-1 hairless mice (106).
UVB irradiation causes an inflammatory response that recruits leukocytes to the site of irradiation. Leukocytes produce ROS that may further induce inflammation. In vitro studies found that pretreatment of EGCG in UVB-exposed keratinocytes decreased H2O2 generation and inhibited phosphorylation of MAPK (107). Pretreatment of mouse skin with EGCG was shown to inhibit UVB-mediated infiltration of leukocytes specifically CD11b+ cells, prevent myeloperoxidase activity, and cause less epidermal structure damage in C3H/HeN mouse skin. CD11b+ cells are monocytes/macrophages involved with the immune response and serve as a source of ROS in response to UVB exposure. By inhibiting the infiltration of leukocytes, EGCG reduced the production of ROS by leukocytes and ROS-induced photocarcinogenesis (43). Pretreatment of EGCG was also found to inhibit UVB-mediated nitric oxide synthase expression and the production of ROS, such as NO and H2O2, in mouse epidermis and dermis (43). Topical application of GTPs in hydrophilic cream to SKH-1 hairless mice was shown to inhibit UVB-mediated reduced expression of GPx, CAT, and GSH levels. The antioxidant properties of GTPs may help to reduce lipid peroxidation, protein oxidation, and UVB-mediated carcinogenesis (108). UVB-exposure induces formation of CPDs which induce inflammatory responses such as upregulation of COX-2 and PGE2. Administration of GTPs in drinking water removed CPDs and inhibited subsequent COX-2 activity and PGE2 formation (50). In addition, GTP treatment to C3H/HeN mice stimulated DNA repair of UVB-induced formation of CPDs. GTP treatment removed or repaired the UVB-induced DNA damage by increasing the expression of NER genes such as XPA, XPC, and RPA1 (109). Thus, GTPs are capable of inducing DNA repair in response to UVB-induced CPD formation.
EGCG acts through various signal transduction pathways to inhibit the damaging effects of UVB exposure. Oral administration of GTPs to SKH-1 hairless mice resulted in increased UV-induced expression of p53, p21, and caspase-3, leading to apoptosis of tumor cells (106,110). EGCG treatment resulted in inhibition of UVB-induced phosphorylation of the MAPK proteins including ERK1/2, JNK, and p38 in normal human epidermal keratinocytes (107). MAPK activation mediates AP-1 activity, and inhibition of UVB-induced MAPK activation by EGCG leads to inhibition of AP-1 activation in HaCaT cells. Therefore, EGCG inhibits c-Fos, a component of AP-1, to inhibit cell proliferation and cell survival (52).
Several studies have tested the efficacy of EGCG in preventing photocarcinogenesis by enhancing its delivery through various mechanisms. The antioxidant effects of EGCG were significantly enhanced when HaCaT cells were treated with the glucosylated form of EGCG, Glc-EGCG. Treatment with Glc-EGCG inhibited ROS formation much more efficiently than EGCG, suggesting that Glc-EGCG may be used to prevent ROS production in UV-exposed skin (111). UVB-exposed SKH-1 hairless mice pretreated with EGCG in a hydrophilic cream have decreased tumor incidence, size, and multiplicity (112). Chitosan microparticles are an additional method that help improve the stability and delivery of phytochemicals (113). Studies have shown that tea catechins may be digested by skin enzymes, and green tea extract delivered in chitosan microparticles resulted in enhanced human skin permeation and decreased metabolism of the active catechins (114–116). Therefore, chitosan microparticles may be used to prevent enzymatic degradation, enhance photochemopreventive effects, and improve the delivery of EGCG. Topical application of oil-in-water emulsions containing EGCG on human skin enhanced the delivery of EGCG into the deeper stratum corneum area (117).
Treatment of human skin with EGCG protected against UVB-induced ROS formation and leukocyte infiltration. Prostaglandins play a role in the production of ROS, and human skin pretreated with EGCG and exposed to UVB irradiation resulted in prominent reduction of PGE2 compared to human skin only exposed to UVB radiation (118). Thus, EGCG acts as an anti-inflammatory and antioxidant to prevent photocarcinogenesis. In addition, studies on human skin have demonstrated the effects of GTPs on the rapid repair of UVB-induced DNA damage. Topical application of GTPs onto human skin resulted in the inhibition of UVB-induced CPD formation in both the dermis and epidermis. In addition, this study found that topical application of GTPs resulted in inhibition of UVB-induced erythema in human skin. UVB-exposed human skin treated with GTPs resulted in decreased DNA damage and inflammation (119). Similar effects were found in UVB-exposed human skin equivalent in which the stimulation of IL-12 production decreased the formation of CPDs (120). Green tea has also been found to protect from UVA-induced DNA adduct formation (121). Topical application of green tea extract to human skin afforded protection from solar simulated UV-induced oxidative DNA damage by decreasing the levels of 8-oxo-dG (122). Therefore, several studies on human skin have demonstrated the effects of GTP as an antioxidant, anti-inflammatory, and DNA repair agent to inhibit photocarcinogenesis.
POMEGRANATE FRUIT EXTRACT
Pomegranate is derived from the Punica granatum tree and contains two main polyphenols, anthocyanins and hydrolysable tannins, which are the primary antioxidant components of the fruit. Anthocyanins include cyanidin and delphinidin while the hydrolysable tannins are punicalin, pedunculagin, punicalagin, and gallagic and ellagic esters of glucose (123). Pomegranate fruit extract (PFE) exerts antioxidant and anti-inflammatory properties (123,124). PFE protects against UVB-induced oxidative stress and ROS formation in human skin fibroblasts (125). In human immortalized HaCaT keratinocytes exposed to UVB-irradiation, PFE protected cells from oxidative stress. PFE further reduced UVB-induced oxidative stress by inhibiting UVB-mediated reduction in GSH concentrations. Thus, PFE exerts its antioxidant properties by maintaining the levels of GSH (126). While UV-irradiation induces cell death and decreases the number of viable cells, PFE application inhibited UV-induced apoptosis (125). Similar effects were found when HaCaT keratinocytes were pretreated with PFE. The proposed mechanism by which PFE protected cells is through inhibition of oxidative stress (126). Oral feeding of PFE to SKH-1 hairless mice resulted in inhibition of UVB-induced increase in ODC, a rate-limiting enzyme of polyamine synthesis (127). Polyamines and ODC activity regulate cell proliferation and photocarcinogenesis, and studies have found a positive relationship between ODC activity and tumor promotion (72,128). The inhibition of ODC by PFE may prevent tumorigenesis by inhibiting cell proliferation (127,128).
PFE acts as an anti-inflammatory agent through various mechanisms. Oral feeding of PFE to SKH-1 hairless mice resulted in inhibition of UVB-induced hyperplasia, skin edema, and infiltration of leukocytes (127). Another study found that oral feeding of PFE to SKH-1 hairless mice yielded similar anti-inflammatory effects and also inhibited UVB-induced COX-2 and iNOS expression and subsequent inflammation. Thus, PFE inhibits tumorigenesis through these mechanisms (33). IL-8 is a pro-inflammatory cytokine that is upregulated in response to UVB-irradiation in human keratinocytes and in vivo (129). Increase in IL-8 leads to angiogenesis, chemotaxis, tumorigenesis, and cell proliferation (130,131). UVB-induced increase in IL-8 was inhibited by pretreatment of HaCaT cells with pomegranate seed oil nanoemulsions (132).
Oral feeding of PFE reduced UVB-induced CPD formation in SKH-1 hairless mice (127). UV exposure induces expression of MMPs which degrade the extracellular matrix, regulate angiogenesis, and aid in tumor invasion in metastasis (133). SKH-1 hairless mice fed with PFE exhibited inhibition of UVB-induced expression of MMPs (33). By inhibiting the expression of MMPs, PFE exerts anti-angiogenic effects and inhibits tumor growth. Several signaling pathways are targeted by topical application of PFE. Activation of the MAPK pathway leads to angiogenesis, tumorigenesis, and invasion (134,135). Pretreatment of HaCaT cells with polyphenol-rich PFE inhibited UVB-induced phosphorylation of MAPK and c-Jun (126). Treatment of PFE inhibited UVB-induced activation of the NFκB pathway and therefore inhibits UV-induced gene transcription in SKU-1064 human skin fibroblasts (125). Oral feeding of PFE in drinking water inhibited UVB-induced skin tumorigenesis in SKH-1 hairless mice. Several human tumors express constitutively active STAT3 and NFκB. In response to UV irradiation, STAT3 and NFκB become phosphorylated and activated and subsequently induce cell proliferation. Compared to a UVB irradiated alone group, mice that received oral feeding of PFE reduced UVB-induced phosphorylation of STAT3 and NFκB and decreased iNOS and COX-2, which are downstream molecules of these signaling pathways. In addition, oral feeding of PFE decreased UVB-induced angiogenic proteins such as HIF-1α, VEGF, and CD31 in mice tumors. Therefore, PFE prevented skin photocarcinogenesis by decreasing UV-induced inflammation and angiogenesis (136).
Pretreatment of UVB-exposed HaCaT cells with pomegranate seed oil nanoemulsions enhanced the delivery and efficacy of the polyphenol constituents of PFE. The nanoemulsions protected cells against UVB-induced DNA damage and decreased secretion of IL-8, a pro-inflammatory cytokine that enhances cell proliferation and angiogenesis. Pomegranate seed nanoemulsions may be a promising delivery method in order to enhance the anti-inflammatory and DNA repair properties of PFE (132).
Pretreatment of human reconstituted skin with pomegranate derived products resulted in a decreased expression and activity of UVB-induced MMPs. In addition, treatment with pomegranate derived products prior to UVB exposure resulted in inhibition of UVB-mediated formation of CPDs and 8-oxo-dG in human reconstituted skin (137). Furthermore, pretreatment of human reconstituted skin with pomegranate derived products inhibited UVB-induced protein oxidation, PCNA protein expression, and phosphorylation of c-Jun. Thus, PFE may be used for photochemoprevention because it exerts antioxidant, anti-proliferative, anti-angiogenic, and anti-tumorigenic properties. A clinical trial on human volunteers tested the effects of oral administration of pomegranate extract on UV-induced pigmentation. Low dose oral administration of pomegranate extract inhibited UV-induced sunburn in volunteers, suggesting the protective effect of pomegranate extract (138).
SILYMARIN
Silymarin is a flavonoid derived from the milk thistle Silybum marianum plant, and the major active component is silibinin, also known as silybin. Several studies demonstrate that silimaryn acts as an antioxidative agent and protects against UVB-induced carcinogenesis (139). The protective effects of silibinin are demonstrated in JB6 mouse epithelial cells treated with silibinin before or immediately after UVB-exposure. Silibinin caused downregulation of UVB-induced phosphorylation of ERK1/2 and AKT. By decreasing phosphorylation of ERK1/2 and AKT, silibinin protects against tumor promotion (140) UVB-induced activation of AP-1 and NFκB leads to cell survival, but silibinin treatment before or immediately after UVB exposure in JB6 cells inhibited UVB-induced activation of these transcription factors. Thus, inhibition of various cell survival signal pathways by silibinin suggests its use as an anti-proliferative agent against skin tumor promotion (141). Topical application or dietary feeding of silibinin to SKH-1 hairless mice afforded protection against photocarcinogenesis by decreasing tumor multiplicity and tumor volume (142). Silibinin also exerted anti-apoptotic effects by inhibiting UVB-induced caspase 9 activation in HaCaT cells. In addition, silibinin protects against UVB-induced apoptosis by increasing the number of cells in the S phase to allow for DNA repair. However, at higher doses of UVB exposure, silibinin was not effective in protecting the cells against UVB-induced apoptosis (143). Treatment with silibinin enhanced UVA-induced apoptosis in HaCaT cells. UVA exposure induced ROS production in HaCaT cells, and cells treated with silbinin prior to UVA exposure showed increased ROS production and increased apoptosis due to oxidative stress (144). Silibinin was also shown to decrease survivin levels and increase activated caspase-3 levels to induce apoptosis in SKH-1 hairless mouse skin. Silibinin treatment also increased p53 and CDKI expression while down-regulating CDK-cyclin expression levels, leading to inhibition of cell growth (142).
Silibinin protects against UVB-induced DNA damage by inducing DNA repair and suppressing apoptosis. E2F transcription factors mediate DNA repair and apoptosis, and E2F1 expression decreases in SKH-1 hairless mouse skin after exposure to UVB radiation. Silibinin reversed the effects of UVB-induced E2F downregulation, and the increased expression of E2F1 inhibited apoptosis and enhanced the repair of UVB-mediated DNA damage (145,146). Treatment with silibinin prior to UVB radiation resulted in increased repair of CPDs through upregulation of p53 in JB6 cells and SKH-1 hairless mouse skin. By increasing p53 expression, silibinin inhibited UVB-induced apoptosis and repaired DNA damage (147). Topical application of silibinin reduced UVB-induced CPD formation in mouse epidermis (148). In addition, silymarin treatment repaired UVB-induced DNA damage by increasing the expression of NER genes in normal human epidermal keratinocytes (149).
Inflammatory responses are mediated through UVB-induced increased expression in COX-2 and iNOS (150). Studies have suggested that photocarcinogenesis may result from increased COX-2 expression in mouse and human skin (39,57). Silibinin exerts anti-inflammatory effects in SKH-1 hairless mouse skin by down regulating COX-2 and iNOS expression, highlighting its potential use as a protective agent against photocarcinogenesis (150). Silymarin exerts significant inhibitory effects on ODC activity in SKH-1 hairless mouse skin, reflecting its ability to inhibit tumor promotion (74). C3H/HeN mice treated with silymarin exhibited decreased levels of UV-induced oxidative stress by inhibiting CD11b+ cell and inflammatory leukocyte infiltration, thereby decreasing levels of MPO in the dermis and epidermis (46). By decreasing induction of these inflammatory factors, silymarin prevents the harmful effects of UVB radiation and may be a promising agent to prevent photocarcinogenesis.
Topical or dietary treatment with silibinin inhibited angiogenesis by regulating levels of STAT3, VEGF, and HIF-1α (150). STAT3 and HIF-1α activation leads to increased expression of VEGF and subsequent angiogenesis and tumor invasion (151). Silibinin treatment decreased protein levels of STAT3, HIF-1α and VEGF, suggesting its use as an antiangiogenic and anti-carcinogenic agent (150). Silymarin oil-in-water microemulsions enhance the stability and solubility of silymarin in pig skin. Thus, microemulsions showed prolonged release compared to silymarin solution, suggesting the use of microemulsion delivery systems to enhance the chemoprevention effects of silymarin (152).
GENISTEIN
Genistein is an isoflavone derived from soybeans that inhibits cellular signaling pathways, inflammation, DNA damage, and skin damage (153–157). While UVB exposure induces the activation of ERK1/2, p38, and JNK, treatment with isoflavone extract inhibited UVB-induced activation of MAPK in HaCaT cells (153). In addition, treatment with soybean isoflavone extract resulted in inhibition of UVB-induced epidermal proliferation and expression of COX-2 (153). In UV-exposed Skh:HR-1 mice, treatment with topical isoflavone extract from soybean resulted in decreased skinfold thickness (154). Therefore, genistein may be used as an anti-inflammatory agent to reduce the effects of UV on skin inflammation.
Genistein inhibited UV-induced DNA damage by inhibiting the formation of 8-oxo-dG in calf thymus DNA. Genistein treatment also exerted antioxidant properties by scavenging H2O2 and inhibiting the formation of superoxide anion (155). These antioxidant effects of genistein may be associated with decreased DNA damage in UV-exposed cells. In addition, genistein treatment inhibited UV-induced DNA damage by inhibiting pyrimidine dimer formation in human reconstituted skin (156). PCNA is vital for DNA replication and nucleotide excision repair. UV irradiation down-regulates PCNA expression and may lead to CPD formation, DNA mutation, and ultimately tumor initiation. Human reconstituted skin samples treated with genistein exhibited increased levels of PCNA activity which inhibited UV-induced DNA damage (156). While UVB exposure induced DNA damage in BJ-5ta human skin cells, genistein treatment significantly reduced UVB-induced DNA damage and rapidly increased DNA repair. Therefore, genistein may be used as a protective agent against UVB-induced DNA damage. A study on human skin biopsies treated with genistein prior to UV exposure demonstrated inhibitory effects on EGFR, ERK1/2, JNK, and c-Jun. Genistein decreased UV-induced skin damage in human by down-regulating c-Jun and subsequent collagenase activation (157).
Several studies have developed a liquid chromatography method in order to accurately determine if genistein has successfully permeated the skin in order to exert its effects. Genistein showed high affinity for porcine skin samples in vitro, and genistein was found in deeper layers of the skin when the amount was increased (158). Liquid chromatography was also used to determine the delivery of genistein from nanoemulsions before and after their incorporation in hydrogels. Results showed that higher amounts of genistein were found in the outer epidermal skin layers rather than the inner layers. In addition, higher amounts of genistein were present in porcine ear skin after incorporation in hydrogels (159). Therefore, liquid chromatography may be used in order to determine the permeation of genistein into the skin, and nanoemulsions incorporated into hydrogels may have promising effects against photocarcinogenesis.
APIGENIN
Apigenin is a flavonoid found in the leaves and stems of various fruits and vegetables including guava, orange, parsley, onion, and chili peppers from Lycopodium clavatum (160). Apigenin is used for its anti-oxidant, anti-inflammatory, and anti-proliferative effects in addition to its ability to activate NER (161). Apigenin exerts anti-tumor effects by inhibiting cellular transformation, angiogenesis, and tumorigenesis and by stimulating gap junctional and intercellular communication (162–164). Various signaling pathways are targeted by apigenin, including AP-1, MAPK, and NF-κB.
Pretreatment of HaCaT cells with apigenin reduced UVA-induced expression of MMP-1 and collagenase production. Apigenin reduced the production of MMP-1 by inhibiting expression of c-Jun and c-Fos, thereby inhibiting the activation of AP-1. Apigenin also interfered with MMP-1 production by inhibiting UVA-induced calcium influx and calcium dependent MAPK and AP-1 signaling (165). Large amounts of MMP-1 are found in UV-exposed skin and basal cell carcinomas, and MMPs mediate collagen degradation to cause skin damage (166,167). Similar effects were found in which treatment with apigenin decreased the UVA-induced expression of MMP-1 at the mRNA and protein levels in human dermal fibroblasts. In addition, apigenin acted as an antioxidant by reducing the production of ROS. Because UV-induced ROS production activates NFκB and enhances MMP gene expression, apigenin may decrease ROS production and subsequent MMP expression (168). A recent in vitro study found that apigenin treatment increased the nuclear translocation and protein expression of Nrf2, and Nrf2 further regulated expression of the downstream antioxidant gene NQO1 (169).
Apigenin treatment induces expression and stability of p53 in mouse 308 keratinocytes, causing reversible G2/M checkpoint arrest during the cell cycle (170). In another study, apigenin was found to bind and suppress Src kinase and subsequently reduce UVB-induced skin inflammation and COX-2 formation in JB6 P+ mouse epidermal cells, HaCaT keratinocytes and SKH-1 hairless mouse skin (171). Another studies also found that apigenin blocked UVB-induced COX-2 expression in mouse keratinocytes (172,173). By inhibiting UVB-induced skin inflammation, apigenin exerted potent photochemoprotective effects. Apigenin also prevents photocarcinogenesis through DNA repair mechanisms. In order to protect DNA from UVB-induced damage, apigenin stimulated NER gene expression and reduced the formation of CPDs in human keratinocytes and mouse skin (161).
Due to its lipophilic characteristics, apigenin is not significantly soluble and becomes trapped in the phospholipid bilayer plasma membrane. Thus, apigenin is loaded in ethosomes containing mainly phospholipids and ethanol in order to enhance its solubility, permeation, and deposition in skin. Increased levels of phospholipid short-chain alcohols improve apigenin’s transdermal absorption and efficacy. By enhancing the delivery of apigenin, ethosomes consequently enhance the anti-inflammatory effects of apigenin. In vitro and in vivo studies demonstrated that apigenin delivery via ethosomes suppressed UVB induced COX-2 levels and thus reduced inflammation. By inhibiting UVB-induced skin inflammation, apigenin may be used to prevent damage from UV radiation (174). Poly (lactic-co-glycolide) (PLGA)-loaded apigenin nanoparticles have also been used to enhance the effects of apigenin against photocarcinogenesis due to their small size and fast movement into the tissues. Treatment of UVB-exposed Swiss albino mice with oral and topical PLGA-loaded apigenin nanoparticles resulted in decreased epidermal hyperplasia, decreased PCNA expression, increased active caspase-9 expression, and increased apoptosis. Therefore, nanoparticles containing apigenin may be used to inhibit skin carcinogenesis with more efficacy than apigenin alone (175).
SULFORAPHANE
Sulforaphane, found in cruciferous vegetables such as broccoli, is a naturally occurring isothiocyanate known for its chemopreventive and antioxidative effects (176). Studies demonstrated sulforaphane’s protective effects against UV-induced skin damage and carcinogenesis in mice through several mechanisms. Sulforaphane acts by inducing the Keap1-Nrf2-ARE pathway, inducing cytoprotective phase II detoxification enzymes, inhibiting inflammation, and acting as a pro-apoptotic agent (42,176,177).
Sulforaphane activates a major transcription factor, Nrf2, which determines the cell’s ability to survive and adapt under oxidative stress by inducing the expression of phase II enzymes (42,178). Keap1 is a protein that binds and negatively regulates Nrf2 through proteasomal degradation (179). Under stress Nrf2 releases from Keap1, translocates to the nucleus, and binds to the ARE site in the promoter of many genes involved in cytoprotection such as phase II enzymes and antioxidants (177,179). Nrf2 deficiency is related to accelerated disease progression and increased sensitivity to carcinogens. Further, in SKH-1 hairless mice with constitutively active Nrf2, UV-induced cutaneous tumors were lower in incidence and multiplicity compared to wild-type mice. In addition, topical application of sulforaphane resulted in increased activation of Nrf2, which reduced UV-induced skin erythema in healthy human subjects (178). Therefore, preventive techniques have been used in mice and human models which activate Nrf2 and protect against UV-induced skin erythema, inflammation and carcinogenesis. Sulforaphane acts as an indirect antioxidant by upregulating the gene expression of antioxidants CAT, superoxide dismutase (SOD), quinone oxidoreductase-1 (QO-1), and glutathione S-transferase (GST) via Nrf2 activation (180). UV exposure also leads to activation of AP-1, a transcription factor that mediates skin photocarcinogenesis. Keratinocytes express increased levels of AP-1 when exposed to UVB irradiation (177). Sulforaphane has been found to inhibit UVB-induced activation of AP-1 in human keratinocytes. Treatment of keratinocytes with sulforaphane was found to inhibit AP-1 activation by blocking AP-1 from binding DNA (181). Topical application of sulforaphane exerted cytoprotective effects by significantly elevating NAD(P)H: quinone oxidoreductase 1 (NQO1) levels in both mice and human subjects (182,183). NQO1 is a phase 2 enzyme which binds and stabilizes p53, and NQO1-deficienct mice are unable to induce p53 and initiate apoptosis, leading to skin tumor formation (184). Sulforaphane application elevated NQO1 expression in mouse skin, which may protect against damaging effects of UV by stabilizing p53 and detoxifying UV-induced ROS. In addition, GSH is a phase 2 enzyme which detoxifies free radicals through several mechanisms. In SKH-1 mouse skin and human keratinocytes, UV exposure has been found to decrease the expression of GSH, and application of sulforaphane inhibited UV-induced depletion of GSH (183). Based on these findings, sulforaphane may be used to protect against the damaging effects of UV irradiation.
Sulforaphane is not chemically stable in aqueous, protic, and polar aprotic environments and becomes rapidly degraded in these solvents. However, sulforaphane exhibited increased stability in organic formulations such as polyethylene glycol (PEG) ointment bases and organic oleaginous bases. In addition, topical application of PEG formulations of sulforaphane significantly decreased AP-1 activation in SKH-1 hairless mouse skin. Proper delivery of sulforaphane in organic preparations may enhance the anti-carcinogenic effects of sulforaphane (177).
HONOKIOL
Honokiol is a natural lignin component from the bark and leaves of Magnolia species with anti-inflammatory and chemopreventive properties (185,186). A study found that treatment with honokiol decreased UVB-induced skin tumor multiplicity and induced intrinsic and extrinsic apoptosis pathways in SKH-1 hairless mice (187). Honokiol treatment decreased UVB-induced skin tumor multiplicity and tumor volume as well as inhibited the transformation of papillomas to carcinomas in SKH-1 hairless mice (185,186). Treatment with honokiol inhibited several inflammatory mediators including COX-2, PGE2, TNF-α, IL-1β, and IL-6 expression in skin and skin tumors of UVB-exposed SKH-1 hairless mice. Honokiol also inhibited photocarcinogenesis by inhibiting cell cycle regulators including cyclins, and CDKs, as well as by increasing expression of p21 and p27. In addition, UVB-mediated activation of PI3K and AKT were attenuated in SKH-1 hairless mouse skin treated with honokiol (186). Treatment with honokiol before or after UVB exposure may inhibit skin photocarcinogenesis through these chemopreventive mechanisms.
LUTEOLIN
Luteolin is a flavonoid found in onions, broccoli, chili, carrot, and celery which exhibits anti-carcinogenic and anti-inflammatory effects (165–191). Studies using HaCaT cells found that luteolin inhibits UVA-induced AP-1 activation as well as c-Fos, c-Jun, and MMP-1 expression (165). Luteolin also inhibited UVB-induced MMP expression by suppressing JNK1 and p90RSK2 activity in HaCaT cells (188). Similar effects were observed when UVA-exposed dermal fibroblasts were treated with luteolin. By inhibiting the MAPK pathway, luteolin decreased UVA-induced MMP-1 expression, which may suggest the protective effects of luteolin (189). Because MMPs play a role in degradation of ECM, angiogenesis, and tumor invasion, luteolin may be used as a protective agent against photocarcinogenesis (192,193). Treatment of UVB-exposed normal human keratinocytes with luteolin resulted in increased levels of Bcl2 and decreased UVB-induced cell death (190). Through this mechanism, luteolin increases the survival of normal human keratinocytes. In SKH-1 hairless mouse skin luteolin treatment inhibited UVB-induced PKCε and Src activation which consequently downregulated MAPK and AKT signaling pathways (191). Studies have shown that PKCε and Src activation participate in angiogenesis, tumor invasion, and the development of NMSC (194,195). Therefore, by inhibiting PKCε and Src pathways, luteolin may inhibit photocarcinogenesis. Treatment of normal human keratinocytes with luteolin reduced UVB-induced release of inflammatory mediators including IL-1α and PGE2 (190). Luteolin also acts as an anti-inflammatory agent by inhibiting UVB-mediated activation of AP-1 and NFκB transcription factors leading to decreased COX-2 expression in JB6P+ cells and SKH-1 hairless mouse skin (191).
QUERCETIN
Quercetin is a natural flavonol found in grapes, berries, apples, onions, tomatoes, seeds, nuts, and tea (196). Various studies have found that quercetin inhibits DNA damage, acts as an antioxidant, and targets various signaling pathways which suggest its use as a chemopreventive agent (197–199). Because quercetin is not easily absorbed, one study determined the effects of a glycosylated form of quercetin known as quercitrin (199). Pretreatment of JB6 mouse epidermal cells and SKH-1 hairless mice with quercitrin resulted in a decrease in UVB-induced ROS production, DNA damage, and apoptosis. While UVB irradiation resulted in increased expression of active caspase 3 and cleaved PARP, pretreatment of quercitrin inhibited these effects, proposing its role in attenuating apoptosis. Quercetrin exerted antioxidant effects by decreasing UVB-induced ROS generation, inhibiting UV-induced decrease in CAT and SOD, and decreasing the ratio of GSH/GSSG (199). Quercetin and ascorbic acid treatment has also been found to inhibit UVB-induced p38, CREB, and PI3K activation as well as c-Fos activity in HaCaT cells (198). UV-induced MMP-1 and TNF-α expression were also inhibited by quercetin treatment in Epiderm™. In addition, quercetin inhibited DNA damage by blocking UV-mediated formation of CPDs (197). Through these mechanisms, quercetin may be used to provide protection against skin photocarcinogenesis.
Although quercetin demonstrates protection against UV-induced DNA damage, oxidative damage, and modulates cell signal transduction pathways, the efficacy of quercetin is limited by its poor penetration and deposition in the skin (200). A recent in vivo study used quercetin-loaded Poly (D,L-lactideco-glycolide) tocopheryl polyethylene glycol 1000 succinate (PLGA-TPGS) nanoparticles to enhance the delivery and bioavailability of quercetin in HaCaT cells and NIH mice. The quercetin-loaded PLGA-TPGS nanoparticles inhibited UVB-induced COX-2 expression and NFκB activation in the HaCaT cells. Quercetin-loaded PLGA-TPGS nanoparticles permeated and deposited in mice skin and decreased UVB-induced skin damage (201). A recent study on human volunteers found that treatment with quercetin phospholipids 1% cream resulted in decreased UV-induced skin inflammation by reducing erythema and wheal diameter. This study demonstrates the anti-inflammatory activity of quercetin and the efficacious delivery of quercetin by using a phospholipids-based delivery system cream of quercetin (202).
DELPHINIDIN
Delphinidin is an anthocyanidin found in berries, grapes, eggplant, tomatoes, carrots, and red onion with antioxidant, anti-proliferative, and anti-inflammatory properties (203). Pretreatment of HaCaT cells with delphinidin inhibited UVB-mediated decrease in cell viability and induction of apoptosis. In addition, pretreatment of HaCaT cells with delphinidin inhibited UVB-induced decrease in PCNA, increase in lipid peroxidation, formation of DNA damage, activation of caspases, increases in pro-apoptotic proteins (Bax, Bid and Bak), as well as decreases in anti-apoptotic proteins (Bcl-2 and Bcl-xL). By inhibiting UVB-mediated decrease in PCNA, delphinidin restored the expression of PCNA, which is a protein involved in DNA replication and repair. These effects suggest that delphinidin inhibits UVB-induced DNA damage and subsequent apoptosis. Similar effects were observed in SKH-1 hairless mouse skin in which delphinidin treatment decreased the formation of CPDs and 8-oxo-dG (203). Delphinidin also significantly inhibited UV-induced ROS formation and NADPH oxidase (NOX) enzyme activity in human dermal fibroblasts. In addition, delphinidin treatment inhibited UVB-induced expression of MMP-1 in a NOX-dependent mechanism. By targeting NOX and suppressing UVB-induced MMP-1 expression, delphinidin inhibited UVB-induced skin damage (204). Delphinidin exhibits anti-inflammatory properties by suppressing UVB-induced COX-2 expression and by inhibiting the MAPK and PI3K pathways (205). Thus, delphinidin exerts photochemopreventive effects by inhibiting UVB-induced ROS formation, inflammation, DNA damage, and apoptosis.
FISETIN
Another promising chemopreventive flavonol found in strawberries, apples, grapes, peaches, mangoes, tomatoes, cucumbers, and onions is fiestin, which possesses antioxidant, anti-proliferative, and anti-inflammatory properties (206,207) Treatment of human fibroblasts with fisetin inhibited UVB-induced COX-2 and MMPs expression. Fisetin treatment also downregulated MAPK and NFκB signaling pathways in UVB exposed human fibroblasts (207). Fisetin treatment in UVB-exposed SKH-1 hairless mouse skin inhibited inflammation by decreasing UVB-induced hyperplasia and infiltration of leukocytes as well as suppressed UVB-induced expression of pro-inflammatory molecules such as MPO, COX-2, PGE2, TNFα, IL-1β, and IL-6. Topical application of fisetin further induced UVB-mediated upregulation of p53 and p21 expression. In addition, fisetin treatment enhanced CPD repair in UVB-exposed SKH-1 hairless mouse skin. Fisetin also downregulated UVB-mediated increase in PI3K/AKT and NFκB pathways (206). By inhibiting these pathways, fisetin treatment inhibited UVB-induced cutaneous inflammation.
LYCOPENE
Lycopene is a carotenoid found in tomatoes with significant antioxidant and anticancer properties (208,209). Topical application of lycopene to SKH-1 hairless mouse skin has been shown to reduce UVB-mediated apoptosis by inhibiting activation of caspase-3. Lycopene exerted anticancer activity by inhibiting UVB-induced ODC activity. Because expression of ODC is induced by UVB and associated with photocarcinogenesis, lycopene may prevent skin cancer by inhibiting ODC. Lycopene also inhibited UVB-induced MPO activity that plays an important role in inflammation. Lycopene inhibited this inflammatory response and may thus inhibit the progression to skin photocarcinogenesis (209). This study suggests that lycopene exerts anti-inflammatory, anti-apoptotic, and anticancer effects. A recent study found that treatment of HaCaT cells with lycopene prior to UVB exposure resulted in a decrease of cells in the G0/G1 phase followed by delay at the S phase of the cell cycle. In addition, lycopene pretreatment increased the expression of Bax in UVB irradiated cells. By modulating these cell cycle events, lycopene may protect cells from photodamage (208). Decylglucoside-based microemulsions of lycopene and ascorbic acid mixed with isopropyl myristate and monocaprylin enhanced the penetration ability of lycopene eightfold compared to a solution of the drug. In addition, the microemulsion exhibited significantly increased antioxidant effects (210). Enhancing the delivery of lycopene through microemulsions may further increase the antiphotocarcinogenic effects of lycopene.
CONCLUSIONS AND FUTURE DIRECTIONS
UV exposure has negative effects on human health and is the main cause of NMSC. Skin is readily accessible to UV exposure and is susceptible to the damaging cellular and molecular changes induced by UV radiation. As the incidence of NMSC continues to increase, the need for effective strategies of reducing population disease burden and containing rising costs of treatment become more urgent. Chemopreventive strategies may be used in order to prevent UV-induced skin carcinogenesis, reducing incidence and decreasing the high costs of treatment. Studies have unequivocally demonstrated that exposure of skin to UV radiation results in inflammation, DNA damage, oxidative stress, immunosuppression, and activation of cell survival pathways leading to development of skin cancer. Phytochemicals have demonstrated promising effects against these adverse effects of UVB radiation, which may lead to inhibition of photocarcinogenesis. Reducing photocarcinogenesis using phytochemicals to target these effects may be a promising approach to reducing NMSC disease burden. Recently, various nanoemulsion techniques have been used to enhance the delivery and effectiveness of phytochemicals. Ongoing research is needed to evaluate the effectiveness of phytochemical nanoemulsions to inhibit UV-induced skin carcinogenesis. Due to the availability and low cost, phytochemicals may be used in creams and other topical products in order to prevent the damaging effects of UV-induced NMSC.
Acknowledgments
This work was supported by NIH Grant 1R21CA173043 to FA.
Biographies

Farrukh Afaq is an Assistant Professor in the Department of Dermatology at the University of Alabama at Birmingham, Alabama, USA. He obtained his M.Phil. and Ph.D. degrees in Biochemistry from A.M.U., Aligarh, India. He worked as a Scientist at the University of Wisconsin, Madison, Wisconsin, USA. Dr. Afaq’s research interests are identifying critical cellular and molecular targets in ultraviolet radiation-induced skin carcinogenesis and exploring the molecular basis for its prevention through the use of commonly consumed dietary phytochemicals. He is also investigating the effects of phytochemicals in combination with BRAF and MEK inhibitors for the treatment of melanoma by utilizing melanoma cells of different genetic backgrounds, tissue-engineered three-dimensional human melanoma skin equivalents, and genetically engineered mouse models. He has published 98 scientific articles in peer-reviewed journals and 14 book chapters.

Sarah F. McClees received her B.S. in Biology with a minor in Chemistry from the University of Alabama, Tuscaloosa, Alabama, in 2015, where she was a member of Dr. Stephen Woski’s research team examining the use of cyanocarbazole derivatives as universal DNA base candidates. Sarah is currently a second year medical student at the University of Alabama School of Medicine in Birmingham, Alabama. After developing an interest in dermatology and skin cancer during her first year of medical school, she became a research intern through the UAB Cancer Research Experiences for Students (CaRES) program under Farrukh Afaq, Ph.D., investigating the efficacy and mechanism of action of certain phytochemicals against skin cancer. Her area of research focuses on the chemotherapeutic potential of phytochemicals on melanoma cells from different genetic backgrounds.

Mary Katherine Montes de Oca received her B.S. degree in Biology at Samford University in Birmingham, Alabama, in 2015. She is currently a second year medical student at University of South Carolina Greenville School of Medicine in Greenville, South Carolina. Her area of research interest is the chemoprevention of skin cancer by phytochemicals. She gained working experience in the laboratory of Dr. Farrukh Afaq.

Ross L. Pearlman obtained his B.S. at Birmingham Southern College, a private liberal arts school in Birmingham, Alabama. While there, he studied membrane trafficking using Schizosaccaromyces pombe (fission yeast) models. As an undergraduate, he received recognition from the Southeastern Regional Yeast Meeting for his work, winning a first-place prize in undergraduate platform presentation. He joined the University of Alabama School of Medicine, in the fall of 2014 and began working in Dr. Farrukh Afaq’s laboratory through the UAB Cancer Research Experiences for Students (CaRES) program. He currently focuses on melanoma and the potential use of phytochemicals as chemotherapeutic and chemopreventive agents. He recently received the CaRES program “Best Research Project” scholarship to support his work.

Rebecca Strickland obtained a Bachelor of Science in Biology from the University of Montevallo in 2015. In 2016 she joined the laboratory of Dr. Afaq to assist with research on chemoprevention of skin cancer, gaining experience in cell culture, western blotting and immunohistochemistry techniques. Her current research is skin cancer chemoprevention.
Footnotes
This article is part of the Special Issue honoring Dr. Hasan Mukhtar’s 70th Birthday and his outstanding contributions to various aspects of photobiology research, including photocarcinogenesis and chemoprevention.
References
- 1.El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 2.Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol. 2013;5:331–347. doi: 10.4161/derm.26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Régnier M, Patwardhan A, Scheynius A, Schmidt R. Reconstructed human epidermis composed of keratinocytes, melanocytes and Langerhans cells. Med. Biol. Eng. Comput. 1998;36:821–824. doi: 10.1007/BF02518889. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016;36:1345–1356. [PubMed] [Google Scholar]
- 5.Bosch R, Philips N, Suárez-Pérez JA, Juarranz A, Devmurari A, Chalensouk-Khaosaat J, González S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants (Basel) 2015;4:248–268. doi: 10.3390/antiox4020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young AR. Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin. Dermatol. 1990;9:25–31. [PubMed] [Google Scholar]
- 7.Katta R, Brown DN. Diet and Skin Cancer: The Potential Role of Dietary Antioxidants in Nonmelanoma Skin Cancer Prevention. J. Skin Cancer. 2015;2015:893149. doi: 10.1155/2015/893149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinert R, de Vries E, Erdmann F, Espina C, Auvinen A, Kesminiene A, Schüz J. European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S75–S83. doi: 10.1016/j.canep.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Lewis KG, Weinstock MA. Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J. Invest. Dermatol. 2007;127:2323–2327. doi: 10.1038/sj.jid.5700897. [DOI] [PubMed] [Google Scholar]
- 10.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;51:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 11.Bower CP, Lear JT, Bygrave S, Etherington D, Harvey I, Archer CB. Basal cell carcinoma and risk of subsequent malignancies: A cancer registry-based study in southwest England. J. Am. Acad. Dermatol. 2000;42:988–991. [PubMed] [Google Scholar]
- 12.Brewster AM, Alberg AJ, Strickland PT, Hoffman SC, Helzlsouer K. XPD polymorphism and risk of subsequent cancer in individuals with nonmelanoma skin cancer. Cancer Epidemiol. Biomarkers Prev. 2004;13:1271–1275. [PubMed] [Google Scholar]
- 13.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 14.Lucas RM, Norval M, Wright CY. Solar ultraviolet radiation in Africa: a systematic review and critical evaluation of the health risks and use of photoprotection. Photochem. Photobiol. Sci. 2016;15:10–23. doi: 10.1039/c5pp00419e. [DOI] [PubMed] [Google Scholar]
- 15.Trakatelli M, Barkitzi K, Apap C, Majewski S, De Vries E. Skin cancer risk in outdoor workers: a European multicenter case-control study. J. Eur. Acad. Dermatol. Venereol. 2016;30(Suppl. 3):5–11. doi: 10.1111/jdv.13603. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 17.Ramos J, Villa J, Ruiz A, Armstrong R, Matta J. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol. Biomarkers Prev. 2004;13:2006–2011. [PubMed] [Google Scholar]
- 18.Banks BA, Silverman RA, Schwartz RH, Tunnessen J, Walter W. Attitudes of teenagers toward sun exposure and sunscreen use. Pediatrics. 1992;89:40. [PubMed] [Google Scholar]
- 19.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouret S, Philippe C, Gracia-Chantegrel J, Banyasz A, Karpati S, Markovitsi D, Douki T. UVA-induced cyclobutane pyrimidine dimers in DNA: a direct photochemical mechanism? Org. Biomol. Chem. 2010;8:1706–1711. doi: 10.1039/b924712b. [DOI] [PubMed] [Google Scholar]
- 21.Vink AA, Roza L. Biological consequences of cyclobutane pyrimidine dimers. J Photochem. Photobiol. B. 2001;65:101–104. doi: 10.1016/s1011-1344(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Ho VC, Mitchell DL, Trotter MJ, Tron VA. Differentiation-dependent p53 regulation of nucleotide excision repair in keratinocytes. Am. J. Pathol. 1997;150:1457–1464. [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura Y, Ananthaswamy HN. Molecular mechanisms of photocarcinogenesis. Front. Biosci. 2002;7:d765–d783. doi: 10.2741/matsumur. [DOI] [PubMed] [Google Scholar]
- 24.Rochette PJ, Lacoste S, Therrien J, Bastien N, Brash DE, Drouin R. Influence of cytosine methylation on ultraviolet-induced cyclobutane pyrimidine dimer formation in genomic DNA. Mutat. Res. 2009;665:7–13. doi: 10.1016/j.mrfmmm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 26.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 27.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 28.Godar DE. UVA1 radiation triggers two different final apoptotic pathways. J. Invest. Dermatol. 1999;112:3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012;67:1013–1024. doi: 10.1016/j.jaad.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Godic A, Poljšak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell Longev. 2014;2014:860479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem. Photobiol. 1996;63:356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 33.Khan N, Syed DN, Pal HC, Mukhtar H, Afaq F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2012;88:1126–1134. doi: 10.1111/j.1751-1097.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deliconstantinos G, Villiotou V, Stavrides JC. Increase of particulate nitric oxide synthase activity and peroxynitrite synthesis in UVB-irradiated keratinocyte membranes. Biochem. J. 1996;320:997–1003. doi: 10.1042/bj3200997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Bowden GT. UVB irradiation regulates Cox-2 mRNA stability through AMPK and HuR in human keratinocytes. Mol. Carcinog. 2008;47:974–983. doi: 10.1002/mc.20450. [DOI] [PubMed] [Google Scholar]
- 36.Ming M, Han W, Zhao B, Sundaresan NR, Deng C, Gupta MP, He Y. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014;74:5925–5933. doi: 10.1158/0008-5472.CAN-14-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu K, Yu D, Cho Y, Bode AM, Ma W, Yao K, Li S, Li J, Bowden GT, Dong Z, Dong Z. Sunlight UV-induced skin cancer relies upon activation of the p38a signaling pathway. Cancer Res. 2013;73:2181–2188. doi: 10.1158/0008-5472.CAN-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang Y, Lo C, Chen Y, Wang S, Yang N, Kuo Y, Shyur L. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 40.An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem. Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Gresham A, Masferrer J, Chen X, Leal-Khouri S, Pentland AP. Increased synthesis of high-molecular-weight cPLA2 mediates early UV-induced PGE2 in human skin. Am. J. Physiol. 1996;270:C1037–C1050. doi: 10.1152/ajpcell.1996.270.4.C1037. [DOI] [PubMed] [Google Scholar]
- 42.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova A. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katiyar SK, Mukhtar H. Green tea polyphenol (™)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 44.Rosin MP, Anwar WA, Ward AJ. Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res. 1994;54:1929s–1933s. [PubMed] [Google Scholar]
- 45.Trush MA, Seed JL, Kensler TW. Oxidant-dependent metabolic activation of polycyclic aromatic hydrocarbons by phorbol ester-stimulated human polymorphonuclear leukocytes: possible link between inflammation and cancer. Proc. Natl. Acad. Sci. U. S. A. 1985;82:5194–5198. doi: 10.1073/pnas.82.15.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katiyar SK, Meleth S, Sharma SD. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem. Photobiol. 2008;84:266–271. doi: 10.1111/j.1751-1097.2007.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 48.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y, He Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014;1:188–198. doi: 10.1016/j.gendis.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magné N, Toillon R, Bottero V, Didelot C, Houtte PV, Gérard J, Peyron J. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–168. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Dong Z, Valcic S, Timmermann BN, Bowden GT. Inhibition of ultraviolet B--induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (™)-epigallocatechin gallate in a human keratinocyte cell line. Mol. Carcinog. 1999;24:79–84. doi: 10.1002/(sici)1098-2744(199902)24:2<79::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 53.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 54.Gonzales M, Bowden GT. The role of PI 3-kinase in the UVB-induced expression of c-fos. Oncogene. 2002;21:2721–2728. doi: 10.1038/sj.onc.1205366. [DOI] [PubMed] [Google Scholar]
- 55.Tang Q, Gonzales M, Inoue H, Bowden GT. Roles of Akt and glycogen synthase kinase 3beta in the ultraviolet B induction of cyclooxygenase-2 transcription in human keratinocytes. Cancer Res. 2001;61:4329–4332. [PubMed] [Google Scholar]
- 56.Syed DN, Afaq F, Mukhtar H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem. Photobiol. 2012;88:1184–1190. doi: 10.1111/j.1751-1097.2012.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, Epstein EH, Jr, Bickers DR. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem. Biophys. Res. Commun. 2001;280:1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 58.Roy P, Madan E, Kalra N, Nigam N, George J, Ray RS, Hans RK, Prasad S, Shukla Y. Resveratrol enhances ultraviolet B-induced cell death through nuclear factor-kappaB pathway in human epidermoid carcinoma A431 cells. Biochem. Biophys. Res. Commun. 2009;384:215–220. doi: 10.1016/j.bbrc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 59.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi J, Choi D, Lee J, Yun S, Lee D, Eun J, Lee S. Ethanol extract of peanut sprout induces Nrf2 activation and expression of antioxidant and detoxifying enzymes in human dermal fibroblasts: implication for its protection against UVB-irradiated oxidative stress. Photochem. Photobiol. 2013;89:453–460. doi: 10.1111/j.1751-1097.2012.01244.x. [DOI] [PubMed] [Google Scholar]
- 61.Chiou Y, Tsai M, Nagabhushanam K, Wang Y, Wu C, Ho C, Pan M. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 62.Sumita N, Bito T, Nakajima K, Nishigori C. Stat3 activation is required for cell proliferation and tumorigenesis but not for cell viability in cutaneous squamous cell carcinoma cell lines. Exp. Dermatol. 2006;15:291–299. doi: 10.1111/j.0906-6705.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 63.Shen Y, Devgan G, Darnell JEJ, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 65.Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I, Heinrich PC, Diehl V, Tesch H. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98:762–770. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- 66.Bito T, Sumita N, Masaki T, Shirakawa T, Ueda M, Yoshiki R, Tokura Y, Nishigori C. Ultraviolet light induces Stat3 activation in human keratinocytes and fibroblasts through reactive oxygen species and DNA damage. Exp. Dermatol. 2010;19:654–660. doi: 10.1111/j.1600-0625.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 67.Ahsan H, Aziz MH, Ahmad N. Ultraviolet B exposure activates Stat3 signaling via phosphorylation at tyrosine705 in skin of SKH1 hairless mouse: a target for the management of skin cancer? Biochem. Biophys. Res. Commun. 2005;333:241–246. doi: 10.1016/j.bbrc.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 68.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GT. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 69.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 70.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 71.Flamigni F, Facchini A, Giordano E, Tantini B, Stefanelli C. Signaling pathways leading to the induction of ornithine decarboxylase: opposite effects of p44/42 mitogen-activated protein kinase (MAPK) and p38 MAPK inhibitors. Biochem. Pharmacol. 2001;61:25–32. doi: 10.1016/s0006-2952(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad N, Gilliam AC, Katiyar SK, O’Brien TG, Mukhtar H. A definitive role of ornithine decarboxylase in photocarcinogenesis. Am. J. Pathol. 2001;159:885–892. doi: 10.1016/S0002-9440(10)61764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elmets CA, Ledet JJ, Athar M. Cyclooxygenases: mediators of UV-induced skin cancer and potential targets for prevention. J. Invest. Dermatol. 2014;134:2497–2502. doi: 10.1038/jid.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 75.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997;378:1247–1257. [PubMed] [Google Scholar]
- 76.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tornaletti S, Rozek D, Pfeifer GP. The distribution of UV photoproducts along the human p53 gene and its relation to mutations in skin cancer. Oncogene. 1993;8:2051–2057. [PubMed] [Google Scholar]
- 78.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J. Dermatol. Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 79.Domann FEJ, Levy JP, Finch JS, Bowden GT. Constitutive AP-1 DNA binding and transactivating ability of malignant but not benign mouse epidermal cells. Mol. Carcinog. 1994;9:61–66. doi: 10.1002/mc.2940090202. [DOI] [PubMed] [Google Scholar]
- 80.Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J. Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 82.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 83.Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- 84.Afaq F, Katiyar SK. Polyphenols: skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011;11:1200–1215. doi: 10.2174/13895575111091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 86.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delmas D, Lançon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 88.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 89.Aziz MH, Ghotra AS, Shukla Y, Ahmad N. Ultraviolet-B radiation causes an upregulation of survivin in human keratinocytes and mouse skin. Photochem. Photobiol. 2004;80:602–608. doi: 10.1562/0031-8655(2004)080<0602:URCAUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 90.Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab. Invest. 1999;79:1121–1126. [PubMed] [Google Scholar]
- 91.Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol. Med. 2001;7:542–547. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- 92.Peter M. The regulation of cyclin-dependent kinase inhibitors (CKIs) Prog. Cell Cycle Res. 1997;3:99–108. doi: 10.1007/978-1-4615-5371-7_8. [DOI] [PubMed] [Google Scholar]
- 93.Sirerol JA, Feddi F, Mena S, Rodriguez ML, Sirera P, Aupí M, Pérez S, Asensi M, Ortega A, Estrela JM. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015;85:1–11. doi: 10.1016/j.freeradbiomed.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 94.Fujimura AT, Martinez RM, Pinho-Ribeiro FA, Lopes Dias da Silva AM, Baracat MM, Georgetti SR, Verri Waldiceu A, Jr, Chorilli M, Casagrande R. Resveratrol-Loaded Liquid-Crystalline System Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Mice. J. Nat. Prod. 2016;79:1329–1338. doi: 10.1021/acs.jnatprod.5b01132. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Chan F, Sun H, Yan J, Fan D, Zhao D, An J, Zhou D. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur. J. Pharmacol. 2011;650:130–137. doi: 10.1016/j.ejphar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Potapovich AI, Kostyuk VA, Kostyuk TV, de Luca C, Korkina LG. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013;62:773–780. doi: 10.1007/s00011-013-0634-z. [DOI] [PubMed] [Google Scholar]
- 97.Zhang G, Flach CR, Mendelsohn R. Tracking the dephosphorylation of resveratrol triphosphate in skin by confocal Raman microscopy. J. Control. Release. 2007;123:141–147. doi: 10.1016/j.jconrel.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y, Jia L, Zheng Y, Xu X, Luo Y, Wang B, Chen JZS, Gao X, Chen H, Matsui M, Li Y. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J. Eur. Acad. Dermatol. Venereol. 2013;27:345–350. doi: 10.1111/j.1468-3083.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- 99.Yutani R, Teraoka R, Kitagawa S. Microemulsion Using Polyoxyethylene Sorbitan Trioleate and its Usage for Skin Delivery of Resveratrol to Protect Skin against UV-Induced Damage. Chem. Pharm. Bull. (Tokyo) 2015;63:741–745. doi: 10.1248/cpb.c15-00378. [DOI] [PubMed] [Google Scholar]
- 100.Yang CS, Wang ZY. Tea and cancer. J. Natl. Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 101.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Katiyar SK, Bergamo BM, Vyalil PK, Elmets CA. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B. 2001;65:109–114. doi: 10.1016/s1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 103.Yang MJ, Lee CS, Chen L, Yang GY. Polyphenols as inhibitors of carcinogenesis. Environ. Health Perspect. 1997;105(Suppl. 4):971–976. doi: 10.1289/ehp.97105s4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (™)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–4419. [PubMed] [Google Scholar]
- 105.Bender K, Blattner C, Knebel A, Iordanov M, Herrlich P, Rahmsdorf HJ. UV-induced signal transduction. J. Photochem. Photobiol. B. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 106.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 107.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (™)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 108.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 109.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev. Res. (Phila) 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu YP, Lou YP, Li XH, Xie JG, Brash D, Huang MT, Conney AH. Stimulatory effect of oral administration of green tea or caffeine on ultraviolet light-induced increases in epidermal wild-type p53, p21(WAF1/CIP1), and apoptotic sunburn cells in SKH-1 mice. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- 111.Nadim M, Auriol D, Lamerant-Fayel N, Lefèvre F, Dubanet L, Redziniak G, Kieda C, Grillon C. Improvement of polyphenol properties upon glucosylation in a UV-induced skin cell ageing model. Int. J. Cosmet. Sci. 2014;36:579–587. doi: 10.1111/ics.12159. [DOI] [PubMed] [Google Scholar]
- 112.Mittal A, Piyathilake C, Hara Y, Katiyar SK. Exceptionally high protection of photocarcinogenesis by topical application of (--)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5:555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaş HS. Chitosan: properties, preparations and application to microparticulate systems. J. Microencapsul. 1997;14:689–711. doi: 10.3109/02652049709006820. [DOI] [PubMed] [Google Scholar]
- 114.Batchelder RJ, Calder RJ, Thomas CP, Heard CM. In vitro transdermal delivery of the major catechins and caffeine from extract of Camellia sinensis. Int. J. Pharm. 2004;283:45–51. doi: 10.1016/j.ijpharm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 115.Sun Y, Hung W, Chen F, Lee C, Huang HW. Interaction of tea catechin (™)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009;96:1026–1035. doi: 10.1016/j.bpj.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wisuitiprot W, Somsiri A, Ingkaninan K, Waranuch N. In vitro human skin permeation and cutaneous metabolism of catechins from green tea extract and green tea extract-loaded chitosan microparticles. Int. J. Cosmet. Sci. 2011;33:572–579. doi: 10.1111/j.1468-2494.2011.00673.x. [DOI] [PubMed] [Google Scholar]
- 117.Scalia S, Trotta V, Bianchi A. In vivo human skin penetration of (™)-epigallocatechin-3-gallate from topical formulations. Acta Pharm. 2014;64:257–265. doi: 10.2478/acph-2014-0017. [DOI] [PubMed] [Google Scholar]
- 118.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (™)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- 119.Katiyar SK, Perez A, Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clin. Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- 120.Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12. Photochem. Photobiol. 2008;84:350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 121.Zhao JF, Zhang YJ, Jin XH, Athar M, Santella RM, Bickers DR, Wang ZY. Green tea protects against psoralen plus ultraviolet A-induced photochemical damage to skin. J. Invest. Dermatol. 1999;113:1070–1075. doi: 10.1046/j.1523-1747.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 122.Camouse MM, Domingo DS, Swain FR, Conrad EP, Matsui MS, Maes D, Declercq L, Cooper KD, Stevens SR, Baron ED. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp. Dermatol. 2009;18:522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 123.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 124.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 125.Pacheco-Palencia L, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott S. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agric. Food Chem. 2008;56:8434–8441. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- 126.Zaid MA, Afaq F, Syed DN, Dreher M, Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007;83:882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 127.Afaq F, Khan N, Syed DN, Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem. Photobiol. 2010;86:1318–1326. doi: 10.1111/j.1751-1097.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verma AK, Shapas BG, Rice HM, Boutwell RK. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979;39:419–425. [PubMed] [Google Scholar]
- 129.Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J. Invest. Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- 130.Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-alpha-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J. Invest. Dermatol. 2005;124:248–255. doi: 10.1111/j.0022-202X.2004.23543.x. [DOI] [PubMed] [Google Scholar]
- 131.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and −8 in pterygia and cultured human pterygium epithelial cells. Invest. Ophthalmol. Vis. Sci. 2002;43:3430–3437. [PubMed] [Google Scholar]
- 132.Baccarin T, Mitjans M, Ramos D, Lemos-Senna E, Vinardell MP. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J. Photochem. Photobiol. B. 2015;153:127–136. doi: 10.1016/j.jphotobiol.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 133.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 134.Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- 135.Zhu W, MacIntyre A, Nicosia RF. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am. J. Pathol. 2002;161:823–830. doi: 10.1016/S0002-9440(10)64242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Afaq F, Zaid MA, Khan N, Syed DN, Yun J-M, Sarfaraz S, Suh Y, Mukhtar H. Inhibitory effect of oral feeding of pomegranate fruit extract on UVB-induced skin carcinogenesis in SKH-1 hairless mice. Proc. Amer. Assoc. Cancer Res. 2008;49:1246. [Google Scholar]
- 137.Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kasai K, Yoshimura M, Koga T, Arii M, Kawasaki S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006;52:383–388. doi: 10.3177/jnsv.52.383. [DOI] [PubMed] [Google Scholar]
- 139.Singh RP, Agarwal R. Flavonoid antioxidant silymarin and skin cancer. Antioxid. Redox Signal. 2002;4:655–663. doi: 10.1089/15230860260220166. [DOI] [PubMed] [Google Scholar]
- 140.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE. 2003;2003:RE2–RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 141.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol. Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 142.Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 143.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25:99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 144.Narayanapillai S, Agarwal C, Tilley C, Agarwal R. Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem. Photobiol. 2012;88:1135–1140. doi: 10.1111/j.1751-1097.2011.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gu M, Singh RP, Dhanalakshmi S, Mohan S, Agarwal R. Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Mol. Cancer Ther. 2006;5:2121–2129. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 146.Berton TR, Mitchell DL, Guo R, Johnson DG. Regulation of epidermal apoptosis and DNA repair by E2F1 in response to ultraviolet B radiation. Oncogene. 2005;24:2449–2460. doi: 10.1038/sj.onc.1208462. [DOI] [PubMed] [Google Scholar]
- 147.Roy S, Deep G, Agarwal C, Agarwal R. Silibinin prevents ultraviolet B radiation-induced epidermal damages in JB6 cells and mouse skin in a p53-GADD45a–dependent manner. Carcinogenesis. 2012;33:629–636. doi: 10.1093/carcin/bgr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 149.Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS One. 2011;6:e21410. doi: 10.1371/journal.pone.0021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 151.Xie T, Huang F, Aldape KD, Kang S, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 152.Panapisal V, Charoensri S, Tantituvanont A. Formulation of microemulsion systems for dermal delivery of silymarin. AAPS Pharm. Sci. Tech. 2012;13:389–399. doi: 10.1208/s12249-012-9762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chiu T, Huang C, Lin T, Fang J, Wu N, Hung C. In vitro and in vivo anti-photoaging effects of an isoflavone extract from soybean cake. J. Ethnopharmacol. 2009;126:108–113. doi: 10.1016/j.jep.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 154.Widyarini S, Spinks N, Husband AJ, Reeve VE. Isoflavonoid compounds from red clover (Trifolium pratense) protect from inflammation and immune suppression induced by UV radiation. Photochem. Photobiol. 2001;74:465–470. doi: 10.1562/0031-8655(2001)074<0465:icfrct>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 155.Wei H, Cai Q, Rahn RO. Inhibition of UV light- and Fenton reaction-induced oxidative DNA damage by the soybean isoflavone genistein. Carcinogenesis. 1996;17:73–77. doi: 10.1093/carcin/17.1.73. [DOI] [PubMed] [Google Scholar]
- 156.Moore JO, Wang Y, Stebbins WG, Gao D, Zhou X, Phelps R, Lebwohl M, Wei H. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis. 2006;27:1627–1635. doi: 10.1093/carcin/bgi367. [DOI] [PubMed] [Google Scholar]
- 157.Kang S, Chung JH, Lee JH, Fisher GJ, Wan YS, Duell EA, Voorhees JJ. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J. Invest. Dermatol. 2003;120:835–841. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 158.Maione-Silva L, Rocha KAD, de Oliveira LC, Taveira SF, Lima EM. Development and validation of a simple and rapid liquid chromatography method for the determination of genistein in skin permeation studies. Biol. Pharm. Bull. 2012;35:1986–1990. doi: 10.1248/bpb.b12-00442. [DOI] [PubMed] [Google Scholar]
- 159.de Vargas BA, Argenta DF, Borghetti G, Koester LS, Bassani VL, Teixeira HF. Validation of an LC method to determine skin retention profile of genistein from nanoemulsions incorporated in hydrogels. J. Chromatogr. Sci. 2012;50:114–118. doi: 10.1093/chromsci/bmr038. [DOI] [PubMed] [Google Scholar]
- 160.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int. J. Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 161.Das S, Das J, Paul A, Samadder A, Khuda-Bukhsh A. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct Meridian Stud. 2013;6:252–262. doi: 10.1016/j.jams.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 162.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 163.Kuo ML, Yang NC. Reversion of v-H-ras-transformed NIH 3T3 cells by apigenin through inhibiting mitogen activated protein kinase and its downstream oncogenes. Biochem. Biophys. Res. Commun. 1995;212:767–775. doi: 10.1006/bbrc.1995.2035. [DOI] [PubMed] [Google Scholar]
- 164.Chaumontet C, Droumaguet C, Bex V, Heberden C, Gaillard-Sanchez I, Martel P. Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells. Cancer Lett. 1997;114:207–210. doi: 10.1016/s0304-3835(97)04664-8. [DOI] [PubMed] [Google Scholar]
- 165.Hwang YP, Oh KN, Yun HJ, Jeong HG. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011;61:23–31. doi: 10.1016/j.jdermsci.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 166.Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Invest. Dermatol. 2001;117:1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 167.Varani J, Hattori Y, Chi Y, Schmidt T, Perone P, Zeigler ME, Fader DJ, Johnson TM. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: comparison with normal skin. Br. J. Cancer. 2000;82:657–665. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sim G, Lee B, Cho HS, Lee JW, Kim J, Lee D, Kim J, Pyo H, Moon DC, Oh K, Yun YP, Hong JT. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007;30:290–298. doi: 10.1007/BF02977608. [DOI] [PubMed] [Google Scholar]
- 169.Paredes-Gonzalez X, Fuentes F, Su Z, Kong AT. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. AAPS J. 2014;16:727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McVean M, Xiao H, Isobe K, Pelling JC. Increase in wild-type p53 stability and transactivational activity by the chemopreventive agent apigenin in keratinocytes. Carcinogenesis. 2000;21:633–639. doi: 10.1093/carcin/21.4.633. [DOI] [PubMed] [Google Scholar]
- 171.Byun S, Park J, Lee E, Lim S, Yu JG, Lee SJ, Chen H, Dong Z, Lee KW, Lee HJ. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis. 2013;34:397–405. doi: 10.1093/carcin/bgs358. [DOI] [PubMed] [Google Scholar]
- 172.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol. Carcinog. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 173.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Mol. Cell. Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shen L, Zhang Y, Wang Q, Xu L, Feng N. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014;460:280–288. doi: 10.1016/j.ijpharm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 175.Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh A. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013;62:670–680. doi: 10.1016/j.fct.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 176.Sikdar S, Papadopoulou M, Dubois J. What do we know about sulforaphane protection against photoaging? J. Cosmet. Dermatol. 2016;15:72–77. doi: 10.1111/jocd.12176. [DOI] [PubMed] [Google Scholar]
- 177.Franklin SJ, Dickinson SE, Karlage KL, Bowden GT, Myrdal PB. Stability of sulforaphane for topical formulation. Drug Dev. Ind. Pharm. 2014;40:494–502. doi: 10.3109/03639045.2013.768634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Knatko EV, Ibbotson SH, Zhang Y, Higgins M, Fahey JW, Talalay P, Dawe RS, Ferguson J, Huang JT, Clarke R, Zheng S, Saito A, Kalra S, Benedict AL, Honda T, Proby CM, Dinkova-Kostova A. Nrf2 Activation Protects against Solar-Simulated Ultraviolet Radiation in Mice and Humans. Cancer Prev. Res. (Phila) 2015;8:475–486. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marrot L, Jones C, Perez P, Meunier J. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 180.Wagner AE, Ernst I, Iori R, Desel C, Rimbach G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2010;19:137–144. doi: 10.1111/j.1600-0625.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 181.Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT. Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol. Carcinog. 2004;41:179–186. doi: 10.1002/mc.20052. [DOI] [PubMed] [Google Scholar]
- 182.Dinkova-Kostova A, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the Phase 2 Response in Mouse and Human Skin by Sulforaphane-containing Broccoli Sprout Extracts. Cancer Epidemiol. Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 183.Dinkova-Kostova A, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 184.Iskander K, Gaikwad A, Paquet M, Long DJ, 3nd, Brayton C, Barrios R, Jaiswal AK. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054–2058. doi: 10.1158/0008-5472.CAN-04-3157. [DOI] [PubMed] [Google Scholar]
- 185.Guillermo RF, Chilampalli C, Zhang X, Zeman D, Fahmy H, Dwivedi C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov. Ther. 2012;6:140–146. [PubMed] [Google Scholar]
- 186.Vaid M, Sharma SD, Katiyar SK. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: development of topical formulation. Carcinogenesis. 2010;31:2004–2011. doi: 10.1093/carcin/bgq186. [DOI] [PubMed] [Google Scholar]
- 187.Chilampalli S, Zhang X, Fahmy H, Kaushik RS, Zeman D, Hildreth MB, Dwivedi C. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010;30:777–783. [PubMed] [Google Scholar]
- 188.Lim SH, Jung SK, Byun S, Lee EJ, Hwang JA, Seo SG, Kim YA, Yu JG, Lee KW, Lee HJ. Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90 RSK2. J. Cell. Mol. Med. 2013;17:672–680. doi: 10.1111/jcmm.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wölfle U, Heinemann A, Esser PR, Haarhaus B, Martin SF, Schempp CM. Luteolin prevents solar radiation-induced matrix metalloproteinase-1 activation in human fibroblasts: a role for p38 mitogen-activated protein kinase and interleukin-20 released from keratinocytes. Rejuvenation Res. 2012;15:466–475. doi: 10.1089/rej.2011.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Verschooten L, Smaers K, Van Kelst S, Proby C, Maes D, Declercq L, Agostinis P, Garmyn M. The flavonoid luteolin increases the resistance of normal, but not malignant keratinocytes, against UVB-induced apoptosis. J. Invest. Dermatol. 2010;130:2277–2285. doi: 10.1038/jid.2010.124. [DOI] [PubMed] [Google Scholar]
- 191.Byun S, Lee KW, Jung SK, Lee EJ, Hwang MK, Lim SH, Bode AM, Lee HJ, Dong Z. Luteolin inhibits protein kinase C (epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010;70:2415–2423. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- 192.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 193.Fisher GJ, Talwar HS, Lin J, Voorhees JJ. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochem. Photobiol. 1999;69:154–157. doi: 10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 194.Verma AK, Wheeler DL, Aziz MH, Manoharan H. Protein kinase Cepsilon and development of squamous cell carcinoma, the nonmelanoma human skin cancer. Mol. Carcinog. 2006;45:381–388. doi: 10.1002/mc.20230. [DOI] [PubMed] [Google Scholar]
- 195.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Kelly GS. Quercetin. Monograph. Altern. Med. Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 197.Maini S, Fahlman BM, Krol ES. Flavonols protect against UV radiation-induced thymine dimer formation in an artificial skin mimic. J. Pharm. Pharm. Sci. 2015;18:600–615. doi: 10.18433/j34w39. [DOI] [PubMed] [Google Scholar]
- 198.Olson ER, Melton T, Dickinson SE, Dong Z, Alberts DS, Bowden GT. Quercetin potentiates UVB-Induced c-Fos expression: implications for its use as a chemopreventive agent. Cancer Prev. Res. (Phila) 2010;3:876–884. doi: 10.1158/1940-6207.CAPR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yin Y, Li W, Son Y, Sun L, Lu J, Kim D, Wang X, Yao H, Wang L, Pratheeshkumar P, Hitron AJ, Luo J, Gao N, Shi X, Zhang Z. Quercetin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013;269:89–99. doi: 10.1016/j.taap.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hung C, Fang C, Al-Suwayeh S, Yang S, Fang J. Evaluation of drug and sunscreen permeation via skin irradiated with UVA and UVB: comparisons of normal skin and chronologically aged skin. J. Dermatol. Sci. 2012;68:135–148. doi: 10.1016/j.jdermsci.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 201.Zhu X, Zeng X, Zhang X, Cao W, Wang Y, Chen H, Wang T, Tsai H, Zhang R, Chang D, He S, Mei L, Shi X. The effects of quercetin-loaded PLGA-TPGS nanoparticles on ultraviolet B-induced skin damages in vivo. Nanomedicine. 2016;12:623–632. doi: 10.1016/j.nano.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 202.Maramaldi G, Togni S, Pagin I, Giacomelli L, Cattaneo R, Eggenhöffner R, Burastero SE. Soothing and anti-itch effect of quercetin phytosome in human subjects: a single-blind study. Clin. Cosmet. Investig. Dermatol. 2016;9:55–62. doi: 10.2147/CCID.S98890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon M, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 204.Lim T, Jung SK, Kim J, Kim Y, Lee HJ, Jang TS, Lee KW. NADPH oxidase is a novel target of delphinidin for the inhibition of UVB-induced MMP-1 expression in human dermal fibroblasts. Exp. Dermatol. 2013;22:428–430. doi: 10.1111/exd.12157. [DOI] [PubMed] [Google Scholar]
- 205.Kwon JY, Lee KW, Kim J, Jung SK, Kang NJ, Hwang MK, Heo Y, Bode AM, Dong Z, Lee HJ. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis. 2009;30:1932–1940. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pal HC, Athar M, Elmets CA, Afaq F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015;91:225–234. doi: 10.1111/php.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chiang H, Chan S, Chu Y, Wen K. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways. J. Agric. Food Chem. 2015;63:4551–4560. doi: 10.1021/jf502500t. [DOI] [PubMed] [Google Scholar]
- 208.Ascenso A, Pedrosa T, Pinho S, Pinho F, de Oliveira JM, Ferreira P, Cabral Marques H, Oliveira H, Simões S, Santos C. The Effect of Lycopene Preexposure on UV-B-Irradiated Human Keratinocytes. Oxid. Med. Cell Longev. 2016;2016:8214631. doi: 10.1155/2016/8214631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fazekas Z, Gao D, Saladi RN, Lu Y, Lebwohl M, Wei H. Protective effects of lycopene against ultraviolet B-induced photodamage. Nutr. Cancer. 2003;47:181–187. doi: 10.1207/s15327914nc4702_11. [DOI] [PubMed] [Google Scholar]
- 210.Pepe D, Phelps J, Lewis K, Dujack J, Scarlett K, Jahan S, Bonnier E, Milic-Pasetto T, Hass MA, Lopes LB. Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. Int. J. Pharm. 2012;434:420–428. doi: 10.1016/j.ijpharm.2012.06.016. [DOI] [PubMed] [Google Scholar]