Abstract
Purpose
To compare cerebrovascular reactivity (CVR) and CVR lagtimes in flow territories perfused by vessels with vs. without proximal arterial wall disease and/or stenosis, separately in patients with atherosclerotic and non-atherosclerotic (moyamoya) intracranial stenosis.
Methods
Atherosclerotic and moyamoya patients with >50% intracranial stenosis and <70% cervical stenosis underwent angiography, VWI, and CVR-weighted imaging (n=36; vessel segments evaluated=396). Angiography and VWI were evaluated for stenosis locations and vessel wall lesions. Maximum CVR and CVR lagtime were contrasted between vascular territories with and without proximal intracranial vessel wall lesions and stenosis, and a Wilcoxon rank-sum was test used to determine differences (criteria: corrected two-sided p<0.05).
Results
CVR lagtime was prolonged in territories with vs. without a proximal vessel wall lesion or stenosis for both patient groups: moyamoya (CVR lagtime=45.5s±14.2s vs. 35.7s±9.7s, p<0.001) and atherosclerosis (CVR lagtime= 38.2s±9.1s vs 35.0s±7.2s, p=0.001). For reactivity, a significant decrease in maximum CVR in the moyamoya group only (maximum CVR=9.8±2.2 vs 12.0±2.4, p<0.001) was observed.
Conclusion
Arterial vessel wall lesions detected on non-invasive, non-contrast intracranial VWI in patients with intracranial stenosis correlate on average with tissue-level impairment on CVR-weighted imaging.
Keywords: Cerebrovascular reactivity, intracranial stenosis, moyamoya, vessel wall imaging
INTRODUCTION
Intracranial atherosclerotic disease (ICAD) is believed to be the cause of 7–24% of new cerebral infarcts (1–3). The appropriate treatment for patients with ICAD remains unclear, particularly in the wake of the recently-halted SAMMPRIS and VISSIT trials (4,5). In these prospective trials, 14–24.1% of patients with ICAD treated with stenting and aggressive medical management (AMM), consisting of anti-platelet and/or statin therapy, experienced a stroke within 30 days, compared to just 5.8–9.4% of patients receiving AMM alone. Final results from both trials revealed that even in the AMM arms, a high 12.2–15.1% of patients experienced recurrent stroke in one year (4,5). As such, there remains a clinical need to understand stroke risk factors in these patients.
Alternatively, moyamoya disease is a non-atherosclerotic cerebrovascular condition characterized by progressive stenosis of the supraclinoid internal carotid arteries (ICAs) and their proximal branches, and the corresponding development of elaborate networks of collateral blood vessels (6). Moyamoya can be idiopathic or secondary to another condition such as sickle cell disease or cranial radiation and places patients at more than a seven-fold risk increase for stroke (7). These patients have variable prognoses, which is hypothesized to depend sensitively on the location and extent of steno-occlusion, hemodynamic compensation mechanisms such as the development of collateral vessels, and associated comorbidities (6). Biomarkers that may stratify new or recurrent stroke risk in patients with moyamoya have not been conclusively established.
Evaluation of patients with these intracranial vasculopathies commonly depends on cerebral angiography for stenosis grading and collateral classification and structural MRI for infarct presence. The extent of intracranial vascular stenosis and the presence of collaterals have traditionally been used as surrogates for disease severity and stroke risk. However, angiography provides no direct information on plaque vulnerability in atherosclerotic patients or how arterial stenosis translates to parenchymal impairment at the tissue level in either class of intracranial stenosis. High spatial resolution intracranial vessel wall imaging (VWI) has recently become available and provides a critical step toward understanding intracranial arterial wall pathology by providing morphologic evaluation of the vessel wall (e.g. wall thickness and signal characteristics). However, the correlation between intracranial vessel wall characteristics and clinical presentation is only beginning to be understood (8–12). Specifically, fundamental gaps exist in our knowledge regarding how intracranial vessel wall pathology correlates with parenchymal impairment, however this information may be fundamental to understanding the utility of vessel wall imaging for portending recurrent stroke risk.
This work aims to improve our understanding of how vessel wall abnormalities manifest in terms of tissue-level cerebrovascular reactivity (CVR) maximum and lagtime. Specifically, it is unclear whether impaired CVR measures in regions perfused by vessels with intracranial stenosis or vessel wall disease represent lower reactivity, delayed reactivity time, or both, and whether these relationships are unique in atherosclerotic vs. non-atherosclerotic intracranial disease. CVR-weighting can be measured using Blood-Oxygenation-Level-Dependent (BOLD) (13–15) or cerebral blood flow (CBF)-weighted arterial spin labeling (ASL) (16–18) MRI and a mild hypercapnic challenge. Here, hypercapnic BOLD MRI is applied together with a novel time regression technique (19) that separately quantifies CVR lagtime (i.e., time for smooth muscle lining arterioles to maximally relax in response to vasoactive stimuli) and maximum CVR (i.e., amount by which arterioles are able to increase cerebral blood flow and volume in response to vasoactive stimuli). In this study, BOLD MRI was used, rather than ASL MRI, as BOLD MRI is more robust over a large range of blood arrival and vascular reactivity times, which are expected in patients with cerebrovascular disease (20,21).
The purpose of this work is to compare CVR lagtime and maximum in flow territories perfused by vessels with vessel wall disease and/or stenosis to territories perfused by vessels without measurable vessel wall disease or stenosis, separately in the two patient groups. The overall hypotheses are that (i) vessel wall pathology co-localizes with arterial stenosis in the majority of patients in both groups, and (ii) lower maximum CVR and prolonged CVR lagtimes are present in flow territories perfused by vessels with vessel wall pathology.
MATERIAL AND METHODS
Patient anonymity, informed consent, and ethics
All study components were reviewed and approved by the Institutional Review Board. Procedures were followed in accordance with the ethical standards of the Human Research Protection Program and the Helsinki Declaration of 1975 (and as revised in 1983). All components of this study were in compliance with the Health Insurance Portability and Accountability Act (HIPAA). All patients provided informed, written consent.
Inclusion criteria were stroke or TIA within the past year, intracranial stenosis>50%, extracranial stenosis<70%, and VWI and CVR-weighted imaging performed within the same session between July 2014 and August 2015. A value of <70% extracranial carotid stenosis was chosen as that is the accepted value for flow-limiting stenosis in the cervical carotid arteries (22) The value for flow-limiting intracranial stenosis is less clear and thus a value of 50% was chosen, including both moderate and severe stenosis.
Structural imaging and angiography
Imaging sessions were performed on a 3T whole-body scanner (Philips Medical Systems, Best, The Netherlands) using body coil transmission and SENSE array reception. FLAIR and T1-weighted MRI were performed during the same session as VWI and CVR imaging. Acquisitions were performed in the axial plane with TR/TE=11000/12 ms and 9.0/4.6 ms, for FLAIR and T1-weighted, respectively. Patients underwent angiographic imaging with digital subtraction angiography (DSA) (n=14), CT angiography (CTA) (n=3), or time-of-flight MR angiography (TOF-MRA) (n=19), of the intracranial vasculature as part of the same session or within 30 days of MRI. TOF-MRA was acquired in the axial plane using a 3D T1-weighted gradient echo (GRE) sequence with TR/TE=13.4/1.7 ms; flip-angle=20°; in-plane FOV=200x200 mm2, imaging matrix=200x200; spatial resolution=1x1x1 mm3. Blinded review of angiography data was performed by a board-certified neuroradiologist (LTD, three years of experience). The right and left supraclinoid ICAs, proximal A1 segment of the anterior cerebral arteries (ACA), proximal M1 segment of the middle cerebral arteries (MCA), proximal P1 segment of the posterior cerebral arteries (PCA), intracranial vertebral arteries, and basilar artery were graded as normal-to-mild (< 50%), moderate (50–69%), or severe (≥70%) stenosis. These approximate categories were used as conservative classifications owing to the differing sensitivity of the angiographic techniques for identifying stenosis.
Vessel wall imaging (VWI)
Identical VWI acquisitions were performed in the coronal plane using a 3D turbo-spin-echo (TSE) proton density-weighted sequence with long TSE readout for blood water nulling and an anti-driven equilibrium module for minimizing CSF water signal. Imaging parameters were spatial resolution=0.6×0.5×0.5mm3, TR/TE=1500/38.5 ms, TSE-factor=56, and duration=6 min 51s. A board-certified neuroradiologist (LTD, three years of experience) performed blinded review of VWI for lesion determination, defined as concentric or eccentric vessel wall thickening or hyperintense signal. The right and left ICAs, proximal ACAs, proximal MCAs, proximal PCAs, vertebral arteries, and basilar artery were evaluated. The locations of vessel wall lesions were compared with the presence of stenosis on angiography. Evaluated vascular segments were grouped as (i) vessel wall lesion with moderate (50–69%) or severe (70–99%) stenosis, (ii) vessel wall lesion with no-to-mild stenosis (0–49%), (iii) moderate or severe stenosis with no vessel wall lesion, or (iv) no vessel wall lesion or stenosis.
Cerebrovascular reactivity (CVR)-weighted MRI
Patients underwent BOLD imaging using hypercapnic stimulation. The stimulus paradigm consisted of three minutes hypercapnic hyperoxia (5% CO2 / 95% O2) administration alternating with three minutes medical grade room air repeated twice. Hypercapnic hyperoxia was used rather than hypercapnic normoxia as hypercapnic hyperoxia (e.g., carbogen-5) is a clinical gas mixture available in hospital settings and is therefore a generalizable stimulus to both specialized and non-specialized centers. Differences in BOLD reactivity between hypercapnic hyperoxia and hypercapnic normoxia stimuli have been investigated in detail in our previous studies (14,23) and these stimuli provide results that are correlated in both healthy volunteers and patients with cerebrovascular disease. BOLD imaging was performed using single-shot gradient echo-planar acquisition, TR/TE=2000/35 ms, spatial resolution=3.0×3.0×3.5 mm3, slices=31. Acquisition parameters were similar as reported and assessed for reproducibility previously (14).
Statistical procedures
CVR lagtime and maximum CVR (Figure 1) were analyzed using Matlab (Mathworks, Natick, MA, USA) and FMRIB Software Library (FSL) routines. CVR-weighted data were registered to a 4 mm isotropic brain atlas (Montreal Neurological Institute, MNI). Similar as has been reported (19), the statistical regressor, which consisted of a boxcar convolved with a single-gaussian was progressed in time, beginning at the start of the hypercapnic stimulus. The maximum z-statistic (e.g., maximum CVR) and time lag to reach the maximum CVR (CVR lagtime), which reflects the total time for blood gas to reach the brain parenchyma from compressed cylinders plus the time for the maximum point of vasodilation, were calculated. For each volunteer the mean and standard deviation of the CVR lagtime and maximum CVR were calculated (i) within the six primary intracranial vascular territories including the right and left ACA, MCA, and PCA territories and within 20 sub-territories (Figure 1) including the ASPECTs territories (24) (M1–M6) and the corresponding ganglionic (A1, P1) and supraganglionic (A2, P2) segments of the ACA and PCA territories. Across 36 patient volunteers, 396 vessel segments were analyzed on VWI and angiography, in addition to (i) 720 separate parenchymal regions for the sub-territories grouping approach and (ii) 216 separate parenchymal regions for the flow territory grouping approach.
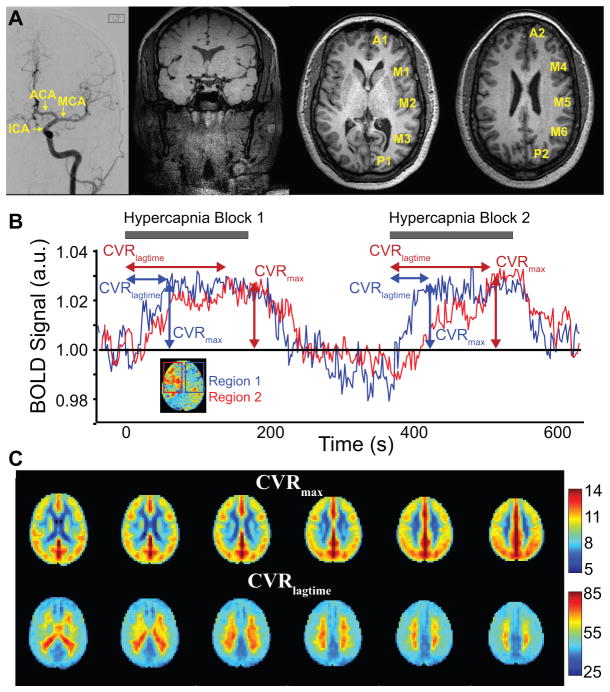
Figure 1.
(A) Representative images including: Digital subtraction angiography (DSA) with left internal carotid artery (ICA) injection showing the anterior territory segments for which degree of stenosis was graded, single coronal slice from vessel wall imaging exam, and T1-weighted images with the sub-territories labeled. (B) Blood-oxygenation-level-dependent (BOLD) cerebrovascular reactivity (CVR)-weighted determination. Time regression analysis is applied to record the time when the stimulus regressor generates the highest z-statistic with the data on a voxel-wise basis. The time shift is recorded as the CVR lagtime and the maximum z-statistic as the maximum CVR. (C) Mean CVR lagtime and maximum CVR values across all patients, which provide a reference standard for individual patients shown in Figure 2.
To test the study hypotheses, first we calculated mean values for CVR lagtime and maximum CVR in each of the vascular territories only in regions without a proximal vessel wall lesion or hemodynamically significant stenosis (> 50% luminal narrowing). The term no proximal vessel disease will be used synonymously with no detectable proximal vessel wall lesion or stenosis. The ACA territories were considered to have a proximal vessel wall lesion if there was an ipsilateral ACA or ICA lesion, MCA territories if there was an ipsilateral MCA or intracranial ICA lesion, PCA territories if there was a lesion of the ipsilateral PCA, vertebral artery or basilar artery.
Next, for each the atherosclerosis and moyamoya groups, the CVR lagtime and maximum CVR were separately compared between territories with no discernible proximal vessel wall lesion or stenosis and territories (i) with a proximal vessel wall lesion or stenosis, (ii) with a proximal vessel wall lesion, and (iii) with a proximal stenosis. Note that the territories with a proximal vessel wall lesion and proximal stenosis are not exclusive of one another. Analysis of territories with a proximal vessel wall lesion only (vessel wall lesion without a corresponding stenosis on angiography) was also included for the atherosclerosis patients. Analysis of this category was not included for the moyamoya patients due to the very small number of territories meeting these criteria in this population, as expected. Similarly, evaluation of territories with a proximal stenosis only (stenosis without discernible vessel wall lesion) was not included for either patient group as this subcategory was very rarely observed and is generally not expected physiologically. The mean, standard deviation, and 95% confidence intervals were calculated for each of the above groups. A non-parametric Wilcoxon rank-sum test was used to determine differences of study variables between vessel types, and corresponding (maximum six comparisons) Bonferroni-corrected two-sided p-values were calculated with criteria for significance being corrected p<0.05. The above comparisons were also performed considering each of the sub-territories individually.
Several additional analyses were performed as well. To evaluate for the effect of prior infarcts on the CVR analysis, the CVR lagtime and maximum CVR were compared between all territories with a proximal vessel wall lesion or stenosis and the same group excluding the territories of encephalomalacia from prior infarct, as determined on FLAIR imaging. This analysis was performed separately for the atherosclerosis and moyamoya patient groups. To evaluate the effect of prior EDAS (encephaloduroarteriosynangiosis), the CVR lagtime and maximum CVR were compared between territories with vs. without a proximal vessel wall lesion or stenosis for all moyamoya patients, moyamoya patients with prior EDAS, and moyamoya patients without prior EDAS.
RESULTS
Patients
36 patients (mean age=51 years; range=25–83 years) comprising 12 males (mean age=53 years, range=25–83 years) and 24 females (mean age=50 years, range=28–74 years) were included and stratified by disease: atherosclerosis (n=16; mean age=64 years, range=49–83 years) or non-atherosclerosis (moyamoya; n=20; mean age=40 years, range=25–60 years). Moyamoya patients carried a diagnosis of idiopathic moyamoya (n=18), moyamoya secondary to childhood radiation (n=1), and moyamoya secondary to sickle cell disease (n=1). In the moyamoya group, 12/20 (60%) patients had undergone prior (duration=17.2 months; range=6–45 months) encephaloduroarteriosynangiosos (EDAS). Per inclusion criteria all patients had prior stroke or TIA. Approximately 80% (13/16) of the atherosclerosis patients had sequela of prior infarcts documented on FLAIR imaging, seven patients with lacunar infarcts and six patients with cortical infarcts involving approximately one of the sub-territories. In the moyamoya group, 14/20 (70%) patients had evidence of prior infarcts of FLAIR imaging including three patients with lacunar infarcts and 11 patients with cortical infarcts involving on average two of the sub-territories.
Vessel wall lesions vs. luminal stenosis
Table 1 shows the number of vessel wall lesions and stenoses detected in each patient group. In both the atherosclerosis and moyamoya groups, the intracranial ICA segments were the most common site of vessel wall lesions. Patients with atherosclerotic disease demonstrated variable patterns including no vessel wall lesion (2/16), single lesion (2/16), two lesions (5/16) and greater than or equal to three lesions (7/16). Of the 176 vascular segments evaluated in the atherosclerosis group, 8 (5%) demonstrated moderate stenosis and 14 (8%) demonstrated severe stenosis. Of the 39 total vessel wall lesions in this group, 17 (44%) had corresponding stenosis on angiography. Findings of moyamoya on vessel wall imaging were unilateral in 6/20 (30%) and bilateral in 14/20 (70%) patients. Of the 220 vascular segments evaluated, 16 (7%) demonstrated moderate stenosis and 67 (30%) demonstrated severe stenosis. In the moyamoya group, 70/82 (85%) of the identified vessel wall lesions demonstrated associated arterial stenosis > 50%, and the cerebral hemispheres(s) involved by vessel wall disease corresponded with findings on angiography in 17/20 (85%) cases. No posterior circulation vessel wall lesions were detected in the moyamoya group, but 5/20 (25%) of these patients had moderate vertebral or basilar artery stenosis on angiography.
Table 1.
Location of vessel wall lesions and stenosis and overlap of the two.
| All patients (36 patients, 396 vessel segments)
| ||
|---|---|---|
| Stenosis | No Stenosis | |
| Vessel wall lesion | 87 | 34 |
| No lesion | 18 | 257 |
| Atherosclerosis patients (16 patients, 176 vessel segments) | ||
|
| ||
| Stenosis | No Stenosis | |
| Vessel wall lesion | 17 | 22 |
| No lesion | 5 | 132 |
| Moyamoya patients (20 patients, 220 vessel segments) | ||
|
| ||
| Stenosis | No Stenosis | |
| Vessel wall lesion | 70 | 12 |
| No lesion | 13 | 125 |
CVR-weighted MRI
Representative patient examples are shown in Figure 2. CVR lagtime and maximum CVR in territories without a discernible proximal vessel wall lesion or hemodynamically significant stenosis are summarized in Table 2. The number of vascular territories without a proximal vessel wall lesion or significant stenosis was 19, 12, and 55 for the ACA, MCA, and PCA territories, respectively.
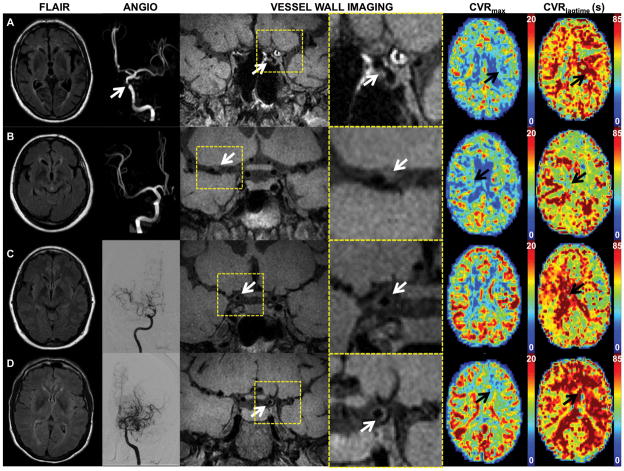
Figure 2.
Axial FLAIR, MRA or DSA, coronal vessel wall imaging (subsection and magnified views), axial maximum CVR and CVR lagtime maps are shown for representative patients. (A) An 83 year male with atherosclerosis and left > right internal carotid artery (ICA) vessel wall lesions and stenosis (white arrows). Left middle cerebral artery (MCA) and anterior cerebral artery (ACA) territories (black arrows) show decreased maximum CVR (CVRmax) and prolonged CVR lagtime (CVRlagtime) compared to the right. (B) 71 year female with atherosclerosis. Vessel wall imaging demonstrates thickening and T1 hyperintensity of the proximal MCA (white arrow). No MCA stenosis is identified on MRA. CVR lagtime is prolonged in the right MCA territory (black arrow). A severe basilar stenosis may account for prolonged CVR lagtimes in the posterior territories bilaterally. (C) Vessel wall imaging in this 44 year female with predominantly right sided moyamoya disease is consistent with concentric thickening of the right terminal ICA (white arrow), proximal ACA, and proximal MCA. DSA right ICA injection shows moyamoya changes of the right anterior circulation. CVR lagtime is prolonged on the right (black arrow), but the maximum CVR is comparable to that on the left. (D) A 49 year female with bilateral, left>right, moyamoya disease and left > right vessel wall thickening of the terminal ICA (white arrow) and moyamoya changes. CVR lagtime is prolonged and maximum CVR decreased in the left ACA and MCA territories (black arrows) compared to the right.
Table 2.
Mean (95% confidence interval) cerebrovascular reactivity (CVR) lagtime and maximum CVR for each of the sub-territories considering territories without a proximal vessel wall lesion or hemodynamically significant stenosis.
| Sub-Territory | CVR lagtime in seconds (95% confidence interval) | Maximum CVR Z-statistic (95% confidence interval) |
|---|---|---|
| A1 | 33.6 (28.9–38.3) | 10.7 (9.5–11.9) |
| A2 | 37.1 (32.4–41.8) | 10.5 (9.5–11.5) |
| M1 | 34.1 (30.0–38.1) | 10.6 (9.6–11.6) |
| M2 | 34.0 (29.6–38.5) | 11.9 (10.4–13.3) |
| M3 | 36.9 (32.5–41.4) | 10.9 (9.8–12.0) |
| M4 | 35.4 (31.0–39.8) | 10.3 (9.3–11.3) |
| M5 | 37.3 (33.2–41.3) | 10.6 (9.6–11.6) |
| M6 | 39.3 (34.4–44.3) | 11.2 (10.1–12.3) |
| P1 | 34.5 (32.4–36.6) | 11.4 (10.8–12.0) |
| P2 | 34.4 (32.1–36.7) | 11.7 (11.2–12.2) |
CVR-weighted MRI: atherosclerosis patients
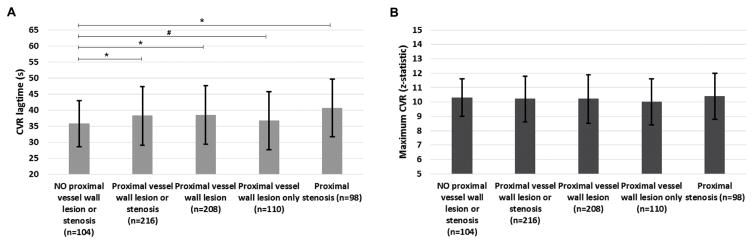
Figure 3 and Supplemental Table 1 summarize the mean CVR lagtime and maximum CVR for all vascular territories comparing those territories without a discernible proximal vessel wall lesion or stenosis to those (i) with a proximal vessel wall lesion or stenosis, (ii) with a proximal vessel wall lesion, (iii) with a proximal vessel wall lesion only, and (iv) with a proximal stenosis for the atherosclerosis patients. CVR lagtime was significantly lengthened in vascular territories with versus without a proximal vessel wall lesion or stenosis in the atherosclerosis group (CVR lagtime= 38.2s±9.1s vs 35.0s±7.2s, p=0.001) and similar results were found when comparing regions with a proximal vessel wall lesion or proximal stenosis to those with no proximal vessel disease. There was no significant difference in the maximum CVR between territories with vs without proximal vessel disease in the atherosclerosis group.
Figure 3.
Mean+/−standard deviation of the CVR lagtime (A) and maximum CVR (B) for atherosclerotic patients comparing territories with no proximal vessel wall lesion or stenosis to those with a proximal wall lesions and/or stenosis. *statistically significant by Wilcoxon rank sum corrected two-sided p-value<0.05. # trend towards significance (p-value < 0.10). The number of subterritories included in each group is included in the x axis labels.
CVR-weighted MRI: moyamoya patients
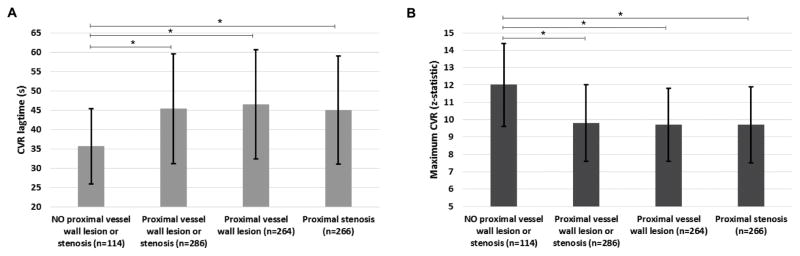
Figure 4 and Supplemental Table 1 summarize the mean CVR lagtime and maximum CVR for all territories comparing those territories without a discernible proximal vessel wall lesion or stenosis to those (i) with a proximal vessel wall lesion or stenosis, (ii) with a proximal vessel wall lesion, and (iii) with a proximal stenosis for the moyamoya patients. The CVR lagtime was prolonged in territories with proximal vessel lesion or stenosis vs in those without (CVR lagtime=45.5s±14.2s vs 35.7s±9.7s, p<0.001). Similar analysis for maximum CVR showed a significant decrease in the maximum CVR in territories with a proximal vessel wall lesion or stenosis compared to regions without (maximum CVR=9.8±2.2 vs 12.0±2.4, p<0.001).
Figure 4.
Mean+/−standard deviation of the CVR lagtime (A) and maximum CVR (B) for non-atherosclerotic (moyamoya) patients comparing territories with no proximal vessel disease to those with proximal vessel wall lesions and/or stenoses. *statistically significant by Wilcoxon rank sum corrected two-sided p-value<0.05. The number of subterritories included in each group is included in the x axis labels.
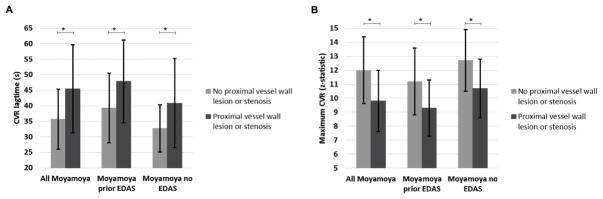
Figure 5 summarizes the mean CVR lagtime and maximum CVR for territories with vs without a proximal vessel wall lesion or stenosis for all moyamoya patients, moyamoya patients with prior EDAS, and moyamoya patients without prior EDAS. In all groups, there is a significant difference between territories with vs without proximal vessel disease. Additionally, there are differences in the CVR lagtime and maximum CVR between patients with and without prior EDAS.
Figure 5.
Mean +/− standard deviation of CVR lagtime (A) and maximum CVR comparing territories with vs without a proximal vessel wall lesion or stenosis, separately for all moyamoya patients, moyamoya patients with prior EDAS, and moyamoya patients without prior EDAS.
CVR-weighted MRI: prior infarcts
Supplemental Table 1 shows the mean CVR lagtime and maximum CVR compared between all territories with a proximal vessel wall lesion or stenosis and all territories with a proximal vessel wall lesion or stenosis excluding territories with evidence of prior infarct. In total only 7/216 (3%) of the sub-territories with a proximal vessel wall lesion in the atherosclerosis patients and 20/286 (7%) of such territories in the moyamoya patients demonstrated changes of prior infarct and were excluded. No significant difference was found in the CVR lagtime or maximum CVR in either patient group after removing the territories with sequela of prior infarcts. This finding is not surprising given the relatively small volume of infarct relative to the total volume considered in analysis.
DISCUSSION
We applied intracranial vessel wall imaging without intravenous contrast in patients with intracranial stenosis. Findings suggest that the presence of vessel wall lesions correlates with functional changes in CVR. The use of a novel time regression technique (19) has allowed for separate calculation of the maximum CVR and CVR lagtime. It was observed that in non-atherosclerotic intracranial disease (e.g., moyamoya), maximum CVR is lower and CVR lagtime longer in flow territories perfused by arteries with vessel wall lesions. In atherosclerotic disease, CVR lagtime is most consistently lengthened in territories perfused by arteries with vessel wall lesions.
When the locations of vessel wall lesions were compared with the locations of hemodynamically significant (>50%) stenosis, the two corresponded in the majority of the moyamoya patients. However, the correlation of vessel wall lesions with stenosis was variable in the atherosclerosis group, with less than half of the detected vessel wall lesions demonstrating greater than 50% stenosis on angiography. Van der Kolk et al. (25) similarly showed a relatively small overlap of vessel wall lesions and stenosis in a group of patients composed primarily of atherosclerotic intracranial stenosis with 14/52 detected vessel wall lesions having corresponding stenosis on MRA. One factor contributing to the underestimation of intracranial plaque burden on angiography may be the presence of arterial remodeling with a compensatory dilatation of the arterial lumen at the plaque location. Even with an extensive plaque, remodeling of the arterial vasculature, with a compensatory enlargement of the vessel size at the location of the plaque, will result in a normal appearance of the lumen on angiography (26,27). The clinical significance of intracranial vessel wall disease that does not result in hemodynamically significant stenosis remains of question but may be important as a long-term vascular risk factor, as shown in other vessels (28). This works shows a strong trend toward prolongation of CVR lagtime in territories with a proximal vessel wall lesion only compared to territories with no proximal vessel disease, which suggests functional significance of vessel lesions in the absence of stenosis. As such, vessel wall imaging may be used as a complement to angiography to understand the spectrum of vascular disease, and future work is required to investigate the clinical importance of this more thoroughly. Studies of coronary and carotid arteries have similarly shown clinical significance of plaque that results in <50% stenosis on angiography (29,30).
Our data provide evidence that CVR lagtime is prolonged in vascular territories with a proximal vessel wall lesion compared to those without in atherosclerosis and moyamoya patients, though the differences are more marked in the moyamoya group. As the majority of vessel wall lesions in the moyamoya patients resulted in severe stenosis, the greater changes in timing in the moyamoya group may be due to greater degree of luminal stenosis. The mechanism by which greater stenosis contributes to a longer CVR lagtime is not well understood. Possible mechanisms include (a) stenosis of larger feeding vessels resulting in reduction in cerebral perfusion pressure and delayed blood arrival time, (b) less efficient response of moyamoya collateral arteriole smooth muscle compared with healthy vessels, and (c) more rigid vessels or vessels operating near reserve capacity in patients with atherosclerosis. Differences between groups may also reflect different mechanisms of endothelial or smooth muscle dysfunction in atherosclerotic versus non-atherosclerotic disease, as recent animal work has revealed an important role of the endothelium in vasodilation (31). Although territories with proximal lesions and/or stenosis appear to react differently based upon disease etiology (atherosclerotic vs non-atherosclerotic), the territories without a proximal vessel wall lesion or stenosis demonstrate similar CVR timing trends.
The maximum CVR is significantly decreased in the presence of a proximal vessel wall lesion in the moyamoya patients only, possibly indicating that it is the CVR lagtime, rather than the commonly-assumed overall reactivity, that is impaired in many, although not all, patients with atherosclerotic disease. However, it is also noted that in territories with no visible proximal vessel wall lesion or hemodynamically significant stenosis, the maximum CVR is significantly lower in the atherosclerosis patient group than in the moyamoya patient group. This likely reflects age-related changes in maximum CVR (32), as the average age in the atherosclerosis group is much higher relative to the moyamoya group (64 years vs 40 years in the presented patients).
Although, analysis of atherosclerosis and moyamoya patients is considered separately, it is noted that the CVR lagtimes in territories without visible proximal vessel disease were not significantly different between the atherosclerosis and moyamoya patients. However, the maximum CVR in territories without proximal vessel disease was greater for the moyamoya group than for the atherosclerosis group. The range and standard deviation of the CVR lagtime and maximum CVR were also greater for the moyamoya group compared to the atherosclerosis group. Such direct comparisons should be interpreted with care however, as disease chronicity and age cannot be matched between groups due to the differing etiology of these diseases.
Approximately one half of the moyamoya patients had received prior EDAS. Separate analysis of the moyamoya patients with and without prior EDAS showed that both groups similarly have prolonged CVR lagtime and decreased maximum CVR in territories with proximal vessel disease. The patients with and without prior EDAS were not directly compared due to inherent differences in the groups including post-surgical physiology as well as differences in disease severity between the groups. Specifically, patients requiring EDAS are inherently more impaired than those not requiring an EDAS, and therefore while the EDAS may improve tissue-level hemodynamics, this improvement may still not lead to an equivalent hemodynamic pattern as an asymptomatic patient never requiring an EDAS. These data imply that patients with prior EDAS have overall prolonged CVR and decreased maximum CVR compared to patients without prior EDAS, but as described above, interpretation of these data is not straightforward
Higher spatial resolution MRI of the vessel wall has been previously shown to provide morphologic evaluation of the vessel wall and assessment of vessel wall pathology. Vessel wall changes including wall thickening, signal changes, and/or enhancement have been shown to be greater in symptomatic intracranial stenosis compared to asymptomatic patients (33,34) and to be able to differentiate a variety of disease conditions including atherosclerosis, moyamoya disease, dissection and vasculitis (35,36). This study adds to the literature by assessing the relationship between intracranial vessel wall plaque or thickening and tissue-level timing and reactivity functioning.
Several limitations should also be considered. Several of the atherosclerosis and moyamoya patients had areas of encephalomalacia from prior cortical infarcts involving on average 1–2 of the ASPECTS territories. As shown, the number of territories affected by prior infarct was small and did not affect the overall results. Approximately one half of the moyamoya patients had received prior EDAS, and it is important to consider these groups separately as discussed above. It is recognized that moyamoya is generally considered to be a bilateral disease of variable severity, and in this study only those segments with a discernible proximal vessel wall lesion or hemodynamically significant stenosis are considered to be diseased. The detection of vessel wall lesions is limited by the sensitivity of the VWI technique, which has been shown to be greater at 7T vs 3T (37). However, at this time is not feasible to routinely perform 7T MRI clinically. Different modalities were used for angiography, primarily DSA for the moyamoya group and MRA for the atherosclerosis group. This was due to DSA being the clinical gold standard for moyamoya collateral grading and classification, but MRA and CTA being used more commonly for atherosclerosis. As both of these modalities are able to accurately detect the presence of a stenosis>50%, this difference is not anticipated to be a major confound given the conservative categorical stenosis classification procedure (see Materials and Methods). The atherosclerosis and non-atherosclerosis groups are not age-matched due to the inherent differences in disease pathology and age of onset, and an age-matched control population was not included in this study. However, the age difference between groups does not impact the primary goal of this work, which was to compare CVR lagtime and maximum between flow territories fed by vessels with measurable wall disease and/or stenosis to flow territories fed by vessels without measurable disease, separately in these patient groups. Finally, the patient group is small, similar to other published work in these populations.
In conclusion, this study provides evidence that vessel wall lesions depicted on vessel wall imaging without intravenous contrast in patients with intracranial stenosis correlate with functional changes of cerebrovascular reactivity on average in both atherosclerotic and non-atherosclerotic (moyamoya) intracranial stenosis. Two disease entities, atherosclerotic and non-atherosclerotic intracranial stenosis, were studied and shown to have different degrees of change in CVR lagtime and maximum CVR in the presence of a proximal vessel wall lesion. These differences may reflect different mechanisms of endothelial or smooth muscle dysfunction.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Institutes of Health NIH/NINDS 5R01NS078828
References
- 1.Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol. 2008;65(6):733–737. doi: 10.1001/archneur.65.6.733. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2010 doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009;40(6):1999–2003. doi: 10.1161/STROKEAHA.108.546150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. Jama. 2015;313(12):1240–1248. doi: 10.1001/jama.2015.1693. [DOI] [PubMed] [Google Scholar]
- 5.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. The New England journal of medicine. 2009;360(12):1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 7.Hallemeier CL, Rich KM, Grubb RL, Jr, et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke; a journal of cerebral circulation. 2006;37(6):1490–1496. doi: 10.1161/01.STR.0000221787.70503.ca. [DOI] [PubMed] [Google Scholar]
- 8.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271(2):534–542. doi: 10.1148/radiol.13122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR American journal of neuroradiology. 2013;34(2):299–304. doi: 10.3174/ajnr.A3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieleman N, van der Kolk AG, van Veluw SJ, et al. Patterns of intracranial vessel wall changes in relation to ischemic infarcts. Neurology. 2014;83(15):1316–1320. doi: 10.1212/WNL.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 11.Lou X, Ma N, Ma L, Jiang WJ. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR American journal of neuroradiology. 2013;34(3):513–517. doi: 10.3174/ajnr.A3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mossa-Basha M, Hwang WD, De Havenon A, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke; a journal of cerebral circulation. 2015;46(6):1567–1573. doi: 10.1161/STROKEAHA.115.009037. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. Journal of visualized experiments : JoVE. 2014;(94) doi: 10.3791/52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke; a journal of cerebral circulation. 2014;45(8):2335–2341. doi: 10.1161/STROKEAHA.114.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandell DM, Han JS, Poublanc J, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke; a journal of cerebral circulation. 2008;39(7):2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- 16.Yun TJ, Paeng JC, Sohn CH, et al. Monitoring Cerebrovascular Reactivity through the Use of Arterial Spin Labeling in Patients with Moyamoya Disease. Radiology. 2015:141865. doi: 10.1148/radiol.2015141865. [DOI] [PubMed] [Google Scholar]
- 17.Blockley NP, Griffeth VE, Simon AB, Dubowitz DJ, Buxton RB. Calibrating the BOLD response without administering gases: comparison of hypercapnia calibration with calibration using an asymmetric spin echo. NeuroImage. 2015;104:423–429. doi: 10.1016/j.neuroimage.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donahue MJ, Strother MK, Hendrikse J. Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke; a journal of cerebral circulation. 2012;43(3):903–915. doi: 10.1161/STROKEAHA.111.635995. [DOI] [PubMed] [Google Scholar]
- 19.Donahue MJ, Strother MK, Lindsey KP, Hocke LM, Tong Y, Frederick BD. Time delay processing of hypercapnic fMRI allows quantitative parameterization of cerebrovascular reactivity and blood flow delays. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1177/0271678X15608643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roach BA, Donahue MJ, Faraco CC, et al. Interrogating the Functional Correlates of Collateralization in Patients with Intracranial Stenosis using Multi-modal ASNR. 53rd Annual Meeting & Symposium; Chicago, IL. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke; a journal of cerebral circulation. 2011;42(9):2485–2491. doi: 10.1161/STROKEAHA.111.616466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division, North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke; a journal of cerebral circulation. 1991;22(6):816–817. doi: 10.1161/01.str.22.6.816. [DOI] [PubMed] [Google Scholar]
- 23.Faraco CC, Strother MK, Siero JC, et al. The cumulative influence of hyperoxia and hypercapnia on blood oxygenation and R. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR American journal of neuroradiology. 2001;22(8):1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 25.van der Kolk AG, Zwanenburg JJ, Brundel M, et al. Intracranial vessel wall imaging at 7. 0-T MRI. Stroke; a journal of cerebral circulation. 2011;42(9):2478–2484. doi: 10.1161/STROKEAHA.111.620443. [DOI] [PubMed] [Google Scholar]
- 26.Babiarz LS, Astor B, Mohamed MA, Wasserman BA. Comparison of gadolinium-enhanced cardiovascular magnetic resonance angiography with high-resolution black blood cardiovascular magnetic resonance for assessing carotid artery stenosis. J Cardiovasc Magn Reson. 2007;9(1):63–70. doi: 10.1080/10976640600843462. [DOI] [PubMed] [Google Scholar]
- 27.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. The New England journal of medicine. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 28.Pasterkamp G, Schoneveld AH, van Wolferen W, et al. The impact of atherosclerotic arterial remodeling on percentage of luminal stenosis varies widely within the arterial system. A postmortem study. Arterioscler Thromb Vasc Biol. 1997;17(11):3057–3063. doi: 10.1161/01.atv.17.11.3057. [DOI] [PubMed] [Google Scholar]
- 29.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman BA, Wityk RJ, Trout HH, 3rd, Virmani R. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke; a journal of cerebral circulation. 2005;36(11):2504–2513. doi: 10.1161/01.STR.0000185726.83152.00. [DOI] [PubMed] [Google Scholar]
- 31.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. Journal of the American Heart Association. 2014;3(3):e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral cortex. 2011;21(6):1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212(2):507–511. doi: 10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Zhao DL, Deng G, Xie B, et al. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2015;22(4):700–704. doi: 10.1016/j.jocn.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72(7):627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 36.Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke; a journal of cerebral circulation. 2012;43(3):860–862. doi: 10.1161/STROKEAHA.111.626184. [DOI] [PubMed] [Google Scholar]
- 37.Harteveld AA, van der Kolk AG, van der Worp HB, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol. 2016 doi: 10.1007/s00330-016-4483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.