Abstract
Introduction
The mdx4cv mouse is a common model to study Duchenne muscular dystrophy (DMD). The most utilized methodology to identify the genotype of these mice is Sanger DNA sequencing.
Methods
Here, we provide a simple, cost-effective alternative approach to identify the wildtype, heterozygous, or homozygous/hemizygous genotypes of these mice, using commonly available laboratory equipment and reagents.
Results
Our technique exploits a restriction fragment length polymorphism (RFLP) that is generated by the point mutation found in exon 53 of mdx4cv mice.
Discussion
This technique can benefit laboratories that require complex breeding strategies involving mdx4cv mice.
Keywords: mdx, mdx4cv, genotyping, PCR-RFLP, restriction fragment length polymorphism, Duchenne muscular dystrophy, DMD
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a common childhood muscle wasting disease that results in the death of patients before 40 years of age. It was discovered in 1987 that DMD is caused by mutations in the dystrophin gene, which plays a canonical role of linking the interior cytoskeleton of the cell with the exterior basal lamina.1-3 Loss of dystrophin functionality results in recurrent bouts of muscle damage, followed by incomplete and/or inefficient repair.4
The originally described mdx mouse model, which harbors a spontaneously-occurring mutation in the dystrophin gene, was identified back in 1984.5 Derivatives of the mdx mouse model (including but not limited to mdx2cv, mdx3cv, mdx4cv, mdx5cv) were created by N-ethyl-N-nitrosourea mutagenesis of mice.6 Regardless of the design, all mdx mice and their variants harbor different point mutations within the dystrophin gene that render the gene products unstable and/or nonfunctional. 7
Genotyping of mdx mouse mutants by DNA sequencing has become a gold standard in the field since the method was described in 2010.8 Although the expense of DNA sequencing has been declining over the years, complicated breeding strategies, in which genetically modified mice harbor alterations in genes in addition to dystrophin, have made the expenditures associated with genotyping these mice constant and repetitive for many laboratories. To overcome this problem, other groups have devised alternative methods to identify mdx mice variants that do not rely on DNA sequencing, all of which exhibit different advantages and disadvantages (briefly contrasted in 9). Such methods include allele-specific hybridization,10 the amplification-resistant mutation system (ARMS), 11-13 high-resolution melt polymerase chain reaction (PCR),9 snapback single-stranded conformation polymorphism,14 and SnAPshot assay.15 The method described in this study is a variation of a traditional PCR-restriction fragment length polymorphism (RFLP) technique described for the original mdx mouse.16 We have optimized this technique for use with the mdx4cv mouse, which harbors a nonsense point mutation in exon 53.7 Under the conditions described in this manuscript, we found this genotyping method to be highly accurate and reproducible.
METHODS
Animals
Mice were bred and housed in accordance with the University of Pennsylvania IACUC committee protocols. Mice used for experiments were mdx4cv (Jackson Labs stock number 002378; Bar Harbor, Maine) males, mdx4cv heterozygous and homozygous females, and wildtype males and females, all from a C57BL/6 background.
Genomic DNA Extraction and PCR Settings
DNA from tails or ear punches was extracted in lysis buffer (100 mM Tris-HCl, pH 8.5, 5 mM EDTA, 200 mM NaCl, 0.2% SDS, and 200 μg/mL proteinase K) overnight at 56°C in a Thermomixer (Eppendorf, Hauppauge, New York) set to agitate at 600 revolutions per minute. Fur was removed by centrifugation and DNA was precipitated with isopropanol, washed with 70% ethanol, and allowed to dry. DNA pellets were resuspended in 50 μL dH20. For PCR reactions, 0.1-0.7 μg of DNA (1μL) was used for each sample, in a final volume of 20 μL. The PCR reaction consisted of 10μL of 2X GoTaq Green Master Mix (Promega, Madison, Wisconsin), 0.5 μL of 150 ng/uL of mdx intron 52 forward primer 5’-TCTAAAGTTGAATTTATATTTCTAAACATG-3’, 0.5μL of 100 ng/μl of mdx exon 53 reverse primer 5’-CTTGGTTTCTGTGATCTTCTTTT-3’ (Sigma-Aldrich, St. Louis, Missouri), and 7 μL water. PCR cycling conditions were as follows: an initial hold of 95°C for 3 min, 36 cycles of 95°C for 30s, 55°C for 30s, 72°C for 45s, followed by a hold at 72°C for 5 min.
Restriction Digest of PCR Products
10μL of PCR reactions were incubated in a digest of 4μl 10X Cutsmart buffer, 1μL BcoDI enzyme (New England Biolabs, Ipswich, Massachusetts), and 25μL water overnight (~16 hours) at 37°C in a Thermomixer (Eppendorf, Hauppauge, New York) agitating at 400 rpm. Note 1: the dye and other components present in the GoTaq Green do not appear to affect enzymatic activity to an extent that makes this approach unfeasible using nonpurified DNA. Note 2: The thermomixer step is crucial, as digestion using a water bath may lead to incomplete digestion. 20μL of digested products were subjected to DNA electrophoresis with a 1% TAE agarose gel containing a 1 in 20,000 dilution of 10 mg/mL Ethidium Bromide (Sigma-Aldrich, St. Louis, Missouri), along side of remaining nondigested DNA (to confirm PCR reaction was successful). Gels were imaged using a GelDoc XR+ System (Bio-Rad, Irvine, California) with accompanying Image Lab 5.1 software. Several isoschizomers of BcoDI exist, including BsmAI, Alw26I, BsoMAI and BstMAI, and it is highly probable that these enzymes could be used interchangeably for our genotyping protocol, but should be first tested by the end user.
Validation of Method by DNA Sequencing
To confirm the validity of this approach, DNA was PCR amplified and processed by DNA sequencing using an ABI 3730 Sequencer by the University of Pennsylvania Genomics Analysis Core. Primers used for amplification were mdx4cvF1: 5’-TCAAGAACAGCTGCAGAACAGGAGA-3’ and mdx4cvR1 5’-GGATTGCATCTACTGTGTGAGGACC-3’, the latter of which was used for sequencing, as described.8 As an additional control, PCR products from this PCR-RFLP method were sequenced before digest, and end products were gel extracted with a Qiaex II gel extraction kit (Qiagen, Valencia, California) and also submitted for DNA sequencing.
RESULTS
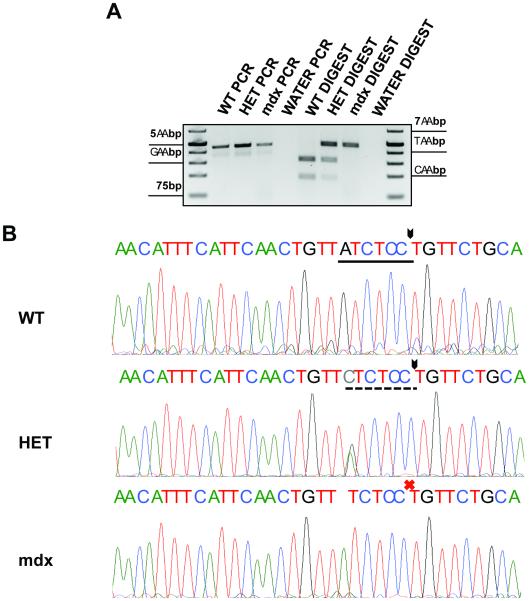
PCR-RFLP has been utilized for genotyping different organisms over several decades 17-22, and here, we have optimized and applied this protocol to genotype the mdx4cv mouse. Briefly, a primer set was identified where the forward primer binds within intron 52 of the dystrophin gene (position 84575312 of X chromosome), and the reverse primer binds within exon 53 (position 84575771 of X chromosome). Primer specificity was confirmed during the design process. Following the PCR, half of the reaction was set aside, while the remaining half was subjected to an overnight restriction enzyme digest with the commercially available BcoDI enzyme. Following digest, the reactions were subjected to gel electrophoresis, and the original PCR was run alongside the digested products to ensure that the PCR reaction was specific and successful (Fig. 1A). The digested product sizes after BcoDI included a band of ~295bp and one of ~165bp for a wildtype mouse. A female heterozygous for mdx4cv would produce bands of ~460bp, ~295bp, and ~165bp. An mdx4cv/mdx4cv homozygous female or mdx4cv hemizygous male reaction would produce a single band of ~460bp, as the BcoDI enzyme is unable digest the mdx4cv PCR product, since it lacks the sequence recognition site of the enzyme.
Figure 1. Polymerase Chain Reaction–Restriction Fragment Length Polymorphism.
(PCR-RFLP) for mdx4cv genotyping. (A) Agarose gel depicting: (left) PCR products with this method using known wildtype, heterozygous, and mdx4cv homozygous/hemizygous mice. (right) Digest of PCR products with the BcoDI enzyme. DNA from the wildtype PCR product completely digests to yield two bands. An mdx4cv heterozygote only partially digests with the enzyme, giving rise to a dark upper band (which does not digest), and two fainter smaller bands. An mdx4cv homozygous or hemizygous mouse does not digest at all with this method. (B) Chromatograms from DNA sequencing to confirm method accuracy. Top: Wildtype mouse chromatogram result. Solid underline represents the BcoDI cut site. Arrow represents the location of the cut. Italicized lettering is the mdx4cv point mutation region. Middle: Heterozygous mouse chromatogram result. Italicized x demonstrates the mdx4cv point mutation site in a heterozygous mouse. Dashed line represents the partial digest performed by the BcoDI enzyme in a heterozygous mouse. Bottom: mdx4cv homozygous/hemizygous chromatogram result. The BcoDI site is not present in these mice, and so no digest occurs (red X).
The specificity of this method was confirmed at two different levels. First, crude DNA preparations from our mice were utilized for traditional mdx genotyping by Sanger sequencing. Using this approach, we found complete agreement of our digest method with the DNA sequencing results when 73 mice were tested (representative samples shown in Fig. 1B). As an additional confirmation of specificity, PCR products prior to digest, as well as digested DNA bands, were gel-extracted and also subjected to DNA sequencing and aligned with 100% identity to murine dystrophin transcripts (data not shown). We highly recommend including known wildtype, mdx4cv heterozygous, and mdx4cv homozygous/hemizygous DNAs as controls for every reaction, to confirm that (1) the restriction digest was complete, and (2) contaminating DNA is not present in reactions.
DISCUSSION
Here, we demonstrate a new strategy of genotyping mdx4cv mice, which is based on a classical, well-accepted and established technique utilized for other genotyping systems. This method provides a simple, cost-effective way to analyze the presence or absence of the mdx4cv mutation, either by itself, or in the context of other mouse genetic mutations bred into the mdx4cv line.
ACKNOWLEDGEMENTS
This work was supported by start up funds, a Thomas B. McCabe and Jeannette E. Laws McCabe Award from the University of Pennsylvania Perelman School of Medicine, and a Pilot and Feasibility Grant from National Institutes of Health (P30 AR069619) to F.M.
ABBREVIATIONS
- ARMS
Amplification resistant mutation system
- DMD
Duchenne muscular dystrophy
- PCR
Polymerase chain reaction
- PCR-RFLP
Polymerase chain reaction restriction fragment length polymorphism
- RFLP
Restriction fragment length polymorphism
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 2.Ozawa E, Yoshida M, Suzuki A, Mizuno Y, Hagiwara Y, Noguchi S. Dystrophin-associated proteins in muscular dystrophy. Hum Mol Genet. 1995;4:1711–1716. doi: 10.1093/hmg/4.suppl_1.1711. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 4.Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep. 2004;5(9):872–876. doi: 10.1038/sj.embor.7400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues M, Echigoya Y, Fukada S, Yokota T. Current Translational Research and Murine Models For Duchenne Muscular Dystrophy. Journal of Neuromuscular Diseases. 2016;3(1):29–48. doi: 10.3233/JND-150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5(8):1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- 8.Banks GB, Combs AC, Chamberlain JS. Sequencing protocols to genotype mdx, mdx(4cv), and mdx(5cv) mice. Muscle Nerve. 2010;42(2):268–270. doi: 10.1002/mus.21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trebbin AL, Hoey AJ. A novel and simple method for genotyping the mdx mouse using high-resolution melt polymerase chain reaction. Muscle Nerve. 2009;39(5):603–608. doi: 10.1002/mus.21215. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain JS, Farwell NJ, Chamberlain JR, Cox GA, Caskey CT. PCR analysis of dystrophin gene mutation and expression. J Cell Biochem. 1991;46(3):255–259. doi: 10.1002/jcb.240460309. [DOI] [PubMed] [Google Scholar]
- 11.Amalfitano A, Chamberlain JS. The mdx-amplification-resistant mutation system assay, a simple and rapid polymerase chain reaction-based detection of the mdx allele. Muscle Nerve. 1996;19(12):1549–1553. doi: 10.1002/(SICI)1097-4598(199612)19:12<1549::AID-MUS4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Pearson-White SH. DMD(mdx3Cv) and DMD(mdx4Cv) dystrophin mutations in mice: rapid polymerase chain reaction genotyping. Neuromuscul Disord. 2002;12(4):366–370. doi: 10.1016/s0960-8966(01)00301-7. [DOI] [PubMed] [Google Scholar]
- 13.Shin JH, Hakim CH, Zhang K, Duan D. Genotyping mdx, mdx3cv, and mdx4cv mice by primer competition polymerase chain reaction. Muscle Nerve. 2011;43(2):283–286. doi: 10.1002/mus.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilton SD, Honeyman K, Fletcher S, Laing NG. Snapback SSCP analysis: engineered conformation changes for the rapid typing of known mutations. Hum Mutat. 1998;11(3):252–258. doi: 10.1002/(SICI)1098-1004(1998)11:3<252::AID-HUMU11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Budowle SA, Gonzalez S, Budowle B, Eisenberg AJ, Grange RW. A novel SNaPshot assay to detect the mdx mutation. Muscle Nerve. 2008;37(6):731–735. doi: 10.1002/mus.21027. [DOI] [PubMed] [Google Scholar]
- 16.Chapman VM, Miller DR, Armstrong D, Caskey CT. Recovery of induced mutations for X chromosome-linked muscular dystrophy in mice. Proc Natl Acad Sci U S A. 1989;86(4):1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20(2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35(2):153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- 20.Pena HF, Vitaliano SN, Beltrame MA, Pereira FE, Gennari SM, Soares RM. PCR-RFLP genotyping of Toxoplasma gondii from chickens from Espirito Santo state, Southeast region, Brazil: new genotypes and a new SAG3 marker allele. Vet Parasitol. 2013;192(1-3):111–117. doi: 10.1016/j.vetpar.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ota M, Fukushima H, Kulski JK, Inoko H. Single nucleotide polymorphism detection by polymerase chain reaction-restriction fragment length polymorphism. Nat Protoc. 2007;2(11):2857–2864. doi: 10.1038/nprot.2007.407. [DOI] [PubMed] [Google Scholar]
- 22.Shia YC, Bradshaw M, Rutherford MS, Lewin HA, Schook LB. Polymerase chain reaction based genotyping for characterization of SLA-DQB and SLA-DRB alleles in domestic pigs. Anim Genet. 1995;26(2):91–100. doi: 10.1111/j.1365-2052.1995.tb02639.x. [DOI] [PubMed] [Google Scholar]