Abstract
Purpose
Multidrug resistance-associated protein 2 (MRP2/ABCC2) is an efflux pump that removes drugs and chemicals from cells. We sought to characterize the expression, trafficking and in vitro activity of seven single nucleotide polymorphisms (SNPs) in the ABCC2 gene.
Methods
ABCC2 SNPs were generated using site-directed mutagenesis and stably expressed in Flp-In HEK293 cells, which allows targeted insertion of transgenes within the genome. Total and cell surface expression of MRP2 as well as accumulation of substrates (calcein AM and 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate, CDCF) were quantified in cells or inverted membrane vesicles expressing wild-type (WT) or variant forms.
Results
The cell surface expression of the C-24T-, G1249A-, G3542T-, T3563A-, C3972T- and G4544A-MRP2 variants was similar to WT-MRP2. While expression was similar, transport of calcein AM was enhanced in cells expressing the G3542T-, T3563A-, C3972T-, and G4544A-MRP2 variants. By comparison, cells expressing the C2366T-MRP2 variant had 40-50% lower surface expression, which increased the accumulation of calcein AM up to 3-fold. Accumulation of CDCF in inverted membrane vesicles expressing the C2366T-MRP2 variant was also reduced by 50%. In addition, the G1249A-MRP2 variant had decreased transport of CDCF.
Conclusions
Taken together, these data demonstrate that genetic variability in the ABCC2 gene influences the in vitro expression, trafficking, and transport activity of MRP2.
Keywords: MRP2, ABCC2, transporter, SNP, variant
Introduction
Human multidrug resistance-associated protein 2 (MRP2, ABCC2) is an efflux transporter that belongs to the ATP-binding cassette transporter, subfamily C. The proposed structure for MRP2 includes 17 transmembrane helices with three membrane-spanning domains (MSD) and two highly conserved nucleotide binding domains (NBD) (Fig 1). The MRP2 protein localizes to the apical membrane of polarized cells, including hepatocytes, proximal tubule cells and enterocytes where it facilitates the excretion of drugs and chemicals. In particular, MRP2 mediates the ATP-dependent efflux of various drugs and chemicals, including glucuronide, sulfate and glutathione conjugates (reviewed in 1, 2).
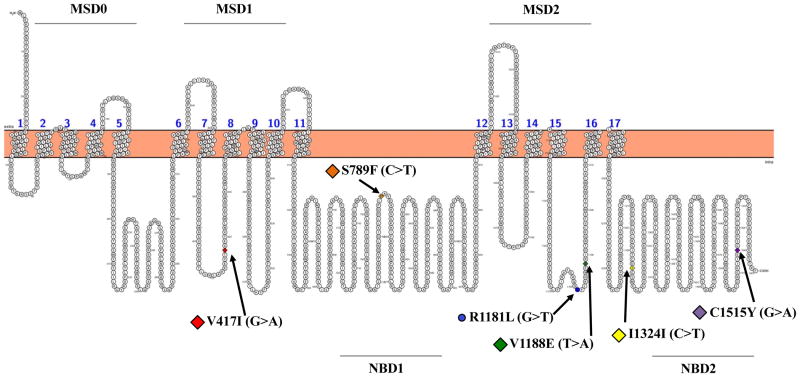
Figure 1. Localization of variants in a predicted topology model of the human MRP2 transporter.
The full-length model of the MRP2 protein was generated using the open source tool Protter (http://wlab.ethz.ch/protter/start/). The C-24T variant is found in the 5′-untranslated region of the ABCC2 promoter and not shown in the figure. MSD: membrane-spanning domain; NBD: nucleotide-binding domain.
Genetic variants in transporter proteins may alter the pharmacokinetics and subsequent pharmacological and toxicological effects of drugs (3-5). For MRP2/ABCC2, both mutations and single nucleotide polymorphisms (SNPs) have been described. Missense, nonsense, and splice-site mutations in ABCC2 cause truncated and dysfunctional MRP2 proteins leading to the genetic disorder, Dubin-Johnson syndrome (6). Individuals afflicted with Dubin-Johnson syndrome exhibit benign conjugated hyperbilirubinemia despite normal liver functioning (7, 8). Beyond these mutations, SNPs in the ABCC2 gene can alter the in vitro activity and/or clinical phenotypes of patients in the absence of overt changes in conjugated bilirubin clearance (5, 9, 10). To date, a number of ABCC2 variants have been identified with differing allele frequencies across ethnic and racial groups (9, 11-14) (Fig. 1, Table 1).
Table 1. Selected Genetic Polymorphisms in the Human ABCC2 Gene.
| db SNP ida | Variant mRNA positionb | Nucleotide change | Amino acid translationc | Exon | Populationd/Chromosome Sample Count | Allele Frequency in HapMape |
|---|---|---|---|---|---|---|
| rs717620 | C-24T | C>T | 5′-UTR | - | CEU/226 | T: 0.18; C: 0.82 |
| HCB/86 | T: 0.22; C: 0.78 | |||||
| JPT/172 | T: 0.19; C: 0.81 | |||||
| YRI/226 | T: 0.03; C: 0.97 | |||||
| rs2273697 | G1249A | G>A | V417I | 10 | CEU/226 | A: 0.24; G: 0.76 |
| HCB/86 | A: 0.07; G: 0.93 | |||||
| JPT/172 | A: 0.13; G: 0.87 | |||||
| YRI/226 | A: 0.22; G: 0.78 | |||||
| rs56220353 | C2366T | C>T | S789F | 18 | Not available | |
| rs8187692 | G3542T | G>T | R1181L | 25 | CEU/120 | T: 0.00; G: 1.00 |
| HCB/90 | T: 0.01; G: 0.99 | |||||
| JPT/172 | T: 0.01; G: 0.99 | |||||
| YRI/226 | T: 0.14; G: 0.86 | |||||
| rs17216324 | T3563A | T>A | V1188E | 25 | Not available | |
| rs3740066 | C3972T | C>T | I1324I | 28 | CEU/120 | T: 0.34; C: 0.66 |
| HCB/90 | T: 0.27; C: 0.73 | |||||
| JPT/88 | T: 0.28; C: 0.72 | |||||
| YRI/120 | T: 0.27; C: 0.73 | |||||
| rs8187710 | G4544A | G>A | C1515Y | 32 | CEU/226 | A: 0.05; G: 0.95 |
| HCB/90 | A: 0.00; G: 1.00 | |||||
| JPT/90 | A: 0.00; G: 1.00 | |||||
| YRI/226 | A: 0.12; G: 0.88 |
Single Nucleotide Polymorphism Database (http://www.ncbi.nlm.nih.gov/snp)
CEU: European; HCB: Han Chinese; JPT: Japanese; YRI: Sub-Saharan African
HapMap data obtained from the Single Nucleotide Polymorphism database (dbSNP)
Prior studies have yielded somewhat inconsistent results regarding the extent to which ABCC2 SNPs alter MRP2 functioning. One example is the G1249A-MRP2 variant. In a clinical report, it was suggested that individuals expressing the G1249A-MRP2 variant have enhanced MRP2 efflux activity in enterocytes (5). This was postulated as a mechanism to explain the reduced absorption of the β1-selective blocker and MRP2 substrate, talinolol, in subjects expressing the G1249A-MRP2 variant. In fact, data from in vitro systems have demonstrated mixed findings. Recent work suggests that the G1249A-MRP2 variant has higher MRP2-stimulated ATPase activity that is associated with greater efflux of the substrate sorafenib (15). In other work, G1249A-MRP2 was shown to decrease the apparent affinity for glutathione and glucuronide-conjugated substrates (16). However, in yet another study, G1249A-MRP2 had no effect on the transport of the MRP2 substrates, 17β-estradiol-D-glucuronide, leukotriene C4 and 2,4-dinitrophenyl-S-glutathione, when expressed in membrane vesicles isolated from LLC-PK1 kidney cells (9). It is possible that these divergent findings result from the utilization of different experimental models. Also, MRP2 may possess multiple orientations for substrates to interact within the substrate binding cavity which could help to explain these results. Nonetheless, additional work is needed to understand the effect of ABCC2 SNPs on MRP2 functioning using a controlled system that allows for the similar integration of variants into the genome and direct comparisons between SNPs.
In the present study, we aimed to clarify the influence of seven ABCC2 SNPs on the expression and function of the MRP2 transporter in vitro using the Flp-In transfection system. The Flp recombinase allows controlled integration and expression of transfected genes in Human Embryonic Kidney (HEK) 293 cells at a specific genomic location called the Flp Recombination Target site (FRT) (17). Stably transfected cells included wild-type (WT-MRP2) and C-24T- (in the 5′-UTR), G1249A- (V417I), C2366T- (S789F), G3542T- (R1181L), T3563A- (V1188E), C3972T- (I1324I) and G4544A-MRP2 (C1515Y). These variants were selected based upon their allele frequencies within populations, associations with clinical phenotypes, locations within the MRP2 protein (MSD, NBD), and/or prior in vitro studies suggesting they impact MRP2 functioning (9, 11, 12). The stably transfected Flp-In cells were used to determine whether SNPs in the ABCC2 gene influenced 1) the total and cell surface expression of MRP2 protein, 2) the intracellular accumulation of the substrate calcein AM (18) and 3) the uptake of a second substrate 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCF) into inverted plasma membrane vesicles (19).
Materials and Methods
Reagents and Chemicals
Lipofectamine LTX and PLUS reagents, hygromycin B and zeocin were purchased from ThermoFisher Scientific (Waltham, MA). Unless specified, all other chemicals and reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Site-Directed Mutagenesis
The full-length human ABCC2 WT cDNA clone was obtained by digestion of a pCMV6-NEO plasmid containing ABCC2 cDNA (Origene, Rockville, MD) using the restriction enzymes SalI and NotI (New England Biolabs, Ipswich, MA). After purification and sequencing, ABCC2 WT cDNA was used as a template to generate variant cDNA clones (C-24T, G1249A, C2366T, G3542T, T3563A, C3972T and G4544A, Table 1) by using a QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). All clones and mutagenesis were confirmed by DNA sequencing at Genewiz (South Plainfield, NJ). Primer sequences for generating ABCC2 variants were provided in Supplemental Table 1.
Cell Culture and Transfection
Flp-In 293 cells (ThermoFisher Scientific) were grown at 37°C with 5% CO2 in Dulbecco's modified eagle medium supplemented with 10% fetal bovine serum, glutamine (2 mM) and zeocin (100 μg/ml). ABCC2 WT and variant cDNAs were inserted into the pcDNA5/FRT vector and transfected into Flp-In 293 cells containing an integrated FRT site. In detail, Flp-In 293 cells (7 × 105/well) were seeded in 6-well plates and allowed to adhere overnight. The opti-MEM I reduced serum medium (ThermoFisher Scientific) was used for the transfection of pcDNA5/FRT/MRP2 (WT or variants) or pcDNA5/FRT (empty vector, EV), pOG44 and Lipofectamine LTX. Stable cell lines were generated using hygromycin B (200 μg/ml) for selection. Colonies were collected and screened for MRP2 expression by Western blot analysis.
Calcein AM Accumulation
The fluorescent substrate calcein AM was used to characterize the transport activity of WT-MRP2 and variants in Flp-In 293 cells (18, 20). In brief, cell suspensions (prepared in medium, 500,000 cells/ml) were loaded with calcein AM (0.5, 2 or 10 μM) for 30 min at 37°C, 5% CO2 (uptake period). After washing with culture medium, cells were incubated in culture medium for 60 min to allow calcein AM efflux from the cells (efflux period). Following washing and centrifugation, the cell pellet was suspended in phosphate-buffered saline (PBS) and relative fluorescence units (RFU) for each cell was quantified on a Cellometer Vision cell counter using filter cube VB-535-402 (excitation/emission: 535/402 nm) (Nexcelom Bioscience LLC, Lawrence, MA).
Cell Surface Protein Isolation and Western Blot Analysis
Localization of WT-MRP2 and variants into the plasma membrane was assessed using the Pierce Cell Surface Protein Isolation kit (ThermoFisher Scientific). A cell-impermeable, cleavable biotinylation reagent (Sulfo-NHS-SS-Biotin) was used to label exposed primary amines of proteins on the surface of cells. The labeled cells were then harvested, lysed and purified using a NeutrAvidin agarose resin. To prepare total cell lysates, cells were placed in lysis buffer containing 20 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton-100 and 1% protease inhibitor cocktail. The protein concentration of cell surface preparations or cell lysates was determined by Pierce 660 nm Protein Assay or the Bicinchoninic Acid (BCA) protein assay kit (ThermoFisher Scientific), respectively. For Western blot analysis, cell surface fractions, cell lysates or vesicles isolated from WT-MRP2 and variants cells (15 μg of protein) were separated by SDS-PAGE electrophoresis and transferred to a nitrocellulose membrane at 4°C overnight. After blocking with 5% nonfat dry milk in PBS with 0.5% of Tween 20 (PBS/T), membranes were incubated with primary antibody against MRP2 (M2III-5, 1:600) (Enzo Life Sciences, Farmingdale, NY) followed by incubation with anti-mouse secondary antibodies (1:2000, Sigma) for 1 to 2 h. The SuperSignal West Dura chemiluminescent substrate (ThermoFisher Scientific) was applied to membranes prior to detection of luminescence using a FluorChem E system (ProteinSimple, San Jose, CA). Target protein band intensities were semi-quantified and normalized to β-ACTIN (Primary ab8227, 1:2000, Secondary 1:2000) or Na+/K+ ATPase (Primary ab76020, 1:20,000, Secnodary 1:2000) (Abcam, Cambridge, MA) levels.
MRP2 Protein Localization by Indirect Immunofluorescence Analysis
Indirect immunofluorescence staining was used to assess human MRP2 localization. Cells (2 × 105) were seeded in chamber slides overnight. After fixation in 4% paraformaldehyde, cells were blocked with 5% goat serum in 0.1% PBS-Triton X for 1 h and then incubated with an anti-MRP2 antibody (M2III-6, 1:150, Enzo Life Sciences) overnight. Sections were washed and incubated with a goat anti-mouse IgG Alexa 488 antibody (green, 1:1000, ThermoFisher Scientific) for 1 h. Images were acquired using a Leica TCS SP5 DMI6000CS inverted confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL). Negative controls without primary antibody were included to ensure minimal nonspecific staining. Images are shown at 63× magnification.
RNA Isolation and mRNA Quantification
Total RNA from Flp-In 293 cell expressing MRP2 WT and variants was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The concentration of total RNA was quantified by UV spectrophotometry at 260/280 nm using a Nanodrop spectrophotometer 2000 (Thermo Fisher Scientific, Wilmington, DE). The messenger RNA (mRNA) expression of human MRP2 was quantified by qPCR assay using Sybr Green as detection of amplified products in the ABI 7900HT PCR system (Applied Biosystems, Carlesbad, CA). Ct values were converted to ΔΔCt by comparing to a reference gene GAPDH. The following primers (forward and reverse, respectively) were used for MRP2 (5′-AGCCATGCAGTTTTCTGAGGCCT-3′ and 5′-TGGTGCCCTTGATGGTGATGTGC-3′), GAPDH (5′-GACATCAAGAAGGTGGTGAA-3′ and 5′-CGTTGTCATACCAGGAAATG-3′). MRP2 primer sequences did not overlap with the SNP variant sites.
Isolation of Membrane Vesicles
Membrane vesicles were isolated from Flp-In 293 WT-MRP2 and variants cells as previously described (21). Briefly, cells were harvested by centrifugation at 3500 × g for 20 min. Cell pellets were then suspended in ice-cold hypotonic buffer (0.3 mM Na2HPO4, 0.2 mM NaH2PO4, 0.1 mM EDTA and 1% protease inhibitor), mixed at 4°C for 20 min and centrifuged at 100,000 × g for 30 min. The obtained pellet was homogenized using a tight-fitting Dounce homogenizer for 25 strokes in ice-cold TS buffer (10 mM Tris-HEPES, 250 mM sucrose, pH7.4) containing 1% protease inhibitor. After centrifugation at 4000 × g at 4°C for 20 min, the supernatant was centrifuged at 100,000 × g at 4°C for 60 min. The membrane pellet was resuspended in ice-cold TS buffer and passed through a 27-gauge needle 25 times to obtain vesicles. Protein concentrations were measured using the BCA protein assay kit (ThermoFisher Scientific). MRP2 protein expression was quantified by Western blotting analysis as described above.
CDCF Transport into Membrane Vesicles
To quantify transport in membrane vesicles, vesicles (50 μg) were incubated with CDCF (2.5, 10 and 50 μM) in reaction buffer (50 mM MOP-Tris, 70 mM KCl, 7.5 mM MgCl2) including 200 mM glutathione and 10 mM MgATP at 37°C for 10 min. Reactions were stopped by adding ice-cold buffer (40 mM MOPS-Tris, 70 mM KCl) and transferred to a 96-well multiscreen filter plate (Millipore, Billerica, MA). After washing, vacuum filtration, and solubilization with 50% methanol, CDCF fluorescence was read at an excitation wavelength of 504 nm and emission wavelength of 529 nm in a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA).
Data Analysis
Quantitative results were expressed as mean ± S.E. and analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA). Student's t test was used for comparison of two groups. One-way ANOVA with a Tukey's multiple comparisons test was used to compare groups of 3 or more. Data were log transformed prior to statistical analysis if they were not normally distributed. Statistical significance was set at p < 0.05.
Results
Expression and activity of the MRP2 transporter using the Flp-In system
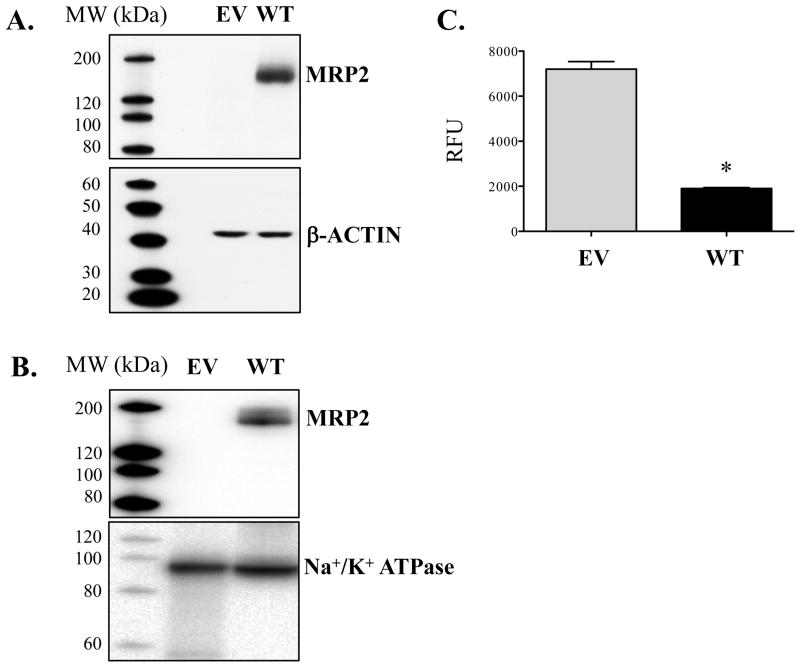
MRP2 protein was not detected in empty vector-containing cells (Figs. 2A and B). Transfection of the human ABCC2-containing plasmid into Flp-In 293 cells led to MRP2 protein expression in both cell lysates and at the plasma membrane (Figs. 2A and B). Accordingly, accumulation of the MRP2 substrate, calcein AM, was 4-fold lower in cells expressing WT-MRP2 compared to empty vector-containing cells (Fig. 2C).
Figure 2. Expression and activity of the MRP2 transporter using the Flp-In system.
Flp-In 293 cells were stably transfected with empty vector (EV) or full-length human ABCC2 plasmids (wild-type, WT). Protein expression of MRP2 in cell lysates (A) and biotinylated membrane fractions (B) was detected by Western blot analysis. β-ACTIN and Na+/K+ ATPase were used as loading controls for total and cell surface expression, respectively. (C) EV and WT-MRP2 cells were treated with calcein AM (0.5 μM) for 30 min (uptake period), washed, and then incubated with fresh culture media for 60 min (efflux period). Intracellular accumulation of calcein AM was determined at the end of the efflux period using a fluorescent cell counter. Data are expressed as relative fluorescence units (RFU) and presented as mean ± SE (n=4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to EV.
Intracellular accumulation of calcein AM in wild-type MRP2 and variant cells
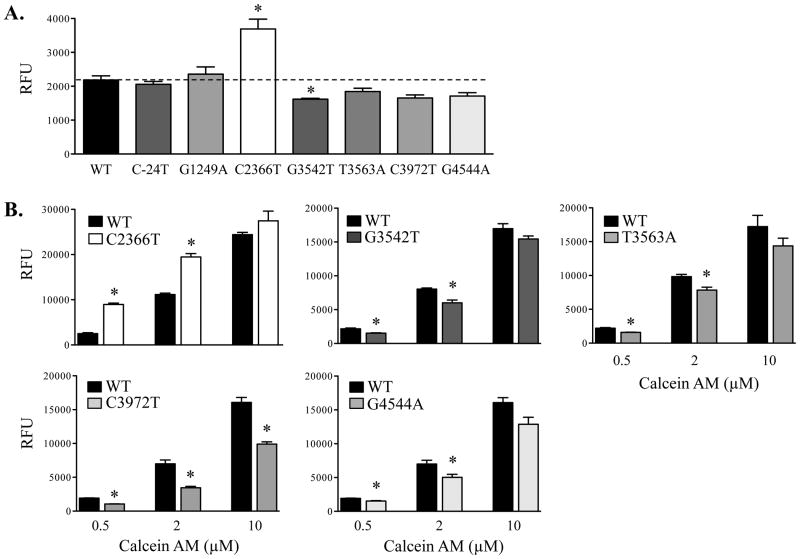
Calcein AM is a fluorescent substrate of MRPs and multidrug resistance protein 1 (18). In the present study, calcein AM was used to evaluate MRP2 transport activity in Flp-In 293 WT-MRP2 and variant cells, as MRP2 was the only efflux transporter expressed. Compared to WT-MRP2 cells, accumulation of calcein AM (0.5 μM) was significantly increased by 1.5-fold in the C2366T-MRP2 cells, while accumulation was 20-30% lower in cells expressing the G3542T-, C3972T- and G4544A-MRP2 variants (Fig. 3A). Further analysis was performed across a range of calcein AM concentrations (0.5, 2, 10 μM) (Figs. 3B). The C2366T-MRP2 variant demonstrated greater calcein AM accumulation at 0.5 and 2 μM (Fig. 3B). By comparison, calcein AM accumulation was lower in the G3542T-, T3563A-, C3972T-, and G4544A-MRP2 variants compared to WT-MRP2 (Figs. 3B).
Figure 3. Transport of calcein AM in MRP2 wild-type and variant cells.
Flp-In 293 cells stably expressing ABCC2 wild-type (WT) or variant plasmids were exposed to calcein AM for 30 min (uptake period), washed, and then incubated with fresh culture medium for 60 min (efflux period). Intracellular calcein AM accumulation was determined at the end of the efflux period using a fluorescent counter. (A) WT-MRP2 and variant cells were exposed to calcein AM (0.5 μM). (B) WT-MRP2 and variants were exposed to calcein AM (0.5, 2, and 10 μM). Data are expressed as relative fluorescence units (RFU) and presented as mean ± SE (n=4-6). Asterisks (*) represent statistically significant differences (p < 0.05) compared to WT.
Protein expression and localization of MRP2 in wild-type and variant cells
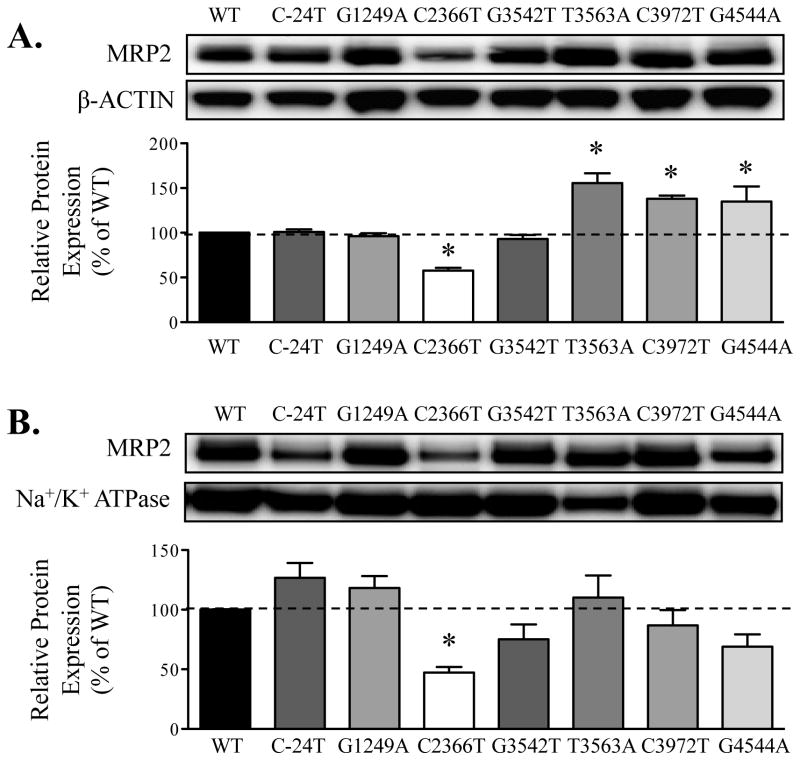
Compared to cells expressing WT-MRP2, total protein expression was significantly lower in cells expressing C2366T-MRP2 (decreased by 50%) and higher in T3563A-, C3972T- and G4544A-MRP2 variants (30-40% increase, Fig. 4A). Trafficking of MRP2 to the cell surface was measured using the biotinylation of amine groups on the exterior of the plasma membrane. As shown in Fig. 4B, protein expression at the cell surface was reduced 60% for the C2366T-MRP2 variant compared to WT-MRP2 cells. Slight, but insignificant, changes of 10-20% were observed in the cell surface expression of MRP2 in cells with other variants. Of note, after normalizing calcein AM transport to MRP2 expression in the plasma membrane, the extent of intracellular accumulation of calcein AM was similar between WT-MRP2 and C2366T-MRP2 variant cells, suggesting that the dysfunction of calcein AM transport in C2366T-MRP2 variant resulted from an overall decrease in MRP2 protein expression.
Figure 4. Protein expression and localization of MRP2 in wild-type and variant cells.
Flp-In 293 cells were stably transfected with ABCC2 wild-type (WT) or variant plasmids. Protein expression of MRP2 in cell lysates (A) and biotinylated membrane fractions (B) was detected by Western blot analysis. β-ACTIN and Na+/K+ ATPase were used as loading controls for total and cell surface expression, respectively. Data are expressed as relative protein expression and presented as mean ± SE (n=3). Asterisks (*) represent statistically significant differences (p < 0.05) compared to WT.
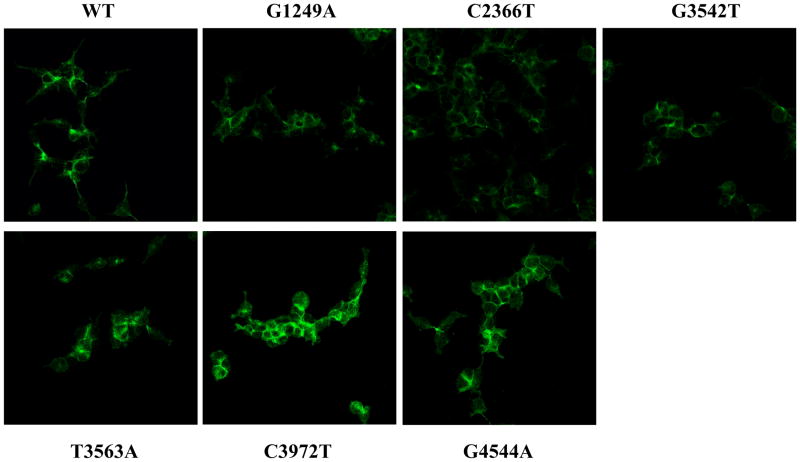
As protein expression was consistently decreased in C2366T-MRP2 cell lysates and cell surface fractions, the subcellular localization and protein expression of MRP2 were also further assessed by indirect immunofluorescence (Fig. 5). Detection of MRP2 protein in WT-MRP2 and C2366T-MRP2 variant cells (green) revealed reduced protein expression in the variant cells that was consistent with the lower expression levels seen in cell lysates and at the cell surface. The staining of MRP2 protein in cells expressing the other MRP2 variants, including G1249A-, G3542T-, T3563A-, C3972T- and G4544A-MRP2 was also performed (Fig. 5). MRP2 was consistently expressed in the plasma membrane of all variant cells (green). The intracellular intensity of fluorescent staining tended to be higher in T3563A-, C3972T- and G4544A-MRP2 variants compared to cells expressing WT-MRP2, which was consistent with the elevated MRP2 protein expression observed in cell lysates (Fig. 4A). For these variants, the increase in MRP2 expression may represent greater protein accumulation in the intracellular compartment, rather than at the cellular surface (Fig. 4B).
Figure 5. Localization of MRP2 protein in wild-type and variant cells.
Flp-In 293 cells were stably transfected with ABCC2 wild-type (WT) or variant plasmids. Staining of MRP2 protein in WT, G1249A, C2366T, G3542T, T3563A, C3972T, and G4544A variant cells was performed using indirect immunofluorescence analysis. Cells were incubated with an anti-MRP2 antibody (M2III-6) (green). Images are shown at 63× magnification.
Because glutathione (GSH) is an important co-substrate that can alter MRP2 activity, we also measured cellular levels. Intracellular GSH concentrations in WT-MRP2 and variant cells were similar (Supplementary Fig. 1).
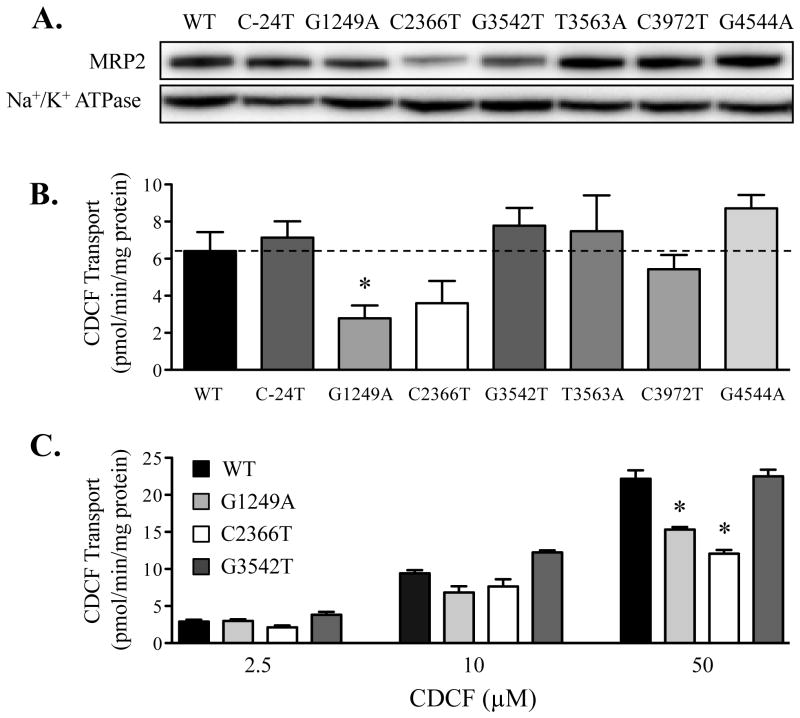
MRP2 expression and CDCF transport in wild-type and variant inverted membrane vesicles
The ability to detect differences in transport activity between MRP2 variants may be dependent upon the selection of substrate. Therefore, the transport of CDCF, another substrate of MRP2 (19), into inverted plasma membrane vesicles isolated from WT-MRP2 and variant cells was also performed. Compared to vesicles containing WT-MRP2, vesicles obtained from cells expressing the C2366T-MRP2 variant exhibited less MRP2 protein (Fig. 6A). In addition, decreased MRP2 protein expression was observed in vesicles containing the G3542T- and G1249A-MRP2 variants. CDCF transport was lower in vesicles expressing the G1249A- and C2366T-MRP2 variants compared to the vesicles with MRP2 WT (Figs. 6B and C). Following normalization of transport rates to MRP2 protein expression in the vesicles, there was no difference in the intrinsic activity of the C2366T-MRP2 variant, further suggesting that the change of MRP2 transport activity in inverted membrane vesicles with the C2366T-MRP2 variant was related to an overall decrease in protein expression. However, this was not the case for the G1249A-MRP2 variant. The transport of CDCF to G1249A vesicles was still approximately 30-50% lower compared to WT-MRP2 after normalizing to its MRP2 protein content, indicating a reduced functional ability of the G1249A-MRP2 variant to transport CDCF (Fig. 6C).
Figure 6. Expression of MRP2 protein and CDCF transport in membrane vesicles isolated from wild-type and variant cells.
Flp-In 293 cells were stably transfected with ABCC2 wild-type (WT) or variant plasmids. (A) Protein expression of MRP2 in membrane vesicles isolated from cells was detected by Western blot analysis. Na+/K+ ATPase was used as a loading control. (B) CDCF (10 μM) transport in membrane vesicles isolated from cells expressing WT-MRP2 and variants. Transport of CDCF (10 μM) in empty vector-transfected vesicles was 4.2 pmol/min/mg protein. (C) CDCF (2.5, 10 and 50 μM) transport in membrane vesicles isolated from cells expressing WT-MRP2 and variants G1249A, C2366T, G3542T. Transport was conducted using vesicles (50 μg) incubated with CDCF at 37°C for 10 min. Data are presented as mean ± SE (n=3-6). Asterisks (*) represent statistically significant differences (p < 0.05) compared to WT.
Discussion
In the present study, we characterized the protein expression, trafficking, and transport activity of seven MRP2 variants in intact cells and inverted membrane vesicles. In Flp-In 293 cells expressing WT-MRP2 and variants, transport of calcein AM was decreased in cells expressing the C2366T-MRP2 variant, while it was increased in G3542T-, T3563A-, C3972T-, and G4544A-MRP2 variant cells. The gain-of-function for the G3542T-, T3563A-, C3972T-, and G4544A-MRP2 variants likely resulted from differences in transporter activity compared to wild-type cells since the cell surface expression of each variant was unchanged. By comparison, the reduced efflux of calcein AM likely resulted from lower expression of C2366T-MRP2 in the plasma membrane rather than changes in intrinsic activity. Supporting this finding, the uptake of CDCF was also decreased in inverted membrane vesicles from cells expressing the C2366T-MRP2 variant. Interestingly, the G1249A-MRP2 variant exhibited decreased CDCF transport in membrane vesicles whereas no change was seen in the cellular extrusion of calcein AM. These divergent results may represent a difference in substrate interactions with G1249A-MRP2, a nonsynonymous variant found in MSD1 on the cytoplasmic loop between transmembranes 7 and 8. It is known that MRP2 possesses at least two drug binding sites, including a site where the substrate is transported and a second site that can modulate transport (22). While four basic amino acids not studied in this paper have been identified as key residues for MRP2 efflux (23), it is possible that SNPs in additional locations can modify transport of some substrates. Taken together, the data suggest that genetic variability in the MRP2 efflux transporter can influence not only its expression, but also its transport activity in a substrate-dependent manner.
MRP2 is responsible for the efflux of GSH as well as GSH conjugates of drugs and chemicals (24, 25). GSH serves as a cofactor that enables MRP-mediated efflux (26, 27). Importantly, we found that the intracellular GSH concentrations were similar between cells expressing WT-MRP2 and the variants (Supplemental Fig 1). While this means that GSH was sufficiently available to serve as a cofactor, the possibility remains that the interaction of GSH with MRP2 was altered in the variant cells. Prior work has pointed to cytoplasmic loop 3 (between transmembrane 5 and 6) as an important region for interaction with GSH (28); however, there is the potential that other portions of the MRP2 protein may also be involved. GSH was included as a cofactor for the inverted vesicle assays, however, other studies suggest that may not be necessary in order to transport CDCF by MRP2 (29, 30). Because of this, the availability and/or interaction of GSH with variant forms of MRP2 may not explain the differences in functional activity that was observed between variants.
Our data are largely consistent with prior work investigating the functional consequences of MRP2 SNPs on in vitro transport activity. Compared to experimentation with other intact cell lines or plasma membrane vesicles, similar results were observed using the Flp-In recombination system. In vesicles obtained from LLC-PK1 kidney cells expressing C2366T-MRP2, the protein expression of MRP2 was significantly lower compared to its expression in WT-MRP2 (9) (Fig 4). This corresponded with reduced transport of leukotriene C4, estradiol-3-glucuronide, estradiol-17β-glucuronide and tauroursodeoxycholic acid in Sf9-derived membrane vesicles containing C2366T-MRP2 (16). By contrast, fibrosarcoma-related Rht14-10 cells expressing the G3542T-MRP2 variant showed decreased efflux of glutathione-monochlorobimane, which was associated with lower total MRP2 protein expression (11). In the current study, we observed only a trend for decreased MRP2 protein expression at the surface of G3542T-MRP2 cells that was not statistically significant. However, cells expressing G3542T-MRP2 exhibited increased transport of calcein AM compared to WT-MRP2. Similar enhancement of transport activity for the G3542T-MRP2 variant has been observed in cells treated with the substrate glutathione-methylfluorescein despite lower MRP2 protein expression (11). It is likely that altered transport activity by MRP2 variants may be substrate-dependent and influenced by the location and nature of the amino acid change in the MRP2 protein.
To date, multiple clinical studies have reported associations between genetic variation in the ABCC2 gene and drug efficacy or adverse drug reactions (5, 31). For example, patients expressing the ABCC2 variant C-24T have reduced clearance of methotrexate, mycophenolic acid and irinotecan (32-35). However, the in vitro findings in the current study did not correspond with these clinical data. This discrepancy points to alternate regulatory mechanisms in vivo that are masked in an overexpression system.
In addition to associations between the C-24T-MRP2 variant and altered pharmacokinetics, studies have also investigated the impact of the G1249A-MRP2 SNP on protein expression in human tissues. In human placentas, carriers of the variant allele (1249AA and 1249GA) had reduced enrichment of MRP2 mRNA early in gestation (36). Conversely, G1249A-MRP2 has been associated with higher MRP2 protein levels in human liver tissues (37). In the current study, the G1249A variant was not associated with significant changes for MRP2 mRNA (Supplemental Fig. 2), protein expression or calcein AM efflux in intact cells. However, in inverted membrane vesicles, lower uptake of CDCF by MRP2 was observed in cells expressing the G1249A-MRP2 variant compared to WT-MRP2s. Thus, further work is needed to understand the tissue-specific influences imparted by polymorphic variants on MRP2 expression in addition to functional changes.
The mechanisms by which genetic variants can influence transporter expression are an avenue of significant research. Several factors, including indirect regulation of DNA-protein binding properties, mRNA secondary structure, mRNA stability, protein expression-posttranscriptional alterations (10), stability of the protein in endoplasmic reticulum, susceptibility to ubiquitin-mediated proteasomal degradation (38), interaction with microRNAs (39) and direct regulation to transport activity, have been suggested as mechanisms for altered expression and/or activity of transporter variants. For example, altered protein expression for the G3542T-MRP2 variant was linked to altered synthesis or stability of the ABCC2 SNP mRNA in fibrosarcoma cells (11). The G4544A-MRP2 variant exhibited impaired ATPase activity and resulted in higher cellular accumulation of lopinavir, calcein and carboxyfluorescein diacetate in HEK293 cells, consistent with reduced transport efficiency (31). In a previous study, the decrease in MRP2 protein levels in LLC-PK1 cells expressing the C2366T-MRP2 variant was related to impaired maturation and trafficking of MRP2 from the endoplasmic reticulum to the Golgi complex and ultimately to the increased degradation of the protein (8, 9). As a result, there are multiple mechanisms by which genetic variants can affect MRP2 trafficking, expression, and activity leading to both tissue- and substrate-dependent differences that affect the overall pharmacokinetics of drugs.
Conclusion
In conclusion, the C2366T-MRP2 variant had lower total and cell surface MRP2 expression as well as decreased efflux of calcein AM compared to WT-MRP2. The accumulation of CDCF in the C2366T-MRP2 variant inverted membrane vesicles was also reduced compared to vesicles containing WT-MRP2. The G1249A-MRP2 variant exhibited decreased CDCF transport. By contrast, the transport of calcein AM was enhanced in cells expressing the G3542T-, T3563A-, C3972T-, and G4544A-MRP2 variants. These data indicate that genetic variability in the ABCC2 gene influences the in vitro expression, trafficking, and transport activity of MRP2.
Supplementary Material
Supplemental Figure 1. Intracellular GSH concentrations in wild-type and variant cells. Flp-In 293 cells were stably transfected with ABCC2 wild-type (WT) or variant plasmids. Intracellular concentrations of reduced GSH were quantified using the GSH/GSSG-Glo assay (Promega, Madison, WI). Data are presented as mean ± SE (n=4).
Supplemental Figure 2. MRP2 mRNA expression in wild-type and variant cells. Total RNA from Flp-In 293 3cells expressing ABCC2 wild-type (WT) and variants was isolated using the RNeasy Mini Kit. The mRNA expression of human ABCC2 was quantified by qPCR assay using Sybr Green to detect amplified products. Ct values were converted to ΔΔCt by comparing to a reference gene GAPDH. Data are presented as mean relative expression ± SE (n=4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to WT.
Supplemental Table 1. Primers Used for ABCC2 Site-Directed Mutagenesis
Acknowledgments
The authors would like to thank Drs. Bo Kong and Shaojun Yang for technical advice. This work was supported by the National Institutes of Health Institute of Diabetes and Digestive and Kidney Diseases [Grant DK080774, DK093903] and the National Institutes of Environmental Health Sciences [Grants ES020522, ES021800, ES005022].
Abbreviations
- BCA
bicinchoninic acid
- CDCF
5(6)-carboxy-2′,7′-dichlorofluorescein diacetate
- EV
empty vector
- Flp-In 293 cells
Flp-In human embryonic kidney 293 cells
- MRP2
multidrug resistance-associated protein 2
- MSD
membrance-spanning domain
- NBD
nucleotide-binding domain
- RFU
relative fluorescence units
- SNPs
single nucleotide polymorphisms
- WT
wild-type
References
- 1.Fardel O, Jigorel E, Le Vee M, Payen L. Physiological, pharmacological and clinical features of the multidrug resistance protein 2. Biomedicine & Pharmacotherapy. 2005;59(3):104–114. doi: 10.1016/j.biopha.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological Reviews. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y. Functional analysis of SNPs variants of BCRP/ABCG2. Pharmaceutical Research. 2004;21(10):1895–1903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 4.Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Ferrin TE, Giacomini KM, Kroetz DL. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. Journal of Pharmacology and Experimental Therapeutics. 2008;325(3):859–868. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haenisch S, May K, Wegner D, Caliebe A, Cascorbi I, Siegmund W. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenetics and Genomics. 2008;18(4):357–365. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]
- 6.Toh S, Wada M, Uchiumi T, Inokuchi A, Makino Y, Horie Y, Adachi Y, Sakisaka S, Kuwano M. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. American Journal of Human Genetics. 1999;64(3):739–746. doi: 10.1086/302292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda D, Takagi H, Kawahara Y, Yata Y, Takakusagi T, Hatanaka T, Yoshinaga T, Iesaki K, Kashiwabara K, Higuchi T, Mori M, Hirota T, Higuchi S, Ieiri I. Novel large-scale deletion (whole exon 7) in the ABCC2 gene in a patient with the Dubin-Johnson syndrome. Drug Metabolism and Pharmacokinetics. 2009;24(5):464–468. doi: 10.2133/dmpk.24.464. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, Sakisaka S, Maniwa F, Amachi T, Ueda K, Kuwano M. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology. 2002;36(5):1236–1245. doi: 10.1053/jhep.2002.36368. [DOI] [PubMed] [Google Scholar]
- 9.Hirouchi M, Suzuki H, Itoda M, Ozawa S, Sawada J, Ieiri I, Ohtsubo K, Sugiyama Y. Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharmaceutical Research. 2004;21(5):742–748. doi: 10.1023/b:pham.0000026422.06207.33. [DOI] [PubMed] [Google Scholar]
- 10.Laechelt S, Turrini E, Ruehmkorf A, Siegmund W, Cascorbi I, Haenisch S. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. The Pharmacogenomics Journal. 2011;11(1):25–34. doi: 10.1038/tpj.2010.20. [DOI] [PubMed] [Google Scholar]
- 11.Arlanov R, Porter A, Strand D, Brough R, Karpova D, Kerb R, Wojnowski L, Schwab M, Lang T. Functional characterization of protein variants of the human multidrug transporter ABCC2 by a novel targeted expression system in fibrosarcoma cells. Human Mutation. 2012;33(4):750–762. doi: 10.1002/humu.22041. [DOI] [PubMed] [Google Scholar]
- 12.Itoda M, Saito Y, Soyama A, Saeki M, Murayama N, Ishida S, Sai K, Nagano M, Suzuki H, Sugiyama Y, Ozawa S, Sawada Ji J. Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: an association between single nucleotide polymorphisms in the 5′-untranslated region and exon 28. Drug Metabolism and Disposition. 2002;30(4):363–364. doi: 10.1124/dmd.30.4.363. [DOI] [PubMed] [Google Scholar]
- 13.Au A, Baba AA, Azlan H, Norsa'adah B, Ankathil R. Clinical impact of ABCC1 and ABCC2 genotypes and haplotypes in mediating imatinib resistance among chronic myeloid leukaemia patients. Journal Clinical Pharmacy and Therapeutics. 2014;39(6):685–690. doi: 10.1111/jcpt.12197. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi MJ, Bahoush G, Zaker F, Ansari S, Rafsanjani KA, Sharafi H. Association of -24CT, 1249GA, and 3972CT ABCC2 gene polymorphisms with methotrexate serum levels and toxic side effects in children with acute lymphoblastic leukemia. Pediatric Hematology and Oncology. 2014;31(2):169–177. doi: 10.3109/08880018.2013.870625. [DOI] [PubMed] [Google Scholar]
- 15.Wei D, Zhang H, Peng R, Huang C, Bai R. ABCC2 (1249G > A) polymorphism implicates altered transport activity for sorafenib. Xenobiotica. 2016:1–7. doi: 10.1080/00498254.2016.1262976. [DOI] [PubMed] [Google Scholar]
- 16.Megaraj V, Zhao T, Paumi CM, Gerk PM, Kim RB, Vore M. Functional analysis of nonsynonymous single nucleotide polymorphisms of multidrug resistance-associated protein 2 (ABCC2) Pharmacogenetics and Genomics. 2011;21(8):506–515. doi: 10.1097/FPC.0b013e328348c786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senecoff JF, Bruckner RC, Cox MM. The FLP recombinase of the yeast 2-micron plasmid: characterization of its recombination site. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(21):7270–7274. doi: 10.1073/pnas.82.21.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essodaigui M, Broxterman HJ, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein. Biochemistry. 1998;37(8):2243–2250. doi: 10.1021/bi9718043. [DOI] [PubMed] [Google Scholar]
- 19.Heredi-Szabo K, Kis E, Molnar E, Gyorfi A, Krajcsi P. Characterization of 5(6)-carboxy-2,′7′-dichlorofluorescein transport by MRP2 and utilization of this substrate as a fluorescent surrogate for LTC4. Journal of Biomolecular Screening. 2008;13(4):295–301. doi: 10.1177/1087057108316702. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CJ, Hossain MM, Richardson JR, Aleksunes LM. Inflammatory regulation of ATP binding cassette efflux transporter expression and function in microglia. Journal of Pharmacology and Experimental Therapeutics. 2012;343(3):650–660. doi: 10.1124/jpet.112.196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozalpour E, Wittgen HG, van den Heuvel JJ, Greupink R, Russel FG, Koenderink JB. Interaction of digitalis-like compounds with p-glycoprotein. Toxicological Sciences. 2013;131(2):502–511. doi: 10.1093/toxsci/kfs307. [DOI] [PubMed] [Google Scholar]
- 22.Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2) Journal of Biological Chemistry. 2003;278(26):23538–23544. doi: 10.1074/jbc.M303504200. [DOI] [PubMed] [Google Scholar]
- 23.Ryu S, Kawabe T, Nada S, Yamaguchi A. Identification of basic residues involved in drug export function of human multidrug resistance-associated protein 2. Journal of Biological Chemistry. 2000;275(50):39617–39624. doi: 10.1074/jbc.M005149200. [DOI] [PubMed] [Google Scholar]
- 24.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Archiv. 2007;453(5):643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Sugiyama Y. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Advanced Drug Delivery Reviews. 2002;54(10):1311–1331. doi: 10.1016/s0169-409x(02)00075-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen HH, Kuo MT. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Metal-Based Drugs. 2010:430939–6. doi: 10.1155/2010/430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338(Pt 2):393–401. [PMC free article] [PubMed] [Google Scholar]
- 28.Bandler PE, Westlake CJ, Grant CE, Cole SP, Deeley RG. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Molecular Pharmacology. 2008;74(1):9–19. doi: 10.1124/mol.108.045674. [DOI] [PubMed] [Google Scholar]
- 29.Colombo F, Armstrong C, Duan J, Rioux N. A high throughput in vitro mrp2 assay to predict in vivo biliary excretion. Xenobiotica. 2012;42(2):157–163. doi: 10.3109/00498254.2011.614021. [DOI] [PubMed] [Google Scholar]
- 30.Colombo F, Poirier H, Rioux N, Montecillo MA, Duan J, Ribadeneira MD. A membrane vesicle-based assay to enable prediction of human biliary excretion. Xenobiotica. 2013;43(10):915–919. doi: 10.3109/00498254.2013.769649. [DOI] [PubMed] [Google Scholar]
- 31.Elens L, Tyteca D, Panin N, Courtoy P, Lison D, Demoulin JB, Haufroid V. Functional defect caused by the 4544G>A SNP in ABCC2: potential impact for drug cellular disposition. Pharmacogenetics and Genomics. 2011;21(12):884–893. doi: 10.1097/FPC.0b013e32834d672b. [DOI] [PubMed] [Google Scholar]
- 32.Naesens M, Kuypers DR, Verbeke K, Vanrenterghem Y. Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation. 2006;82(8):1074–1084. doi: 10.1097/01.tp.0000235533.29300.e7. [DOI] [PubMed] [Google Scholar]
- 33.Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clinical Pharmacology and Therapeutics. 2006;80(5):468–476. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, Suzuki T, Habuchi T. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. European Journal of Clinical Pharmacology. 2007;63(12):1161–1169. doi: 10.1007/s00228-007-0380-7. [DOI] [PubMed] [Google Scholar]
- 35.Han JY, Lim HS, Yoo YK, Shin ES, Park YH, Lee SY, Lee JE, Lee DH, Kim HT, Lee JS. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–147. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 36.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, Cascorbi I, Kroemer HK. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metabolism and Disposition. 2005;33(7):896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]
- 37.Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD. Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): quantification by liquid chromatography/tandem mass spectrometry. Drug Metabolism and Disposition. 2012;40(5):852–855. doi: 10.1124/dmd.111.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa T, Wakabayashi K, Tamura A, Nakagawa H, Morishima Y, Osawa Y, Ishikawa T. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharmaceutical Research. 2009;26(2):469–479. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werk AN, Bruckmueller H, Haenisch S, Cascorbi I. Genetic variants may play an important role in mRNA-miRNA interaction: evidence for haplotype-dependent downregulation of ABCC2 (MRP2) by miRNA-379. Pharmacogenetics and Genomics. 2014;24(6):283–291. doi: 10.1097/FPC.0000000000000046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Intracellular GSH concentrations in wild-type and variant cells. Flp-In 293 cells were stably transfected with ABCC2 wild-type (WT) or variant plasmids. Intracellular concentrations of reduced GSH were quantified using the GSH/GSSG-Glo assay (Promega, Madison, WI). Data are presented as mean ± SE (n=4).
Supplemental Figure 2. MRP2 mRNA expression in wild-type and variant cells. Total RNA from Flp-In 293 3cells expressing ABCC2 wild-type (WT) and variants was isolated using the RNeasy Mini Kit. The mRNA expression of human ABCC2 was quantified by qPCR assay using Sybr Green to detect amplified products. Ct values were converted to ΔΔCt by comparing to a reference gene GAPDH. Data are presented as mean relative expression ± SE (n=4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to WT.
Supplemental Table 1. Primers Used for ABCC2 Site-Directed Mutagenesis