Abstract
Human papillomavirus (HPV)-negative cervical carcinomas are uncommon and typically encompass unusual histologic subtypes. Mesonephric adenocarcinoma is one such subtype. Mesonephric tumors in the female genital tract are thought to arise from Wolffian remnants, and are extremely rare tumors with widely variable morphology. Sarcomatoid dedifferentiation has been previously described in a few cases, but other forms of dedifferentiation have not been reported. Neuroendocrine carcinoma of the cervix (e.g. small cell carcinoma) is associated with HPV infection, typically HPV 18. These tumors often arise in association with a conventional epithelial component such as squamous cell carcinoma or usual-type endocervical adenocarcinoma. We describe a case of mesonephric adenocarcinoma of the uterine cervix associated with an HPV-negative high-grade neuroendocrine carcinoma at the morphologic and immunophenotypic level, for which we performed targeted massively parallel sequencing analysis of the two elements. Both components shared identical mutations in U2AF1 p.R156H (c.467G>A) and GATA3 p.M422fs (c.1263dupG), as well as MYCN amplification. In addition, the neuroendocrine carcinoma harbored TP53 and MST1R mutations not present in the mesonephric carcinoma. Our data suggest a clonal origin of the two components of this rare entity, rather than a collision tumor.
Keywords: Mesonephric, Neuroendocrine, Human papillomavirus, Uterine cervix, Molecular
INTRODUCTION
The role of human papillomavirus (HPV) in the etiopathogenesis of cervical carcinoma is underscored by the extremely high percentage of HPV-positive cervical carcinomas (up to 99.7% in some studies) (1). This, in turn, is affected by the histologic tumor types evaluated. HPV-associated cervical carcinomas are overrepresented by squamous cell carcinomas. Adenocarcinomas (including adenosquamous carcinomas) are the second most common histologic type linked to HPV infection, specifically usual-type endocervical, mucinous intestinal and villoglandular subtypes, followed by high-grade neuroendocrine carcinomas (HGNEC). Non-HPV-associated cervical carcinomas are rare, and predominantly comprise unusual adenocarcinoma variants: mesonephric adenocarcinoma, minimal deviation/gastric-type mucinous adenocarcinoma, and clear cell carcinoma (2, 3).
Among non-HPV-related carcinomas of the uterine cervix, mesonephric carcinomas are extremely rare. These tumors arise from mesonephric remnants and hyperplasia, embryonic vestiges of the mesonephric (or Wolffian) duct in the female genital tract and their benign proliferative counterpart, respectively. These tumors are characterized by a wide morphologic array and have been reported to occasionally harbor a sarcomatoid component (carcinosarcoma or mixed malignant mesonephric tumor) (4–7). However, other forms of dedifferentiation have not been described to date. Here we describe a case of an HPV-negative mixed mesonephric adenocarcinoma and HGNEC of the uterine cervix, a previously unreported entity, and discuss its clinical, morphologic and molecular features.
MATERIALS AND METHODS
Immunohistochemistry (IHC)
Automated IHC using Ventana Benchmark Ultra (Ventana Medical Systems, Tucson, AZ) and Leica Bond III (Leica, Buffalo Grove, IL) was performed on formalin-fixed paraffin-embedded (FFPE) tissue sectioned at 4 μm for the following antibodies: cytokeratin 18, epithelial membrane antigen (EMA), estrogen receptor (ER), progesterone receptor (PR), p16, chromogranin, synaptophysin, CD56, calretinin, GATA-3, PAX8, CD10, TIF-1, and S100. Their clones, dilutions and respective vendors are specified in Table 1. Either Optiview or iView (Ventana Medical Systems) or Bond polymer refine (Leica) were used as detection systems, depending on the antibody. Positive and negative controls were performed and evaluated for all reactions.
Table 1.
Immunohistochemistry antibodies
| ANTIBODY | CLONE | VENDOR | DILUTION |
|---|---|---|---|
| Calretinin | SP65 | VENTANA (Tucson, AZ) | RTU |
| CD10 | SP67 | VENTANA | RTU |
| CD56 | MRQ42 | CELL MARQUE (Rocklin, CA) | RTU |
|
| |||
| CK18 | DC10 | DAKO (Carpinteria, CA) | 1:1000 |
|
| |||
| Chromogranin A | LK2H10 | VENTANA | RTU |
|
| |||
| EMA | 43B2 | VENTANA | RTU |
|
| |||
| ER | 6F11 | LEICA (Buffalo Grove, IL) | RTU |
|
| |||
| GATA-3 | L50.823 | BIO CARE (Concord, CA) | 1:200 |
|
| |||
| PR | 16 | LEICA | RTU |
|
| |||
| PAX8 | Polyclonal | PROTEINTECH (Chicago, IL) | 1:100 |
|
| |||
| P16 | E6H4 | VENTANA | RTU |
|
| |||
| S100 | Polyclonal | DAKO | 1:8000 |
|
| |||
| TTF-1 | 8G7G3/1 | VENTANA | RTU |
Abbreviations: CK18, Cytokeratin 18; EMA, Epithelial membrane antigen; ER, estrogen receptor; PR, progesterone receptor; RTU, ready to use
HPV in situ hybridization
Automated Chromogenic in situ Hybridization (CISH) (Ventana Benchmark XT, Ventana Medical Systems) using probes against high-risk HPV types 16, 18, 31, 33 and 51 (PATHO-GENE, ENZO Life Sciences Inc., Farmingdale, NY) was performed on representative 4 μm thick FFPE sections. External positive (showing discrete dot-like stippled nuclear labeling) and negative controls were used as appropriate.
HPV polymerase chain reaction (PCR)
DNA extraction
Representative 8 μm-thick FFPE sections were subjected to microdissection of the mesonephric and HGNEC components by a pathologist (AMS) using a sterile needle under a stereomicroscope (Olympus SZ61, Center Valley, PA) (8). DNA was extracted separately from each component using the DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD) and quantified using the Qubit Fluorometer (Invitrogen, Life Technologies, Grand Island, NY).
HPV detection
Primers for the most common HPV types in cervical cancer (i.e. HPV 16 and HPV 18) were designed. To investigate the HPV status for each tumor component, standard PCR was performed using the Amplitaq Gold 360 DNA Polymerase (Applied Biosystems, Life Technologies) following the manufacturer’s protocol. In brief, forward and reverse primers (HPV 16 Forward GTACTGCAAGCAACAGTTACTGCGACGT, Reverse CGACCGGTCCACCGACCCCT; HPV 18 Forward AACCTGTGTATATTGCAAGACAGTATTGGAACTTACA, Reverse GATTCAACGGTTTCTGGCACCGC) were used to amplify amplicons of 312 bp (HPV 16) and 251 bp (HPV 18) in the stable domains of HPV. DNA from the HPV 16-positive CaSki and HPV 18-positive HeLa cell lines were used as positive controls (9).
Targeted Next-Generation Sequencing
After review of the hematoxylin & eosin (H&E) slides, macrodissection was performed on unstained FFPE sections. Tumor DNA from each component (neuroendocrine carcinoma and mesonephric adenocarcinoma) was extracted separately from areas with no intermixing of tumor cells. The two tumor DNA samples and matched normal DNA obtained from peripheral blood were assessed for the presence of somatic mutations and copy number alterations in 410 key cancer-associated genes (Supplementary Material) using solution-phase exon hybridization capture and next-generation sequencing (MSK-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT)) (10). In brief, bar-coded sequences were prepared and captured by hybridization with custom biotinylated DNA probes for all exons and selected introns of 410 oncogenes and tumor suppressor genes using 100–250 ng of input DNA. Captured libraries were sequenced on the Illumina HiSeq2500 platform (Illumina, San Diego, CA) (2 × 100 bp paired-end reads). Reads were aligned to the human genome (hg19) using BWA-MEM (v 0.7.5a); duplicate read removal, base recalibration, and insertion and deletions (indel) realignment using GATK (v 2.6–5), following best practices; variant calling using MuTect (v 1.1.4) for single nucleotide variants; and Somatic Indel Detector (GATK 2.3–9) for indels. Threshold for variant calling was set at 2% for known hotspot mutations, which were considered as high-confidence calls, and 5% for other variants, similar to previous description (10). Annovar was used to annotate the variants for cDNA and amino acid changes as well as presence in dbSNP database (v137) and COSMIC database (v68) and 1000 Genomes minor allele frequencies. Copy number variation calling was performed by comparing sequencing coverage, adjusted for GC content at different gene regions relative to diploid normal control samples. Because DNA is extracted from samples with a mixture of tumor and normal cells, the degree of gene amplification or loss detected is proportional to tumor purity.
Fluorescence In Situ Hybridization (FISH)
FISH analysis was performed separately on each component on FFPE tissue, using commercially available locus-specific and centromeric probes: LSI N-MYC (2p24)/ CEP 2 (2p11.1-q11.1) (Abbott Molecular, Abbott Park, IL). FFPE tumor sections (4 μm) were pretreated by de-paraffinizing in xylene and dehydrating in ethanol. Dual-color FISH was performed according to Abbott Molecular protocol for FFPE sections, with a few minor modifications. FISH analysis and signal capture were performed using fluorescence microscopes (Axio; Carl Zeiss AG, Jena, Germany) coupled with an ISIS FISH Imaging System (MetaSystems GmbH, Altlussheim, Germany). Two hundred interphase nuclei were evaluated for each tumor specimen. The ratio of Spectrum Green (LSI N-MYC) to Spectrum Orange (CEP 2) signals was determined per nucleus. The percentage of cells showing a ratio greater than 2 was also determined. Nuclei of normal cells present outside the area of interest were used as a normal control (2:2 ratio).
CASE DESCRIPTION
A 59-year-old female patient presented with a 12-day history of postmenopausal bleeding. Her last Pap smear had been 4 years ago and revealed no abnormalities. Her pelvic exam was normal. A sonohysterogram showed a 6.3 cm uterus with a 1.3 mm endometrial stripe and polypoid tissue at the cervical os. Endometrial biopsy revealed a poorly differentiated carcinoma with neuroendocrine features. On PET-CT performed at presentation, a hypermetabolic low-attenuation lesion in the cervix was seen, measuring approximately 2.3 × 1.7 cm (SUV 11.1), with no evidence of regional or distant metastatic disease. The patient underwent robotic radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymph node dissection. Intraoperatively, tissue protruding from the external cervical os was noted, and sampled separately. The gross and microscopic findings of the preoperative biopsy and surgical specimen, as well as the results of ancillary studies, are described below.
Gross findings
The uterus weighed 86.5 g. The cervix measured 3.4 cm in length by 3.8 cm in diameter and showed a smooth ectocervix with marked hemorrhage surrounding the os. Sectioning revealed a 3.6 × 3.5 × 1.5 cm white-gray firm tumor involving the left posterior cervix. The tumor had a gross depth of invasion of 1.5 cm, and extended into the left parametrium. The endometrium, myometrium and bilateral adnexa were grossly unremarkable, except for the presence of two simple cysts in the right ovary. The lesion protruding from the cervical os was received separately in multiple pieces measuring 3.5 × 3.0 × 1.0 cm in aggregate, and composed of tan-brown soft tissue.
Microscopic and immunohistochemical findings
The initial endometrial biopsy demonstrated solid sheets of round, intermediate-sized, discohesive cells with open chromatin and prominent nucleoli (single or multiple), as well as numerous mitotic figures and apoptotic bodies (Figure 1). No other component was seen on the biopsy specimen. The tumor undermined the epithelium of the lower uterine segment, which showed reactive/metaplastic changes but was otherwise unremarkable. IHC demonstrated rare EMA-positive tumor cells, whereas CK18 was entirely negative. Synaptophysin and CD56 were both diffusely and strongly positive; chromogranin was patchy, as was S100 protein. The findings were consistent with HGNEC. Identical morphologic findings were seen in the tumor protruding from the cervical os, sent at the time of surgery, and in the most superficial portions of tumor in the hysterectomy specimen.
Figure 1. High-grade neuroendocrine component.
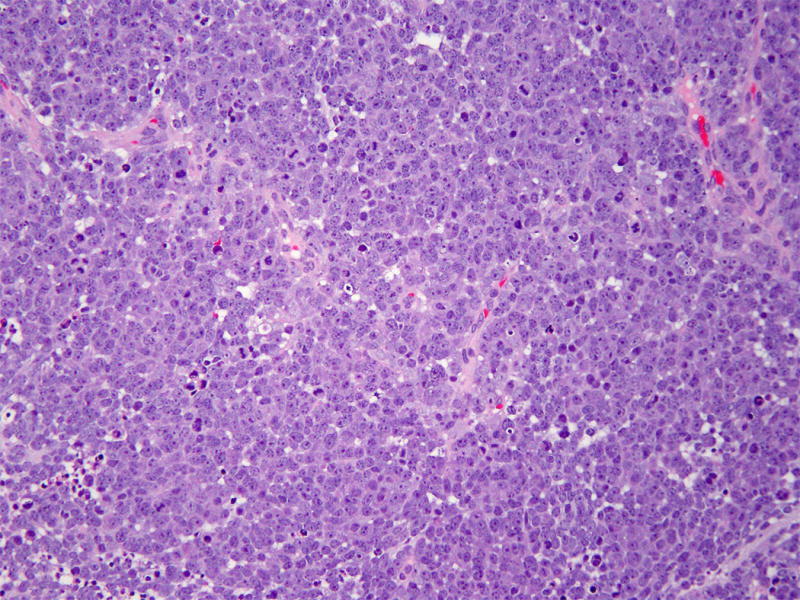
High-grade neuroendocrine component with intermediate-sized cells arranged in solid sheets. The cells have scant cytoplasm and prominent nucleoli in addition to brisk mitotic activity and abundant apoptotic bodies. (Hematoxylin and eosin, 200X)
The resection specimen revealed a second distinct component, deep to the previously described neuroendocrine carcinoma. This second component was predominantly composed of small tubules, most of which were lined by a single layer of cuboidal cells with scant, pale eosinophilic cytoplasm, round-to-oval nuclei with fine chromatin, and small, conspicuous nucleoli. Up to 8 mitoses per 10 high-power fields (HGF) were detected. Small foci showed cystically dilated tubules (Figure 2). When present, intraluminal contents varied from eosinophilic hyaline material to necrotic with cellular debris, the latter being more common (Figure 3). Towards the center of the tumor, the tubules were back-to-back and at times coalesced; the periphery of the tumor tended to show more dispersed tubules with an infiltrative appearance. As a minor component of the neoplasm, a ductal pattern was also present. This was characterized by larger and elongated glands with irregular contours and intraluminal papillary formations. Multifocally, the glands/tubules displayed slightly taller cells with pseudostratified nuclei, reminiscent of endometrioid glands (Figure 3). A minimal but noticeable stromal response associated with most of the tubular component was observed, as well as moderate lymphocytic infiltrate. The two major tumor components (superficial solid and deep tubular) intermingled in several areas of juxtaposition; however, obvious merging of these components was not seen (Figure 4).
Figure 2. Mesonephric adenocarcinoma component.
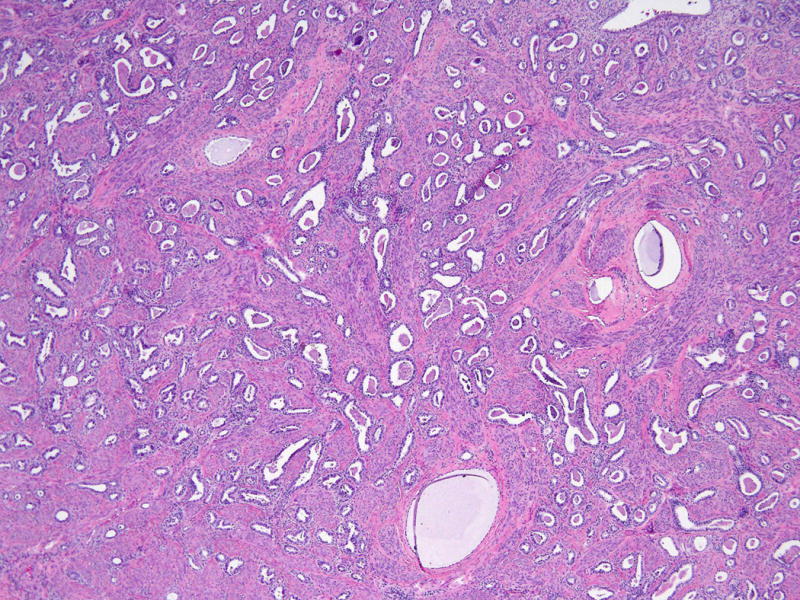
Mesonephric adenocarcinoma component composed of tubules and duct-like structures filled with eosinophilic secretions. Occasional cystic dilatation of the tubules is seen. (Hematoxylin and eosin, 100X)
Figure 3. Different morphologic patterns of mesonephric component.
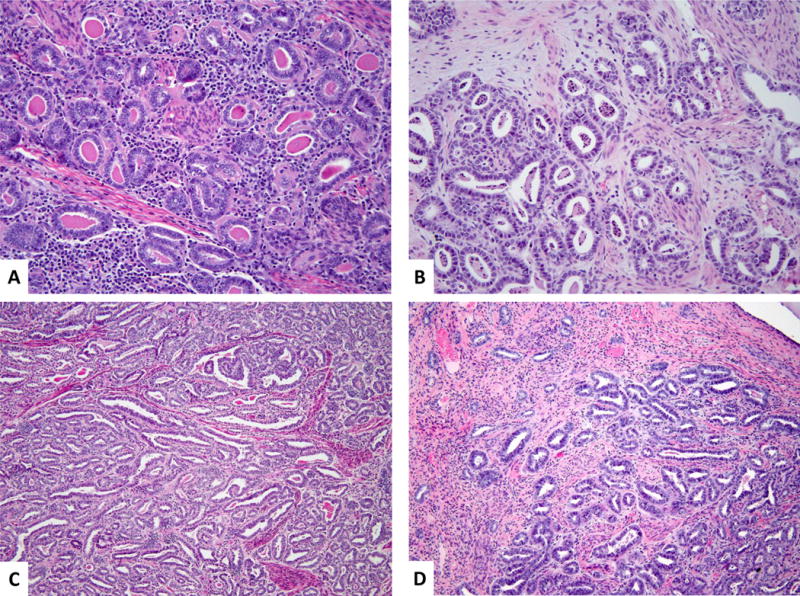
Higher magnification shows small tubules lined by cuboidal to low columnar cells with conspicuous nucleoli. The intraluminal contents were either densely eosinophilic (A) or composed of necrotic cellular debris (B). Note the mild chronic peritumoral inflammatory infiltrate (A) and the subtle myxoid/edematous stromal changes (B). Areas with elongated, cleft-like spaces and occasional intraluminal papillary formations (C) and foci displaying cytomorphologic features reminiscent of endometrioid differentiation characterized by tubules with taller cells and pseudostratified nuclei (D) compared to the classic mesonephric component in the upper left corner of the image. (Hematoxylin and eosin, 400X and 200X)
Figure 4. Juxtaposition of high-grade neuroendocrine and mesonephric carcinomas.
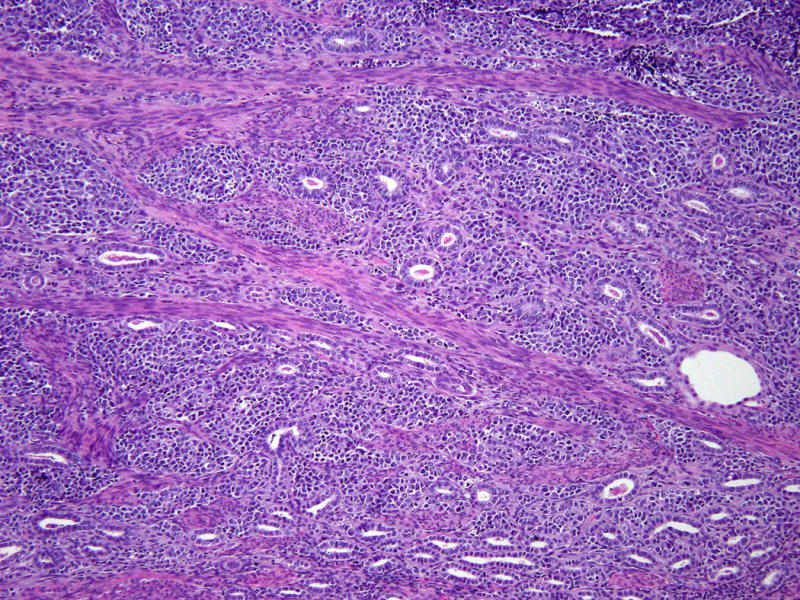
There is intermingling of both components, but they are clearly distinct from one another. (Hematoxylin and eosin, 200X)
Also identified were a few foci of mesonephric glands, which were subtly different from the remainder of the adenocarcinoma. These were small, measuring up to 1.5 mm and characterized by closely packed tubules, some in a lobular arrangement, with bland cytologic features. These were located at the periphery of the tumor, situated deeply in the cervical wall or intermixed with tumor, showing smaller nuclei (tumor nuclei were 1.5–2 x larger) and inconspicuous nucleoli. Mitotic figures were not readily identifiable in these areas. The tubules did not display intraluminal content or dense hyaline material; no necrotic debris was seen (Figure 5).
Figure 5. Small lobules of mesonephric tubules with bland cytologic features.
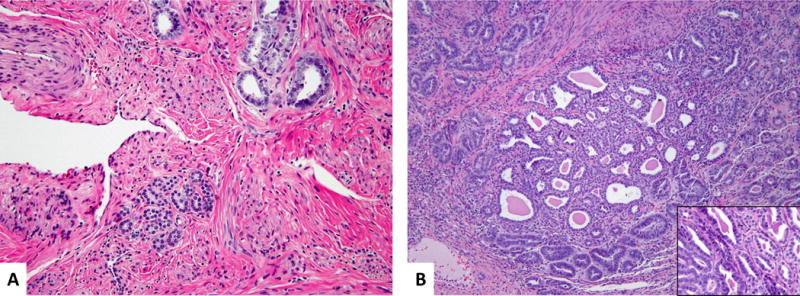
Small lobules of mesonephric tubules showing more bland cytologic features present in the deep cervical wall (A) and intermixed with the bulk of the tumor (B). Compare the cytology of these areas to the adjacent mesonephric adenocarcinoma in the upper right corner (A) and left bottom corner of inset (B). (Hematoxylin and eosin, 200X and 400X)
As previously noted, the intramural portion of the tumor was composed of the mesonephric carcinoma extending close to the endocervical surface, while the neuroendocrine carcinoma emerged from the superficial cervical stroma and formed an exophytic polypoid lesion protruding into the endocervical canal (Figure 6). The tumor extended distally to involve the subepithelial stroma of the ectocervix; proximally to involve the posterior lower uterine segment; and radially penetrated into the entire thickness of the cervical wall, with a maximum stromal invasion of 15 mm. Both ecto- and endocervical epithelia were unremarkable. No vascular or perineural invasion was identified. Surgical margins and all submitted pelvic lymph nodes (10 in total) were negative. The uterine corpus and bilateral adnexa showed no significant pathologic abnormalities. Final FIGO stage was IB1.
Figure 6. Polypoid tumor protruding into endocervical canal.
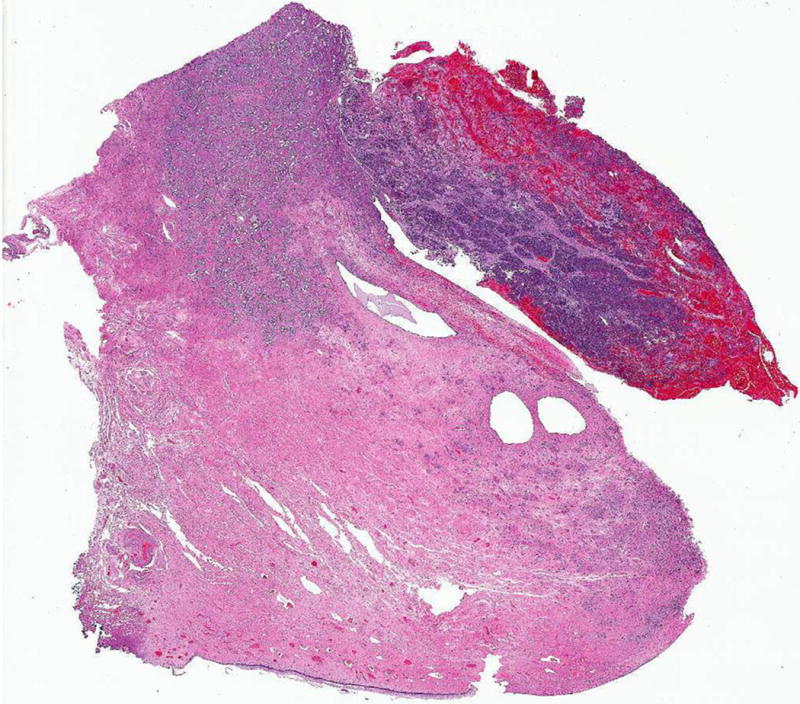
Polypoid tumor protruding into the endocervical canal formed by the high-grade neuroendocrine carcinoma, and deeper tumor infiltrating into the cervical wall composed of the mesonephric component. (Hematoxylin and eosin, 10X)
Immunohistochemically, the neuroendocrine component demonstrated diffuse and strong positivity for synaptophysin, CD56, calretinin and p16, and focal positivity for chromogranin, PAX8 (weak) and GATA-3. ER, PR, CD10, and TIF-1 were negative. However, the mesonephric component was diffusely positive for PAX8 and showed luminal CD10 staining; p16 was patchy positive, as was GATA-3. Calretinin and chromogranin revealed no more than 5% reactivity. Of note, chromogranin showed a cytoplasmic pattern staining of a few individual tubules entirely, among which scattered cells stained more strongly. ER, PR, synaptophysin, CD56, and TIF-1 were negative (Figure 7).
Figure 7. Immunohistochemistry results.
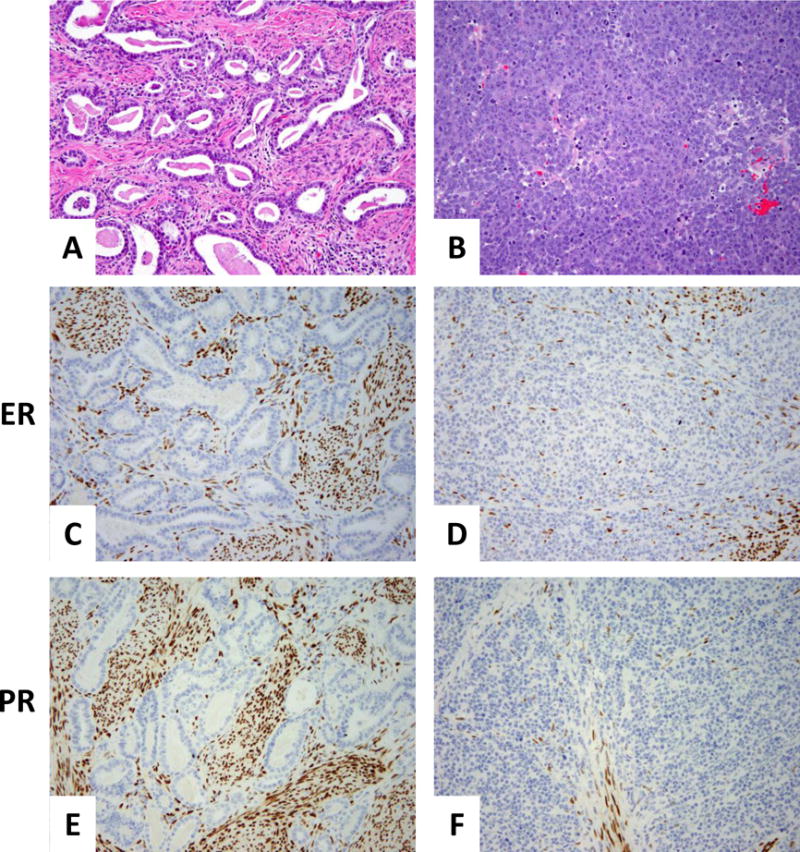
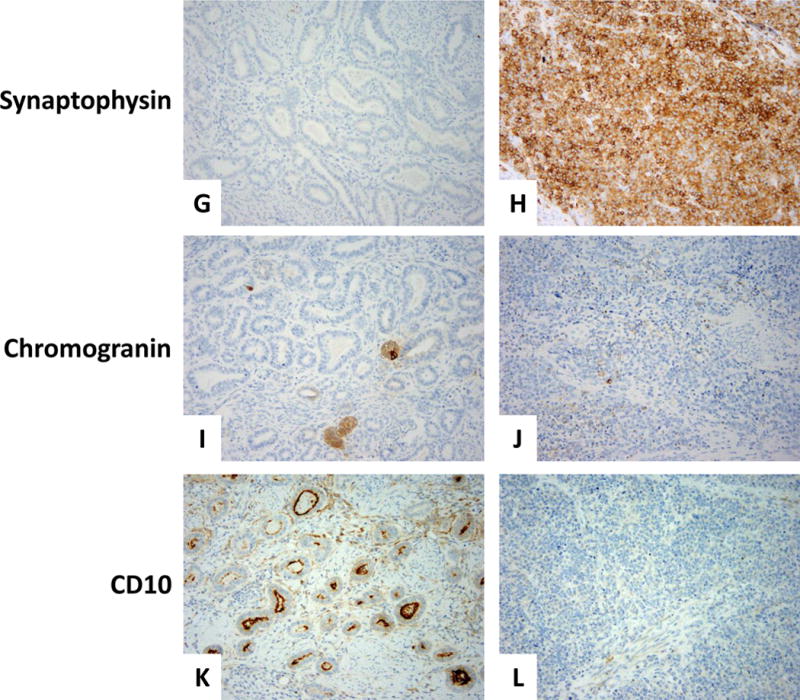
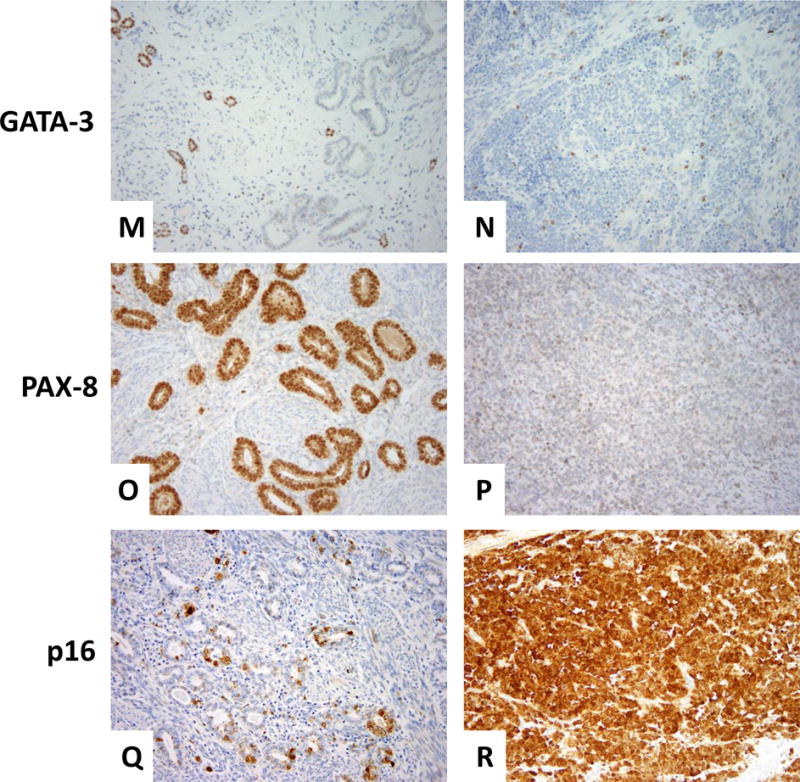
Mesonephric component (A) showing negative ER (C), PR (E), and synaptophysin (G). Rare tubules were chromogranin-positive (I) and CD10 showed the typical intraluminal pattern of staining (K). Patchy positivity for GATA-3 was seen mainly in the peripheral areas of the tumor (M); diffuse positivity for PAX-8 (O). P16 positivity was focal (Q). The neuroendocrine component (B) was negative for ER and PR (D and F, respectively), whereas synaptophysin was strong and diffuse (H). Chromogranin was focal (J); CD10 was entirely negative (L). GATA-3 and PAX-8 both showed weak, focal staining (N and P, respectively). P16 was diffusely and strongly positive (R).
HPV studies
In situ hybridization for high-risk HPV subtypes and PCR for HPV 16 and 18 showed that both the mesonephric and the HGNEC were HPV-negative (Figure 8).
Figure 8. HPV 16 and HPV 18 PCR results.
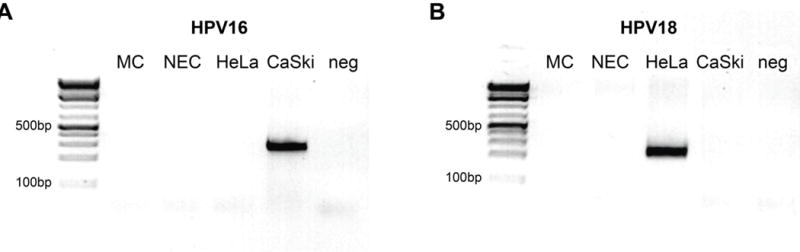
Both mesonephric and neuroendocrine components were negative for HPV 16 (A) and HPV 18 (B) subtypes. Compare to positive controls for HPV 16 and 18 (CaSki and HeLa, respectively).
MC, mesonephric adenocarcinoma component; NEC, neuroendocrine carcinoma component; HeLa, HPV 18-positive cervical carcinoma cell line; CaSki, HPV 16-positive cervical carcinoma cell line; neg, negative control
Molecular characterization
Based on morphologic assessment, the mesonephric and neuroendocrine carcinomas were estimated to have 10% and 80% tumor purity, respectively. High-depth targeted next-generation sequencing with a mean coverage of 749X and 702X in the mesonephric and the neuroendocrine portions, respectively, revealed amplification of the MYCN gene (2p24.3) in both components. Both tumors shared a somatic missense mutation in exon 6 of U2AF1 (p.R156H; c.467G>A), as well as a single nucleotide duplication resulting in frameshift in exon 6 of GATA3 (p.M422fs; c. 1263dupG). Interestingly, however, an FBXW7 (p.R465C; c. 1393C>T) exon 9 missense mutation was found to be restricted to the mesonephric carcinoma, while the neuroendocrine component harbored an IRS2 (13q34) amplification and mutations in TP53 (exon 7 p.G244S; c.730G>A) and MST1R (exon 12 p.V935I; c.2803G>A) which were not present in the mesonephric tumor. The variant frequencies ranged from 5–7% in the mesonephric portion and 40–54% in the neuroendocrine portion. No structural alterations, including large gene deletion, duplication, inversion, or translocation were detected in either component. It should be noted, however, that the low tumor purity in the mesonephric carcinoma limited the detection of variants that might be present at frequencies below the variant calling threshold (2% for known hotspot mutations, 5% for other variants) (Figure 9).
Figure 9. Non-synonymous somatic mutations and amplifications.
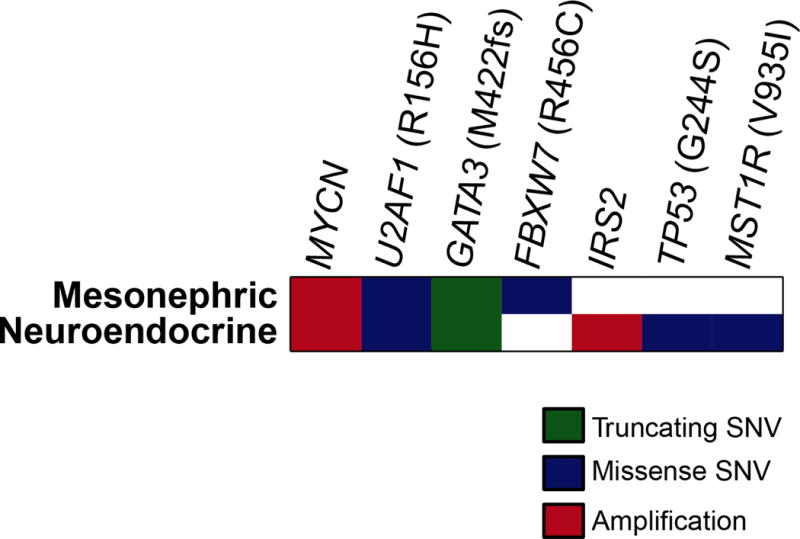
Non-synonymous somatic mutations and amplifications were identified in the mesonephric carcinoma and HGNEC components using targeted next-generation sequencing. Each row represents one tumor component; altered genes are reported in columns. Alteration types are color-coded according to the legend.
SNV, Single Nucleotide Variant
FISH results
Both mesonephric and neuroendocrine carcinomas showed amplification of MYCN gene. The green signals corresponding to the LSI MYCN probe were too numerous to count in both components; thus, a formal ratio of LSI:CEP signals was not determined (Figure 10).
Figure 10. FISH on mesonephric and neuroendocrine components.
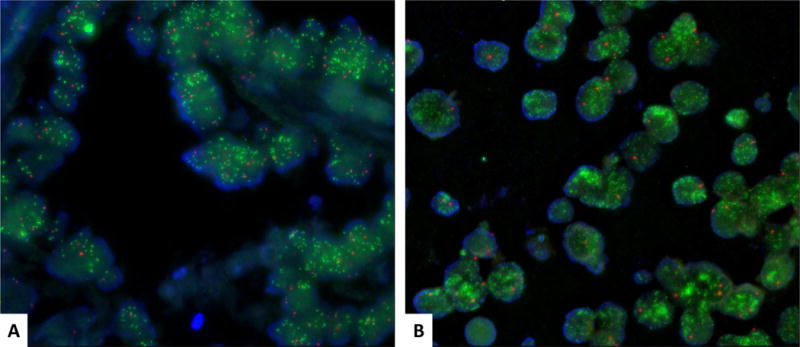
FISH on the mesonephric and neuroendocrine components showed the mesonephric component (A) and the neuroendocrine component (B). Both demonstrated high level amplification of MYCN gene in 96% and 100% of the analyzed cells, respectively. The number of green signals was too numerous to count; therefore, a formal ratio of LSI:CEP signals was not determined. Green represents the LSI MYCN probe. Red represents the chromosome 2 centromeric probe.
FISH, Fluorescence in situ Hybridization; LSI, Locus-Specific Identifier; CEP, Chromosome Enumeration Probe
Follow-up
The immediate postoperative course was uneventful. However, three months later the patient presented with acute renal failure and lower back pain. Imaging studies revealed recurrent pelvic disease. Whole pelvis radiation therapy with nodal boost was initiated, with subsequent addition of chemotherapy (carboplatin and etoposide). The patient had an initial response to treatment, but after three cycles her disease progressed in the form of peritoneal carcinomatosis. The chemotherapeutic regimen was switched to paclitaxel. Despite that, the patient continued to show worsening peritoneal carcinomatosis and died of disease 14 months after initial diagnosis.
DISCUSSION
Mesonephric adenocarcinomas are considered to be the malignant transformation of mesonephric remnants. In early embryologic development, during the sexually indifferent stage, both mesonephric and paramesonephric ducts are present and run parallel to one another, the former being the most lateral structure distally. The mesonephric ducts (also known as Wolffian ducts) are the embryologic structures that give rise to the epididymis, vas deferens, seminal vesicles and ejaculatory ducts in males. In females, the internal genital system is mainly derived from the paramesonephric or Müllerian ducts and, in the absence of testosterone, the mesonephric ducts regress (11). Nonetheless, small remnants may persist anywhere along their trajectory: broad ligaments, lateral uterine and cervical walls, and vagina, the most common sites being the cervix and ovarian hilum (12).
The frequency of persistent mesonephric duct remnants varies from less than 1% to 22% in adults, and up to 40% in children (12, 13). Despite their relatively high frequency, malignant transformation is rare. Fewer than 100 cases of mesonephric adenocarcinomas have been reported in the English literature thus far, usually as case reports or small series. Remnants are small lesions composed of tubules arranged in well-circumscribed lobules with or without a central duct, usually embedded deep within the cervical wall. The tubules are typically lined by a single layer of cuboidal cells with scant cytoplasm and indistinct cell borders, often containing dense eosinophilic intraluminal material. Mesonephric hyperplasia is classified as lobular or diffuse. The distinction between remnants and lobular hyperplasia is somewhat arbitrary; it is mainly based on size and contour of the lobules and, to a lesser degree, on the variation in size and shape of individual tubules. Diffuse mesonephric hyperplasia is characterized by an extensive proliferation of evenly spaced tubules (12). Overall, both remnants and hyperplasia show bland cytology, and no or minimal mitotic activity. Stromal response surrounding the tubules is not typically seen. Tubules lined by endometrioid-like glands can be found in either scenario. In our case, a few tubules showing bland cytology were observed, some of which were located in the periphery of the tumor, deep in the cervical wall; these most likely represent benign remnants. Other tubules with similar cytologic features were seen within the tumor, composed of tightly packed glands; the interpretation of these as remnants or hyperplasia is hindered by their back-to-back arrangement and location in the cervical wall, and would therefore be speculative.
Mesonephric adenocarcinomas are thought to arise from these benign remnants and hyperplastic lesions. In fact, many of the carcinoma cases reported in the literature have been shown to have an accompanying benign component intimately associated with the malignant glands (4, 6, 12, 14). Compared to their benign precursors, the malignant counterparts display more pronounced cytologic atypia and increased mitotic activity, an infiltrative growth pattern, and back-to-back or complex architectural arrangements, and may have a stromal response. When pure, adenocarcinomas may exhibit a variety of patterns even within the same tumor, previously described as tubular, ductal, retiform, sex-cord-like, and solid (4). Twelve cases associated with a sarcomatoid component have been described (4–7, 14, 15), three of which exhibited heterologous differentiation in the form of osteosarcoma, rhabdomyosarcoma, and chondrosarcoma (4, 5). Like many of the more unusual types of cervical adenocarcinomas, they are HPV-negative (2, 16). Immunohistochemically these tumors are usually vimentin-, EMA-, PAX8-, calretinin- and CD10 (luminal)-positive; they are negative for ER, PR, CEA and p16 (17). These markers are useful as a panel for distinction of the main differential diagnoses, which include endometrioid adenocarcinoma, clear cell adenocarcinoma, usual-type endocervical adenocarcinoma, and minimal deviation /gastric-type endocervical adenocarcinoma. Accordingly, our case showed diffuse PAX8 positivity, luminal CD10, and patchy p16, and was negative for ER. Calretinin was among the negative markers. This must be kept in mind, because it is traditionally thought of as positive in this scenario (88% of cases positive in the two largest series) (14, 16). More recently, GATA-3 immunoexpression has been demonstrated in benign and malignant mesonephric proliferations, and may be a helpful marker in the appropriate setting (7, 18, 19). Conversely, TTF-1 and HNF1-beta positivity has been identified in a proportion of mesonephric adenocarcinomas, and may be misleading (16). In the present case, GATA-3 staining was patchy, mostly limited to the peripheral tubules, and included one of the foci with bland cytology, presumably representing a benign remnant; the frankly malignant adenocarcinoma and the neuroendocrine tumor exhibited very minimal and weak GATA-3 staining. TTF-1 and HNF1-beta were not performed. Of interest, we also found positivity for chromogranin in the mesonephric component, albeit very focally; this raises the possibility that these endocrine cells could be the origin of the neuroendocrine carcinoma. To our knowledge, only one previous case report has demonstrated the presence of scattered endocrine cells in a pure mesonephric adenocarcinoma by Grimelius histochemical stain and IHC for chromogranin and serotonin. In that single report, the adjacent hyperplastic lesion showed a larger number of the same cells (20). Another study from 1985 also demonstrated serotonin-producing cells in mesonephric rests in the fallopian tube (21). These findings indicate that endocrine serotonin-producing cells are a normal constituent of mesonephric lesions. This may explain rare instances of neuroendocrine (de)differentiation in these neoplasms, as seen in the current case.
HGNECs of the cervix are uncommon tumors, comprising approximately 1% of invasive cervical carcinomas (22). They are histologically indistinguishable from HGNEC occurring in other organs, and may be of small or large cell type. As with HGNEC arising in other sites, they are characterized by aggressive clinical behavior, often present at an advanced stage, and lead to high mortality rates (22). Pure and mixed forms may be encountered; more often, the latter contains either squamous cell carcinoma or adenocarcinoma as the additional component. High-risk HPV has been detected in the vast majority of these tumors, depending on the assay used for detection (i.e. HPV in situ hybridization or HPV-PCR); thus, these tumors have traditionally been classified within the broad category of HPV-associated cervical carcinomas (23–26). However, unlike cervical squamous cell carcinomas, the most common subtype is HPV 18 (27).
Although few studies have specifically addressed genetic alterations in small cell carcinomas of the uterine cervix, pulmonary tumors have been extensively studied from a molecular standpoint. As in small cell carcinomas from many anatomic sites, TP53 and RB1 inactivating mutations have been described (28–30). In the present case, a mutation in TP53 was present solely in the neuroendocrine component, whereas mutations in or deletions of RB1 were not detected in either component. The TP53 missense mutation (p.G244S, c.730G>A) has been reported in various neoplasms, in different organ systems in the COSMIC database (http://cancer.sanger.ac.uk/cosmic). This mutation alters the DNA-binding domain of the protein and likely affects its normal function of DNA repair activation. On the other hand, due to their rarity, only few molecular studies on mesonephric adenocarcinomas have been reported. Recently, Mirkovic et al. performed targeted next-generation sequencing analysis of 18 mesonephric adenocarcinomas from 16 patients, and 1 female adnexal tumor of Wolffian origin. They identified several recurrent genetic alterations, distinct from those found in more common forms of cervical and endometrial adenocarcinomas (31). Mesonephric adenocarcinomas were found to harbor KRAS or NRAS hotspot mutations in 81% of cases, mutations in chromatin remodeling genes including ARID1A, ARID1B and SMARCA4 in almost two-thirds of cases, mutations in BCOR/BCORL1 in 31% of cases, and mutations in FBXW7, MYCN and GATA3 in 2 cases. Additionally, recurrent 1q gains were found in 75% of cases. Conversely, TP53 mutation occurred in only 1 case, and no mutations were detected in PIK3CA or PTEN (31). Interestingly, our case did not harbor mutations in KRAS, NRAS or chromatin remodeling genes even after manual review in Integrated Genomic Viewer (IGV) for hotspot mutations in KRAS (p.G12/G13 and p.Q61) and NRAS (p.Q61)—mutations detected in the Mirkovic study. Instead, identical mutations in GATA3 (p.M422fs, c.1263dupG) and U2AF1 (p.R156H, c.467G>A) were detected in both the mesonephric and neuroendocrine components of the tumor, which provides some support for a clonal relationship between the two components. GATA-3 is a transcription factor essential to the development and differentiation of multiple human tissues, including T-lymphocytes and luminal cells of the breast and kidney, among others (32). This detected GATA3 frameshift mutation affects the 3′ end of the gene. Its functional significance is uncertain. As previously mentioned, two recent studies have demonstrated immunoexpression of GATA-3 protein in mesonephric lesions (benign and malignant), with diminished expression in carcinosarcomas (18, 19). U2AF1 encodes for the small subunit of the U2 small nuclear ribonucleoprotein auxiliary factor (U2AF) involved in mRNA splicing (33). Mutations in this gene have been observed mainly in myelodysplastic syndrome (34) and lung adenocarcinomas (35), but in only 3 of 191 cervical carcinomas (1.5%) from the provisional The Cancer Genome Atlas (TCGA) Research Network dataset (2 missense mutations and 1 deletion); additionally, there was 1 case with gene amplification (www.cBioPortal.org, accessed on 2/18/2016) (36, 37). The detected U2AF1 missense mutation in the current case affects one of the zinc finger domains of the protein, which likely alters target molecule binding, hence mRNA splicing. Also shared by both components was the presence of MYCN amplification. Although the detected MYCN fold change differed significantly between the two components (30.2X for the former versus 2.4X for the latter), this could be at least partially explained by the difference in tumor purity of the DNA samples (only 10% in the mesonephric tumor). FISH using a probe for MYCN confirmed amplification in both tumors. MYCN protooncogene encodes for one of the constituent transcription factors of the Myc family (C-myc, N-myc and L-myc), which in turn regulates cell cycle, proliferation, apoptosis and differentiation. While C-myc and L-myc are normally expressed during early embryo development and proliferating adult tissues, N-myc expression is more limited, being expressed in some tissues during embryogenesis but downregulated thereafter (38). MYCN amplification has been associated with aggressive behavior and poor prognosis in several tumors (38), including lung small cell carcinomas (in which this genetic alteration has been described in 2–20% of cases) (39, 40). MYCN amplification has also been observed in tumors in other anatomic locations, many of which exhibit neuroendocrine differentiation (38). Thus, it is not surprising that we found high copy numbers of MYCN in the neuroendocrine component of the current case. Furthermore, the presence of MYCN amplification in the mesonephric component—a tumor type not typically associated with this finding—leads us to hypothesize that MYCN amplification was one of the initiating events that led to dedifferentiation of the mesonephric adenocarcinoma to neuroendocrine carcinoma. This is similar to the described driver role of MYCN in neuroendocrine transdifferentiation of prostate adenocarcinomas (41).
FBXW7 mutation, as was observed in the mesonephric carcinoma, is present in about 6% of human tumors (42). A tumor suppressor gene, FBXW7 (F-box and WD repeat domain-containing 7) encodes a member of the F-box protein family, which mediates the ubiquitination and subsequent proteasomal degradation of target phosphorylated proteins including Cyclin E, Myc and Notch. Notch, in turn, activates several downstream genes, among them GATA3; interaction with the latter is a crucial step during Th2 cell differentiation (43). The exact role of all of these molecular interactions in the setting of cervical carcinomas is unknown. However, the missense mutation detected in our case (p.R465C; c. 1393C>T) is a hotspot mutation known to affect the substrate-binding domain of the protein, resulting in decreased degradation of target proteins (44). One might speculate that a diminished degradation of the N-Myc protein secondary to FBXW7 mutation, in conjunction with increased N-Myc protein expression due to MYCN amplification, may have had a synergistic effect. This further supports the hypothesis that a deregulation of the pathway was the triggering event for differentiation towards the neuroendocrine phenotype.
IRS2 (insulin receptor substrate-2) gene alterations, as seen in the HGNEC portion of our case, have been observed in 3% (5 of 191) of cervical carcinomas (squamous and endocervical adenocarcinoma - provisional TCGA data) (www.cBioPortal.org, accessed on 2/18/2016) (36, 37) and 1.8% of small cell lung carcinomas (30). Of the 5 cervical carcinoma cases from TCGA, 3 were amplified (as in our case), 1 showed a deletion, and 1 had a missense mutation. The IRS2 protein has been implicated in tumor progression and metastasis (45, 46), similar to RON (47), the protein byproduct of the MST1R (macrophage stimulating 1 receptor) gene, which was also mutated in the present case. However, the missense mutation detected in the latter gene did not involve a characterized domain of the protein, nor is it a hotspot mutation; thus, its functional significance is unclear. Nonetheless, it may be logical to conclude that both proteins associated with tumor progression and metastasis were mutated exclusively in the most aggressive component of the tumor. However, because of the low tumor content of the mesonephric adenocarcinoma, subclonal gene alterations may have been missed in this component.
The unique case reported herein is the first description of an HGNEC arising in association with an HPV-negative tumor, i.e. mesonephric adenocarcinoma in the uterine cervix. The assumption that these tumors indeed have a clonal derivation is strongly supported by the overlapping molecular alterations (MYCN amplification, GATA3 mutation and U2AF1 mutation) and negative HPV studies (by in situ hybridization and PCR) in both, as well as their intimate intermingling on histology. Therefore, we believe these findings are best explained as a phenomenon of dedifferentiation in the form of neuroendocrine carcinoma arising from a mesonephric adenocarcinoma, as opposed to a collision tumor. This case, albeit a single one, adds to the scant literature on the molecular profile of mesonephric neoplasms.
Supplementary Material
Acknowledgments
Sources of Funding:
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
AMS is supported by a stipend from the German Cancer Aid (Deutsche Krebshilfe (DE)) (Dr. Mildred Scheel Stiftung).
Footnotes
Conflicts of Interest
None of the authors declare any other sources of funding or conflicts of interest.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 3.Ueno S, Sudo T, Oka N, et al. Absence of human papillomavirus infection and activation of PI3K-AKT pathway in cervical clear cell carcinoma. Int J Gynecol Cancer. 2013;23:1084–1091. doi: 10.1097/IGC.0b013e3182981bdc. [DOI] [PubMed] [Google Scholar]
- 4.Clement PB, Young RH, Keh P, et al. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol. 1995;19:1158–1171. doi: 10.1097/00000478-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bague S, Rodriguez IM, Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2004;28:601–607. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Meguro S, Yasuda M, Shimizu M, et al. Mesonephric adenocarcinoma with a sarcomatous component, a notable subtype of cervical carcinosarcoma: a case report and review of the literature. Diagn Pathol. 2013;8:74. doi: 10.1186/1746-1596-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roma AA. Mesonephric carcinosarcoma involving uterine cervix and vagina: report of 2 cases with immunohistochemical positivity For PAX2, PAX8, and GATA-3. Int J Gynecol Pathol. 2014;33:624–629. doi: 10.1097/PGP.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 8.Marchio C, Iravani M, Natrajan R, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol. 2008;215:398–410. doi: 10.1002/path.2368. [DOI] [PubMed] [Google Scholar]
- 9.Meissner JD. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J Gen Virol. 1999;80(Pt 7):1725–1733. doi: 10.1099/0022-1317-80-7-1725. [DOI] [PubMed] [Google Scholar]
- 10.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson BM. Human Embryology and Developmental Biology. Philadelphia, PA: Elsevier/Saunders; 2014. [Google Scholar]
- 12.Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol. 1990;14:1100–1111. doi: 10.1097/00000478-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wepfer JF, Boex RM. Mesonephric duct remnants (Gartner’s duct) AJR Am J Roentgenol. 1978;131:499–500. doi: 10.2214/ajr.131.3.499. [DOI] [PubMed] [Google Scholar]
- 14.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, et al. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. 2001;25:379–387. doi: 10.1097/00000478-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Zhang L, Cao W, et al. Mesonephric adenocarcinoma of the uterine corpus. Int J Clin Exp Pathol. 2014;7:7012–7019. [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny SL, McBride HA, Jamison J, et al. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- 17.Goyal A, Yang B. Differential patterns of PAX8, p16, and ER immunostains in mesonephric lesions and adenocarcinomas of the cervix. Int J Gynecol Pathol. 2014;33:613–619. doi: 10.1097/PGP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 18.Roma AA, Goyal A, Yang B. Differential expression patterns of GATA3 in uterine mesonephric and nonmesonephric lesions. Int J Gynecol Pathol. 2015;34:480–486. doi: 10.1097/PGP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 19.Howitt BE, Emori MM, Drapkin R, et al. GATA3 is a sensitive and specific marker of benign and malignant mesonephric lesions in the lower female genital tract. Am J Surg Pathol. 2015;39:1411–1419. doi: 10.1097/PAS.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 20.Stewart CJ, Taggart CR, Brett F, et al. Mesonephric adenocarcinoma of the uterine cervix with focal endocrine cell differentiation. Int J Gynecol Pathol. 1993;12:264–269. doi: 10.1097/00004347-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Fetissof F, Berger G, Dubois MP, et al. Endocrine cells in the female genital tract. Histopathology. 1985;9:133–145. doi: 10.1111/j.1365-2559.1985.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Macdonald OK, Gaffney DK. Incidence, mortality, and prognostic factors of small cell carcinoma of the cervix. Obstet Gynecol. 2008;111:1394–1402. doi: 10.1097/AOG.0b013e318173570b. [DOI] [PubMed] [Google Scholar]
- 23.Masumoto N, Fujii T, Ishikawa M, et al. P16 overexpression and human papillomavirus infection in small cell carcinoma of the uterine cervix. Hum Pathol. 2003;34:778–783. doi: 10.1016/s0046-8177(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 24.Carlson JW, Nucci MR, Brodsky J, et al. Biomarker-assisted diagnosis of ovarian, cervical and pulmonary small cell carcinomas: the role of TTF-1, WT-1 and HPV analysis. Histopathology. 2007;51:305–312. doi: 10.1111/j.1365-2559.2007.02790.x. [DOI] [PubMed] [Google Scholar]
- 25.Horn LC, Lindner K, Szepankiewicz G, et al. p16, p14, p53, and cyclin D1 expression and HPV analysis in small cell carcinomas of the uterine cervix. Int J Gynecol Pathol. 2006;25:182–186. doi: 10.1097/01.pgp.0000185406.85685.df. [DOI] [PubMed] [Google Scholar]
- 26.Nofech-Mozes S, Khalifa MM, Ismiil N, et al. Detection of HPV-DNA by a PCR-based method in formalin-fixed, paraffin-embedded tissue from rare endocervical carcinoma types. Appl Immunohistochem Mol Morphol. 2010;18:80–85. doi: 10.1097/PAI.0b013e3181ae7240. [DOI] [PubMed] [Google Scholar]
- 27.Atienza-Amores M, Guerini-Rocco E, Soslow RA, et al. Small cell carcinoma of the gynecologic tract: a multifaceted spectrum of lesions. Gynecol Oncol. 2014;134:410–418. doi: 10.1016/j.ygyno.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Liu D, Fallon JT, et al. Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp Hematol Oncol. 2015;4:2. doi: 10.1186/2162-3619-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirkovic J, Sholl LM, Garcia E, et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol. 2015;28:1504–1514. doi: 10.1038/modpathol.2015.103. [DOI] [PubMed] [Google Scholar]
- 32.Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–1188. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Romfo CM, Nilsen TW, et al. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature. 1999;402:832–835. doi: 10.1038/45590. [DOI] [PubMed] [Google Scholar]
- 34.Graubert TA, Shen D, Ding L, et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2012;44:53–57. doi: 10.1038/ng.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beltran H. The N-myc oncogene: maximizing its targets, regulation, and therapeutic potential. Mol Cancer Res. 2014;12:815–822. doi: 10.1158/1541-7786.MCR-13-0536. [DOI] [PubMed] [Google Scholar]
- 39.Hwang DH, Sun H, Rodig SJ, et al. Myc protein expression correlates with MYC amplification in small-cell lung carcinoma. Histopathology. 2015;67:81–89. doi: 10.1111/his.12622. [DOI] [PubMed] [Google Scholar]
- 40.Alves Rde C, Meurer RT, Roehe AV. MYC amplification is associated with poor survival in small cell lung cancer: a chromogenic in situ hybridization study. J Cancer Res Clin Oncol. 2014;140:2021–2025. doi: 10.1007/s00432-014-1769-1. [DOI] [PubMed] [Google Scholar]
- 41.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 43.Fang TC, Yashiro-Ohtani Y, Del Bianco C, et al. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King B, Trimarchi T, Reavie L, et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell. 2013;153:1552–1566. doi: 10.1016/j.cell.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Day E, Poulogiannis G, McCaughan F, et al. IRS2 is a candidate driver oncogene on 13q34 in colorectal cancer. Int J Exp Pathol. 2013;94:203–211. doi: 10.1111/iep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuveni H, Flashner-Abramson E, Steiner L, et al. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013;73:4383–4394. doi: 10.1158/0008-5472.CAN-12-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camp ER, Liu W, Fan F, et al. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol. 2005;12:273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


