Abstract
Our meta-analysis published in 2010 provided evidence that low levels of serum albumin (hypoalbuminemia) are a significant independent predictor of acute kidney injury (AKI) and death following AKI. Since then, a large volume of additional data from observational clinical studies has been published further evaluating the relationship between serum albumin and AKI occurrence. This is an updated review of the literature to re-evaluate the hypothesis that hypoalbuminemia is independently associated with increased AKI risk. Eligible studies published from September 2009 to December 2016 were sought in PubMed (MEDLINE) and forty-three were retained, the great majority being retrospective observational cohort studies. These included a total of about 68000 subjects across a diverse range of settings, predominantly cardiac surgery and acute coronary interventions, infectious diseases, transplant surgery, and cancer. Appraisal of this latest data set served to conclusively corroborate and confirm our earlier hypothesis that lower serum albumin is an independent predictor both of AKI and death after AKI, across a range of clinical scenarios. The body of evidence indicates that hypoalbuminemia may causally contribute to development of AKI. Furthermore, administration of human albumin solution has the potential to prevent AKI; a randomized, controlled study provides evidence that correcting hypoalbuminemia may be renal-protective. Therefore, measurement of serum albumin to diagnose hypoalbuminemia may help identify high-risk patients who may benefit from treatment with exogenous human albumin. Multi-center, prospective, randomized, interventional studies are warranted, along with basic research to define the mechanisms through which albumin affords nephroprotection.
Keywords: Acute kidney injury, Acute renal failure, Hypoalbuminemia, Mortality, Prevention
Core tip: The relationship between hypoalbuminemia and acute kidney injury (AKI)-related morbidity/mortality is now confirmed. This association is consistently evident in a wealth of observational studies conducted across a wide range of clinical settings, and suggests a causal link. Prospective studies adequately powered to assess severe AKI, mortality and causality are needed, as is evaluation of the trigger and appropriate target serum levels and albumin dose necessary to confer renal protection. Basic research is also warranted to define the mechanisms through which albumin affords nephroprotection. Serum albumin should be measured to identify patients with increased AKI risk who may benefit from treatment correcting underlying hypoalbuminemia.
INTRODUCTION
Acute kidney injury (AKI), formerly referred to as acute renal failure (ARF), is a syndrome in which kidney function deteriorates rapidly over a period of hours or days. It is characterized by increased serum creatinine level (of ≥ 0.3 mg/dL in 48 h and/or 1.5-fold within 7 d) and decreased urine output. The staging system for AKI has evolved from the risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) criteria, to the acute kidney injury network (AKIN) scheme, and most recently to the Kidney Disease Improving Global Outcomes (KDIGO) score (for a review of AKI diagnosis, see[1]).
AKI is an acute systemic disease with major consequences for other organs besides the kidney, and is associated with significant short-term effects (e.g., fluid, electrolyte, and acid-base abnormalities, uremic toxin accumulation, cytokine elevation, systemic inflammation) and long-term adverse outcomes (e.g., myocardial infarction, chronic kidney disease, end-stage renal disease, mortality)[2,3]. Need for dialysis and transplantation are increased, as is length of hospital stay[4,5].
AKI is recognized as a major global health problem, with increasing incidence in both high- and low-income nations, and high associated healthcare costs[6,7]. It is a common disorder encountered in multiple settings, occurring in 21% of hospital admissions worldwide and in more than 13 million people each year[5], especially critically ill patients[1], where incidence rates well above 50% were reported in the recent prospective AKI-EPI study[8]. The International Society of Nephrology’s 0by25 initiative sets out a framework to eradicate preventable death from AKI by 2025 based on the 5 Rs: Risk, Recognition, Response, Renal Support, and Rehabilitation[5].
The etiology of AKI includes community-acquired causes (common in developing countries), such as infections (malaria, dengue, gastroenteritis, pneumonia), acute glomerular disease, underlying chronic disease (kidney, cardiac, diabetes), and trauma, as well as hospital-acquired causes (especially in industrialized nations), such as surgery, hemorrhage, infection, septic shock, drug toxicity, and underlying chronic disease (reviewed in[7]). The pathogenesis of AKI is multifactorial and several risk factors have been identified, both modifiable (e.g., dehydration, intravascular volume depletion, hypotension, anemia, hypoxia, body mass index) and non-modifiable (e.g., age, sex, prior invasive procedures, high-risk surgery, and comorbid disorders such as cancer and lung, liver or gastrointestinal disease)[5,9].
Hypoalbuminemia, or low levels of serum albumin (often defined as < 3.5-4.0 g/dL or ≤ 3.5 mmol/L), is a well-established risk factor for increased morbidity and mortality[10] and has also been associated with an increased risk of AKI occurrence; it is modifiable by infusion of human albumin solution. Our systematic review and meta-analysis published in 2010 found evidence, from observational studies in surgical and ICU patients, that low serum albumin is a significant independent predictor of AKI [pooled odds ratio (OR) = 2.34, 95%CI: 1.74-3.14] and of death following AKI (pooled OR = 2.47, 95%CI: 1.51-4.05)[9].
Since then, a large volume of additional clinical data has been published on hypoalbuminemia and AKI, across an expanded range of settings. Therefore, we performed an updated review of the literature to define the role of hypoalbuminemia and albumin administration in the development and prevention of AKI. Detailed discussion of the potential nephroprotective mechanisms of albumin is beyond the scope of the present review but was the subject of a separate review[11].
RESEARCH
Literature was sourced by conducting a systematic search of the PubMed (MEDLINE) database using phrases and synonyms for “kidney injury”, “albumin”, “hypoalbuminemia” and “mortality” (Table 1). The search was limited to articles published from September 2009 to December 2016 (inclusive), i.e., since the end of the search period used in our 2010 systematic review[9].
Table 1.
PubMed (MEDLINE) search strategy
| 1 Search: "acute kidney injury" OR AKI OR "acute renal failure" OR ARF |
| 2 Search: mortality OR survival OR death |
| 3 Search: "serum albumin" OR hypoalbuminemia* OR hypoalbuminaemia* |
| 4 Filter: Publication date from 2009/09/01 to 2016/12/31 |
| 5 Search: #1 AND #2 AND #3 AND #4 |
The search was conducted without applying restrictions to language or publication type.
Only studies meeting the following selection criteria were retained for inclusion in the present review: Reported original data on associations between serum albumin levels (absolute levels, variously defined by authors as < 3.5 or < 3.0 or < 2.5 g/dL, or percentage prevalence of hypoalbuminemia) and the development of AKI (during the different observation periods, depending on the indication), or on mortality in patients with both AKI and hypoalbuminemia.
From included studies, data were extracted on clinical setting/population, study design and size, assessment of albumin levels/hypoalbuminemia, AKI occurrence/risk, and mortality (Table 2, Table 3, Table 4 and Table 5).
Table 2.
Included studies on cardiac surgery and acute coronary interventions
| Ref. | Population/setting | Study design | Overall study size | Albumin measurement |
Hypoalbuminemia-related outcomes |
|
| AKI/ARF | Mortality | |||||
| Lee et al[13] | OPCAB surgery | Prospective RCT | 220 | Postoperative albumin 3.5-3.9 vs < 3.0 g/dL | Increased rate: 29.5% vs 41.7%. AKI rate lower with albumin vs control (13.7% vs 25.7%; P = 0.048) | ND |
| Grodin et al[20] | Acute heart failure | Prospective, observational | 456 | Admission albumin level (continuous and stratified by median ≥ 3.5 g/dL) | NS | NS |
| Moguel-González et al[16] | Cardiac surgery | Prospective, observational, longitudinal | 164 | Preoperative albumin < 4.0 g/dL | Increased risk: OR = 3.852 (95%CI: 1.101-13.473; P = 0.063) | ND |
| Lee et al[14] | OPCAB surgery | Retrospective, observational, propensity score matching | 1182 (incl. 323 matched pairs) | Preoperative albumin < 4.0 g/dL | Increased risk: OR = 1.83 (95%CI: 1.27-2.64); P = 0.001; propensity analysis: OR = 1.62 (95%CI: 1.12-2.35); P = 0.011 | ND |
| Murat et al[21] | ACS and PCI | Retrospective, observational | 890 | Albumin level at hospitalization | Low albumin (3.52 g/dL vs 3.94 g/dL) predictive of CI-AKI: OR = 0.177 (95%CI: 0.080-0.392; P < 0.001) | ND |
| Kim et al[17] | Thoracic aorta repair with CPB | Retrospective, observational, propensity score matching | 702 (incl. 183 matched pairs) | Preoperative albumin < 4.0 g/dL | Increased risk: OR = 2.50 (95%CI: 1.39-4.50; P = 0.002) | ND |
| Findik et al[15] | CAB surgery | Retrospective, observational | 530 | Preoperative albumin < 3.5 g/dL | Increased rate: OR = 1.661 (95%CI: 1.037-2.661); P = 0.035 | ND |
| Go et al[19] | LVAD implantation | Retrospective, observational | 200 | < 2.5 g/dL (low) vs 2.5-3.5 g/dL (mid-range) vs > 3.5 g/dL (normal) | Increased ARF: 42.9% vs 16.5% vs 17.3%; P = 0.05 | NS |
ACS: Acute coronary syndromes; AKI: Acute kidney injury; ARF: Acute renal failure; CI-AKI: Contrast-induced acute kidney injury; CPB: Cardiopulmonary bypass; LVAD: Left ventricular assist device; ND: Not disclosed; NS: Not significant; OPCAB: Off-pump coronary artery bypass; OR: Odds ratio; PCS: Percutaneous coronary intervention; RCT: Randomized, controlled trial.
Table 3.
Included studies on infectious diseases
| Ref. | Population/setting | Study design | Overall study size | Albumin measurement |
Hypoalbuminemia-related outcomes |
|
| AKI/ARF | Mortality | |||||
| Prakash et al[22] | HIV | Prospective, observational | 3540 | Albumin level at hospitalization | ND | 2.14 g/dL in patients who died vs 3.2 g/dL in survivors; P < 0.001 |
| Vannaphan et al[34] | Severe falciparum malaria | Retrospective, observational | 915 | Albumin < 3.5 g/dL | Associated with ARF (P < 0.001) | ND |
| Lee et al[39] | Acute viral hepatitis A | Retrospective, observational | 391 | Albumin < 3.0 g/dL | OR = 8.24 (95%CI: 2.53-26.86; P < 0.0001) | ND |
| Lee et al[35] | Scrub typhus | Retrospective, observational | 246 | Admission albumin < 3.0 g/dL vs ≥ 3.0 g/dL | Increased rate of non-oliguric ARF (40.4% vs 11.1%; P < 0.001) | ND |
| Mehra et al[40] | Dengue fever | Retrospective, observational | 223 | Admission Albumin level | Lower albumin (2.65 g/dL) in patients with vs without AKI (3.09 g/dL; P < 0.001) | ND |
| Vikrant et al[36] | Scrub typhus | Retrospective, observational | 174 | Admission albumin level | ND | 2.4 g/dL in patients who died vs 2.9 g/dL in survivors; P < 0.001 |
| Ceylan et al[41] | Antibiotic therapy | Retrospective, observational | 112 | Albumin level at start of colistin therapy | Lower albumin (2.4 g/dL vs 2.7 g/dL) predicts colistin-induced AKI: OR = 0.643 (95%CI: 0.415-0.994; P = 0.047) | ND |
| Trimarchi et al[37] | H1N1 pneumonia | Retrospective, observational | 22 | Albumin level at study inclusion | NS | ARF in 10 of 12 deaths: 1.82 g/dL in patients who died vs 2.61 g/dL in survivors; P < 0.01 |
AKI: Acute kidney injury; ARF: Acute renal failure; ND: Not disclosed; OR: Odds ratio; NS: Not significant.
Table 4.
Included studies on transplant surgery
| Ref. | Population/setting | Study design | Overall study size | Albumin measurement |
Hypoalbuminemia-related outcomes |
|
| AKI/ARF | Mortality | |||||
| Tinti et al[45] | Liver transplantation | Prospective, observational | 24 | Preoperative albumin level | Lower albumin (3.1 g/dL vs 3.7 g/dL) predictive of ARF (P = 0.02) | ND |
| Moore et al[48] | Renal transplantation | Retrospective, observational | 2763 | Albumin < 4.0 g/dL | Predictive of transplant failure: HR = 1.71 (95%CI: 1.18-2.49; P < 0.001) | ND |
| Sang et al[46] | LDLT | Retrospective, observational, propensity score matching | 998 (incl. 249 matched pairs) | Albumin < 3.0 g/dL vs ≥ 3.0 g/dL before surgery | Albumin < 3.0 g/dL associated with increased AKI: OR = 0.42 (95%CI: 0.28-0.64; P < 0.001) | Survival rate lower with postoperative albumin < 3.0 g/dL (P = 0.02) |
| Park et al[47] | LDLT | Retrospective, observational | 538 | Preoperative albumin level | Albumin < 3.5 g/dL: OR = 1.76 (95%CI: 1.05-2.94; P = 0.032) | ND |
| Yang et al[49] | Renal transplantation | Retrospective, observational | 375 | Preoperative albumin < 3.5 g/dL vs 3.5-3.9 g/dL vs 4.0-4.4 g/dL vs ≥ 4.5 g/dL | Lowest risk of graft failure with ≥ 4.5 g/dL: HR = 0.536 (P = 0.029) vs < 3.5 g/dL | ND |
| Chen et al[44] | Liver transplantation | Retrospective, observational, matching | 334 (incl. 118 matched pairs) | Preoperative albumin ≤ 3.5 g/dL | OR = 2.785 (95%CI: 1.427-5.434; P = 0.003); risk factor for posttransplantation AKI or ARF | ND |
AKI: Acute kidney injury; ARF: Acute renal failure; HR: Hazard ratio; LDLT: Living donor liver transplantation; ND: Not disclosed; OR: Odds ratio.
Table 5.
Included studies on cancer
| Ref. | Population/setting | Study design | Overall study size | Albumin measurement |
Hypoalbuminemia-related outcomes |
|
| AKI/ARF | Mortality | |||||
| Hsu et al[51] | HCC with ascites | Prospective, observational | 591 | Albumin < 3.3 g/dL | Independently associated with ARF: OR = 7.3 (95%CI: 1.47-35.7; P = 0.009) | ND |
| Kim et al[50] | Gastric cancer surgery | Retrospective, observational | 4718 | Preoperative albumin < 4.0 g/dL | Independent predictor of AKI: OR = 1.40 (95%CI: 1.11-1.77; P = 0.005) | ND |
| Mizuno et al[55] | Chemotherapy-induced hypotension | Retrospective, observational | 972 | Hypoalbuminemia defined as ≤ 3.5 g/dL | Associated with low BP: OR = 1.497 (95%CI: 1.070-2.095; P = 0.019). Low BP associated with AKI | ND |
| Lahoti et al[56] | AML or HR-MDS | Retrospective, observational | 537 | Albumin level at baseline (median 3.3 g/dL) | Hypoalbuminemia predictive of AKI: OR = 0.7 (95%CI: 0.5-0.99; P = 0.049) | ND |
| Haynes et al[57] | Multiple myeloma | Retrospective, observational | 107 | Albumin ≥ 3.5 g/dL vs < 3.5 g/dL | ND | Higher albumin predictive of survival: HR = 0.56 (95%CI: 0.35-0.91; P = 0.02) |
| Fischler et al[59] | Cancer | Retrospective, observational | 103 | Albumin level at start of CVVHDF | ND | Low albumin (median 2.5 g/dL vs 3.05 g/dL) associated with mortality: OR = 3.341 (95%CI: 1.229-9.077); P = 0.02 |
AKI: Acute kidney injury; AML: Acute myelogenous leukemia; ARF: Acute renal failure; BP: Blood pressure; CVVHDF: Continuous venovenous hemodiafiltration; HCC: Hepatocellular carcinoma; HR: Hazard ratio; HR-MDS: High-risk myelodysplastic syndrome; ND: Not disclosed; OR: Odds ratio.
INCLUDED STUDIES
Our search retrieved a total of 206 entries on PubMed (MEDLINE). The titles and abstracts of all hits were screened and research articles that did not report relevant data as stated in the selection criteria were discarded, along with any review/opinion articles. A total of 43 research articles were retained for inclusion in the present review (Figure 1). The great majority of these were retrospective in nature (37 of 43 studies), except for a single interventional randomized controlled trial (RCT), conducted in off-pump coronary artery bypass (OPCAB) surgery, and five prospective observational studies: Two in cardiac settings, one in HIV-infected patients, one in liver transplant recipients, and one in patients with hepatocellular carcinoma (HCC) with ascites. In total, the data set comprises approximately 68000 patients across a range of clinical settings, as described and discussed below.
Figure 1.
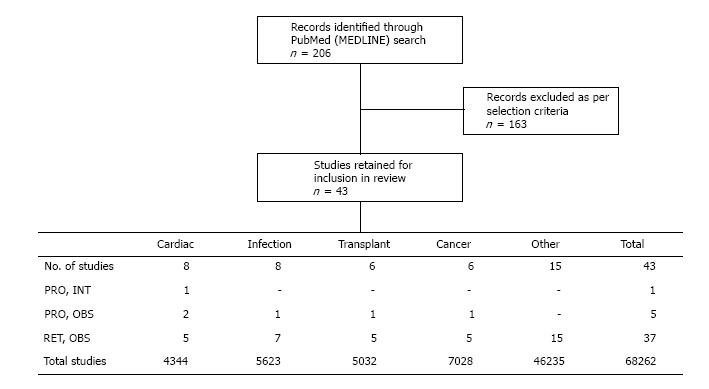
Flow diagram summarizing the literature screening and the designs and settings of the included studies. The numbers presented for the sizes of the data sets include control populations and do not represent only patients with acute kidney injury/acute renal failure and/or hypoalbuminemia. OBS: Observational; INT: Interventional; PRO: Prospective; RET: Retrospective.
CARDIAC SURGERY AND ACUTE CORONARY INTERVENTIONS
AKI is a common complication following cardiac surgery, and is associated with significant morbidity, mortality, and hospital costs (reviewed in[12]). We identified eight studies, involving more than 4000 subjects, which explored the role of albumin in AKI occurring after cardiac surgery or coronary intervention (Table 2).
In the only interventional drug study among the 43 retained articles, Lee et al[13] recently performed a single-center, randomized, parallel-arm, double-blind trial which evaluated the effects of exogenous 20% human albumin solution vs saline on the incidence of postoperative AKI in adult patients with hypoalbuminemia (< 4.0 g/dL) undergoing off-pump coronary artery bypass (OPCAB) surgery. Dose was 100, 200, or 300 mL immediately before surgery, stratified according to preoperative serum albumin level of 3.5-3.9, 3.0-3.4, or < 3.0 g/dL, respectively. In the saline group, rate of postoperative AKI (KDIGO-criteria patients) appeared to increase as postoperative serum albumin level decreased (29.5%, 31.1%, 41.7% for 3.5-3.9, 3.0-3.4, < 3.0 g/dL, respectively). The incidence of postoperative AKI (KDIGO criteria) was lower in the albumin group compared with the saline group (17.6% vs 31.7%; P = 0.031). Multivariate logistic regression revealed a renal-protective effect of albumin therapy (OR = 0.42, 95%CI: 0.21-0.83; P = 0.012). Administration of albumin increased urine output during surgery (median 550 mL vs 370 mL; P = 0.006). No differences were observed between the two groups in the incidence of severe AKI, need for renal replacement therapy (RRT), or mortality.
These findings are interesting for a number of reasons. First, the inverse relationship apparent between serum albumin level and postoperative AKI rate, in the setting of a randomized double-blind trial, provides the highest-quality and most compelling evidence yet that serum albumin level is an independent driver of AKI risk. This corroborates an earlier retrospective analysis in 1182 consecutive adult patients undergoing OPCAB, conducted by the same group[14]. Moreover, the data from Lee et al[13] further underline the importance of the relationship between albumin level and renal health, as correction of hypoalbuminemia by exogenous albumin supplementation resulted in smaller increases in serum creatinine and conferred a degree of protection against AKI occurrence. That no significant treatment effect on severe AKI was observed (≥ KDIGO stage 2) might reflect an underpowered analysis due to the relatively low sample size/event rate. Alternatively, either albumin supplementation is beneficial only in milder AKI, or the dosing regimen was insufficient.
Lee et al[13] did not investigate the mechanism(s) underlying the renal-protective effect they observed with albumin administration but speculated whether this might be attributable to augmentation of intravascular volume over correction of hypoalbuminemia. Indeed, both hemodynamic and pharmacodynamic mechanisms may contribute to the beneficial renal effects of albumin, supporting a possible causal link. Pharmacodynamic properties of human albumin with renal-protective potential include mitigation of nephrotoxicity of medications, restoration of balanced net fluid balance, protection against loss of glycocalyx, and maintenance of glomerular filtration (reviewed in[11]). Further studies are needed to ascertain in which clinical indications such properties might be beneficial. In addition, data from large-scale RCTs are needed to define trigger and target levels and dosing for pre-emptive albumin therapy as a strategy for protecting against postoperative renal morbidity and mortality.
Whereas in the RCT performed by Lee et al[13] no differences were evident between the albumin and saline treatment groups with respect to subsequent need for RRT or mortality, precedent does exist for the impact of albumin level on both of these outcomes. Findik et al[15] recently performed a retrospective review of data collected prospectively from 530 adults with normal renal function who underwent isolated CAB surgery. Their analysis divided the patient population based on preoperative serum albumin level and found that RRT (P = 0.018) and death within 30 d (6.8% vs 2.4%; P = 0.037) after surgery were more frequent in the group with albumin < 3.5 g/dL. Mean duration of ventilatory support (7.9 h vs 11.4 h; P = 0.001), ICU stay (66.0 h vs 59.0 h; P = 0.026), and hospital stay (7.7 d vs 7.1 d; P = 0.022) were also greater in the lower albumin group.
Beyond CAB surgery, a prospective, observational, longitudinal study of 164 adult patients undergoing any type of elective cardiac surgery used univariate logistic regression analysis to identify low serum albumin (< 4 g/dL), among other variables [high preoperative blood urea nitrogen (BUN), creatinine, and uric acid], as a major risk factor for postoperative development of AKI[16]. Similarly, Kim et al[17] also identified preoperative albumin level < 4.0 g/dL as an independent risk factor for AKI in 183 patients who underwent surgery on the thoracic aorta with cardiopulmonary bypass (CPB) and subsequently developed AKI, matched by propensity score with controls without AKI. The authors suggested that correction of preoperative hypoalbuminemia might protect against AKI in the studied population. However, when patients converted to CPB were included in the randomized analysis performed by Lee et al[13], the effect of albumin treatment was unclear. Further research is needed to evaluate the effects of exogenous albumin treatment in patients undergoing cardiac surgery with CPB, though such studies will be complicated by the fact that CPB itself is associated with AKI and contributes to its pathogenesis (reviewed in[18]).
In a single-center, retrospective review, Go et al[19] aimed to establish the impact of different serum albumin strata (< 2.5 g/dL, low; 2.5-3.5 g/dL, mid-range; > 3.5 g/dL, normal) on outcomes after left ventricular assist device (LVAD) implantation in 200 patients. Consistent with findings in cardiac surgery patients, lower albumin was associated with significantly increased rates of postoperative ARF (Table 2) and prolonged hospitalization (median 28.5 d vs 16 d vs 15.5 d; P = 0.008). Survival at 6 mo, 1 year, and 5 years appeared to reflect albumin levels (79%, 79%, 49% with low; 84%, 78%, 51% with mid-range; 94%, 88%, 60% with normal), though this trend was not statistically significant (P = 0.22). The authors concluded that hypoalbuminemia (in this case defined as < 2.5 g/dL) should not be considered a contraindication to LVAD candidacy, and called for more data on the utility of albumin levels for predicting morbidity and mortality after LVAD implantation.
Two studies reported data on albumin levels in patients undergoing acute coronary interventions. In a prospective, observational study of 456 acute heart failure patients undergoing decongestive therapy, no significant associations were found between serum albumin levels at admission and clinical outcomes, either short-term (worsening renal function, worsening heart failure, clinical decongestion by 72 h) or longer-term (60-d mortality, re-hospitalization, unscheduled emergency room visits)[20]. The authors concluded that serum albumin levels might not be relevant in guiding decongestion strategies. They also acknowledged that their post-hoc analysis used a carefully selected cohort drawn from the DOSE-AHF and ROSE-AHF trials that were inadequately powered to detect clinical end points according to baseline albumin, and that their findings may not be generalizable. A separate study, by Murat et al[21], retrospectively looked at the impact of serum albumin levels on AKI occurrence in a cohort of 890 patients with acute coronary syndromes (ACS) treated with percutaneous coronary intervention (PCI). Serum albumin was inversely associated with AKI risk and, along with a number of other variables [age, female gender, creatinine kinase-myocardial band, and glomerular filtration rate (GFR)], was independently predictive of AKI occurrence. This was the first such report in ACS patients receiving PCI and requires confirmation by prospective, randomized trial. Nonetheless, these preliminary findings suggest that measurement of serum albumin, widely available and relatively inexpensive, should be included in the risk stratification of ACS patients before undergoing PCI.
INFECTIOUS DISEASES
Our review identified eight studies conducted across a total of more than 5500 subjects with infections and data on albumin levels and renal injury (Table 3). The only prospective study was an observational analysis of AKI in HIV-seropositive adults[22]. AKI was noted in 138/3540 (3.9%) patients and in most cases was AKIN stage II (42.1%) or III (48.5%). Mean serum albumin at baseline was 2.92 g/dL. Low serum albumin was one of few variables found to be significantly associated with death of HIV-infected patients following AKI. Prerenal factors (e.g., clinical/laboratory evidence of volume depletion or reduced renal blood flow), ischemic acute tubular necrosis (ATN), and sepsis were among the most frequent causes of AKI in this population. These observations are consistent with clinical trials suggesting efficacy and mortality benefit of albumin therapy for volume resuscitation in sepsis patients[23-30], as well as mechanistic data indicating a role for albumin in preservation of renal tubular cells[31-33].
The largest study on AKI occurrence in an infectious disease setting to be published during the review period was a 10-year retrospective analysis of AKI occurrence in 915 severe falciparum malaria patients[34]. AKI is a major contributor to morbidity and mortality in severe malaria infection, and hypalbuminemia (< 3.5 g/dL) was significantly more prevalent in patients with AKI (135/195; 69%) vs those without AKI (308/720; 43%). The authors concluded that although causality could not be deduced, correction of ARF risk factors such as hypoalbuminemia should be incorporated into the management of patients with severe malaria.
Two retrospective studies reported associations between serum albumin level and AKI outcomes in scrub typhus infection. Lee et al[35] divided a population of 246 adults with scrub typhus into two groups based on serum albumin level. Their analysis revealed that serum albumin < 3.0 g/dL was closely related to AKI occurrence as well as various other complications (e.g., confusion, pulmonary edema, pleural effusion, arrhythmia), leading to longer mean hospital stay (additional 5.5 d) and higher direct hospital costs (additional United States $1222). Whereas this study found no difference in mortality with lower vs higher albumin (overall deaths 9/246; 3.7%), a subsequent study by Vikrant et al[36] did detect a difference. This later study was smaller overall than that conducted by Lee et al[35] but provided a larger cohort of scrub typhus patients with AKI and substantially more deaths occurred (28/174; 16.1%), providing greater power to assess mortality. Mean serum albumin was 2.8 g/dL and hypoalbuminemia, again defined as < 3.0 g/dL, was present in 56.9% of study subjects. Serum albumin was significantly higher in survivors vs non-survivors (mean 2.9 g/dL vs 2.4 g/dL; P = 0.001).
An association between lower serum albumin and mortality has also been observed in critically compromised patients with H1N1 pneumonia. In a 2-mo, retrospective, ICU study, both hypoalbuminemia and AKI were significantly associated with death (P < 0.01), and serum albumin appeared to be lower in those with vs without AKI (1.95 g/dL vs 2.59 g/dL), though this difference did not reach statistical significance in this small cohort (n = 22)[37]. Larger, multivariate analysis is required to confirm these results. The authors suggested that rhabdomyolysis (life-threatening muscle disintegration) is likely to be the main pathophysiologic mechanism of renal dysfunction in this setting, and a separate study subsequently linked hypoalbuminemia with AKI in patients with severe rhabdomyolysis[38].
Other reports on low serum albumin as a predictive factor for AKI include a chart review and multivariate analysis of data from 391 patients with acute viral hepatitis A[39]. AKI was present in 45 (11.5%) patients, and the AKI group had significantly decreased albumin levels compared with the non-AKI group at presentation (mean 3.3 g/dL vs 3.8 g/dL; P < 0.0001) and at peak during illness (mean 2.7 g/dL vs 3.4 g/dL; P < 0.0001). Among 223 patients with dengue fever, AKI developed in 24 (10.8%) and was associated with lower serum albumin (2.65 g/dL vs 3.09 g/dL; P < 0.001)[40]. Low serum albumin (median 2.4 g/dL vs 2.7 g/dL) has also been shown to be independently predictive of acute renal injury in patients receiving antibiotic therapy with colistin (n = 112)[41], consistent with previous observations[42,43].
TRANSPLANT SURGERY
Hypoalbuminemia is common before and after liver transplantation, especially in patients with cirrhosis[44], and there is a growing body of research exploring the implications of this. Our searches retrieved six relevant studies (including > 5000 subjects) published since September 2009 that reported albumin levels in relation to AKI occurrence after transplantation (Table 4). Four of these were in the setting of liver transplantation (approximately 2000 subjects), while 2 studies focused on renal transplant recipients. All six studies were non-interventional and all except one were conducted retrospectively.
In the only prospective study, data on 24 patients were collected from health records before, during and after deceased donor orthotopic liver transplantation (OLT)[45]. Reduced pre-OLT serum albumin level was found to be associated with ARF (RIFLE classification), whereas no other pre-OLT parameters (e.g., creatinine, GFR, serum sodium, serum bilirubin) were. Interestingly though, higher Model for End-Stage Liver Disease (MELD) score (mean 22 vs 18; P = 0.02) was also associated with AKI, leading the authors to speculate that the significance of hypoalbuminemia and higher MELD score as risk factors in this population might reflect the close relationship between renal and hepatic function in cirrhosis.
The largest of the three retrospective, observational studies assessing hypoalbuminemia in liver transplantation was a propensity score analysis of 998 consecutive living donor liver transplantation (LDLT) patients in a single center[46]. This analysis aimed to ascertain the influence of early postoperative serum albumin level on subsequent development of AKI. Serum albumin < 3.0 g/dL within 48 h postoperatively was identified by multivariate analysis as an independent risk factor for AKI (AKIN or RIFLE classification; Table 4), the first such report in the setting of LDLT. Furthermore, ICU (P = 0.006) and hospital (P < 0.001) stays were prolonged in the low albumin group and overall mortality was also higher (P = 0.02), making this one of the few studies retained from our literature review to report data associating serum albumin level with mortality. The findings of Sang et al[46] are consistent with an earlier, smaller retrospective study by Chen et al[44] in which multivariable analysis of 118 matched pairs of liver transplantation patients with/without postoperative renal injury demonstrated that preoperative hypoalbuminemia (≤ 3.5 g/dL) was strongly predictive of AKI within the first week after surgery. Similarly, Park et al[47] also identified by multivariate analysis that preoperative serum albumin < 3.5 g/dL was an independent, modifiable risk factor for AKI (RIFLE classification) in patients (n = 538) undergoing LDLT. Interestingly, the authors also identified MELD score > 20 as a significant risk factor for post-LDLT AKI (OR = 2.01, 95%CI: 1.17-3.44; P = 0.011), as in the study by Tinti et al[45], providing further evidence of renal-hepatic interactions in patients undergoing liver transplantation.
Notwithstanding the inherent limitations of retrospective, observational analysis, Sang et al[46] postulated that, when considering their results together with evidence accruing from other studies, hypoalbuminemia may be one of the major contributors to AKI development. Park et al[47] noted the reported nephroprotective capacity of albumin, through enhanced renal perfusion and reduced apoptosis/increased proliferation of renal tubular cells[31-33]. However, they questioned whether exogenous augmentation of serum albumin could modify renal dysfunction within a short timeframe and reduce the risk of postoperative AKI. Sufficiently powered, prospective, interventional, randomized trials will be required to answer this question. Additional research will also be needed to further elucidate the mechanism(s) by which albumin can positively influence renal function, in LDLT surgery and other settings.
Fewer studies provide evidence on albumin levels in renal transplantation; however, these studies enrolled more subjects than those discussed above in the setting of liver transplantation. One large retrospective study, by Moore et al[48], analyzed data from 2763 adult kidney transplant recipients who were enrolled into the Long Term Efficacy and Safety Surveillance study and survived for ≥ 12 mo post transplantation. Multiple regression analysis revealed that hypoalbuminemia in the preceding 6 mo was independently associated with renal transplant failure, both in the death-censored analysis [< 3.5 g/dL, hazard ratio (HR) = 2.19, 95%CI: 1.58-3.05; P < 0.001] and overall (Table 4). Yang et al[49] also found a relationship between serum albumin level and likelihood of renal graft failure. In their single-center study of 375 renal transplant recipients, the relative risk of graft failure was lowest in the group with highest serum albumin (≥ 4.5 g/dL) before transplantation. Chronic rejection (36.2%) and delayed graft function (12.8%) were most frequent in patients with albumin < 3.5 g/dL, though these results were not statistically significant. The authors concluded that hypoalbuminemia before kidney transplantation is associated with more serious complications and worse short- and long-term graft outcomes.
CANCER
An association between hypoalbuminemia and AKI morbidity/mortality has also been noted in cancer studies. We identified six relevant studies published during the search period, involving a total of more than 7000 patients, the majority of whom had gastric cancer[50] (Table 5).
One of these studies was prospective (n = 591) and included data on incidence and risk factors of AKI in a subset of 87 patients with HCC with ascites undergoing transarterial chemoembolization (TACE) at a single center[51]. Lower serum albumin was more common in HCC patients with vs without ascites (mean 3.2 g/dL vs 3.8 g/dL; P < 0.001). Furthermore, hypoalbuminemia (< 3.3 g/dL) occurred in 82% vs 38% of ascitic HCC patients who did (n = 11) vs did not (n = 76) develop AKI after TACE (P = 0.009). Logistic regression analysis among HCC patients with ascites found that hypoalbuminemia was the only risk factor independently predictive of post-TACE AKI. Based on earlier reports, the authors speculated whether nephroprotection by albumin might result from increased renal blood flow and GFR[52], decreased changes in electrolyte and serum creatinine levels[52], or plasma expansion[53,54].
The largest retained study in cancer patients was a retrospective analysis of AKI occurrence after partial or total gastrectomy for gastric cancer (n = 4718)[50]. Multivariate analysis identified hypoalbuminemia (< 4 g/dL), along with male gender, hypertension, chronic obstructive pulmonary disease, use of diuretics, vasopressors, or contrast agents, and packed red blood cell transfusions, as an independent predictor of postoperative AKI (Table 5). Prevalence of hypoalbuminemia tended to increase with severity of AKI (KDIGO staging). The authors acknowledged that the mechanisms through which hypoalbuminemia causes AKI are not fully understood, but noted that albumin plays a critical role in maintaining the integrity and function of renal tubular cells[32,33].
Smaller retrospective studies also found significant associations between serum albumin level and AKI development in cancer settings. Mizuno et al[55] demonstrated significantly lower serum albumin in patients receiving cisplatin as first-line chemotherapy who had low (n = 229) vs normal (n = 743) blood pressure (mean 3.73 g/dL vs 3.87 g/dL; P = 0.001), suggesting that hypoalbuminemia associates with low blood pressure, leading to renal hypoperfusion and thereby promoting ischemic AKI. Hypoalbuminemia was similarly associated with AKI in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome undergoing induction chemotherapy (n = 537; 187 with AKI)[56]. In a 20-year, single-center, retrospective study of patients with multiple myeloma and acute severe renal failure (n = 107), serum albumin ≥ 3.5 g/dL was one of three factors (along with use of chemotherapy, and dialysis independence) found to be independently associated with survival[57], though serum albumin is already incorporated into the International Staging System for multiple myeloma[58]. A recent multivariate analysis of 103 consecutive ICU patients with cancer (any type) and AKI revealed low albumin level to be statistically associated with in-hospital mortality, leading to the conclusion that hypoalbuminemia (and presumably correction thereof) must be considered before initiating RRT in cancer patients[59].
OTHER INDICATIONS
The majority of the recent studies generating data on hypoalbuminemia and AKI occurrence were conducted in the settings of cardiac/coronary interventions, infectious diseases, transplantation, or cancer, as described above. However, a further fifteen studies, all retrospective and observational in nature and involving a total of more than 46000 patients, also reported data on albumin levels and kidney outcomes, across numerous other patient populations.
By far the largest albumin data set among all of the literature retained for this review was a retrospective analysis of 37143 patients in the American College of Surgeons National Surgical Quality Improvement Program who underwent primary total knee arthroplasty (TKA) and had serum albumin data available[60]. Mortality was higher in patients with albumin < 3.5 g/dL vs ≥ 3.5 g/dL (0.64% vs 0.15%; OR = 3.17, 95%CI: 1.58-6.35; P = 0.001), as were AKI (0.32% vs 0.06%, OR = 5.19, 95%CI: 1.96-13.73; P = 0.001) and a range of other major perioperative complications. The authors drew encouragement from these findings since hypoalbuminemia may be more easily modifiable than other risk factors (e.g., morbid obesity). Results from two subsequent studies also involving thousands of patients undergoing TKA (primary[61] or revision[62]) were consistent with these findings.
In a recent analysis of 408 patients with amyloidosis, serum albumin < 2.5 g/dL at admission was highly associated with requirement for RRT within 30 d of autologous stem cell transplantation (P < 0.001)[63]. A smaller study in amyloidosis patients receiving high-dose melphalan with stem cell transplantation yielded similar results[64]. Recent evidence in other clinical scenarios comes from isolated studies involving a total of more than 2500 patients. Hypoalbuminemia has been associated with AKI-related morbidity/mortality in patients with severe rhabdomyolysis[38], pyogenic liver abscess[65], contrast-induced nephropathy[66], hospital-acquired AKI[67,68], and in critically ill patients requiring continuous RRT[69], geriatric patients[70-72], and those undergoing open ventral hernia repair[73].
Taken together, the recent evidence discussed herein clearly demonstrates that hypoalbuminemia is an important consideration for AKI risk in a broad range of patients. However, each clinical scenario is multifactorial and presents its own complexities, and comorbidities that might also impact the development of AKI and thus be confounders in assessing the relative importance/contribution of serum albumin level in AKI. In diverse clinical settings there is a need for controlled, interventional studies to evaluate exogenous albumin therapy aimed at correcting hypoalbuminemia and reducing the risk of subsequent AKI, mortality, and other adverse outcomes. Also required will be mechanistic studies to define the pathways involved in nephroprotection by albumin.
LIMITATIONS
Limitations of this review include the fact that most of the included studies were observational with patient populations in the various clinical settings that are still quite heterogeneous. In addition definitions of AKI were often creatinine-dependent and based on different classification systems for AKI including RIFLE, AKIN, and KDIGO. As the systematic search of the literature was restricted to PubMed (MEDLINE), additional studies may be missing.
CONCLUSION
The association between hypoalbuminemia and development of AKI and subsequent morbidity/mortality can be regarded as confirmed. This robust association is consistently evident in a wealth of observational studies conducted across a wide range of clinical settings and involving tens of thousands of patients, and may be interpreted as an indication of a causal link.
Furthermore, a prospective RCT conducted in cardiac surgery patients, demonstrated for the first time that correction of low albumin level is associated with lower increase in creatinine, suggesting improved renal function with human albumin therapy and also supporting a causal link. These observations justify further interventional studies with albumin therapy, in cardiac surgery (including CPB) and other settings, such as transplantation other than liver or renal. Multi-center, prospective studies adequately powered to assess severe AKI, mortality and causality would be valuable, as would evaluation of the appropriate trigger and target serum levels and albumin dose necessary to confer renal protection. Basic research is also warranted to define the mechanisms through which albumin affords protection from renal injury. Moreover, because the development of AKI in high-risk patients is multifactorial, modification of a single risk factor might not be sufficient for prevention. Therefore, further research is needed to advance our understanding of the combinatorial nature of AKI pathogenesis.
In the meantime, the large volume of data already available underscores the need to be alert to risk factors for AKI. Serum albumin level should be monitored to aid early identification of patients who may be at increased risk and who may stand to benefit from treatment to correct hypoalbuminemia.
ACKNOWLEDGMENTS
The authors are indebted to Ms Rajam Csordas for critical reading of the manuscript.
Footnotes
Conflict-of-interest statement: Christian J Wiedermann has received travel cost reimbursements from CSL Behring. Wolfgang Wiedermann has no conflict of interest to report. Michael Joannidis has received speaker’s honoraria from Astute Medical, Fresenius and Ortho Clinical Diagnostics.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 14, 2017
First decision: April 14, 2017
Article in press: May 6, 2017
P- Reviewer: Stavroulopoulos A, Trimarchi H S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20:299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singbartl K, Joannidis M. Short-term Effects of Acute Kidney Injury. Crit Care Clin. 2015;31:751–762. doi: 10.1016/j.ccc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 6.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 7.Ponce D, Balbi A. Acute kidney injury: risk factors and management challenges in developing countries. Int J Nephrol Renovasc Dis. 2016;9:193–200. doi: 10.2147/IJNRD.S104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 9.Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010;36:1657–1665. doi: 10.1007/s00134-010-1928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–334. doi: 10.1097/01.SLA.0000055547.93484.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiedermann CJ, Joannidis M. Nephroprotective Potential of Human Albumin Infusion: A Narrative Review. Gastroenterol Res Pract. 2015;2015:912839. doi: 10.1155/2015/912839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, Choo SJ, Chung CH, Lee JW, Choi IC. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less Than 4.0 g/dl. Anesthesiology. 2016;124:1001–1011. doi: 10.1097/ALN.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 14.Lee EH, Baek SH, Chin JH, Choi DK, Son HJ, Kim WJ, Hahm KD, Sim JY, Choi IC. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012;38:1478–1486. doi: 10.1007/s00134-012-2599-8. [DOI] [PubMed] [Google Scholar]
- 15.Findik O, Aydin U, Baris O, Parlar H, Alagoz GA, Ata Y, Turk T, Kunt AT. Preoperative Low Serum Albumin Levels Increase the Requirement of Renal Replacement Therapy after Cardiac Surgery. Heart Surg Forum. 2016;19:E123–E127. doi: 10.1532/hsf.1577. [DOI] [PubMed] [Google Scholar]
- 16.Moguel-González B, Wasung-de-Lay M, Tella-Vega P, Riquelme-Mc-Loughlin C, Villa AR, Madero M, Gamba G. Acute kidney injury in cardiac surgery. Rev Invest Clin. 2013;65:467–475. [PubMed] [Google Scholar]
- 17.Kim WH, Park MH, Kim HJ, Lim HY, Shim HS, Sohn JT, Kim CS, Lee SM. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: a propensity score matched case-control study. Medicine (Baltimore) 2015;94:e273. doi: 10.1097/MD.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar AB, Suneja M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology. 2011;114:964–970. doi: 10.1097/ALN.0b013e318210f86a. [DOI] [PubMed] [Google Scholar]
- 19.Go PH, Hodari A, Nemeh HW, Borgi J, Lanfear DE, Williams CT, Paone G, Morgan JA. Effect of Preoperative Albumin Levels on Outcomes in Patients Undergoing Left Ventricular Device Implantation. ASAIO J. 2015;61:734–737. doi: 10.1097/MAT.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 20.Grodin JL, Lala A, Stevens SR, DeVore AD, Cooper LB, Abou Ezzeddine OF, Mentz RJ, Groarke JD, Joyce E, Rosenthal JL, et al. Clinical Implications of Serum Albumin Levels in Acute Heart Failure: Insights From DOSE-AHF and ROSE-AHF. J Card Fail. 2016;22:884–890. doi: 10.1016/j.cardfail.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murat SN, Kurtul A, Yarlioglues M. Impact of Serum Albumin Levels on Contrast-Induced Acute Kidney Injury in Patients With Acute Coronary Syndromes Treated With Percutaneous Coronary Intervention. Angiology. 2015;66:732–737. doi: 10.1177/0003319714551979. [DOI] [PubMed] [Google Scholar]
- 22.Prakash J, Gupta T, Prakash S, Rathore SS, Usha S. Acute kidney injury in patients with human immunodeficiency virus infection. Indian J Nephrol. 2015;25:86–90. doi: 10.4103/0971-4065.138696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 24.Charpentier J, Mira JP. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: the EARSS study. Intensive Care Med. 2011;37(Suppl 1):S115. [Google Scholar]
- 25.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–391. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 26.Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 28.Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11:123–130.e1. doi: 10.1016/j.cgh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, De Backer D, Wiedermann CJ. Fluid management in sepsis: The potential beneficial effects of albumin. J Crit Care. 2016;35:161–167. doi: 10.1016/j.jcrc.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Wiedermann CJ, Joannidis M. Albumin replacement in severe sepsis or septic shock. N Engl J Med. 2014;371:83. doi: 10.1056/NEJMc1405675. [DOI] [PubMed] [Google Scholar]
- 31.Dixon R, Brunskill NJ. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: implications for the pathophysiology of proteinuric states. J Am Soc Nephrol. 1999;10:1487–1497. doi: 10.1681/ASN.V1071487. [DOI] [PubMed] [Google Scholar]
- 32.Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. 1999;277:F711–F722. doi: 10.1152/ajprenal.1999.277.5.F711. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann MA, Castelli I, Pargger H, Drop LJ. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: the role of serum albumin. J Pharmacol Exp Ther. 1995;273:855–862. [PubMed] [Google Scholar]
- 34.Vannaphan S, Walters N, Saengnedsawang T, Tangpukdee N, Kham-In P, Klubprasit M, Wilairatana P, Looareesuwan S. Factors associated with acute renal failure in severe falciparum [corrected] malaria patients. Southeast Asian J Trop Med Public Health. 2010;41:1042–1047. [PubMed] [Google Scholar]
- 35.Lee CS, Min IS, Hwang JH, Kwon KS, Lee HB. Clinical significance of hypoalbuminemia in outcome of patients with scrub typhus. BMC Infect Dis. 2010;10:216. doi: 10.1186/1471-2334-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vikrant S, Dheer SK, Parashar A, Gupta D, Thakur S, Sharma A, Kaushal SS, Kanga A. Scrub typhus associated acute kidney injury--a study from a tertiary care hospital from western Himalayan State of India. Ren Fail. 2013;35:1338–1343. doi: 10.3109/0886022X.2013.828257. [DOI] [PubMed] [Google Scholar]
- 37.Trimarchi H, Greloni G, Campolo-Girard V, Giannasi S, Pomeranz V, San-Roman E, Lombi F, Barcan L, Forrester M, Algranati S, et al. H1N1 infection and the kidney in critically ill patients. J Nephrol. 2010;23:725–731. [PubMed] [Google Scholar]
- 38.Rodríguez E, Soler MJ, Rap O, Barrios C, Orfila MA, Pascual J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8:e82992. doi: 10.1371/journal.pone.0082992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Choi MS, Gwak GY, Lee JH, Koh KC, Paik SW, Yoo BC. [Clinical features and predictive factors of acute hepatitis A complicated with acute kidney injury] Korean J Gastroenterol. 2010;56:359–364. doi: 10.4166/kjg.2010.56.6.359. [DOI] [PubMed] [Google Scholar]
- 40.Mehra N, Patel A, Abraham G, Reddy YN, Reddy YN. Acute kidney injury in dengue fever using Acute Kidney Injury Network criteria: incidence and risk factors. Trop Doct. 2012;42:160–162. doi: 10.1258/td.2012.120023. [DOI] [PubMed] [Google Scholar]
- 41.Ceylan B, Taniş M, Akkoyunlu ME, Çınar A, Kurt AR, Akkoyunlu Y, Ozkan D, Ozcelik HK, Aslan T, Fincancı M, et al. Variables determining the development of colistin-associated renal impairment. Wien Klin Wochenschr. 2016;128:614–619. doi: 10.1007/s00508-015-0773-z. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Lee KH, Yoo S, Pai H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int J Antimicrob Agents. 2009;34:434–438. doi: 10.1016/j.ijantimicag.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Kwon JA, Lee JE, Huh W, Peck KR, Kim YG, Kim DJ, Oh HY. Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents. 2010;35:473–477. doi: 10.1016/j.ijantimicag.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Singhapricha T, Hu KQ, Hong JC, Steadman RH, Busuttil RW, Xia VW. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011;91:348–353. doi: 10.1097/TP.0b013e31820437da. [DOI] [PubMed] [Google Scholar]
- 45.Tinti F, Umbro I, Meçule A, Rossi M, Merli M, Nofroni I, Corradini SG, Poli L, Pugliese F, Ruberto F, et al. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transplant Proc. 2010;42:1233–1236. doi: 10.1016/j.transproceed.2010.03.128. [DOI] [PubMed] [Google Scholar]
- 46.Sang BH, Bang JY, Song JG, Hwang GS. Hypoalbuminemia Within Two Postoperative Days Is an Independent Risk Factor for Acute Kidney Injury Following Living Donor Liver Transplantation: A Propensity Score Analysis of 998 Consecutive Patients. Crit Care Med. 2015;43:2552–2561. doi: 10.1097/CCM.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 47.Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, Kim CS, Gwak MS, Kim GS. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One. 2015;10:e0136230. doi: 10.1371/journal.pone.0136230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore J, He X, Shabir S, Hanvesakul R, Benavente D, Cockwell P, Little MA, Ball S, Inston N, Johnston A, et al. Development and evaluation of a composite risk score to predict kidney transplant failure. Am J Kidney Dis. 2011;57:744–751. doi: 10.1053/j.ajkd.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Yang SW, Choi JY, Kwon OJ. The impact of pretransplantation serum albumin levels on long-term renal graft outcomes. Transplant Proc. 2013;45:1379–1382. doi: 10.1016/j.transproceed.2012.10.063. [DOI] [PubMed] [Google Scholar]
- 50.Kim CS, Oak CY, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kweon SS, Kim SW. Incidence, predictive factors, and clinical outcomes of acute kidney injury after gastric surgery for gastric cancer. PLoS One. 2013;8:e82289. doi: 10.1371/journal.pone.0082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu CY, Huang YH, Su CW, Lin HC, Chiang JH, Lee PC, Lee FY, Huo TI, Lee SD. Renal failure in patients with hepatocellular carcinoma and ascites undergoing transarterial chemoembolization. Liver Int. 2010;30:77–84. doi: 10.1111/j.1478-3231.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 52.Wong PY, Carroll RE, Lipinski TL, Capone RR. Studies on the renin-angiotensin-aldosterone system in patients with cirrhosis and ascites: effect of saline and albumin infusion. Gastroenterology. 1979;77:1171–1176. [PubMed] [Google Scholar]
- 53.Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 54.Luca A, García-Pagán JC, Bosch J, Feu F, Jiménez W, Ginés A, Fernández M, Escorsell A, Arroyo V, Rodés J. Beneficial effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology. 1995;22:753–758. [PubMed] [Google Scholar]
- 55.Mizuno T, Hayashi T, Shimabukuro Y, Murase M, Hayashi H, Ishikawa K, Takahashi K, Yuzawa Y, Yamada S, Nagamatsu T. Lower Blood Pressure-Induced Renal Hypoperfusion Promotes Cisplatin-Induced Nephrotoxicity. Oncology. 2016;90:313–320. doi: 10.1159/000446371. [DOI] [PubMed] [Google Scholar]
- 56.Lahoti A, Kantarjian H, Salahudeen AK, Ravandi F, Cortes JE, Faderl S, O’Brien S, Wierda W, Mattiuzzi GN. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer. 2010;116:4063–4068. doi: 10.1002/cncr.25306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant. 2010;25:419–426. doi: 10.1093/ndt/gfp488. [DOI] [PubMed] [Google Scholar]
- 58.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 59.Fischler R, Meert AP, Sculier JP, Berghmans T. Continuous Renal Replacement Therapy for Acute Renal Failure in Patients with Cancer: A Well-Tolerated Adjunct Treatment. Front Med (Lausanne) 2016;3:33. doi: 10.3389/fmed.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson CL, Elkassabany NM, Kamath AF, Liu J. Low Albumin Levels, More Than Morbid Obesity, Are Associated With Complications After TKA. Clin Orthop Relat Res. 2015;473:3163–3172. doi: 10.1007/s11999-015-4333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HJ, Koh WU, Kim SG, Park HS, Song JG, Ro YJ, Yang HS. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: A retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016;95:e4489. doi: 10.1097/MD.0000000000004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamath AF, Nelson CL, Elkassabany N, Guo Z, Liu J. Low Albumin Is a Risk Factor for Complications after Revision Total Knee Arthroplasty. J Knee Surg. 2017;30:269–275. doi: 10.1055/s-0036-1584575. [DOI] [PubMed] [Google Scholar]
- 63.Leung N, Kumar SK, Glavey SV, Dispenzieri A, Lacy MQ, Buadi FK, Hayman SR, Dingli D, Kapoor P, Zeldenrust SR, et al. The impact of dialysis on the survival of patients with immunoglobulin light chain (AL) amyloidosis undergoing autologous stem cell transplantation. Nephrol Dial Transplant. 2016;31:1284–1289. doi: 10.1093/ndt/gfv328. [DOI] [PubMed] [Google Scholar]
- 64.Lee SY, Meehan RS, Seldin DC, Sloan JM, Quillen K, Shelton A, Brauneis D, Sanchorawala V. Effect of severe hypoalbuminemia on toxicity of high-dose melphalan and autologous stem cell transplantation in patients with AL amyloidosis. Bone Marrow Transplant. 2016;51:1318–1322. doi: 10.1038/bmt.2016.132. [DOI] [PubMed] [Google Scholar]
- 65.Yun SE, Jeon DH, Kim MJ, Bae EJ, Cho HS, Chang SH, Park DJ. The incidence, risk factors, and outcomes of acute kidney injury in patients with pyogenic liver abscesses. Clin Exp Nephrol. 2015;19:458–464. doi: 10.1007/s10157-014-1016-8. [DOI] [PubMed] [Google Scholar]
- 66.Banda J, Duarte R, Dickens C, Dix-Peek T, Muteba M, Paget G, Mngomezulu V, Manga P, Naicker S. Risk factors and outcomes of contrast-induced nephropathy in hospitalised South Africans. S Afr Med J. 2016;106:699–703. doi: 10.7196/SAMJ.2016.v106i7.10429. [DOI] [PubMed] [Google Scholar]
- 67.Qian J, Fang J, Zhu Q, Ma S, Wang W, Zheng Y, Hao G, Deng B, Zhao X, Ding F. Serum Protein Thiol Levels in Patients with Hospital-Acquired Acute Kidney Injury. Kidney Blood Press Res. 2015;40:623–629. doi: 10.1159/000368538. [DOI] [PubMed] [Google Scholar]
- 68.Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H, Ma S, Hao C, Gu Y, Lin S, et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol. 2011;12:30. doi: 10.1186/1471-2369-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kritmetapak K, Peerapornratana S, Srisawat N, Somlaw N, Lakananurak N, Dissayabutra T, Phonork C, Leelahavanichkul A, Tiranathanagul K, Susantithapong P, et al. The Impact of Macro-and Micronutrients on Predicting Outcomes of Critically Ill Patients Requiring Continuous Renal Replacement Therapy. PLoS One. 2016;11:e0156634. doi: 10.1371/journal.pone.0156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu W, He W, Liu W, Fang X, Wu Y, Yu F, Hao W. Risk Factors and Prognosis of Cardiorenal Syndrome Type 1 in Elderly Chinese Patients: A Retrospective Observational Cohort Study. Kidney Blood Press Res. 2016;41:672–679. doi: 10.1159/000447936. [DOI] [PubMed] [Google Scholar]
- 71.Li QL, Cheng QL, Ma Q, Wang XD, Ao QG, Zhao JH, Du J, Liu S, Zhang XY. [Risk factors and short-term prognosis of acute kidney injury in elderly patients] Zhonghua Yixue Zazhi. 2013;93:2715–2718. [PubMed] [Google Scholar]
- 72.Shin MJ, Rhee H, Kim IY, Song SH, Lee DW, Lee SB, Kwak IS, Seong EY. RIFLE classification in geriatric patients with acute kidney injury in the intensive care unit. Clin Exp Nephrol. 2016;20:402–410. doi: 10.1007/s10157-015-1165-4. [DOI] [PubMed] [Google Scholar]
- 73.Chung CU, Nelson JA, Fischer JP, Wink JD, Serletti JM, Kovach SJ. Acute kidney injury after open ventral hernia repair: an analysis of the 2005-2012 ACS-NSQIP datasets. Hernia. 2016;20:131–138. doi: 10.1007/s10029-015-1395-0. [DOI] [PubMed] [Google Scholar]


