Abstract
The incidence of skin cancers, both non-melanoma as well as melanoma, are increasing in the United States. The ultraviolet radiation, mainly from sun, is considered the major cause for these neoplasms. While non-melanoma skin cancers are far more numerous, melanoma remains the most challenging. This is because melanoma can become extremely aggressive and its incidence is increasing worldwide due to lack of effective early detection, as well as disease recurrence, following both surgery and chemotherapy. Therefore, in addition to better treatment options, newer means are required to prevent melanomas from developing. Chemoprevention is a reasonable cost-effective approach to prevent carcinogenesis by inhibiting the processes of tumor initiation, promotion and progression. Melanoma is a progressive disease, which makes it very suitable for chemopreventive interventions, by targeting the processes and molecular pathways involved in the progression of melanoma. This review discusses the roles of various chemopreventive agents such as NSAIDs, statins, vitamins and dietary agents in melanoma and highlights current advancements and our perspective on future of melanoma chemoprevention. Although considerable preclinical data suggest that melanoma may be prevented or delayed by a numerous chemopreventive agents, we realize there are insufficient clinical studies evaluating their efficacy and long-term safety for human use.
Graphical abstract
Exposure to ultraviolet radiation is a leading cause of skin cancers, including melanoma. Current preventive strategies and therapies are not sufficient to reduce incidence or mortality associated with melanoma. Therefore, it is necessary to explore new therapeutic, as well as chemopreventive strategies for melanoma management. Here, we discuss studies aimed at chemoprevention of melanoma. To date, limited clinical studies have been done in the field of melanoma chemoprevention, and no agent has so far been approved, for clinical use. However, encouraging results from preclinical studies, support further exploration and clinical trials to identify novel agents for melanoma prevention in humans.
INTRODUCTION
Melanoma is one of the most aggressive forms of skin cancer, and may be fatal if not diagnosed and treated early (1). According to recent predictions from the American Cancer Society, 87,110 new cases of melanoma will be diagnosed in 2017, which is significantly up from previous years (2). Further, despite advancements in targeted therapies (e.g. BRAF and MAPK inhibitors) as well as immune-based approaches (e.g. immune checkpoint inhibitors), approximately 9,730 melanoma-related deaths are estimated for 2017 (2). Additionally, melanoma recurrence rate is high after successful treatment, often with resistance to the initial treatment (3). Although melanoma development in many people is tied to a genetic predisposition, several other risk factors have been found that increase the risk of developing this cancer, including eye and hair color and ultraviolet radiation (UVR) exposure. UVR exposure may be the easiest risk factor to address, and research suggests that both DNA damage from UVB exposure and cellular damage from UVA-induced reactive oxygen species (ROS) may play roles in melanomagenesis. The current status quo of melanoma management, including the recommended lifestyle changes, such as the use of sunscreens and sun avoidance, as well as the available therapeutic options, have not been very successful in either decreasing the incidence or affecting the mortality associated with melanoma. The consistently increasing global incidence of melanoma coupled with therapeutic difficulties in the treatment of advanced disease has spurred research efforts towards identifying novel means to prevent melanoma. In this review, we have provided a succinct and critical account of chemoprevention of melanoma via a variety of means, including sunscreens, synthetic agents and dietary approaches.
ULTRAVIOLET RADIATION AND RISK OF MELANOMA
Solar light is made up of a wide range of wavelengths including the UV and visible light spectrum. The most potent risk factor for skin cancer is solar radiation in the UV range (200–400 nm) (4, 5). The UV range can be further divided into three spectral regions on the basis of their biological effects. As shown in Fig. 1, UVC (200–290 nm) rays are mostly blocked by the atmospheric ozone layer before they reach the Earth’s surface. However, concern about ozone depletion makes UVC a potentially dangerous risk factor (6). UVB (290–320 nm) radiation is traditionally thought to be the spectrum responsible for most of the cutaneous damage inflicted by the sun (7). The longest UV wavelengths, those from 320–400 nm, are referred as UVA. While originally thought to have minimal effects on the body, UVA radiation has been shown to be involved in many detrimental processes, including carcinogenesis, due to their ability to impact deeper skin layers and generate ROS (7). In addition to UVR from the sun, man-made light bulbs can emit low levels of UV radiation (8). Although the levels emitted from light bulbs may be minimal, it is worth noting that they are present and increase overall UV exposure levels. Additionally, frequent use of UV lamp and tanning beds has been shown to be associated with development of skin cancers, especially melanoma, likely due to the overwhelming ratio of UVA to UVB radiation levels in the lamp outputs (9, 10).
Figure 1.
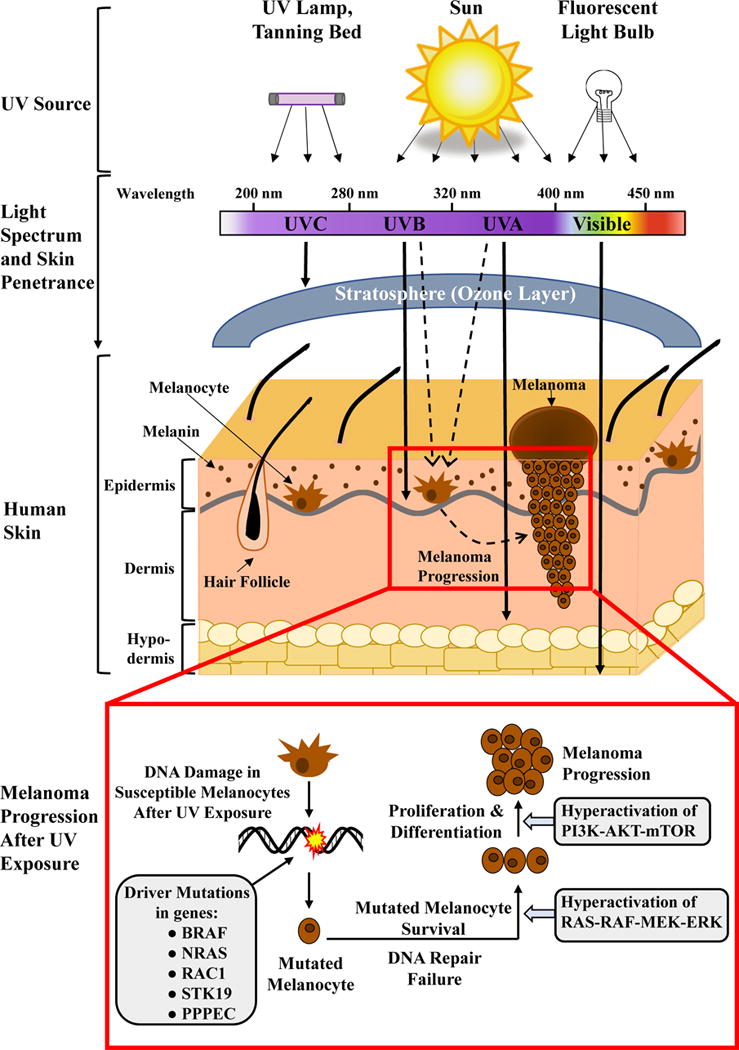
Schematic representation of ultraviolet (UV) radiation transmission in human skin and a proposed mechanism of melanoma progression from melanocytes after UV exposure. Solar radiation, tanning beds, UV lamps and other artificial light bulbs are all sources of UV exposure to human skin. UVA and UVB rays are mainly responsible for mutagenic effects on melanocytes and promotion of melanoma (indicated by the dashed lines). The inset box indicates the path of melanoma progression following exposure of UV light. The key genes that have been identified as having driver mutations in melanoma are outlined in the grey box on the left, including BRAF and NRAS, as well as other newly identified genes. Hyperactivation of RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathway genes have been suggested to be responsible for melanoma progression (grey boxes on the right).
The skin, being the outermost part of the body, is the first line of defense against environmental insults to body, including harmful radiations. Therefore, skin has evolved multiple methods to provide protection from the external insults, including specialized cells and cellular structures. The melanin producing cells or ‘melanocytes’ are a very important cell type in cutaneous defense mechanisms (11). Melanocytes are located in the basal layer of the epidermis where they transfer the melanin to the lower layers of actively dividing keratinocytes, conferring protection to these actively dividing cells (12). This protection ability stems from the fact that melanin has strong broad-spectrum UV-blocking abilities (13). Although melanocytes produce the sun-blocking pigments melanin, they are not fully protected against UVR, as melanomas arise from these pigment-producing cells (depicted in Fig. 1).
UVR has been shown to exert a variety of acute and chronic adverse cutaneous responses such as sunburn, photoaging and skin carcinogenesis (including melanoma and non-melanoma skin cancers). Skin cancers have been found to have complex mechanisms for their instigation and development, encompassing multiple signaling pathways and the immune system, occurring over years or decades. Typically, it consists of three distinct stages: (i) initiation, an essentially irreversible step consisting of genotoxic effects in normal cells leading to DNA mutations, (ii) promotion, a reversible stage of clonal expansion of initiated cells and (iii) progression, the malignant transformation of initiated cells into carcinomas (depicted in Fig. 2) (14). In the skin, UVR has been shown to play roles in all three steps, making it a complete carcinogen, meaning that it can induce skin cancers without any further outside influence. Epidemiologic and biologic evidence suggest that skin exposure to UVR is the most significant environmental risk factor and a well-recognized etiological agent for development of the main types of skin cancer including melanoma (15). Although it is established that UV exposure is a contributing factor in developing melanoma, its exact mechanism remains unclear. Recent studies suggest that small, frequent exposures to UV are less melanomagenic than intermittent, high doses of UVR, especially as UVA (16–18). This contrasts with non-melanoma skin cancers, where UVB and frequent exposure are the main etiological components. This underlines the importance of understanding the mechanisms of melanocytic transformation, to find novel strategies for melanoma management. Fig. 1 depicts the process of melanocytic transformation and melanoma progression and outlines multiple melanoma-specific mutations and signaling pathways involved in the process. For several years, BRAF and NRAS mutations have been frequently reported in melanomas. However, Hodis et al. recently reported new additions to the list of driver mutations by identifying six new melanoma genes (PPP6C, RAC1, SNX31, TACC1, STK19, and ARID2), based on their large-scale melanoma exome data. Out of these three genes (RAC1, PPP6C, and STK19) were suggested to harbor recurrent and potentially targetable melanoma mutations (19). The broad spectrum of these driver mutations in melanoma also provides strong genomic evidence for a direct mutagenic role of UVR in melanoma pathogenesis (19). These newly identified genes provide novel targets for melanoma treatment, which needs to be carefully investigated in well-planned studies. At this time, successful management of melanoma depends on its stage and location. Treatments range from simple excision of an early stage cancer, to systemic treatment of late-stage disease with dacarbazine, BRAF/MEK-targeted therapies, or immunotherapy (reviewed in (20)). Although many researchers are focused on discovering newer melanoma therapeutics, preventing melanoma from occurring may be a more useful strategy to curtail increasing melanoma incidence.
Figure 2.
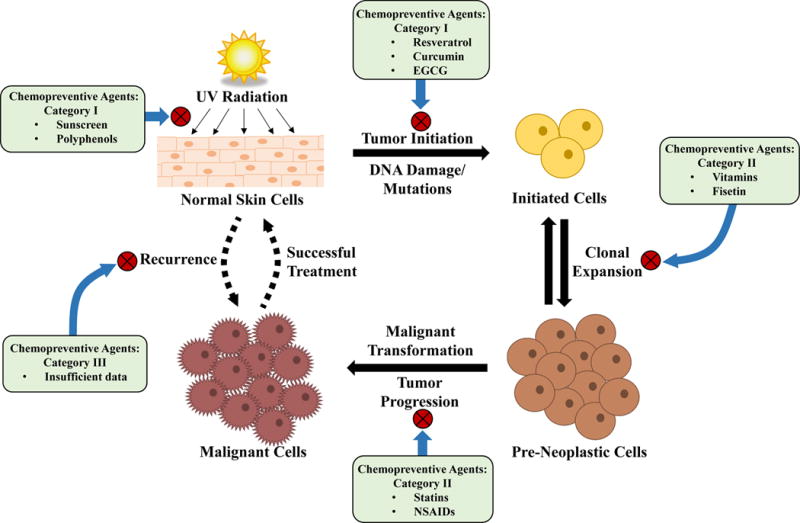
Schematic representation of processes involved in ultraviolet (UV) radiation-mediated development of skin cancer, and examples of chemopreventive agents reported to be useful at different stages of the carcinogenic process. Normally, when skin is exposed to UV radiation, it initiates tumor formation by DNA damage, mutagenesis and release of reactive oxygen species. After damage to the cells, the initiated cells with mutations inferring greater growth potential expand to become pre-neoplastic lesions. If these lesions are not found and removed, they may progress through transformation and become malignant skin cancers and require treatment, which is not always successful (as indicated by the dashed up arrow). Additionally, many melanomas reoccur after successful treatment (dashed down arrow). Chemopreventive agents can be applied topically that directly block the UV rays from reaching to the skin, or can be used to reverse or delay the process of multi-stage carcinogenesis at one or at all of the stages, depending on their efficacy. The ideal chemoprevention regimen would target all stages of cancer development and may include a combination of two or more agents in order to obtain the greatest preventive effects against UV-induced melanoma.
CHEMOPREVENTION OF MELANOMA
The increasing incidence of melanoma together with mortality due to advanced disease complications necessitates the attention of the scientific community towards discovering new approaches for prevention of melanoma (21). Advanced research in the complex biology of melanin-producing cells and the molecular mechanisms of melanocytic transformation have facilitated the research and possibilities in chemoprevention of melanoma. The term “chemoprevention” refers to efforts to prevent, delay or suppress the process of carcinogenesis with the help of dietary means, natural agents, synthetic agents, vitamins, etc. (22). Additionally, chemoprevention also refers to the use of the same types of agents to reduce the risk of reoccurrence of cancer in patients who have undergone successful primary cancer treatment and are in remission (22, 23).
In general, chemopreventive agents for melanoma can be divided in three categories; 1) agents aimed at preventing the occurrence of melanoma, 2) agents intended to prevent development of malignant melanomas from pre-malignant lesions, and 3) agents that can prevent melanomas from re-occurring after successful treatment of primary melanomas (24). In this review, we have discussed these possibilities, which are also depicted in Fig. 2. Unfortunately, there is a lack of available studies on chemopreventive strategies aimed at preventing the re-occurrence of melanoma following a successfully treatment. Studies stemming from the late 1980’s suggested that the anticoagulant drug coumarin may have potential to prevent early recurrence of melanoma, but these studies need to be further validated (25, 26). Further, although several agents have been shown to be effective for melanoma chemoprevention, no agent/drug has been approved yet for melanoma prevention. In fact, very limited agents have received the approval of the Food and Drug Administration (FDA) for prevention of any cancer. In this regard, the first FDA-approved chemopreventive agent, Tamoxifen, has been shown to effectively reduce breast cancer incidences, and has been in use for this purpose since 1999 (27, 28). This is an encouraging development in the field of clinical chemoprevention, opening an avenue to explore chemopreventive strategies for clinical management of other cancers, including melanoma.
Chemoprevention of melanoma is clearly an underdeveloped area and requires more attention by the scientific community (29). The main reasons behind this under-exploration are associated with difficulties in clinical trials of chemopreventive agents in melanoma and unknown mechanisms responsible for UV-mediated transformation of melanocytes to melanomas (30, 31). Current research is focused on identifying genetic and non-genetic risk factors and the molecular pathways that trigger the UV-mediated transformation of melanocytes to melanomas, that could be targeted for chemoprevention (32, 33). This review focuses on various promising chemopreventive agents that show potential for melanoma management. Below, we have outlined several groups of melanoma chemopreventive agents, including sunscreens, synthetic and dietary agents, as well as vitamins. It is important to note that the reported chemopreventive agents have been tested for efficacy against several different cancer types and are not melanoma-specific.
SUNSCREENS
The easiest way to prevent UV exposure mediated damages, including UV-related cancers, is topical application of sun screening/blocking agents to sun-exposed areas, thus preventing damaging radiations from penetrating the skin. Current research on this subject focuses on inorganic particulate compounds that have sun blocking and filtering properties, as well as organic chemical and natural compounds that have sunscreen abilities. In this section, we have discussed the different classes of sunscreen agents and their efficacy in melanoma prevention.
Inorganic UV filters
A popular and widely used class of sunscreen agents is inorganic particles that exhibit a combination of absorptive, reflective, and light-scattering properties so that UV radiation does not reach and/or penetrate skin (34, 35). In this category, the two inorganic agents currently approved by the FDA are titanium dioxide (TiO2) and zinc oxide (ZnO) (36). These agents are considered to be valuable because of their wide range of protection abilities, encompassing both UVB and UVA wavelengths, as well as their photostability (36). Although these molecules provide excellent sunblock protection, until recently they were less frequently used, as they left an unappealing visible layer over the skin. Recent attempts to microencapsulate and microparticulate these moieties have been very successful, and many cosmetics and sunscreens now contain these ingredients (36). These formulations were not immediately embraced, as initial evidence suggested that there may be toxicity due to free-radical generation or bioaccumulation issues if the nanoparticles penetrate the skin and become systemic (37–39). Bioaccumulation as a key safety concern is evidenced in a study completed by Gao et al., which describes the accumulation of titanium dioxide nanoparticles in mouse ovaries and subsequent dysregulation of key ovarian genes (37). However, this and many other studies that showed bioaccumulation were done via oral administration of the TiO2, which may not be relevant to skin administration. Newer formulations, when applied to the skin, have not been found to have significant skin penetrance activity (40–43). One study, however, found that although an aqueous suspension of ZnO did not penetrate the epidermis, certain formulations of ZnO were hydrolyzed on the skin, thereby leading to Zn2+ ions crossing into systemic circulation (40). The authors of this study theorized that this was the reason why some other studies have found high Zn levels in the blood and urine of study subjects, which is one of the main concerns in using ZnO as a topical sunscreen. This has led other researchers to explore more inert or traditional agents to use as sunscreens, including traditional muds and clays (44, 45), as well as formulating new crystal nanoparticles (46). However, much more research needs to be done on these alternate methods of blocking UVR.
Organic UV filters
The other group of sunscreen agents, organic filters, generally stop UVR from reaching the skin by absorbing it via certain chemical structures and converting it into heat. There are currently 15 active organic sunscreen ingredients approved by the FDA (36). Each of these molecules have different filtering ranges and capabilities, as well as unique safety and stability profiles, which results in most companies using a combination of multiple agents to formulate sunscreens to their desired specifications ((47), discussed in (36)). As mentioned above, until recently UVB was thought to be the most harmful UVR component, which resulted in most research being focused on UVB-blocking chemicals. However, it has since been found that not only are UVA rays harmful, they maybe the spectrum of radiation most likely to induce melanomagenesis. Thus, researchers and manufacturers have increased their efforts towards developing UVA-filtering sunscreen. It is important to note that many of the organic molecules used in sunscreen can be absorbed into the skin, enabling them to become systemic entities, which may have other biological effects inside the body, such as endocrine disruption (48, 49). Different formulation methods are being explored to reduce the absorption properties of the sunscreen molecules, including nanoformulation, emulsions, and usage in combination with other chemicals (50, 51). This underlines the importance of determining the best formulation with the least detrimental impact on the rest of the body.
All sunscreens undergo specific testing to determine the stability and photoprotective levels when exposed to sunlight, which determines their sun protection factor (SPF), but recent research suggests that sunscreens should be screened using artificial light as well, as one study found that several commercial sunscreen formulations had significantly decreased absorbance curves after fluorescent light exposure (52). Because of the broad exposure to non-solar light in today’s society, this may present a large challenge, as sunscreens may not be as effective as consumers believe, especially in the case of those used in cosmetics. This is especially true with UVA-blocking organic sunscreens, which has excellent UVA-filtering capabilities but may not be very photostable. To broaden the effectiveness of agents that may not be photostable, researchers are exploring a strategy of using additives, such as natural antioxidants. One such recent study showed that two natural compounds found in several fruits, mangiferin and naringenin, were able to increase the photostability of avobenzone in cell cultures (53). Although this group was able to increase photostability of an organic filter, most groups studying natural agents for sun protection focus on using natural agents without synthetic agents for best effect. Several reviews have been written discussing many compounds that have been explored for their potential UV-absorbing and filtering properties, including silymarin, resveratrol from grapes, and green tea polyphenols including EGCG (54–58). Although this area of research shows promise, there is much more to be studied, especially regarding sunscreen formulation, which could be relevant to melanoma, such as broad-spectrum sunscreen effects and supplementing the sunscreens with natural agents and antioxidants. Interestingly, although they have not been FDA-approved, many companies are already using natural-derived agents as additives in sunscreens and cosmetics. It remains to be scientifically evaluated whether these natural additives impart better protection against harmful UV radiation.
Sunscreens and melanoma
Using sunscreens to prevent melanoma has resulted in considerable debate. Since the 1970s, melanoma occurrences have increased, despite recommendations to use sunscreen to prevent skin cancer. This led many to question the efficacy of sunscreens, and even the possibility of sunscreen uses as a causative factor in melanoma incidence. However, it is likely that the early rise in melanoma incidence was due to a combination of poor UVA filtering capabilities of early sunscreens and increased sun exposure due to reduced sunburn and erythema due to UVB exposure (59). Since then, there have been several epidemiological studies focused on melanoma incidence after sun exposure with and without sunscreen, and the results have been inconsistent (60, 17). Interestingly, early meta-analyses have suggested that there were either no preventive effects of sunscreens or that in some cases, sunscreen increased melanoma incidence ((61, 62), discussed in (63)). One of such analysis focused on elucidating the differences between studies performed in different latitudes. In this study, it was found that overall, there was no significant effect on melanoma incidence with sunscreen use. However, when filtered by latitude, it appeared that those individuals at high latitudes had an increased risk of melanoma incidence (62). The authors postulated that this is likely because of the reduced UVA blocking abilities of common sunscreen formulations. On the other hand, other studies have found a beneficial effect of suncreens, including a recent study of Norwegian women which found that high SPF decreases risk of melanoma development, but not low SPF sunscreen (64). Another study found that regular sunscreen use may lead to reduced melanoma invasiveness (65). It is likely that the conflicting data are due to the inconsistent use of and underapplication of sunscreen, a greater tendency of people to stay in the sun longer when wearing sunscreen, and the lower UVA-blocking potential of most sunscreens as compared to UVB, especially those from earlier studies (66–68). Thus, it is important to determine the proper formulation and use profile to enhance the anti-melanoma activities of sunscreens, both organic and inorganic.
Synthetic Agents
Although topical application of sunscreens is the most direct route to block UVR, the ideal mode for chemoprevention is believed to be via oral supplementation. Many researchers believe that administration of chemopreventive agents by either supplementation or dietary changes may be a better or at least supplementary route for protection (69, 70). This section is focused on some of the important candidate agents with potential chemopreventive activity against melanoma, based on published literature.
Non-steroidal Anti-Inflammatory Drugs (NSAIDs)
The fact that a major cutaneous response of UVR is inflammation (in the form of erythema and edema), leads to the idea that NSAIDs could be used for preventing or treating sunburn and other UV-induced inflammatory skin conditions. NSAIDs are drugs with analgesic, antipyretic and anti-inflammatory effects (71), and are being investigated for use in chemoprevention of skin inflammatory conditions and cancers, as well. NSAIDs are reported to function through various mechanisms, targeting both canonical and non-canonical pathways. One mechanism by which NSAIDs work is via inhibition of nuclear factor-κB (NF-κB) (72). A study by McNulty et al. found that NF-κB-related protein signaling is upregulated in melanoma and nevus biopsies, as compared to normal skin melanocytes (72). Diclofenac has been shown to increase intracellular ROS and mitochondrial dysfunction leading to enhanced apoptosis in melanoma lines with no significant effects on normal fibroblasts (73). NSAIDs are also able to exert reversible inhibition of the cyclooxygenase (COX) enzymes (COX-1, COX-2), leading to decreased synthesis of prostaglandins and thromboxane, which are implicated in invasion, proliferation, and angiogenesis of cancer cells (74). Interestingly, COX-2 is overexpressed in melanomas (75, 76), and increased COX-2 levels have been positively correlated with melanoma progression (77), and negatively with patient survival (78). In a small retrospective cohort study with different COX inhibitors, a decrease in the number of new tumors, disease recurrence, and metastases has been observed in melanoma patients (79). This suggests that COX-2 specific targeting NSAIDs, such as celecoxib, etoricoxib, and rofecoxib, may be used as chemopreventive agents in patients with high susceptibility to melanoma.
Although the reasoning behind using NSAIDs as chemopreventive agents against melanoma is sound, their optimal treatment regimen, safety, and efficacy as long-term chemopreventive agents is an issue for debate and investigation. In a case–control study, Harris and colleagues observed a significant decrease in the relative risk of malignant melanoma with regular intake of common NSAIDs such as aspirin and ibuprofen, suggesting that NSAIDs may be useful in melanoma prevention (80). In another study, reduction in melanoma incidence was observed in women taking daily low doses of salicylic acid for a period of 3 years (81). Additionally, a 2012 study of retroactive patient data found a reduced risk malignant melanomas with use of NSAIDs (82). These studies suggest that long-term use of NSAIDs may be useful in the prevention of malignant melanoma. However, results obtained by other research groups do not confirm the chemopreventive potential of NSAIDs on melanoma. Cook et al. did not observe a significant effects of low doses of acetylsalicylic acid on the risk of cutaneous melanoma in women (83). Further, protective influences of other NSAIDs on melanoma have not been observed, even after long-term use (84). Asgari et al. showed no effect of NSAIDs on cancer cell invasion, tumor thickness, or the risk of metastasis (85). Another recent study did not find any effect of NSAIDs on skin cancers, including melanoma, even with frequent use (86). Still yet another study found that there was a potential protective effect for SCC and basal cell carcinoma, but only when NSAIDs were used short term (87). Although promising studies using NSAIDs have been reported in various cancer types, the efficacy and exact usefulness of NSAIDS in melanoma chemoprevention has yet to be demonstrated.
Statins
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase, and were originally prescribed to prevent cardiovascular diseases by reducing cholesterol levels (88). Since then, statins have also been reported to possess antiproliferative, anti-invasive and immunomodulatory properties by inhibiting Ras pathway proteins as well as tumorigenic activities (89, 90). Mechanistically, statins have also been shown to decrease protein prenylation, a key step in the mevalonate pathway, which is critical for cellular functions such as membrane integrity, cell signaling, protein synthesis, and cell cycle progression (91, 92). A number of different statins such as lovastatin (93), simvastatin (94), atorvastatin (95) and more recently, rosuvastatin (96), have been utilized to reduce proliferation and induce apoptosis in human melanoma cell lines. Although preclinical studies support the exploration of statins as an anti-melanoma therapy, clinical studies are yet to confirm these observations. A meta-analysis of published literature done in 2014 found that there was no evidence of reduced melanoma incidence in statin-using patients (97). Another study published in 2014 found the same non-association, although there was evidence that there may be a slightly better outcome for male statin users than female (98). Although statin use does not appear to be an appropriate chemopreventive strategy for general use, further evaluation may be helpful to determine if it may be appropriate for certain sub-populations.
DIETARY AGENTS
Dietary agents have been widely investigated for their potential chemopreventive effects. The benefits of using dietary agents for chemoprevention are that they tend to be non-toxic and easily available, and may target multiple pathways involved in tumor development and progression. These agents have been found to work through induction of apoptosis, inhibition of signaling proteins, inhibition of angiogenesis and modulation of the immune system. Common dietary chemopreventive agents that have been tested for preventing melanomas include resveratrol, curcumin, and green tea polyphenols (EGCG). Some selected dietary chemopreventive agents and their mechanism of actions at various stages of melanoma development are discussed below.
Resveratrol
Dietary polyphenols have been studied extensively for their photoprotective effects (4). It is believed that they have strong antioxidant properties that are useful in scavenging ROS, which are the molecules responsible for the damaging effects of UVR (99–101). Resveratrol, chemically known as 3,5,4′-trihydroxy-trans-stilbene, is a polyphenolic phytoalexin found in many dietary sources including grapes, red wines, mulberries and peanuts (102). Resveratrol has been shown to inhibit various cellular events associated with initiation, promotion and progression of skin cancer (102, 103). Topical resveratrol has been demonstrated to suppress UV-induced skin carcinogenesis through downregulation of COX-2 and by inhibiting the RICTOR component of mTORC2 and hydrogen peroxide formation (104, 105). Afaq et al. found that a single application of resveratrol was able to block much of the UVB-induced skin damage in a hairless mouse model (106). Further, topical resveratrol treatment has been found to inhibit UV-mediated increase in cyclins (D1 and D2), cyclin-dependent kinases (CDK 2, 4 and 6), proliferating cell nuclear antigen, mitogen-activated protein kinase (MAPK), and the mitogen-activated protein kinase kinase (MAPKK) in SKH-1 hairless mouse skin (107). Similarly, treatment with resveratrol was also shown to inhibit UVB-induced increases in cellular proliferation and markers of tumor promotion, possibly via its effect on the anti-apoptosis factor, survivin (108). These studies all suggest that resveratrol would be an ideal chemopreventive agent against UV-mediated tumorigenesis. Resveratrol induced apoptosis in melanoma cells has been shown to be mediated via increased phosphorylation of extracellular signal-regulated kinases (ERK) and mitogen-activated protein kinases (109). More recently, resveratrol was shown to be effective in overcoming resistance against the BRAF inhibitor Vemurafenib, through dephosphorylation of AKT-serine/threonine kinase 1 (110). This suggests that beyond using resveratrol to prevent melanoma from occurring in the first place, resveratrol may have potential as an agent to prevent recurrence in patients who have had their melanoma successfully treated with Vemurafenib.
Thus far, the results from preclinical studies using resveratrol have failed to be replicated in humans, possibly due to the short half-life, low bioavailability in vivo and lipophilic nature of resveratrol (111, 112). To improve bioavailability and anti-melanomagenesis activity, synthetic resveratrol derivatives and nanoformulations are now being explored as chemopreventive agents to prevent melanoma (113–117). A recent study by Sanna et al. found that chitosan- and anionic alginate-coated poly(d,l0lactide-co-glycolide (PLGA) nanoparticles were able to preserve bioactivity of resveratrol up to 6 months and allow for a controlled release formulation (118). Furthermore, resveratrol solubility, stability and intracellular delivery were shown to be increased when used in the form of solid lipid nanoparticles (119). In this study, resveratrol-lipid nanoparticles had an improved cell permeability over traditional resveratrol, and significantly decreased immortalized keratinocyte proliferation (119). Moreover, resveratrol-loaded nanocapsules have recently been investigated in murine melanoma model in which they prevented metastasis and pulmonary hemorrhage (120). Although these preclinical findings with resveratrol and its derivatives are encouraging, further studies are required to determine the optimal formulation to use towards their clinical applications in melanoma prevention.
Curcumin
Spices have been found to be a major source of bioactive compounds, and many chemopreventive and chemotherapeutic molecules have been discovered from these common household ingredients. Curcumin, also known as diferuloylmethane, is one such agent derived from a commonly used spice, turmeric (Curcuma longa) (121). Curcumin has strong antioxidant properties and has been shown to protect cultured human cells from radiation induced DNA damage (122). Curcumin has been reported to protect hairless mice from UVB induced photocarcinogenesis by triggering DNA repair mechanisms (123), and topical application of curcumin significantly decreased UVA and TPA-induced ornithine decarboxylase activity in mice (124). The chemopreventive effects of curcumin against UV radiation have been suggested to be due to decreases in superoxide radical formation in curcumin-treated normal human keratinocytes, leading to inhibition of cytotoxic hydrogen peroxide (125). This was further elucidated when Oguro and Yoshida suggested that the chemopreventive effects of curcumin may be due to its ROS scavenger activity, as topical application of curcumin inhibited UVA and TPA-induced gene expression of metalloprotein in the mouse skin (126). Despite these excellent preclinical results, clinical utilization of curcumin is restricted due to its limited bioavailability, low water solubility and excessive metabolism in the colon and liver (127, 128). To counter this issue, researchers have been working on new formulations to increase its clinical applicability. Recently, chitosan-coated curcumin nanoparticles have been shown to decrease pulmonary tumor formation in a murine model of melanoma metastasis (129). When orally administered, these curcumin nanoparticles attenuated metastatic melanoma in the lungs of treated mice, indicating the potential use of curcumin in prevention melanoma metastasis (129). Although these preclinical trials are encouraging, there have been no clinical trials published to date exploring the prevention of melanoma using curcumin. However, the preclinical success definitely warrants future studies to determine the safety, efficacy and feasibility of translating these preclinical mouse studies into human chemopreventive therapies.
Epigallocatechin-3-gallate (EGCG)
Green tea contains several polyphenolic compounds, including catechin and epicatechin, as well as their derivatives (−)-epicatechin, (−)-epigallocatechin, and (−)-epigallocatechin-3-gallate (EGCG) (130). In 1992, a study by Agarwal et al. found that a polyphenolic fraction isolated from green tea was able to inhibit induction of skin tumorigenesis in mice (131). Since then, it has been determined that EGCG is the major cancer preventive agent in green tea (130), which works to inhibit UV-induced tumor initiation by inhibiting DNA damage and oxidative stress (132, 133). Both in vitro and in vivo, topical application of EGCG provided protection from oxidative stress associated with photocarcinogenesis by maintaining antioxidants such as glutathione peroxidase and catalase in the epidermis (133, 134). Green tea polyphenols, including EGCG, were shown by Katiyar et al. to prevent a large portion of UV-induced immunosuppression in mice, suggesting that dietary supplementation with green tea may allow a measure of safety from sun damage (135). Further, human epidermal keratinocytes have also been used to evaluate the chemopreventive effects of EGCG against UVB-induced oxidative stress, which is known to be involved in tumor promotion (136). In these studies, treatment of EGCG in normal human epidermal keratinocytes was found to inhibit UVB-induced intracellular release of hydrogen peroxide concomitant with the inhibition of phosphorylation of cell signaling proteins, such as epidermal growth factor receptor (EGFR) and ERK1/2, JNK and p38 proteins of the MAPK family. Additionally, other studies have demonstrated that EGCG protected against the adverse effects of UV radiation by modulating pRb-E2F/DP and NF-κB pathways (137, 138).
EGCG is has also been shown to prevent UV-induced immunosuppression via inducing IL-12 production (139). Moreover, in melanoma murine model, EGCG has been found to induce apoptosis and cell cycle arrest while reducing cell migration and metastatic properties of the cells, thereby inhibiting melanoma tumor growth (140, 141). The advantages of EGCG, such as low cost and negligible toxicity, makes it an ideal chemopreventive agent, and nanotechnology-based encapsulation of EGCG shows potential to lower doses even further. A study published in 2013 found that EGCG-loaded PLGA nanoparticles were able to protect against DNA damage in mouse skin, in ~30-fold less of a dose than EGCG alone (142). Overall, the tremendous of EGCG success in preclinical studies, combined with its strong chemopreventive properties, make this dietary agent ideal for further studies and clinical trials in preventing melanomas.
Fisetin
Fisetin (3,3′,4′,7-Tetrahydroxyflavone) is a naturally occurring flavonoid, commonly found in several fruits and vegetables including mangoes, strawberries, apples, kiwis, grapes, onions and cucumbers (143). Fisetin has been reported to possess antioxidant, anti-proliferative, and anti-inflammatory properties against various cancers including melanoma and non-melanoma skin cancers (144, 145). Syed et al. reported that fisetin has the potential to inhibit human melanoma by disrupting the WNT/β-catenin/MITF signaling in 451Lu human melanoma cells (146). More interestingly, they were also able to demonstrate these in vitro effects in in vivo studies, which showed that fisetin significantly inhibited tumor growth in 451Lu melanoma xenografts, and this was associated with decreased MITF levels (146). Further, Pal et al. have reported photochemopreventive effects of fisetin in the manangement of UVB-induced skin cancer in an SKH-1 hairless mouse model. According to this study, topical application of fisetin to SKH-1 mice skin after UVB exposure resulted in significant decrease in inflammatory markers (MPO, COX2, and PGE2), cytokines (TNF-α, IL-1β, and IL-6) and proliferation markers (PCNA and cyclin D1) (147). Fisetin was also reported to inhibit the PI3K/AKT/NF-κB signaling which is associated with UVB-induced inflammation, cell survival, and proliferation (147). Collectively, these studies suggest that fisetin may be a strong and potential candidate in melanoma chemoprevention and further warrants preclinical and clinical studies using fisetin to prevent melanoma.
VITAMINS
Recently, the role of vitamins as chemopreventive agents has been an area of increasing focus in the scientific community. One of the classic hallmarks of cancer cells is uncontrolled cell proliferation, with a dysregulation of cellular metabolism being a key component of this. The enzymatic mechanism of many vitamins is a key element for metabolism homeostasis and cellular differentiation of multiple cell types in various organs (148, 149). Studies conducted in recent decades have explored the use of vitamins against melanoma and other cancers (reviewed in (150)). The focus of this section is to outline research that has been reported to show the potential usefulness of vitamins in melanoma chemoprevention. Vitamins A, C, D, E, and K have been shown to exert chemotherapeutic effects against several cancers (151, 150), but only limited number of studies have suggested a potential usefulness of these vitamins in melanoma prevention. These studies and the suspected anti-cancer mechanisms in melanoma that have been reported are discussed below.
Vitamin A
One of the most well-studied vitamins, vitamin A is important for cell differentiation, immune response and cell development (152). Vitamin A is found in many foods, including milk, butter, and eggs, and is vitally important in vision, immunity, and childhood growth (153). Although “vitamin A” is technically a term used to describe any number of compounds with the same retinyl group, there are two principal groups of this vitamin: retinoids and carotenoids. Here we specifically focus on the retinoids, as they are the most commonly studied vitamin A derivative. The use of vitamin A derivatives on the skin has been prevalent for many years, and includes the anti-acne compound isotretinoin. Interestingly, although vitamin A deficiency in children can lead to immune problems and blindness, use of isotretinoin in pregnant women has been known to be a very strong teratogen since the 1980s (154). Despite this, researchers have continued research into in the anticancer and antioxidant activities of vitamin A, in the hope of finding a form and dosing regimen that will be safe and effective. Early on, the antioxidant properties of vitamin A were found to reduce UV-light induced skin tumors (155), suggesting the role of vitamin A as a possible chemopreventive agent in skin cancers, including melanoma.
As the most active and prevalent forms of vitamin A, over the years, retinoids have been widely investigated for their effectiveness against melanoma. Many of the effects of vitamin A have been associated with the activation of retinoic acid receptor (RAR) or retinoid X receptor (RXR) (156, 157, 150). The activation of this retinoid pathway is responsible for cell growth arrest and cell differentiation, which can lead to apoptosis (158, 156, 157, 150). In a 2012 study, Asgari et al. found that supplementation with retinol, at both low and high doses, reduced the risk of melanoma development, especially in sun-exposed anatomic sites (159). However, Miura and Green reviewed prospective data and found that the limited number of relevant clinical trials did not show sufficient evidence of a beneficial effect of vitamin A on melanoma prevention (160). Interestingly, UVR has been shown to change the metabolism of retinoic acid, which has been shown to reduce retinol in melanocytes (161). Further, another study demonstrated that UV-response is metabolite-specific, and both UVA and UVB promoted dehydroretinol biosynthesis in keratinocytes, which may reduce UVA/B driven apoptosis more effectively than retinol (162). This may play a role in the apparent discrepancy in chemopreventive efficacy of retinols in melanoma. This controversy also holds true in retinoid treatment of melanomas as well (163–169). In humans, a clinical trial displayed promising effects, with a response rate of 20% in patients that were treated with isotretinoin in combination with interferon α-2a, but ultimately the treatments did not have any influence on overall survival of melanoma patients (170). Nonetheless, it is likely that testing with a different retinoid compound may provide better effects in melanoma, as has been seen in other cancers (171). Future studies should aim to uncover the mechanistic differences between the different forms of retinoids to explore their exact role in melanoma proliferation, which may lead to an effective chemoprevention agent.
Vitamin C
Structurally a six-carbon lactone with a base of ascorbic acid, vitamin C has been found to be an important antioxidant. Vitamin C is most well known in its two forms: ascorbic acid (its reduced form) and dehydroascorbic acid (its oxidized form). Because of the involvement of oxidative stress in melanoma development and progression, the antioxidant activity of vitamin C could be utilized for melanoma chemoprevention. Indeed, it has been shown in vitro that ascorbic acid can prevent UVA-induced cytotoxicity (172, 173). However, issues with stability in air and movement into the skin when taken orally still need to be addressed (discussed in (174)). An early study showed that topical application of ascorbic acid in pigs was able to protect the skin from UVA-mediated phototoxicity, but repeated applications were needed to give optimal protection (175). A recent study published in 2015 found that liposomal encapsulation of ascorbate was able to significantly increase penetrance into the skin, as well as increase the antioxidant and anti-inflammatory competence of human skin (176). Interestingly, a study by Campbell et al. recently found that supplementation of high levels of ascorbate was able to decrease tumor formation ability in a xenograft mouse model (177). This suggests that using vitamin C may be a good chemopreventive strategy, especially since it has a well-documented safety profile (150). This holds true in regards to established and metastatic melanoma as well, as melanoma cells have shown susceptibility to acidic ascorbic toxicity (178). In 2014, Venturelli et al. evaluated the epigenetic impacts of ascorbate on human metastatic melanoma cells and found pharmacological doses of ascorbate shifted melanoma cells towards sub-G1 fraction after an initial G2/M arrest, suggesting secondary apoptosis induction (179). Furthermore, a recent study demonstrated that ascorbate supplementation impedes invasion, inflammatory cytokine secretion and melanoma tumor growth (180). Collectively, the aforementioned studies support vitamin C as a potential chemopreventive agent in melanoma, both before development of melanoma, and to prevent recurrence.
Vitamin D
The “sunshine vitamin”, as it is sometimes referred to in the literature, is biologically inert in its basic form found in the skin, 7-dehydrocholesterol. Syntheses of the bioactive forms start after the skin is exposed to UVB rays. Interestingly, vitamin D requires two hydroxylations outside the skin to gain activity and become its most active biological form, 1,25-dihydroxycholecalciferol (calcitriol) (181). Calcitriol is known to be essential in the skin for normal calcium-induced keratinocyte differentiation, as well as in the body for maintenance of overall calcium homeostasis. Although there is inconsistent evidence that vitamin D supplementation has an effect on melanoma incidence or outcomes, many researchers have focused on exploring this vitamin with ideal chemopreventive properties, including ease of production and well tolerated oral dosing (182). Furthermore, a recent study by Piotrowska et al. showed the antiproliferative effects of vitamin D analogs against human malignant melanoma cell lines (183). Vitamin D has been suggested to be associated with primary melanomas and could potentially influence the outcome of melanoma (184). Known melanoma-related signaling pathways have been shown to be regulated by vitamin D signaling, and many biological mechanisms of vitamin D3 action are directly associated with the vitamin D receptor (VDR) (185). VDR heterodimerizes with RXR, activating the retinoid pathway, which can in turn affect cell progression, differentiation and apoptosis (185, 158). Although more research is needed to elucidate the mechanisms involved, polymorphisms of VDR genes have been correlated with prognosis and/or susceptibility of melanoma (186–189). While the role of vitamin D in melanoma remains unclear, studies seem to support the possibility of chemopreventive and anti-melanoma effects of proper levels/doses of systemic vitamin D.
Vitamin E
Vitamin E, an extensively studied vitamin and strong antioxidant, consists of a group of four tocopherols (D-α, D-β, D-γ, D-δ) and four tocotrienols (D-α, D-β, D-γ, D-δ). As a peroxyl radical-scavenging antioxidant, vitamin E is suggested to prevent the generation of free radicals and inhibit lipid peroxidation (182). Further, the ability of vitamin E to modulate signal transduction and gene expression has led researchers to focus on vitamin E analogs in search of new targets for treatments in melanoma development (190). A clinical trial conducted by Placzek et al. found a photoprotective effect after three months of supplementation with α-tocopherol, and the participants seemed to have increased resistance to UVB-induced sunburn and protection from DNA damage (191). In a topical application study of 5% vitamin E, application of the cream twenty-four hours prior to UV exposure inhibited human macrophage metalloelastase (192). These studies provide evidence for the potential photoprotective role of vitamin E. Moreover, α-tocopherol has been shown to inhibit growth and survival of melanoma cells in vitro (193–196). Mechanistically, it is thought that several factors are responsible for the chemopreventive and chemotherapeutic effects of vitamin E, including protein kinase modulation, regulation of transforming growth factor beta (TGF-β), G1 cell cycle blockage, induction of apoptosis and DNA synthesis arrest (193–196). The diversity of these mechanisms and the preclinical data suggests that vitamin E may be a good chemopreventive agent against melanoma.
Vitamin K
Vitamin K consists of a family of molecules with a similar 2-methyl-1,4-napthoquinone backbone. There are three main members, phylloquinone (K1), menaquinone (K2), and menadione (K3), as well as several other natural and synthetically made derivatives. For many years, vitamin K has been studied mainly as an important blood-clotting factor, but some researchers have focused on its role in cancer, as well (reviewed in (197, 150)). Although most of the research has focused on the role of vitamin K as a chemotherapeutic agent, based on available data, it appears that it can have chemopreventive effects against melanoma. The proposed mechanisms of melanoma development and progression involve activation of hypoxia signaling pathways, as well as MAPK (198). Interestingly, one of the most studied forms in cancer, menodione, has been found to be a specific inhibitor of the E3 ubiquitin ligase Siah2, which is involved in hypoxia signaling and regulation of the MAPK pathway, and is known to be upregulated in melanoma (199, 200). The antineoplastic role of vitamin K appears to involve several mechanisms, including: cell cycle arrest, cell death through inhibition of protein kinase, antioxidative effects, depletion of glutathione and regulation of transcription factors that lead to proto-oncogenes (197, 201). As a chemotherapy agent in melanoma, vitamin K has been found to exhibit antiproliferative and apoptosis-inducing effects in metastatic human A375 melanoma cells (202). The same study also showed significant antiproliferative effects at very low concentrations of both vitamins K3 and K5 (2-Methyl-4-amino-1-naphthol), which were able to inhibit the growth of decarbazine resistant melanoma cells (202). Interestingly, it was noted early on that vitamin K antagonists such as warfarin may exhibit anticancer activity, and although the evidence is not conclusive, this opens another area of potential chemopreventive agents linked to vitamin K (203, 204). These studies showing the success of using vitamin K as a chemotherapeutic agent, when combined with its roles in hypoxia and MAPK signaling, suggest that further exploration of vitamin K as a chemopreventive agent for melanoma is warranted.
CONCLUSION
Ultraviolet rays from the sun can significantly damage the skin, resulting in a myriad of skin diseases and conditions, from photoaging to skin cancer, including both melanoma and non-melanoma skin cancers. Chemoprevention strategies for melanoma may be useful in preventing melanocytic transformation as well as melanoma progression, ultimately reducing the incidence and mortality associated with this deadly cancer. In this review, we have summarized chemoprevention strategies, different categories of melanoma chemopreventive agents, recent progress in the field, and our perspective on future research that may be useful in identifying novel means for melanoma management. Conventional commercial sunscreens have been proven to block significant amounts of solar radiation, but also have the capability to exert potential harmful side effects. It is becoming apparent that some natural chemopreventive agents have more potential as additives to existing chemopreventive agents instead of having significant effects as a single agent to prevent melanomas. This has led scientific community to identify new combinatorial and nanoparticle based chemoprevention strategies to obtain more efficacy and potency. Preclinical studies warrant the use of naturally occurring, cost effective agents such as resveratrol, curcumins and EGCGs, as well as vitamins to determine the efficacy and safety for melanoma prevention. Future studies using chemopreventive agents should also target melanoma re-occurrence after successful treatment and should consider the specific patient pool that are at higher risk of melanoma after organ transplant.
Acknowledgments
This work was partially supported by funding from the NIH (grant numbers R01AR059130 and R01CA176748) and the Department of Veterans Affairs (VA Merit Review Award number 1I01BX001008). We also acknowledge the core facilities supported by the Skin Diseases Research Center (SDRC) Core Grant P30AR066524 from NIH/NIAMS.
Footnotes
This article is part of the Special Issue honoring Dr. Hasan Mukhtar’s 70th Birthday and his outstanding contributions to various aspects of photobiology research, including photocarcinogenesis and chemoprevention.
References
- 1.Spagnolo F, Queirolo P. Upcoming strategies for the treatment of metastatic melanoma. Arch Dermatol Res. 2012;304:177–184. doi: 10.1007/s00403-012-1223-7. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand JL, Laman SD, Kottschade LA, Maples WJ, Pittelkow MR, Pulido JS, Cameron JD, Creagan ET, C. Melanoma Study Group of the Mayo Clinic Cancer Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- 4.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almutawa F, Buabbas H. Photoprotection: clothing and glass. Dermatol Clin. 2014;32:439–448, x. doi: 10.1016/j.det.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Timares L, Katiyar SK, Elmets CA. DNA damage, apoptosis and langerhans cells–Activators of UV-induced immune tolerance. Photochem Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golmohammadzadeh S, Jaafarixx MR, Khalili N. Evaluation of liposomal and conventional formulations of octyl methoxycinnamate on human percutaneous absorption using the stripping method. Journal of cosmetic science. 2008;59:385–398. [PubMed] [Google Scholar]
- 8.Moseley H, Ferguson J. The risk to normal and photosensitive individuals from exposure to light from compact fluorescent lamps. Photodermatol Photoimmunol Photomed. 2011;27:131–137. doi: 10.1111/j.1600-0781.2011.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Gershenwald JE, Halpern AC, Sondak VK. Melanoma Prevention-Avoiding Indoor Tanning and Minimizing Overexposure to the Sun. JAMA. 2016;316:1913–1914. doi: 10.1001/jama.2016.16430. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99–106. doi: 10.1111/ajd.12145. [DOI] [PubMed] [Google Scholar]
- 11.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoang-Minh Thao, L TL, Kasbohm Jörn, Gieré Reto. Substituting non-natural agents in UV-protection cream by a mixture of clay with Ganoderma pfeifferi extract. Applied Clay Science. 2011;53:66–72. [Google Scholar]
- 13.Hoang-Minh Thao, L TL, Kasbohm Jörn, Gieré Reto. UV-protection characteristics of some clays. Applied Clay Science. 2010;48:349–357. [Google Scholar]
- 14.Nishisgori C. Current concept of photocarcinogenesis. Photochem Photobiol Sci. 2015;14:1713–1721. doi: 10.1039/c5pp00185d. [DOI] [PubMed] [Google Scholar]
- 15.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moan JE, Baturaite Z, Dahlback A, Porojnicu AC. Ultraviolet radiation and cutaneous malignant melanoma. Adv Exp Med Biol. 2014;810:359–374. doi: 10.1007/978-1-4939-0437-2_20. [DOI] [PubMed] [Google Scholar]
- 17.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Autier P, Dore JF, Eggermont AM, Coebergh JW. Epidemiological evidence that UVA radiation is involved in the genesis of cutaneous melanoma. Curr Opin Oncol. 2011;23:189–196. doi: 10.1097/CCO.0b013e3283436e5d. [DOI] [PubMed] [Google Scholar]
- 19.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashim PW, Friedlander P, Goldenberg G. Systemic Therapies for Late-stage Melanoma. J Clin Aesthet Dermatol. 2016;9:36–40. [PMC free article] [PubMed] [Google Scholar]
- 21.Dellavalle RP, Nicholas MK, Schilling LM. Melanoma chemoprevention: a role for statins or fibrates? Am J Ther. 2003;10:203–210. doi: 10.1097/00045391-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 23.Sporn MB, Dunlop NM, Newton DL, Henderson WR. Relationships between structure and activity of retinoids. Nature. 1976;263:110–113. doi: 10.1038/263110a0. [DOI] [PubMed] [Google Scholar]
- 24.Lao CD, Demierre MF, Sondak VK. Targeting events in melanoma carcinogenesis for the prevention of melanoma. Expert Rev Anticancer Ther. 2006;6:1559–1568. doi: 10.1586/14737140.6.11.1559. [DOI] [PubMed] [Google Scholar]
- 25.Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E, Henley J, Healy T, Keane F, Lennon F, McMurray N, O'Loughlin S, Shine M, Tanne A. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol. 1994;120(Suppl):S32–34. doi: 10.1007/BF01377122. [DOI] [PubMed] [Google Scholar]
- 26.Thornes D, Daly L, Lynch G, Browne H, Tanner A, Keane F, O’Loughlin S, Corrigan T, Daly P, Edwards G, Breslin B, Browne Hy M, Shine F, Lennon J, Hanley N, McMurray E, Gaffney E. Prevention of early recurrence of high risk malignant melanoma by coumarin. Irish Melanoma Group. Eur J Surg Oncol. 1989;15:431–435. [PubMed] [Google Scholar]
- 27.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–532. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 28.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–446. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demierre MF, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. 2003;21:158–165. doi: 10.1200/JCO.2003.07.173. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Malek ZA, Kadekaro AL, Swope VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–186. doi: 10.1111/j.1755-148X.2010.00679.x. [DOI] [PubMed] [Google Scholar]
- 31.Lund LP, Timmins GS. Melanoma, long wavelength ultraviolet and sunscreens: controversies and potential resolutions. Pharmacol Ther. 2007;114:198–207. doi: 10.1016/j.pharmthera.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Wang HT, Choi B, Tang MS. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc Natl Acad Sci U S A. 2010;107:12180–12185. doi: 10.1073/pnas.1005244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaath NA. Ultraviolet filters. Photochem Photobiol Sci. 2010;9:464–469. doi: 10.1039/b9pp00174c. [DOI] [PubMed] [Google Scholar]
- 35.Cole C, Shyr T, Ou-Yang H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol Photoimmunol Photomed. 2016;32:5–10. doi: 10.1111/phpp.12214. [DOI] [PubMed] [Google Scholar]
- 36.Sambandan DR, Ratner D. Sunscreens: an overview and update. J Am Acad Dermatol. 2011;64:748–758. doi: 10.1016/j.jaad.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Gao G, Ze Y, Li B, Zhao X, Zhang T, Sheng L, Hu R, Gui S, Sang X, Sun Q, Cheng J, Cheng Z, Wang L, Tang M, Hong F. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61:685–692. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 39.Tan MH, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas J Dermatol. 1996;37:185–187. doi: 10.1111/j.1440-0960.1996.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 40.Holmes AM, Song Z, Moghimi HR, Roberts MS. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano. 2016;10:1810–1819. doi: 10.1021/acsnano.5b04148. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123:264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 42.Sadrieh N, Wokovich AM, Gopee NV, Zheng J, Haines D, Parmiter D, Siitonen PH, Cozart CR, Patri AK, McNeil SE, Howard PC, Doub WH, Buhse LF. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156–166. doi: 10.1093/toxsci/kfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senzui M, Tamura T, Miura K, Ikarashi Y, Watanabe Y, Fujii M. Study on penetration of titanium dioxide (TiO(2)) nanoparticles into intact and damaged skin in vitro. J Toxicol Sci. 2010;35:107–113. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- 44.Rifkin RF, Dayet L, Queffelec A, Summers B, Lategan M, d’Errico F. Evaluating the Photoprotective Effects of Ochre on Human Skin by In Vivo SPF Assessment: Implications for Human Evolution, Adaptation and Dispersal. PLoS One. 2015;10:e0136090. doi: 10.1371/journal.pone.0136090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dlova NC, Nevondo FT, Mwangi EM, Summers B, Tsoka-Gwegweni J, Martincigh BS, Mulholl DA. Chemical analysis and in vitro UV-protection characteristics of clays traditionally used for sun protection in South Africa. Photodermatol Photoimmunol Photomed. 2013;29:164–169. doi: 10.1111/phpp.12042. [DOI] [PubMed] [Google Scholar]
- 46.Truffault L, Choquenet B, Konstantinov K, Devers T, Couteau C, Coiffard LJ. Synthesis of nano-hematite for possible use in sunscreens. J Nanosci Nanotechnol. 2011;11:2413–2420. doi: 10.1166/jnn.2011.3524. [DOI] [PubMed] [Google Scholar]
- 47.Hojerova J, Medovcikova A, Mikula M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int J Pharm. 2011;408:27–38. doi: 10.1016/j.ijpharm.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 48.Maipas S, Nicolopoulou-Stamati P. Sun lotion chemicals as endocrine disruptors. Hormones (Athens) 2015;14:32–46. doi: 10.1007/BF03401379. [DOI] [PubMed] [Google Scholar]
- 49.Benson HA. Assessment and clinical implications of absorption of sunscreens across skin. Am J Clin Dermatol. 2000;1:217–224. doi: 10.2165/00128071-200001040-00003. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert E, Roussel L, Serre C, Sandouk R, Salmon D, Kirilov P, Haftek M, Falson F, Pirot F. Percutaneous absorption of benzophenone-3 loaded lipid nanoparticles and polymeric nanocapsules: A comparative study. Int J Pharm. 2016;504:48–58. doi: 10.1016/j.ijpharm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez J, Maibach HI. Percutaneous penetration and pharmacodynamics: Wash-in and wash-off of sunscreen and insect repellent. J Dermatolog Treat. 2016;27:11–18. doi: 10.3109/09546634.2015.1050350. [DOI] [PubMed] [Google Scholar]
- 52.Romanhole RC, Ataide JA, Cefali LC, Moriel P, Mazzola PG. Photostability study of commercial sunscreens submitted to artificial UV irradiation and/or fluorescent radiation. J Photochem Photobiol B. 2016;162:45–49. doi: 10.1016/j.jphotobiol.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Kawakami CM, Gaspar LR. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J Photochem Photobiol B. 2015;151:239–247. doi: 10.1016/j.jphotobiol.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Saewan N, Jimtaisong A. Natural products as photoprotection. J Cosmet Dermatol. 2015;14:47–63. doi: 10.1111/jocd.12123. [DOI] [PubMed] [Google Scholar]
- 55.Saraf S, Kaur CD. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn Rev. 2010;4:1–11. doi: 10.4103/0973-7847.65319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radice M, Manfredini S, Ziosi P, Dissette V, Buso P, Fallacara A, Vertuani S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia. 2016;114:144–162. doi: 10.1016/j.fitote.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Saric S, Sivamani RK. Polyphenols and Sunburn. Int J Mol Sci. 2016;17(9):1521. doi: 10.3390/ijms17091521. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Couteau C, Cheignon C, Paparis E, Coiffard LJ. Silymarin, a molecule of interest for topical photoprotection. Nat Prod Res. 2012;26:2211–2214. doi: 10.1080/14786419.2011.637219. [DOI] [PubMed] [Google Scholar]
- 59.Garland CF, Garland FC, Gorham ED. Rising trends in melanoma. An hypothesis concerning sunscreen effectiveness. Ann Epidemiol. 1993;3:103–110. doi: 10.1016/1047-2797(93)90017-x. [DOI] [PubMed] [Google Scholar]
- 60.Caini S, Gandini S, Sera F, Raimondi S, Fargnoli MC, Boniol M, Armstrong BK. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur J Cancer. 2009;45:3054–3063. doi: 10.1016/j.ejca.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol. 2011;29:e557–558. doi: 10.1200/JCO.2011.35.5727. author reply e859. [DOI] [PubMed] [Google Scholar]
- 62.Gorham ED, Mohr SB, Garland CF, Chaplin G, Garl FC. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol. 2007;17:956–963. doi: 10.1016/j.annepidem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735–739. doi: 10.3122/jabfm.2011.06.100178. [DOI] [PubMed] [Google Scholar]
- 64.Ghiasvand RE, Weiderpass E, Green AC, Lund E, Veierod MB. Sunscreen Use and Subsequent Melanoma Risk: A Population-Based Cohort Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.5934. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 66.Cohen LE, Grant RT. Sun Protection: Current Management Strategies Addressing UV Exposure. Clin Plast Surg. 2016;43:605–610. doi: 10.1016/j.cps.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol. 2009;161(Suppl 3):40–45. doi: 10.1111/j.1365-2133.2009.09448.x. [DOI] [PubMed] [Google Scholar]
- 68.Autier P, Dore JF, Reis AC, Grivegnee A, Ollivaud L, Truchetet F, Chamoun E, Rotmensz N, Severi G, Cesarini JP. Sunscreen use and intentional exposure to ultraviolet A and B radiation: a double blind randomized trial using personal dosimeters. Br J Cancer. 2000;83:1243–1248. doi: 10.1054/bjoc.2000.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen AC, Damian DL, Halliday GM. Oral and systemic photoprotection. Photodermatol Photoimmunol Photomed. 2014;30:102–111. doi: 10.1111/phpp.12100. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Garcia E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014;5:1994–2003. doi: 10.1039/c4fo00280f. [DOI] [PubMed] [Google Scholar]
- 71.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 72.McNulty SE, del Rosario R, Cen D, Meyskens FL, Jr, Yang S. Comparative expression of NFkappaB proteins in melanocytes of normal skin vs. benign intradermal naevus and human metastatic melanoma biopsies. Pigment Cell Res. 2004;17:173–180. doi: 10.1111/j.1600-0749.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 73.Albano F, Arcucci A, Granato G, Romano S, Montagnani S, De Vendittis E, Ruocco MR. Markers of mitochondrial dysfunction during the diclofenac-induced apoptosis in melanoma cell lines. Biochimie. 2013;95:934–945. doi: 10.1016/j.biochi.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Allaj V, Guo C, Nie D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013;3:8. doi: 10.1186/2045-3701-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denkert C, Kobel M, Berger S, Siegert A, Leclere A, Trefzer U, Hauptmann S. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61:303–308. [PubMed] [Google Scholar]
- 76.Kuzbicki L, Lange D, Straczynska-Niemiec A, Chwirot BW. The value of cyclooxygenase-2 expression in differentiating between early melanomas and histopathologically difficult types of benign human skin lesions. Melanoma Res. 2012;22:70–76. doi: 10.1097/CMR.0b013e32834defec. [DOI] [PubMed] [Google Scholar]
- 77.Kuzbicki L, Sarnecka A, Chwirot BW. Expression of cyclooxygenase-2 in benign naevi and during human cutaneous melanoma progression. Melanoma Res. 2006;16:29–36. doi: 10.1097/01.cmr.0000194430.77643.a0. [DOI] [PubMed] [Google Scholar]
- 78.Becker MR, Siegelin MD, Rompel R, Enk AH, Gaiser T. COX-2 expression in malignant melanoma: a novel prognostic marker? Melanoma Res. 2009;19:8–16. doi: 10.1097/CMR.0b013e32831d7f52. [DOI] [PubMed] [Google Scholar]
- 79.Ramirez CC, Ma F, Federman DG, Kirsner RS. Use of cyclooxygenase inhibitors and risk of melanoma in high-risk patients. Dermatol Surg. 2005;31:748–752. doi: 10.1097/00042728-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Harris RE, Beebe-Donk J, Namboodiri KK. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol Rep. 2001;8:655–657. doi: 10.3892/or.8.3.655. [DOI] [PubMed] [Google Scholar]
- 81.Joosse A, Koomen ER, Casparie MK, Herings RM, Guchelaar HJ, Nijsten T. Non-steroidal anti-inflammatory drugs and melanoma risk: large Dutch population-based case-control study. J Invest Dermatol. 2009;129:2620–2627. doi: 10.1038/jid.2009.201. [DOI] [PubMed] [Google Scholar]
- 82.Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sørensen HT. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer. 2012;118:4768–4776. doi: 10.1002/cncr.27406. [DOI] [PubMed] [Google Scholar]
- 83.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 84.Jeter JM, Bonner JD, Johnson TM, Gruber SB. Nonsteroidal anti-inflammatory drugs and risk of melanoma. J Skin Cancer. 2011;2011:598571. doi: 10.1155/2011/598571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asgari MM, Maruti SS, White E. A large cohort study of nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst. 2008;100:967–971. doi: 10.1093/jnci/djn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeter JM, Han J, Martinez ME, Alberts DS, Qureshi AA, Feskanich D. Non-steroidal anti-inflammatory drugs, acetaminophen, and risk of skin cancer in the Nurses’ Health Study. Cancer Causes Control. 2012;23:1451–1461. doi: 10.1007/s10552-012-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clouser MC, Roe DJ, Foote JA, Harris RB. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol Drug Saf. 2009;18:276–283. doi: 10.1002/pds.1718. [DOI] [PubMed] [Google Scholar]
- 88.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–621. doi: 10.1517/14740331003662620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41:554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Bonovas S, Nikolopoulos G, Filioussi K, Peponi E, Bagos P, Sitaras NM. Can statin therapy reduce the risk of melanoma? A meta-analysis of randomized controlled trials. Eur J Epidemiol. 2010;25:29–35. doi: 10.1007/s10654-009-9396-x. [DOI] [PubMed] [Google Scholar]
- 91.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 92.Khosravi-Far R, Cox AD, Kato K, Der CJ. Protein prenylation: key to ras function and cancer intervention? Cell Growth Differ. 1992;3:461–469. [PubMed] [Google Scholar]
- 93.Shellman YG, Ribble D, Miller L, Gendall J, Vanbuskirk K, Kelly D, Norris DA, Dellavalle RP. Lovastatin-induced apoptosis in human melanoma cell lines. Melanoma Res. 2005;15:83–89. doi: 10.1097/00008390-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Saito A, Saito N, Mol W, Furukawa H, Tsutsumida A, Oyama A, Sekido M, Sasaki S, Yamamoto Y. Simvastatin inhibits growth via apoptosis and the induction of cell cycle arrest in human melanoma cells. Melanoma Res. 2008;18:85–94. doi: 10.1097/CMR.0b013e3282f60097. [DOI] [PubMed] [Google Scholar]
- 95.Collisson EA, Kleer C, Wu M, De A, Gambhir SS, Merajver SD, Kolodney MS. Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis in human melanoma cells. Mol Cancer Ther. 2003;2:941–948. [PMC free article] [PubMed] [Google Scholar]
- 96.Maj M, Czajkowski R, Zegarska B, Kowaliszyn B, Pokrywczynska M, Drewa T. Anti-proliferative and cytotoxic activity of rosuvastatin against melanoma cells. Postepy Dermatol Alergol. 2016;33:257–262. doi: 10.5114/ada.2016.61601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Wu XB, Chen Q. Statin use is not associated with reduced risk of skin cancer: a meta-analysis. Br J Cancer. 2014;110:802–807. doi: 10.1038/bjc.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Livingstone E, Hollestein LM, van Herk-Sukel MP, van de Poll-Franse L, Joosse A, Schilling B, Nijsten T, Schadendorf D, de Vries E. Statin use and its effect on all-cause mortality of melanoma patients: a population-based Dutch cohort study. Cancer Med. 2014;3:1284–1293. doi: 10.1002/cam4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection (Review) Int J Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 100.Matsui MS, Hsia A, Miller JD, Hanneman K, Scull H, Cooper KD, Baron E. Non-sunscreen photoprotection: antioxidants add value to a sunscreen. J Investig Dermatol Symp Proc. 2009;14:56–59. doi: 10.1038/jidsymp.2009.14. [DOI] [PubMed] [Google Scholar]
- 101.Stevanato R, Bertelle M, Fabris S. Photoprotective characteristics of natural antioxidant polyphenols. Regul Toxicol Pharmacol. 2014;69:71–77. doi: 10.1016/j.yrtph.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 103.Wu Z, Liu B, E C, Liu J, Zhang Q, Liu J, Chen N, Chen R, Zhu R. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol Med Rep. 2015;11:400–404. doi: 10.3892/mmr.2014.2716. [DOI] [PubMed] [Google Scholar]
- 104.Back JH, Zhu Y, Calabro A, Queenan C, Kim AS, Arbesman J, Kim AL. Resveratrol-mediated downregulation of Rictor attenuates autophagic process and suppresses UV-induced skin carcinogenesis. Photochem Photobiol. 2012;88:1165–1172. doi: 10.1111/j.1751-1097.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 106.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 107.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 108.Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 109.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–163. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 110.Luo H, Umebayashi M, Doi K, Morisaki T, Shirasawa S, Tsunoda T. Resveratrol Overcomes Cellular Resistance to Vemurafenib Through Dephosphorylation of AKT in BRAF-mutated Melanoma Cells. Anticancer Res. 2016;36:3585–3589. [PubMed] [Google Scholar]
- 111.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 112.Smoliga JM, Blanchard O. Enhancing the delivery of resveratrol in humans: if low bioavailability is the problem, what is the solution? Molecules. 2014;19:17154–17172. doi: 10.3390/molecules191117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Q, Kim C, Jo YH, Kim SB, Hwang BY, Lee MK. Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules. 2015;20:16933–16945. doi: 10.3390/molecules200916933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park S, Seok JK, Kwak JY, Choi YH, Hong SS, Suh HJ, Park W, Boo YC. Anti-melanogenic effects of resveratryl triglycolate, a novel hybrid compound derived by esterification of resveratrol with glycolic acid. Arch Dermatol Res. 2016;308:325–334. doi: 10.1007/s00403-016-1644-9. [DOI] [PubMed] [Google Scholar]
- 115.Androutsopoulos VP, Fragiadaki I, Spandidos DA, Tosca A. The resveratrol analogue, 3,4,5,4′trans-tetramethoxystilbene, inhibits the growth of A375 melanoma cells through multiple anticancer modes of action. Int J Oncol. 2016;49:1305–1314. doi: 10.3892/ijo.2016.3635. [DOI] [PubMed] [Google Scholar]
- 116.Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci. 2015;1348:20–31. doi: 10.1111/nyas.12811. [DOI] [PubMed] [Google Scholar]
- 117.Summerlin N, Soo E, Thakur S, Qu Z, Jambhrunkar S, Popat A. Resveratrol nanoformulations: challenges and opportunities. Int J Pharm. 2015;479:282–290. doi: 10.1016/j.ijpharm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Sanna V, Roggio AM, Siliani S, Piccinini M, Marceddu S, Mariani A, Sechi M. Development of novel cationic chitosan-and anionic alginate-coated poly(D,L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int J Nanomedicine. 2012;7:5501–5516. doi: 10.2147/IJN.S36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2010;390:61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 120.Carletto B, Berton J, Ferreira TN, Dalmolin LF, Paludo KS, Mainardes RM, Farago PV, Favero GM. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf B Biointerfaces. 2016;144:65–72. doi: 10.1016/j.colsurfb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 121.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parshad R, Sanford KK, Price FM, Steele VE, Tarone RE, Kelloff GJ, Boone CW. Protective action of plant polyphenols on radiation-induced chromatid breaks in cultured human cells. Anticancer Res. 1998;18:3263–3266. [PubMed] [Google Scholar]
- 123.Li H, Gao A, Jiang N, Liu Q, Liang B, Li R, Zhang E, Li Z, Zhu H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem Photobiol. 2016;92:808–815. doi: 10.1111/php.12628. [DOI] [PubMed] [Google Scholar]
- 124.Ishizaki C, Oguro T, Yoshida T, Wen CQ, Sueki H, Iijima M. Enhancing effect of ultraviolet A on ornithine decarboxylase induction and dermatitis evoked by 12-o-tetradecanoylphorbol-13-acetate and its inhibition by curcumin in mouse skin. Dermatology. 1996;193:311–317. doi: 10.1159/000246276. [DOI] [PubMed] [Google Scholar]
- 125.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 126.Oguro T, Yoshida T. Effect of ultraviolet A on ornithine decarboxylase and metallothionein gene expression in mouse skin. Photodermatol Photoimmunol Photomed. 2001;17:71–78. doi: 10.1034/j.1600-0781.2001.017002071.x. [DOI] [PubMed] [Google Scholar]
- 127.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 128.Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, Salehi H, Peyvandi M, Pawelek JM, Sahebkar A. Curcumin: A new candidate for melanoma therapy? Int J Cancer. 2016;139:1683–1695. doi: 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- 129.Loch-Neckel G, Santos-Bubniak L, Mazzarino L, Jacques AV, Moccelin B, Santos-Silva MC, Lemos-Senna E. Orally Administered Chitosan-Coated Polycaprolactone Nanoparticles Containing Curcumin Attenuate Metastatic Melanoma in the Lungs. J Pharm Sci. 2015;104:3524–3534. doi: 10.1002/jps.24548. [DOI] [PubMed] [Google Scholar]
- 130.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. discussion 1703S–1694S. [DOI] [PubMed] [Google Scholar]
- 131.Agarwal R, Katiyar SK, Zaidi SI, Mukhtar H. Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by polyphenolic fraction isolated from green tea and its individual epicatechin derivatives. Cancer Res. 1992;52:3582–3588. [PubMed] [Google Scholar]
- 132.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Mittal A, Piyathilake C, Hara Y, Katiyar SK. Exceptionally high protection of photocarcinogenesis by topical application of (–)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5:555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 135.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res (Phila) 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Afaq F, Ahmad N, Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 137.Ahmad N, Adhami VM, Gupta S, Cheng P, Mukhtar H. Role of the retinoblastoma (pRb)-E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch Biochem Biophys. 2002;398:125–131. doi: 10.1006/abbi.2001.2704. [DOI] [PubMed] [Google Scholar]
- 138.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 139.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 140.Taniguchi S, Fujiki H, Kobayashi H, Go H, Miyado K, Sadano H, Shimokawa R. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65:51–54. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- 141.Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Ahmad N, Wood GS. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle. 2009;8:2057–2063. doi: 10.4161/cc.8.13.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Srivastava AK, Bhatnagar P, Singh M, Mishra S, Kumar P, Shukla Y, Gupta KC. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int J Nanomedicine. 2013;8:1451–1462. doi: 10.2147/IJN.S26364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 144.Pal HC, Baxter RD, Hunt KM, Agarwal J, Elmets CA, Athar M, Afaq F. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6:28296–28311. doi: 10.18632/oncotarget.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pal HC, Diamond AC, Strickland LR, Kappes JC, Katiyar SK, Elmets CA, Athar M, Afaq F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2016;7:1227–1241. doi: 10.18632/oncotarget.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, Setaluri V, Mukhtar H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/beta-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–1299. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pal HC, Athar M, Elmets CA, Afaq F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFkappaB signaling pathways in SKH-1 hairless mice. Photochem Photobiol. 2015;91:225–234. doi: 10.1111/php.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64–67. doi: 10.1016/j.clindermatol.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 149.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 150.Russo I, Caroppo F, Alaibac M. Vitamins and Melanoma. Cancers (Basel) 2015;7:1371–1387. doi: 10.3390/cancers7030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Degos L. All-trans-retinoic acid treatment and retinoic acid receptor alpha gene rearrangement in acute promyelocytic leukemia: a model for differentiation therapy. Int J Cell Cloning. 1992;10:63–69. doi: 10.1002/stem.5530100202. [DOI] [PubMed] [Google Scholar]
- 152.van Berkel TJ. Bringing retinoid metabolism into the 21st century. J Lipid Res. 2009;50:2337–2339. doi: 10.1194/jlr.E002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Semba RD. On the ‘discovery’ of vitamin A. Ann Nutr Metab. 2012;61:192–198. doi: 10.1159/000343124. [DOI] [PubMed] [Google Scholar]
- 154.Rosa FW. Teratogenicity of isotretinoin. Lancet. 1983;2:513. doi: 10.1016/s0140-6736(83)90538-x. [DOI] [PubMed] [Google Scholar]
- 155.Santamaria L, Bianchi A, Arnaboldi A, Ravetto C, Bianchi L, Pizzala R, Andreoni L, Santagati G, Bermond P. Chemoprevention of indirect and direct chemical carcinogenesis by carotenoids as oxygen radical quenchers. Ann N Y Acad Sci. 1988;534:584–596. doi: 10.1111/j.1749-6632.1988.tb30149.x. [DOI] [PubMed] [Google Scholar]
- 156.di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 157.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 159.Asgari MM, Brasky TM, White E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J Invest Dermatol. 2012;132:1573–1582. doi: 10.1038/jid.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miura K, Green AC. Dietary Antioxidants and Melanoma: Evidence from Cohort and Intervention Studies. Nutr Cancer. 2015;67:867–876. doi: 10.1080/01635581.2015.1053499. [DOI] [PubMed] [Google Scholar]
- 161.Sorg O, Tran C, Saurat JH. Cutaneous vitamins A and E in the context of ultraviolet- or chemically-induced oxidative stress. Skin Pharmacol Appl Skin Physiol. 2001;14:363–372. doi: 10.1159/000056370. [DOI] [PubMed] [Google Scholar]
- 162.Tafrova JI, Pinkas-Sarafova A, Stolarzewicz E, Parker KA, Simon M. UVA/B exposure promotes the biosynthesis of dehydroretinol in cultured human keratinocytes. Mol Cell Biochem. 2012;364:351–361. doi: 10.1007/s11010-012-1237-7. [DOI] [PubMed] [Google Scholar]
- 163.Bertram JS, Vine AL. Cancer prevention by retinoids and carotenoids: independent action on a common target. Biochim Biophys Acta. 2005;1740:170–178. doi: 10.1016/j.bbadis.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 164.Emionite L, Galmozzi F, Raffo P, Vergani L, Toma S. Retinoids and malignant melanoma: a pathway of proliferation inhibition on SK MEL28 cell line. Anticancer Res. 2003;23:13–19. [PubMed] [Google Scholar]
- 165.Helige C, Smolle J, Zellnig G, Hartmann E, Fink-Puches R, Kerl H, Tritthart HA. Inhibition of K1735-M2 melanoma cell invasion in vitro by retinoic acid. Clin Exp Metastasis. 1993;11:409–418. doi: 10.1007/BF00132984. [DOI] [PubMed] [Google Scholar]
- 166.Huen AO, Kim EJ. The Role of Systemic Retinoids in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin. 2015;33:715–729. doi: 10.1016/j.det.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 167.Liu X, Chan SY, Ho PC. Comparison of the in vitro and in vivo effects of retinoids either alone or in combination with cisplatin and 5-fluorouracil on tumor development and metastasis of melanoma. Cancer Chemother Pharmacol. 2008;63:167–174. doi: 10.1007/s00280-008-0763-1. [DOI] [PubMed] [Google Scholar]
- 168.Ohashi E, Inoue K, Kagechika H, Hong SH, Takayuki Nakagawa, Takahashi T, Mochizuki M, Nishimura R, Sasaki N. Effect of natural and synthetic retinoids on the proliferation and differentiation of three canine melanoma cell lines. J Vet Med Sci. 2002;64:169–172. doi: 10.1292/jvms.64.169. [DOI] [PubMed] [Google Scholar]
- 169.Edward M, Gold JA, MacKie RM. Modulation of melanoma cell adhesion to basement membrane components by retinoic acid. J Cell Sci. 1989;93(Pt 1):155–161. doi: 10.1242/jcs.93.1.155. [DOI] [PubMed] [Google Scholar]
- 170.Sondak VK, Liu PY, Flaherty LE, Fletcher WS, Periman P, Gandara DR, Taylor SA, Balcerzak SP, Meyskens FL., Jr A phase II evaluation of all-trans-retinoic acid plus interferon alfa-2a in stage IV melanoma: a Southwest Oncology Group study. Cancer J Sci Am. 1999;5:41–47. [PubMed] [Google Scholar]
- 171.Ortiz MA, Bayon Y, Lopez-Hernandez FJ, Piedrafita FJ. Retinoids in combination therapies for the treatment of cancer: mechanisms and perspectives. Drug Resist Updat. 2002;5:162–175. doi: 10.1016/s1368-7646(02)00050-x. [DOI] [PubMed] [Google Scholar]
- 172.Petruk G, Raiola A, Del Giudice R, Barone A, Frusciante L, Rigano MM, Monti DM. An ascorbic acid-enriched tomato genotype to fight UVA-induced oxidative stress in normal human keratinocytes. J Photochem Photobiol B. 2016;163:284–289. doi: 10.1016/j.jphotobiol.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 173.Tebbe B, Wu S, Geilen CC, Eberle J, Kodelja V, Orfanos CE. L-ascorbic acid inhibits UVA-induced lipid peroxidation and secretion of IL-1alpha and IL-6 in cultured human keratinocytes in vitro. J Invest Dermatol. 1997;108:302–306. doi: 10.1111/1523-1747.ep12286468. [DOI] [PubMed] [Google Scholar]
- 174.Burke KE. Interaction of vitamins C and E as better cosmeceuticals. Dermatol Ther. 2007;20:314–321. doi: 10.1111/j.1529-8019.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 175.Darr D, Combs S, Dunston S, Manning T, Pinnell S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br J Dermatol. 1992;127:247–253. doi: 10.1111/j.1365-2133.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 176.Serrano G, Almudever P, Serrano JM, Milara J, Torrens A, Exposito I, Cortijo J. Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clin Cosmet Investig Dermatol. 2015;8:591–599. doi: 10.2147/CCID.S90781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Campbell EJ, Vissers MC, Dachs GU. Ascorbate availability affects tumor implantation-take rate and increases tumor rejection in Gulo−/− mice. Hypoxia (Auckl) 2016;4:41–52. doi: 10.2147/HP.S103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bram S, Froussard P, Guichard M, Jasmin C, Augery Y, Sinoussi-Barre F, Wray W. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980;284:629–631. doi: 10.1038/284629a0. [DOI] [PubMed] [Google Scholar]
- 179.Venturelli S, Sinnberg TW, Berger A, Noor S, Levesque MP, Bocker A, Niessner H, Lauer UM, Bitzer M, Garbe C, Busch C. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front Oncol. 2014;4:227. doi: 10.3389/fonc.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cha J, Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013;42:55–64. doi: 10.3892/ijo.2012.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77:51–58. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 182.Beer TM, Munar M, Henner WD. A Phase I trial of pulse calcitriol in patients with refractory malignancies: pulse dosing permits substantial dose escalation. Cancer. 2001;91:2431–2439. [PubMed] [Google Scholar]
- 183.Piotrowska A, Wierzbicka J, Nadkarni S, Brown G, Kutner A, Zmijewski MA. Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D(2) Against Human Malignant Melanoma Cell Lines. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O’Shea SJ, Davies JR, Newton-Bishop JA. Vitamin D, vitamin A, the primary melanoma transcriptome and survival. Br J Dermatol. 2016;175(Suppl 2):30–34. doi: 10.1111/bjd.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bandera Merchan B, Morcillo S, Martin-Nunez G, Tinahones FJ, Macias-Gonzalez M. The Role of Vitamin D and Vdr in Carcinogenesis: Through Epidemiology and Basic Sciences. J Steroid Biochem Mol Biol. 2016;167:203–218. doi: 10.1016/j.jsbmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 186.Halsall JA, Osborne JE, Potter L, Pringle JH, Hutchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibilty and prognosis in malignant melanoma. Br J Cancer. 2004;91:765–770. doi: 10.1038/sj.bjc.6602006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hutchinson PE, Osborne JE, Lear JT, Smith AG, Bowers PW, Morris PN, Jones PW, York C, Strange RC, Fryer AA. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res. 2000;6:498–504. [PubMed] [Google Scholar]
- 188.Zhao XZ, Yang BH, Yu GH, Liu SZ, Yuan ZY. Polymorphisms in the vitamin D receptor (VDR) genes and skin cancer risk in European population: a meta-analysis. Arch Dermatol Res. 2014;306:545–553. doi: 10.1007/s00403-014-1464-8. [DOI] [PubMed] [Google Scholar]
- 189.Orlow I, Reiner AS, Thomas NE, Roy P, Kanetsky PA, Luo L, Paine S, Armstrong BK, Kricker A, Marrett LD, Rosso S, Zanetti R, Gruber SB, Anton-Culver H, Gallagher RP, Dwyer T, Busam K, Begg CB, Berwick M, G. E. M. S. Group Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: a population-based study. Carcinogenesis. 2016;37:30–38. doi: 10.1093/carcin/bgv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zingg JM. Modulation of Signal Transduction and Gene Expression by Vitamin E via PI3Kgamma/PKB and hTAP1/SEC14L2-Mediated Lipid Exchange. J Nutr Sci Vitaminol (Tokyo) 2015;61(Suppl):S76–77. doi: 10.3177/jnsv.61.S76. [DOI] [PubMed] [Google Scholar]
- 191.Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005;124:304–307. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- 192.Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, Fisher GJ, Voorhees JJ. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol. 2002;119:507–512. doi: 10.1046/j.1523-1747.2002.01844.x. [DOI] [PubMed] [Google Scholar]
- 193.Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145–1151. doi: 10.1016/s0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- 194.Ottino P, Duncan JR. The role of adenylate cyclase, cAMP and PGE2 in the in vitro growth regulation of murine melanoma cells by vitamin E. Prostaglandins Leukot Essent Fatty Acids. 1996;54:375–383. doi: 10.1016/s0952-3278(96)90052-6. [DOI] [PubMed] [Google Scholar]
- 195.Prasad KN, Hernandez C, Edwards-Prasad J, Nelson J, Borus T, Robinson WA. Modification of the effect of tamoxifen, cis-platin, DTIC, and interferon-alpha 2b on human melanoma cells in culture by a mixture of vitamins. Nutr Cancer. 1994;22:233–245. doi: 10.1080/01635589409514349. [DOI] [PubMed] [Google Scholar]
- 196.Malafa MP, Fokum FD, Mowlavi A, Abusief M, King M. Vitamin E inhibits melanoma growth in mice. Surgery. 2002;131:85–91. doi: 10.1067/msy.2002.119191. [DOI] [PubMed] [Google Scholar]
- 197.Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev. 2003;8:303–318. [PubMed] [Google Scholar]
- 198.Lopez-Bergami P, Fitchman B, Ronai Z. Understanding signaling cascades in melanoma. Photochem Photobiol. 2008;84:289–306. doi: 10.1111/j.1751-1097.2007.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shah M, Stebbins JL, Dewing A, Qi J, Pellecchia M, Ronai ZA. Inhibition of Siah2 ubiquitin ligase by vitamin K3 (menadione) attenuates hypoxia and MAPK signaling and blocks melanoma tumorigenesis. Pigment Cell Melanoma Res. 2009;22:799–808. doi: 10.1111/j.1755-148X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nakayama K, Qi J, Ronai Z. The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res. 2009;7:443–451. doi: 10.1158/1541-7786.MCR-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ming M, Zhao B, Shea CR, Shah P, Qiang L, White SR, Sims DM, He YY. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J Allergy Clin Immunol. 2015;135:936–945 e934. doi: 10.1016/j.jaci.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ishibashi M, Arai M, Tanaka S, Onda K, Hirano T. Antiproliferative and apoptosis-inducing effects of lipophilic vitamins on human melanoma A375 cells in vitro. Biol Pharm Bull. 2012;35:10–17. doi: 10.1248/bpb.35.10. [DOI] [PubMed] [Google Scholar]
- 203.Pengo V, Denas G, Jose SP, Pengo MF. Cancer prevention and vitamin K antagonists: an overview. Thromb Res. 2010;125(Suppl 2):S103–105. doi: 10.1016/S0049-3848(10)70025-6. [DOI] [PubMed] [Google Scholar]
- 204.Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]


