Abstract
Matrix maturation within cortical bone is an important but oft-neglected component of bone remodeling because of the lack of a suitable small animal model. Intra-cortical remodeling can be induced in rodents by feeding virgin or lactating animals a low calcium diet. The current study aimed to determine which of these two models is most suitable for studying intra-cortical matrix maturation. We compared intra-cortical remodeling in female rats fed a normal calcium diet (virgin/normal Ca), a low calcium diet (virgin/low Ca) or a low calcium diet during lactation (lactation/low Ca). The low calcium diet was administered for 23 days (induction phase) followed by return to normal calcium for 30 days (recovery phase). At the end of induction, the virgin/normal Ca and virgin/low Ca animals had no difference in cortical porosity, but the lactation/low Ca animals had elevated cortical porosity at various diaphyseal sites in the femur and tibia. The distal femoral site had the greatest amount of induced porosity in the size range of rat secondary osteons. Neither global mineralization nor tissue-age-specific mineral-to-matrix ratio in the bone formed during recovery were affected in the lactation/low Ca rats. Serum calcium levels did not differ from controls, but phosphate levels were slightly elevated, consistent with the rapid recovery of lost bone mass. We conclude that the lactation/low Ca model represents a means to increase intra-cortical remodeling in adult rats with no apparent detrimental effect on matrix maturation. This model will provide researchers with a new tool to study matrix maturation throughout the cortex.
Keywords: Matrix mineralization, Bone modeling and remodeling, Animal Models, Lactation, Rat
1. Introduction
Cortical bone makes up nearly 80% of the total skeletal mass and contributes significantly to the mechanical strength of long bones. Lifelong maintenance of cortical bone mechanical competence depends on bone remodeling to replace damaged and aged tissue. Bone remodeling is a coupled process initiated when osteoclasts remove old or damaged tissue. Resorption is followed by osteoblast-mediated production of osteoid, which gradually matures, a process most easily identified by examining mineralization. How the newly formed cortical bone matrix matures in the adult has received little attention partly because rodents, the most commonly used animal model in bone research, are frequently noted to have minimal intra-cortical remodeling [1, 2].
Scientists lack a versatile and easily manipulated small animal model in which bone matrix maturation during cortical remodeling can be studied. However, previous experiments have pointed to potential models, including feeding rats a low calcium diet during lactation and examining the bones of the dams [3, 4]. Early characterization of the low calcium during lactation model highlighted the increase in cortical porosity [3]. Following weaning and return of the dams to a normal calcium diet, the animals begin to recover lost bone [3–5], suggesting an active intra-cortical remodeling process. Nevertheless, the only mention of matrix maturation in the newly formed tissue is the observation that this tissue is qualitatively less mineralized than tissue from control animals after 3 weeks of recovery [5]. Low calcium diet alone has also been reported to increase skeletal remodeling in rats [6–9]. However, the primary sites of remodeling noted have been limited to the trabecular [10, 11] and endocortical compartments [12], with very few reports noting an increase in the cortical porosity [8, 9].
The current study was designed to determine if using a low calcium diet in virgin or lactating rats is suitable for studying intra-cortical matrix maturation within haversian-like remodeling units. To this end, we examined cortical porosity and matrix mineralization. We hypothesized that the low calcium diet during lactation would induce intra-cortical remodeling and that the low calcium diet alone would induce endocortical remodeling, however, the low calcium diet alone would not be a sufficiently high calcium stress to induce intra-cortical remodeling and further that the matrix maturation process would be largely unaffected by the induced elevated remodeling.
2. Methods
The materials and methods are outlined here with more details provided in supplementary material.
2.1 Study Design
30 female Sprague-Dawley rats were purchased from Envigo Labs at 12 weeks of age (Table 1). 20 of the 30 animals were virgins, while 10 animals were shipped on a timed pregnancy basis and arrived at E15, 6–7 days before parturition. After arrival, all animals were immediately placed on a normal calcium diet (0.6% calcium, 0.4% phosphate, Teklad Diets, TD.97191). The experiment was designed with two phases: induction (23 days) and recovery (30 days). The induction phase was initiated when the pregnant animals assigned to the lactation during low calcium group (lactation/low Ca) gave birth. At this point the lactation/low Ca and the virgin/low Ca groups were placed on a low calcium diet (0.01% calcium, 0.4% phosphate, TD.9507). After the 23 day induction phase, the pups were weaned and a cohort of dams was sacrificed to measure baseline parameters. The remaining animals were placed onto the normal calcium diet for a 30 day recovery phase.
Table 1.
Experimental Design
| Group | Induction (23 days) |
Recovery (30 days) |
|---|---|---|
| Virgin/normal Ca induction control | Normal Ca diet ■ | |
| Virgin/normal Ca recovery control | Normal Ca diet | Normal Ca diet ■ |
| Virgin/low Ca induction | Low Ca diet ■ | |
| Virgin/low Ca recovery | Low Ca diet | Normal Ca diet ■ |
| Lactation/low Ca induction | Low Ca diet during lactation ■ | |
| Lactation/low Ca recovery | Low Ca diet during lactation | Normal Ca diet ■ |
Each group included 5 animals. The black box indicates the time of sacrifice.
2.2 Rat Husbandry
All animals were individually housed under an institutionally approved protocol. Animals were allowed free access to water and food throughout the course of the experiment. Health was assessed daily by monitoring behavior, food intake, and signs of stress. The average litter size was 12 pups per dam, with 8 of the 10 dams delivering litters of more than 12 pups and two delivering litters of 6. The day after parturition, the larger litters were reduced to 10 pups per dam, while the two animals that only gave birth to 6 pups were split between the induction and recovery groups to minimize the effects of the small litters. The remaining 8 dams were randomized to either induction or recovery groups, while the 20 virgin controls were randomized to the virgin/normal Ca or virgin/low Ca groups. Blood samples were taken via tail vein from animals in the recovery endpoint groups at days 2 and 3 of recovery and weekly thereafter until sacrifice.
Fluorochrome injections were given subcutaneously to differentiate bone of known tissue-ages. Oxytetracycline hydrochloride (25 mg/kg, Sigma), calcein (25 mg/kg, Sigma), xylenol orange (20 mg/kg, Alfa Aesar) and alizarin red (25 mg/kg, Acros) were injected on days 2, 14, 21, and 26 of the recovery phase, respectively. Animals were sacrificed via CO2 asphyxiation, and the long bones of the lower limbs (femurs and tibiae) were removed, dissected free of soft tissue, and fixed in 70% ethanol.
2.3 Micro-Computed Tomography (μCT)
μCT scanning was performed using a tube voltage of 70 kVp, current of 57 μA, integration time of 1500 ms, and a voxel size of 2 μm (μCT50, Scanco Medical). Cortical geometry and porosity were assessed at 6 regions of interest (ROIs) consisting of 250 slices in the femur and the tibia (Fig. 1a). Femoral regions were at 35%, 50%, and 75% of the total length, while tibial regions were at 25%, 50%, and 75% of the total length.
Figure 1.
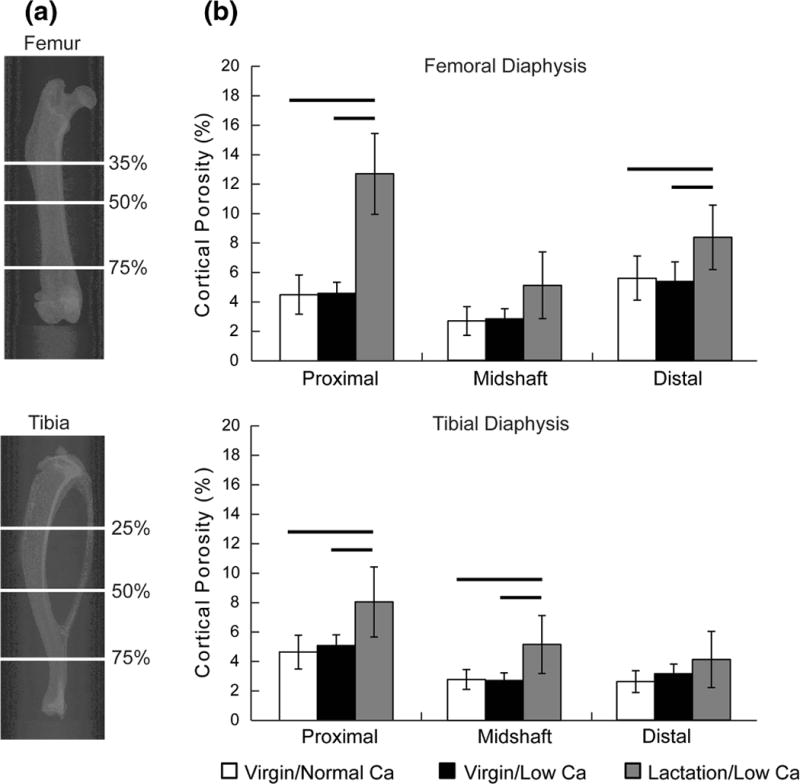
(a) Representative two-dimensional X-ray images demonstrating the skeletal sites used to characterize cortical porosity in the diaphysis of the femur and tibia. In the femur, the sites were at 35, 50, and 75% of the total length, while in the tibia the sites were at 25, 50, and 75% of the total length. (b) Cortical porosity at all 6 skeletal sites at the end of the induction phase (means and standard deviations). The data demonstrate the dramatic increase in cortical porosity induced by the lactation/low Ca model, but no significant increase in the virgin/low Ca model compared to virgin/normal Ca. There were significant between-group differences at all sites investigated with the exception of the distal tibia (p<0.001, p=0.041, and p=0.031, for the distal, midshaft, and distal femur and p=0.010, p=0.013, and p=0.194, for the proximal, midshaft, and distal tibia, respectively). Bars represent significant between-group differences (p<0.05 for the post-hoc tests).
In addition to the total cortical porosity, pore diameter distributions were used to gate the amount of porosity within specified ranges. Literature values for healthy rat cortical tissue indicate that the diameter of secondary osteons ranges between 30 and 60 μm [13, 14]. Therefore, in addition to comparing the total cortical porosity, the amount of porosity with pore diameters between 30 and 60 μm was also compared across groups.
2.4 Sample Preparation
After μCT scanning, femurs were dehydrated in a graded series of alcohol and embedded in an epoxy resin (EpoThin, Buehler). Roughly 1 mm thick slabs at the distal femoral ROI were cut from the embedded specimens (Isomet 5000, Buehler) and fixed to plastic slides. The slabs were ground to a thickness of roughly 500 μm and polished to a mirror finish (Phoenix 4000, Buehler), using a series of colloidal diamond suspensions (9, 3, 1 μm average particle size, Metadi, Buehler) with ethylene glycol as a lubricant.
2.5 Confocal Microscopy
Distal femoral diaphyseal sections were examined by confocal microscopy (LSM7000, Zeiss). Only the tetracycline and calcein labels were consistently found in intra-cortical sites. The number of intra-cortical remodeling sites with single or double label were counted and normalized to the μCT cortical area values. Although the majority of the intra-cortical remodeling units were in the direction of the long axis of the bone, pores transverse to the cut plane surrounded by fluorochrome labeling were also counted.
2.6 Fourier Transform Infrared Microspectroscopy (FTIRM)
FTIRM measurements of the mineral-to-matrix ratio were made using fluorochrome labels to differentiate tissue of various ages and to assess the mineralization kinetics as described in detail previously [15] (additional information provided in the supplementary material).
2.7 Backscattered Scanning Electron Microscopy (bSEM)
Bone mineral density distributions (BMDD) were assessed using quantitative backscattered scanning electron microscopy (bSEM). Embedded and polished samples used for FTIRM were carbon coated (Crestington) and imaged at 25 keV, a working distance of 19 mm, and a magnification of 150× (Zeiss Sigma HDVP) using techniques previously described elsewhere [16].
2.8 Histology
The sections were further ground and polished to a thickness of 100 μm and examined using polarized light microscopy to identify concentric lamellae (Nikon Eclipse 80i). Cement lines were then stained with toluidine blue[17] and imaged with bright field imaging.
2.9 Calcium and Phosphate Levels
Ionic calcium and phosphate concentrations were measured in serum using commercially available colorimetric assays (Biovision).
2.10 Statistical Analysis
Data were assumed to be normally distributed and a significance level of 0.05 was used in all tests.
Determining the appropriate model (virgin/low Ca or lactation/low Ca)
Cortical porosity was examined in the virgin/normal Ca, virgin/low Ca, and lactation/low Ca groups at the end of induction at each of the 6 sites examined using one-way analyses of variance (ANOVA), followed by post-hoc least significant difference comparisons. The selection criterion used to determine the most appropriate model (virgin/low Ca or lactation/low Ca) was the presence of increased cortical porosity compared to virgin/normal Ca controls.
Identifying the preferred skeletal site
After the most suitable model was determined, the preferred skeletal site was identified by examining the volume of porosity with pore diameters consistent with the size of secondary osteons in rats [13, 14] at each of the six sites sampled by μCT. The site with the highest fraction of induced porosity within the size range of secondary osteons was used for all subsequent investigation.
Assessing bone matrix maturation during recovery
Once the skeletal site was identified, all statistical analyses of μCT-derived porosity measures and bSEM-derived global mineralization parameters were performed using two-way ANOVAs with group (virgin/normal Ca v. lactation/low Ca) and time (induction v. recovery) as the factors, followed by independent samples post hoc t-tests. Tissue-age specific data were also examined at the intra-cortical and endocortical surfaces to assess mineralization kinetics. Only one tissue age was consistently identified in the intra-cortical compartment so we used an independent samples t-test to compare the virgin/normal Ca and lactation/low Ca groups. Three tissue ages were consistently identified at the endocortical surface so we used a repeated measures ANOVA (tissue-age as the within-subjects factor and group as the between-subjects factor).
Assessing circulating ionic calcium and phosphate levels during recovery
These values were assessed using repeated measures ANOVAs with time as the within–subjects factor and group as the between-subjects factor.
3. Results
Animals tolerated the lactation and the low calcium diet without visible signs of stress and there were no losses during the experiment.
3.1 Model Selection
Cortical porosity of the virgin/low Ca group was not different than the virgin/normal Ca controls at any of the 6 skeletal sites investigated (Fig. 1b). Therefore, the low calcium diet alone was determined to be insufficient to induce intra-cortical remodeling and was excluded from further investigation. The lactation/low Ca group, on the other hand, had increased cortical porosity at four of the six skeletal sites investigated (Fig. 1b).
3.2 Skeletal Site Selection
The extent of induced porosity varied across the sites investigated, with the proximal femur showing the largest increase in porosity. However, the pores formed in the proximal femur were primarily located within the third trochanter (Supplemental Fig. 1 & 2), and had a median diameter larger than 100 μm, considerably larger than the size of secondary osteons in rats (30–60 μm [13, 14]). Therefore, the amount of porosity induced within the size range of rat secondary osteons was compared among the sites (Fig. 2). Of the 4 sites which had differences in porosity, the distal femoral site had the largest absolute amount and the largest fraction of pores consistent with the size of rat secondary osteons (Fig. 2).
Figure 2.
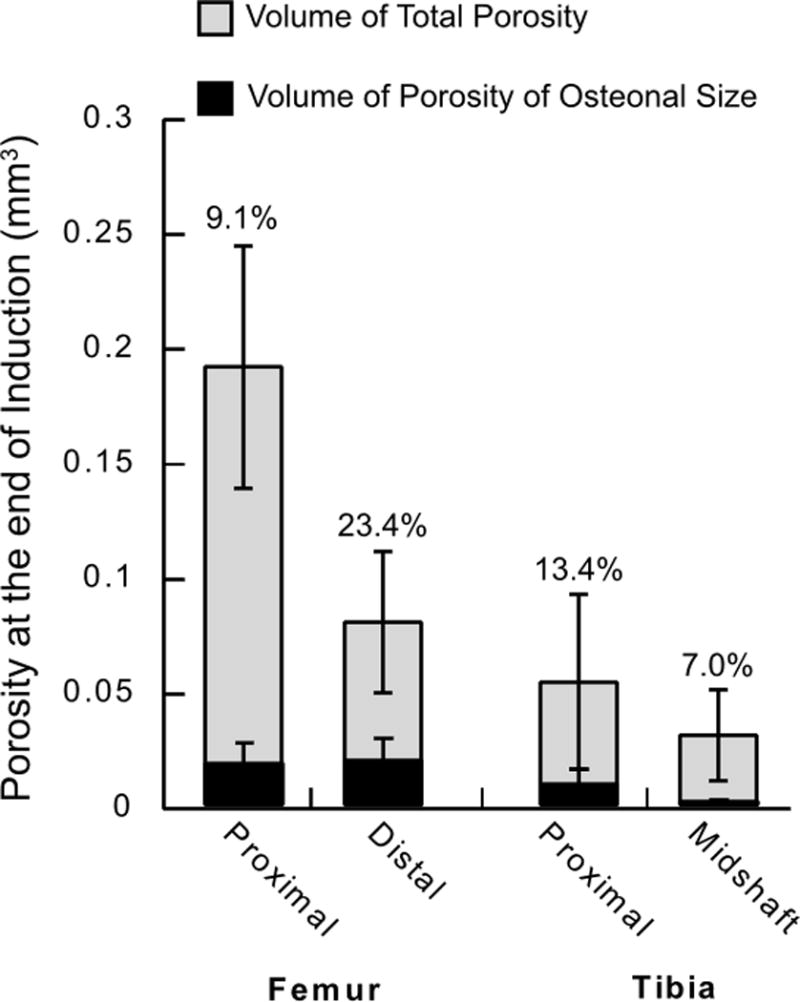
Amount of cortical porosity (gray bars) and the amount of porosity within the size range of secondary osteons in rats (black bars, 30–60 μm [13, 14]). The porosity was measured at the end of the induction phase in animals of the lactation/low Ca group and presented as the mean ± standard deviation. The fraction of the total porosity within the size range of secondary osteons is also noted above the data bars.
3.3 Recovery of Lost Skeletal Mass
After weaning and return to a normal calcium diet, the cortical porosity in the lactation/low Ca group was not different than the virgin/normal Ca group (Fig. 3). Post-hoc analysis revealed that the distal femoral diaphyseal region was one of 2 sites to show both an increase in porosity at induction and a complete return to control levels after recovery. The tibial midshaft region also had increased cortical porosity at induction and return to normal after recovery, but the cortical porosity measured at the end of recovery was highly variable (Supplemental Table 1). Despite, the increased cortical porosity in the proximal femoral and proximal tibial diaphyseal regions at the end of induction, the values did not return to control levels (Supplemental Table 1).
Figure 3.
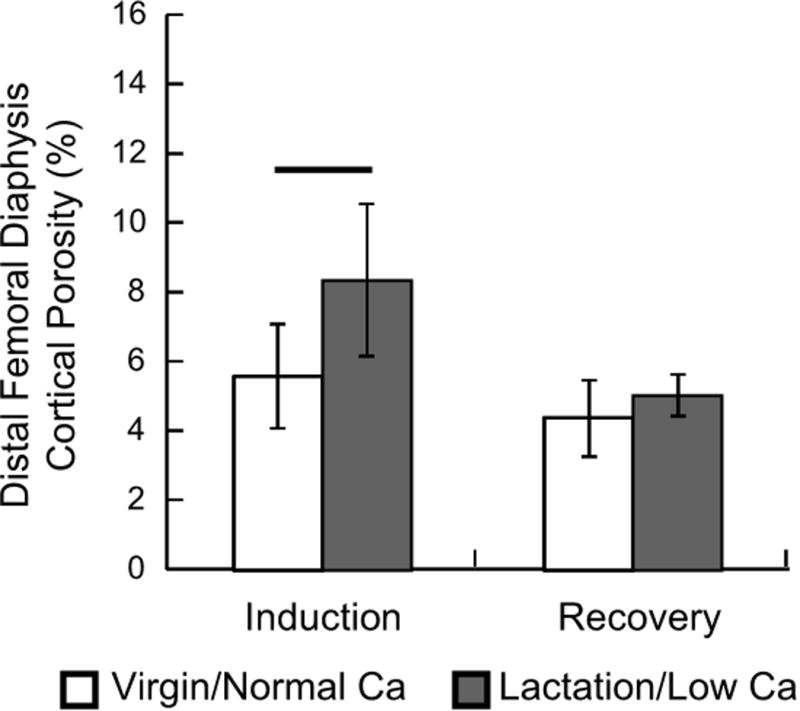
Cortical porosity measured in the distal femoral diaphysis at the end of the induction phase and after recovery. The data are presented as the means and standard deviations. The two-way ANOVA indicated significant group (p=0.019) and phase (p=0.003) effects without a significant interaction term (p=0.128). Horizontal bar indicates that the difference between the two groups was significant at the end of the induction phase (p<0.05).
At the end of induction, the lactation/low Ca diet group had increased medullary area, decreased total and decreased cortical area at the distal femoral site compared to the virgin/normal Ca controls (Supplemental Table 2). All three parameters were back to virgin/normal Ca levels after recovery, except the cortical area, which remained significantly reduced compared to virgin/normal Ca controls. In general, the other 5 skeletal sites showed similar patterns of bone loss and a return to near virgin/normal Ca levels after recovery (Supplemental Table 2).
3.4 Intra-Cortical Remodeling
Qualitative imaging of the distal femoral sections demonstrate the presence of intra-cortical remodeling units based on the presence of polarized concentric lamellae and stained cement lines surrounding remodeled osteons (Fig. 4). As identified using the fluorochrome labels, there was an increase in the number of active intra-cortical remodeling events in the lactation/low Ca animals compared to virgin/normal Ca animals (p=0.007). Specifically, the virgin/low Ca animals had an average of 3 events per mm2 (range: 1–5), while the lactation/low Ca had an average of 10 events per mm2 (range: 5–15).
Figure 4.
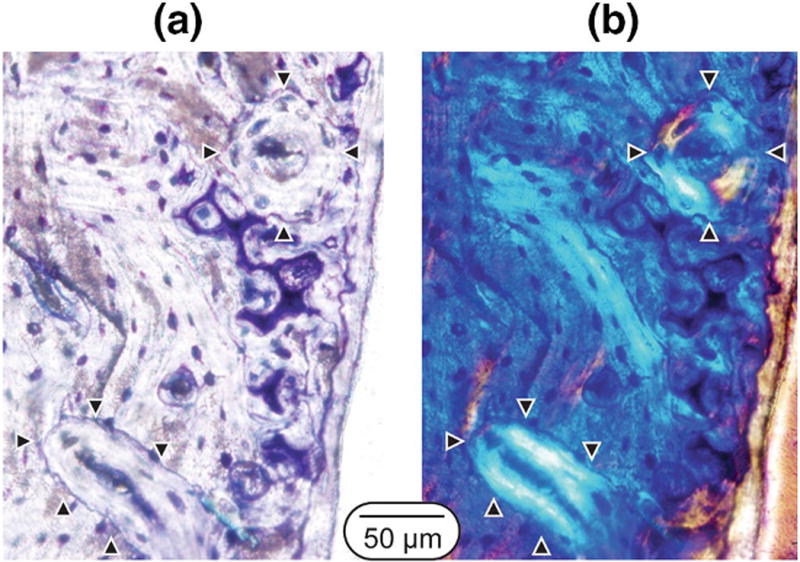
Example of secondary osteons in a lactation/low Ca rat at the end of the recovery phase. The images are from transverse sections taken at the distal femoral site. (a) Toluidine blue stained image showing cement lines and remnant calcified cartilage within the cortex and (b) matched polarized light images of concentric lamellae. Arrowheads point to secondary osteons within the field of view.
3.5 Tissue Mineralization
Tissue mineralization was assessed using FTIRM and bSEM to measure the tissue-age-specific mineralization kinetics and the global mineralization levels, respectively. Fluorochrome labels were used to differentiate tissue of known tissue-age (Fig. 5). The intra-cortical remodeling units had only tetracycline and calcein labels, limiting the FTIRM comparison to a single known tissue-age (16–28 days). There was no difference in mineralization for this tissue-age between the two groups (p=0.717). All four injected fluorochrome labels were present at the endocortical surface. Therefore, three tissue-age specific measurements of the mineral-to-matrix ratio were made (4–9, 9–16, and 16–28 days). In this compartment, there was no group-effect (p=0.602), however there was a significant tissue-age effect (p=0.031) and interaction term (p=0.013). Specifically, the lactation/low Ca group had reduced mineral-to-matrix ratios at tissue-ages between 9–16 and 16–28 days compared to the virgin/normal Ca group (Fig. 5). Despite these main ANOVA effects, the post-hoc between-group comparisons for specific tissue-ages failed to reach significance. Although there was staining on the periosteal surface in the lactation/low Ca group, the virgin/normal Ca animals had very limited periosteal labeling (Fig. 5) and, therefore, it was not possible to compare the maturation rates between groups at this surface.
Figure 5.
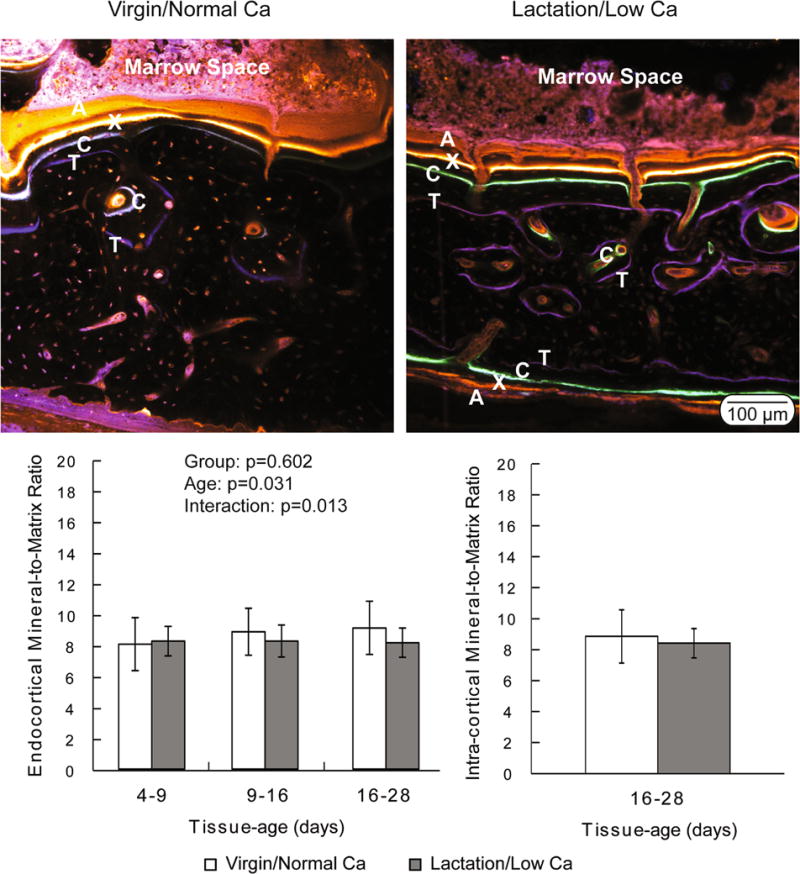
(Top) Representative confocal microscopy images demonstrating the presence of fluorochrome labels in the endocortical and intra-cortical compartments of both virgin/normal Ca and lactation/low Ca groups. The virgin/normal Ca controls lacked fluorochrome labels in the periosteal compartment. Oxytetracycline (labeled ‘T’ in the images) was given on day 2, calcein (‘C’) was given on day 14, xylenol orange (‘X’) was given on day 21, and alizarin (‘A’) was given on day 26 of the recovery phase. (Bottom) Degree of mineralization (mineral-to-matrix ratio) plotted as a function of tissue-age and compartment. Data are presented as the means and standard deviations. There was not a significant difference between groups in the intra-cortical compartment. Endocortically, the two-way ANOVA indicated a significant tissue-age effect (p=0.031) and a significant interaction term (p=0.013), with no significant group effect (p=0.602).
There appeared to be more regions of younger bone tissue in the lactation/low Ca group compared to the virgin/normal Ca group, as evidenced by the lower grayscale values near the endocortical and periosteal surfaces and surrounding intra-cortical porosity in the bSEM images (Supplemental Fig. 3). Despite this qualitative appearance, the mean mineralization and mineralization heterogeneity were not significantly different between the lactation/low Ca and the virgin/normal Ca groups (Table 2).
Table 2.
Bone mineral density distribution parameters.
| Group | Mean Mineralization (Z) | Mineralization Heterogeneity (Z) |
|---|---|---|
| Virgin/Normal Ca | 11.64 (0.23) | 0.79 (0.09) |
| Lactation/Low Ca | 11.37 (0.35) | 0.69 (0.08) |
Reported as the mean (standard deviation)
3.6 Circulating calcium and phosphate levels
The circulating levels of ionic calcium were not different in the lactation/low Ca and virgin/normal Ca recovery groups (Fig. 6). The circulating phosphate levels, however, varied as different as a function of time in the two groups (p=0.013 for the time-by-group interaction term). Specifically, the phosphate levels were elevated at days 2 and 7 post-weaning in the lactation/low Ca group compared to the virgin/normal Ca group. Overall, the group (p=0.002) and time (p<0.001) effects were also significant.
Figure 6.
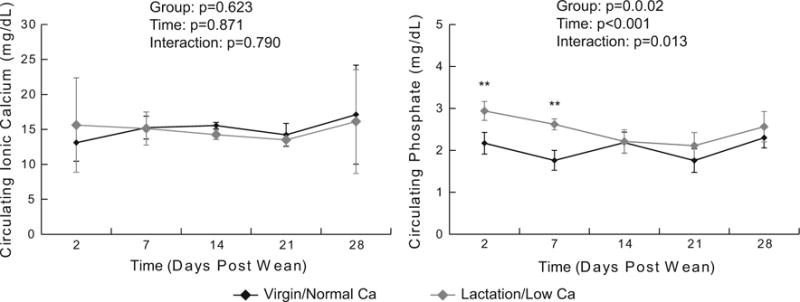
Circulating calcium and phosphate concentration measured longitudinally during the recovery phase (means and standard deviations, n=4–5 per group per time point). Results from the repeated measures ANOVA are presented in the legend.
4. Discussion
The current study sought to evaluate two potential rat models of induced intra-cortical remodeling (virgin/low Ca v. low calcium during lactation) in terms of the induced cortical porosity and bone matrix maturation following return to a normal calcium diet. The low calcium diet alone did not cause a significant increase in cortical porosity and was, therefore, excluded from consideration as a model and from additional analysis. Administering a low calcium diet during lactation triggered a substantial increase in cortical porosity throughout the femur and tibia. Of the sites with increased cortical porosity at the end of the induction phase, the distal femoral diaphysis demonstrated the largest increase in porosity consistent with the size of secondary osteons in rats [13, 14]. The porosity returned to baseline levels at the end of the recovery period, implying that intra-cortical resorption during induction was followed by formation during recovery. This interpretation is consistent with the 3-fold increase in the number of intra-cortical remodeling units in the lactation/low Ca group compared to the virgin/normal Ca group and the presence of remodeling sites with cement lines and polarized lamellae. The degree of mineralization in tissue of the same age did not differ in the lactation/low Ca and the virgin/normal Ca groups during the earliest time point measured. Although there was evidence of slight secondary mineralization rate reduction endocortically, the specific comparison between matrix of the same tissue-age were not significant, suggesting that the experimental manipulation did not dramatically alter the matrix maturation process. Therefore, the combination of lactation with a low calcium diet induces haversian-like remodeling process in the rat. This model should prove useful for studies of intra-cortical matrix maturation in the adult skeleton.
Bone matrix maturation is an important determinant of the mechanical competence of the skeleton. Variations in the organic and inorganic phases of bone matrix affect material properties such as strength, modulus and toughness. These properties, in combination with the spatial distribution of the matrix determine the strength, stiffness and energy-to-failure of bone as a tissue and organ. Bone matrix maturation can be characterized by studying mineralization of the newly formed osteoid, a process controlled by extra-cellular proteins expressed by osteoblasts and osteocytes [18, 19]. Primary mineralization occurs within days and results in the tissue achieving ~50% to 70% of its maximal mineralization level, while secondary mineralization occurs on a time scale of weeks to months or even years [20–25].
Our primary goal in the current study was to establish a rat model for the study of intra-cortical matrix maturation during haversian remodeling. Direct comparison of the mineral-to-matrix ratio at matched tissue-ages suggests that the lactation/low Ca model did not impact primary intra-cortical matrix maturation, although remodeling was significantly elevated. Despite the injection of four fluorochrome labels during the recovery period, only two were present within the intra-cortical remodeling units, limiting the intra-cortical maturation measurement to a single tissue-age. The lack of xylenol orange and alizarin (injected 21 and 26 days into the recovery phase, respectively) suggests that the induced cortical porosity infilled within the first 3 weeks of weaning and return of the dams to a normal calcium diet. Future experiments will need to use an alternate fluorochrome injection schedule to more completely assess mineralization over both primary and secondary phases.
In addition to inducing intra-cortical remodeling, feeding lactating rats a low Ca diet induced remodeling in the endocortical compartment. Although, the lactation/low Ca group had fluorochrome labeling on the periosteal surface, suggesting periosteal remodeling as well, the virgin/normal Ca controls lacked periosteal labels and, therefore, it wasn’t possible to compare the mineral-to-matrix ratio between groups at this surface. The lack of periosteal labeling in the virgin controls is likely because the animals were relatively skeletal mature at the time of the first fluorochrome injection (~16 weeks of age), limiting the amount of bone formation at the periosteal surface. This interpretation is consistent with the lack of difference in μCT-derived total area, a measurement of the periosteal surface, at the distal femoral diaphysis in the virgin/normal calcium controls at induction and recovery. At the endocortical surface, our data suggest that primary mineralization was not affected in the lactation/low Ca group. Specifically, at the earliest tissue-age studied (4–9 days) there were no differences in the mineral-to-matrix ratio between the virgin/normal Ca and the lactation/low Ca groups. However, there was evidence of delayed secondary mineralization in the lactation/low Ca group, as evidenced by a significant tissue-age effect and interaction term, primarily attributed to lower mineral-to-matrix ratio at tissue-ages greater than 9 days in the lactation/low Ca group. This delay in the endocortical compartment may be related to the rapid bone formation rates following weaning [26, 27], as we recently demonstrated that the rapid bone formation in mice following intermittent parathyroid treatment reduced the tissue-age matched mineral-to-matrix ratio [28]. Despite this slowed secondary mineralization, the bone mineral density distribution, a global measurement impacted by the remodeling rate and the mineralization kinetics [29], was not different between groups at the end of recovery. The bone mineral density distribution measurement includes the entire cortical cross-section and, thus, is a composite of remodeling and non-remodeling tissue, presumably leading to a less sensitive measure than the tissue-age-specific measurements.
The process of matrix maturation is inherently difficult to study in adult animals. Indeed, most studies investigating matrix maturation have concentrated on the periosteal surface of the long bones of growing animals [30, 31] and animals treated with pharmacological agents [32]. Direct studies of matrix maturation in the adult skeleton are rare [15, 25, 33]. However, the present study shows that the lactation/low Ca model can be used to study bone maturation throughout the cortical compartment, including intra-cortically. The extensive quantity of tissue undergoing maturation will allow for mechanistic studies into the process of intra-cortical matrix maturation. Further, by having well-defined periods of resorption and formation (the induction and recovery phases, respectively), researchers will now be able to use the lactation/low Ca model to determine the effects of pharmacological or mechanical agents on matrix maturation independent of the effects of these agents on bone resorption.
Although, this is the first study to characterize bone matrix maturation in the lactation/low Ca model, several groups have used this model in both rats and mice to investigate the dynamics of bone remodeling [3–5, 34–39]. The model was originally reported by Ellinger et al [4] and Ruth [3] in the early 1950s. The authors in these early studies noted the remarkable bone loss induced in the lactation/low Ca, followed by recovery to near baseline bone mass when the animals were returned to a normal calcium diet after weaning. Ruth [3] was the first to note the presence of haversian-like systems following recovery, a finding that was later confirmed by de Winter et al. [5]. We similarly note the presence of haverisan-like systems. Using a combination of fluorochrome labeling, cement line staining, and polarized light microscopy, we conclude that the lactation/low rat calcium model has elevated intra-cortical remodeling and a greater number of secondary osteons. Further, although several authors have reported increase bone formation rates following weaning, limited data existed on the matrix-level recovery of the bone formed post-lactation. In the current study we determined that primary mineralization is not affected by the combination of a low calcium diet during lactation and despite evidence of a slowed secondary mineralization, the global mineralization level is comparable to virgin/controls by the end of a 30 day recovery process.
We were somewhat surprised that the virgin animals fed a normal calcium diet showed signs of active intra-cortical remodeling, albeit at a much lower incidence than lactating rats fed a low calcium diet. Thus, the commonly held viewpoint that rodents do not remodel intra-cortically should be re-evaluated. It seems more accurate to state that rodents do not normally have extensive intra-cortical remodeling and that this process can be stimulated [2].
Aberrant mineral metabolism has been shown to impact the mineralization process. Indeed, calcium [6, 40] and phosphate [41] deficiency can lead to reduced bone mineral content. To determine if mineral metabolism was normal during the recovery phase, we studied the circulating levels of ionic calcium and phosphate. Our findings show that ionic calcium levels remain unchanged compared to controls and were well within the physiological range of adult rats [42]. Although previous studies have reported reduced calcium levels at weaning in lactating animals on a low calcium diet [35], those findings were based on assessment of the total calcium content and not ionic calcium, which was measured in the current study. Phosphate levels were slightly elevated in the lactation/low Ca group early in the recovery phase, likely due to the increased bone formation because phosphate levels are known to be elevated during periods of rapid bone growth [43].
The low calcium diet alone did not induce increased cortical porosity during the 23 day induction period. Although these results seem contrary to previously published studies, it is worth noting that the animals in this experiment were relatively skeletally mature (12 weeks of age) by the time of dietary manipulation, while previous studies that noted increased porosity tend to use young rats [9, 12]. A similar study in aged rats did not show increased porosity in response to 6 weeks of dietary manipulation [44]. In the current study, our goal was to find a practical model to induce intra-cortical remodeling; therefore we matched the length of calcium deprivation to the lactation period in the lactation/low Ca group. While it is possible that increasing the length of calcium deprivation in the absence of lactation may eventually lead to increased cortical porosity, the long period of induction and possible variable time of appearance of intra-cortical porosity would render the model less useful for experimental manipulation than the lactation/low Ca model.
The study had a few limitations that will need to be addressed in subsequent follow-up experiments. The fluorochrome labeling strategy likely only captured the secondary mineralization process, as the first mineral-to-matrix measurement made in the endocrotical compartment was over 90% of the oldest tissue measured. Further, as noted above, we were only able to make a single tissue-age-specific matrix measurement in the intra-cortical compartment due to the rapid pace of infilling. Although we report that circulating calcium levels during recovery were not different than virgin controls, future studies will seek to understand how the hormones regulating mineral metabolism are impacted.
In conclusion, we demonstrate the utility of the lactation/low Ca model as a means to study intra-cortical bone matrix maturation during haversian-like remodeling in the adult rat. As the combination of lactation and low calcium increases cortical remodeling throughout cortical bone, including intra-cortically, and has no impact on early mineralization, this model represents a means to study matrix maturation during remodeling and should prove useful in developing an improved understanding of this important process.
Supplementary Material
Acknowledgments
The authors would like to thank Maleeha Mashiatulla, Meghan Moran, and Diana Goldstein for their help with the animal husbandry. The authors would like to thank Dr. Mitch Schaffler for pointing out the early publications by Ellinger et al. and Ruth. Micro-Computed Tomography data were collected at the Rush University microCT/Histology Core. Scanning electron imaging was performed at the Rush University Internal Medicine Research and Drug Discovery Imaging Core. Synchrotron Fourier Transform Infrared Microspectroscopy was collected at the Advanced Light Source at the Lawrence Berkeley National Laboratory. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR065604. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions. RDR contributed substantially to the research design, data acquisition, analysis and interpretation of the data, drafting and revising manuscript and approving the final version to be published. DRS contributed substantially to the research design, interpretation of the data, drafting and revising manuscript and approving the final version to be published. DRS is responsible for the content of the manuscript.
References
- 1.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- 2.Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001;1:193–207. [PubMed] [Google Scholar]
- 3.Ruth EB. Bone studies. II. An experimental study of the Haversian-type vascular channels. Am J Anat. 1953;93:429–455. doi: 10.1002/aja.1000930306. [DOI] [PubMed] [Google Scholar]
- 4.Ellinger GM, Duckworth J, Dalgarno AC, Quenouille MH. Skeletal changes during pregnancy and lactation in the rat: effect of different levels of dietary calcium. British Journal of Nutrition. 1952;6:235–253. doi: 10.1079/bjn19520027. [DOI] [PubMed] [Google Scholar]
- 5.de Winter FR, Steendijk R. The effect of a low-calcium diet in lactating rats; observations on the rapid development and repair of osteoporosis. Calcif Tissue Res. 1975;17:303–316. doi: 10.1007/BF02546602. [DOI] [PubMed] [Google Scholar]
- 6.Stauffer M, Baylink D, Wergedal J, Rich C. Decreased bone formation, mineralization, and enhanced resorption in calcium-deficient rats. Am J Physiol. 1973;225:269–276. doi: 10.1152/ajplegacy.1973.225.2.269. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer M, Baylink D, Wergedal J, Rich C. Bone repletion in calcium deficient rats fed a high calcium diet. Calcif Tissue Res. 1972;9:163–172. doi: 10.1007/BF02061954. [DOI] [PubMed] [Google Scholar]
- 8.Sissons HA, Kelman GJ, Marotti G. Mechanisms of bone resorption in calcium-deficient rats. Calcif Tissue Int. 1984;36:711–721. doi: 10.1007/BF02405394. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Hayakawa D, Emura S, Ozawa Y, Okumura T, Shoumura S. Effect of low or high dietary calcium on the morphology of the rat femur. Histology & Histopathology. 2002;17:1129–1135. doi: 10.14670/HH-17.1129. [DOI] [PubMed] [Google Scholar]
- 10.Seto H, Aoki K, Kasugai S, Ohya K. Trabecular bone turnover, bone marrow cell development, and gene expression of bone matrix proteins after low calcium feeding in rats. Bone. 1999;25:687–695. doi: 10.1016/s8756-3282(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 11.Geng W, DeMoss DL, Wright GL. Effect of calcium stress on the skeleton mass of intact and ovariectomized rats. Life Sci. 2000;66:2309–2321. doi: 10.1016/s0024-3205(00)00562-2. [DOI] [PubMed] [Google Scholar]
- 12.Drivdahl RH, Liu CC, Baylink DJ. Regulation of bone repletion in rats subjected to varying low-calcium stress. Am J Physiol. 1984;246:R190–R196. doi: 10.1152/ajpregu.1984.246.2.R190. [DOI] [PubMed] [Google Scholar]
- 13.Martiniakova M, Chovancova H, Omelka R, Grosskopf B, Toman R. Effects of a single intraperitoneal administration of cadmium on femoral bone structure in male rats. Acta Vet Scand. 2011;53:49. doi: 10.1186/1751-0147-53-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duranova H, Martiniakova M, Omelka R, Grosskopf B, Bobonova I, Toman R. Changes in compact bone microstructure of rats subchronically exposed to cadmium. Acta Vet Scand. 2014;56:64. doi: 10.1186/s13028-014-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross RD, Edwards LH, Acerbo AS, Ominsky MS, Virdi AS, Sena K, Miller LM, Sumner DR. Bone matrix quality following sclerostin antibody treatment. Journal of Bone and Mineral Research. 2014;29:1597–1607. doi: 10.1002/jbmr.2188. [DOI] [PubMed] [Google Scholar]
- 16.Acerbo AS, Carr GL, Judex S, Miller LM. Imaging the material properties of bone specimens using reflection-based infrared microspectroscopy. Analytical Chemistry. 2012;84:3607–3613. doi: 10.1021/ac203375d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne DL, Curtis J. A protocol for the staining of cement lines in adult human bone using toluidine blue. The Journal of Histotechnology. 2005;28:73–79. [Google Scholar]
- 18.Boskey AL. Matrix proteins and mineralization: an overview. Connective Tissue Research. 1996;35:357–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 19.Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci. 2012;17:1891–1903. doi: 10.2741/4026. [DOI] [PubMed] [Google Scholar]
- 20.Marotti G, Favia A, Zallone AZ. Quantitative analysis on the rate of secondary bone mineralization. Calcif Tissue Res. 1972;10:67–81. doi: 10.1007/BF02012537. [DOI] [PubMed] [Google Scholar]
- 21.Boivin G, Meunier PJ. Methodological considerations in measurement of bone mineral content. Osteoporosis International. 2003;14(Suppl 5):S22–S27. doi: 10.1007/s00198-003-1469-1. [DOI] [PubMed] [Google Scholar]
- 22.Boivin G, Meunier PJ. Changes in bone remodeling rate influence the degree of mineralization of bone. Connective Tissue Research. 2002;43:535–537. doi: 10.1080/03008200290000934. [DOI] [PubMed] [Google Scholar]
- 23.Bala Y, Farlay D, Delmas PD, Meunier PJ, Boivin G. Time sequence of secondary mineralization and microhardness in cortical and cancellous bone from ewes. Bone. 2010;46:1204–1212. doi: 10.1016/j.bone.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18:1012–1019. doi: 10.1359/jbmr.2003.18.6.1012. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs RK, Allen MR, Ruppel ME, Diab T, Phipps RJ, Miller LM, Burr DB. In situ examination of the time-course for secondary mineralization of Haversian bone using synchrotron Fourier transform infrared microspectroscopy. Matrix Biol. 2008;27:34–41. doi: 10.1016/j.matbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Bowman BM, Siska CC, Miller SC. Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res. 2002;17:1954–1960. doi: 10.1359/jbmr.2002.17.11.1954. [DOI] [PubMed] [Google Scholar]
- 27.Miller SC, Bowman BM. Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:143–149. doi: 10.1002/ar.a.10138. [DOI] [PubMed] [Google Scholar]
- 28.Ross RD, Mashiatulla M, Robling AG, Miller LM, Sumner DR. Bone matrix composition following PTH treatment is not dependent on sclerostin status. Calcif Tissue Int. 2015 doi: 10.1007/s00223-015-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Wergedal JE, Baylink DJ. Electron microprobe measurements of bone mineralization rate in vivo. Am J Physiol. 1974;226:345–352. doi: 10.1152/ajplegacy.1974.226.2.345. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly E, Boskey AL, Baker SP, van der Meulen MC. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A. 2010;92:1048–1056. doi: 10.1002/jbm.a.32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40:1308–1319. doi: 10.1016/j.bone.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs RK, Faillace ME, Allen MR, Phipps RJ, Miller LM, Burr DB. Bisphosphonates do not alter the rate of secondary mineralization. Bone. 2011;49:701–705. doi: 10.1016/j.bone.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen P. Calcium deficiency, pregnancy, and lactation in rats. Microscopic and microradiographic observations on bones. Calcif Tissue Res. 1977;23:95–102. doi: 10.1007/BF02012772. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen P. Calcium deficiency, pregnancy, and lactation in rats. Some effects on blood chemistry and the skeleton. Calcif Tissue Res. 1977;23:87–94. doi: 10.1007/BF02012771. [DOI] [PubMed] [Google Scholar]
- 36.Wong KM, Singer L, Ophaug RH, Klein L. Effect of lactation and calcium deficiency, and of fluoride intake, on bone turnover in rats: isotopic measurements of bone resorption and formation. Journal of Nutrition. 1981;111:1848–1854. doi: 10.1093/jn/111.10.1848. [DOI] [PubMed] [Google Scholar]
- 37.Brommage R, DeLuca HF. Regulation of bone mineral loss during lactation. Am J Physiol. 1985;248:E182–E187. doi: 10.1152/ajpendo.1985.248.2.E182. [DOI] [PubMed] [Google Scholar]
- 38.Lozupone E, Favia A. Distribution of resorption processes in the compacta and spongiosa of bones from lactating rats fed a low-calcium diet. Bone. 1988;9:215–224. doi: 10.1016/8756-3282(88)90034-8. [DOI] [PubMed] [Google Scholar]
- 39.Gruber HE, Stover SJ. Maternal and weanling bone: the influence of lowered calcium intake and maternal dietary history. Bone. 1994;15:167–176. doi: 10.1016/8756-3282(94)90704-8. [DOI] [PubMed] [Google Scholar]
- 40.Harrison M, Fraser R. Bone structure and metabolism in calcium-deficient rats. J Endocrinol. 1960;21:197–205. doi: 10.1677/joe.0.0210197. [DOI] [PubMed] [Google Scholar]
- 41.Cuisnier-Gleizes P, Thomasset M, Sainteny-Debove F, Mathieu H. Phosphorus deficiency, parathyroid hormone and bone resorption in the growing rat. Calcif Tissue Res. 1976;20:235–249. doi: 10.1007/BF02546412. [DOI] [PubMed] [Google Scholar]
- 42.Sharp PE, LaRegina MC. The Laboratory Rat. CRC Press LLC; 1998. [Google Scholar]
- 43.Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27:2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C, Park D. The effect of restriction of dietary calcium on trabecular and cortical bone mineral density in the rats. J Exerc Nutrition Biochem. 2013;17:123–131. doi: 10.5717/jenb.2013.17.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


