Abstract
l-Amino acid oxidases (LAO) are widely distributed enzymes but those from snake venoms have been studied the most. We describe a method for in-gel detection of LAO activities based on H2O2 detection by a horseradish peroxidase-coupled reaction using o-phenylenediamine. Complex substrates and single l-amino acids were used successfully for screening LAO activities in higher fungi using crude aqueous extracts of fruiting bodies of 22 basidiomycetes and 1 ascomycete. Half of these samples exhibited one to two bands of LAO activities with mostly broad substrate specificities and a variety of apparent molecular masses ranging from 25 to 200 kDa that were generally more active at pH 5.5 than at pH 8.0. Mushrooms are shown to be a rich source of LAOs that could find use in various medical and biotechnological applications.
Keywords: Fungi, Fruiting body, l-Amino acid oxidase, In-gel detection, Mushroom, Horseradish peroxidase
Introduction
l-Amino acid oxidases (LAO, EC 1.4.3.2) catalyze the stereospecific oxidative deamination of l-amino acids to α-keto acids, producing ammonia and hydrogen peroxide (Hossain et al. 2014). They play a wide range of biological functions either in basal amino acid catabolism or in reactions related to generation of H2O2, a reactive oxygen species. The most extensively studied LAOs are from crotalid snake venoms, in which they constitute an important toxic component (Tan and Fung 2008). Their effects, mediated mainly by H2O2 production, encompass antiviral, antibacterial, antiparasitic, anticoagulant, apoptosis inducing, and other effects (Izidoro et al. 2014). Based on these activities LAOs have been considered for various medical and biotechnological applications, including as catalysts in biotransformations of l-amino acids, as tools in biosensors in medical, biological, and food technological analyses, as anti-cancer and anti-tumor drugs, as biofertilizers for nitrogen acquisition, and as biocontrol agents (Hossain et al. 2014; Pollegioni et al. 2013; Izidoro et al. 2014).
Mushrooms are recognized as a promising source of new enzymes with unique features (Erjavec et al. 2012; Sabotič et al. 2007). However, only a handful of LAOs have been identified in mushrooms. An l-tryptophan oxidase was purified from Coprinus sp. (Furuya et al. 2000), various LAO activities have been identified in Hebeloma strains and in Laccaria bicolor (Nuutinen et al. 2012; Nuutinen and Timonen 2008), and cytotoxic LAOs were isolated from Amanita phalloides, Amanita virosa and Infundibulicybe geotropa (Antonyuk et al. 2010; Stasyk et al. 2010; Pišlar et al. 2016) .
Methods to measure LAO activity are based on detecting the production of ammonia, α-keto acids or H2O2, or on measuring the consumption of oxygen or amino acids. Those most widely used have been methods that measure H2O2, with horseradish peroxidase (HRP) as an H2O2-sensitive probe. Although spectrophotometric assays using microplates are preferred for high-throughput screening, in-gel detection methods provide additional information on the number and approximate molecular masses of detected LAO activities (Rau and Fischer 2011; Yu et al. 2014).
Here we describe the riches of LAO activities in fungal fruiting bodies, using a modified method adapted from a spectrophotometric microplate assay (Kishimoto and Takahashi 2001) for in-gel detection of LAO activity based on the HRP-coupled reaction that uses o-phenylenediamine (OPD) as the substrate.
Materials and methods
Materials
Casein enzymatic hydrolysate from bovine milk (NZ-Case-Plus) and peptone from animal tissue, Type I were from Sigma-Aldrich and Bacto™ Tryptone (pancreatic digest of casein) was from BD. Complete supplement mixture (CSM, used at 790 mg/l) comprised of adenine (10 mg/l), l-Arg (50 mg/l), l-Asp (80 mg/l), l-His (20 mg/l), l-Ile (50 mg/l), l-Leu (100 mg/l), l-Lys (50 mg/l), l-Met (20 mg/l), l-Phe (50 mg/l), l-Thr (100 mg/l), l-Trp (50 mg/l), l-Tyr (50 mg/l), Uracil (20 mg/l), and Val (140 mg/l) was from Formedium, l-amino acids l-Ala, l-Glu, l-Lys, l-Phe were from Serva, and l-Asn, l-Arg, l-Leu, OPD, horseradish peroxidase, catalase, and lysine oxidase from Trichoderma viride was from Sigma-Aldrich and the other stuff (chemicals and enzymes mentioned before) were also from Sigma-Aldrich and not from Trichoderma viride.
Preparation of aqueous extracts of fruiting bodies
Fruiting bodies of 23 fungal species from 14 families of 5 different orders (Table 1) were collected at various locations in Slovenia, identified to species level and frozen at −20 °C. Crude aqueous extracts were prepared by homogenizing thawed fruiting bodies by a ricer and removed insoluble material by centrifugation at 16,000g for 5 min at 4 °C. Approximate protein content in different samples was assessed by SDS-PAGE analysis and visual inspection of Coomassie brilliant blue stained polyacrylamide gels as described (Sabotič et al. 2007). Extracts with very low protein content were concentrated by ultrafiltration (3 kDa cut-off) prior to storing aliquots at −20 °C.
Table 1.
Presence of in-gel-detected LAO activities in crude aqueous extracts of fruiting bodies of selected basidiomycete and ascomycete species
| Order and family | Species | Ecology | Edibility | In-gel LAO activity | Tryptone | CSM | l-Lys | l-Asn | l-Phe | l-Leu | App. M w (kDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 5.5 | pH 8.0 | pH 5.5 | pH 8.0 | pH 5.5 | pH 8.0 | pH 5.5 | pH 8.0 | pH 5.5 | pH 8.0 | pH 5.5 | pH 8.0 | ||||||
| Agaricales (B) | |||||||||||||||||
| Agaricaceae | Agaricus bisporus | s | e | Yesa | + | − | + | + | + | + | + | + | + | + | + | + | 25 |
| Coprinus comatus | s | e | Yesb | + | − | + | − | + | − | ++ | − | + | − | + | − | 30, 60, 180 | |
| Macrolepiota procera | s | e | Yesa | +++ | ++ | − | − | − | − | − | − | − | − | − | − | 35, 50, 70 | |
| Psathyrellaceae | Coprinopsis cinerea | s | i | Noa | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – |
| Amanitaceae | Amanita citrina | m | e* | Nob | − | − | − | − | − | − | − | − | − | − | − | − | – |
| Amanita muscaria | m | p | Nob | − | − | − | − | − | − | − | − | − | − | − | − | – | |
| Amanita phalloides | m | p | Yesc | +++ | +++ | +++ | ++ | + | ++ | ++ | + | +++ | ++ | +++ | +++ | 120 | |
| Amanita rubescens | m | e* | Noa | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – | |
| Hygrophoraceae | Hygrophorus erubescens | m | i | Nob | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – |
| Physalacriaceae | Armillaria borealis | s | e* | Noa | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – |
| Pleurotaceae | Pleurotus ostreatus | s | e | Nob, f | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | (40–55)f |
| Strophariaceae | Hypholoma fasciculare | s | i | Yesb | ++ | + | + | + | − | − | − | − | − | − | − | − | 80 |
| Tricholomataceae | Infundibulicybe geotropa e | s | e | Yesc | +++ | +++ | +++ | +++ | +++ | +++ | − | ++ | ++ | + | +++ | ++ | 80 |
| Clitocybe nebularis | s | e* | Noc, f | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | (30)f | |
| Lepista nuda | s | e | Yesb | +++ | + | +++ | + | +++ | + | +++ | + | +++ | + | +++ | + | 100 | |
| Tricholoma saponaceum | m | e* | Nob | − | − | − | − | − | − | − | − | − | − | − | − | – | |
| Hydnangiaceae | Laccaria amethystina | m | e | Yesb | ++ | ++ | ++ | ++ | + | ++ | − | + | ++ | ++ | ++ | ++ | 120, 200 |
| Auriculariales (B) | |||||||||||||||||
| (Hyaloriaceae) | Pseudohydnum gelatinosum | s | e | Nob | − | − | − | − | − | − | − | − | − | − | − | − | – |
| Boletales (B) | |||||||||||||||||
| Boletaceae | Imleria badia | m | e | Yesb | ++ | + | ++ | ++ | − | − | − | − | − | − | − | − | 25, 30 |
| Suillaceae | Suillus variegatus | m | e | Nob | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – |
| Russulales (B) | |||||||||||||||||
| Russulaceae | Lactarius torminosus | m | e* | Yesb | ++ | − | ++ | + | ++ | − | +++ | − | ++ | − | ++ | − | 35, (90)f |
| Russula ochroleuca | m | e* | Noa | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | – | |
| Pezizales (A) | |||||||||||||||||
| Tuberaceae | Tuber mesentericum | m | e | Yesb | ++ | ++ | ++ | ++ | ++ | ++ | − | + | + | + | + | + | 55, (180)f |
| Hypocreales (A) | |||||||||||||||||
| Hypocreaceae | Trichoderma viride lysine oxidased | na | na | Yes | +++ | +++ | ++ | ++ | +++ | +++ | − | + | ++ | + | − | + | 55 |
Taxonomic classification follows the Index Fungorum database (http://www.indexfungorum.org)
Plus and minus signs represent strength of LAO activity: − no activity, + weak activity, ++ moderate activity, +++ strong activity, nd not determined, na not applicable, B basidiomycetes, A ascomycetes, s saprotrophic, m mycorrhizal, e edible, e* conditionally edible, i inedible, p poisonous
aNon-concentrated crude protein extract
bTwo- to tenfold concentrated crude protein extract
cTwo- to tenfold diluted crude protein extract
dLysine oxidase from Trichoderma viride (Sigma) analyzed at ~2 U
ePreviously named Clitocybe geotropa
fOPD-oxidizing activity detected without the addition of substrate (app. M w of this non-LAO enzymatic activity is indicated in brackets)
In-gel detection of LAO activity
Samples for SDS-PAGE were prepared by dilution in sample buffer (0.15 M Tris–HCl, pH 6.8, 20% glycerol, 4% SDS with bromophenol blue), incubated at room temperature for 15 min, and electrophoresed in 10% polyacrylamide resolving gels containing 0.1% SDS. Approximately, equal loading of proteins in samples was achieved by dilution or aforementioned concentration as indicated in Table 1. Following electrophoresis, gels were washed in the buffer of choice (0.1 M) for 10 min, then incubated in the dark in the same buffer supplemented with substrate (5 mM l-amino acid, 0.1% CSM or 1% tryptone), 1 mM OPD and 0.5 U/ml HRP at room temperature for 1–24 h. The reaction was stopped by adding 2 M H2SO4 and incubating for 10 min. The results were documented using an image scanner. Gels can be stored in water for extended periods of time without losing the intensity of the brown bands of LAO activity.
Results and discussion
Optimization of LAO activity assay conditions
To optimize assay conditions, each component of the reaction mixture was analyzed separately at concentrations guided by published parameters (Kishimoto and Takahashi 2001), using A. phalloides and I. geotropa extracts (Stasyk et al. 2010; Pišlar et al. 2016) as the enzyme source. Both extracts yielded comparable results in all optimization conditions. The presence of SDS was beneficial for detecting the LAO activity, since the use of native PAGE conditions or washing gels in 2.5% Triton-X-100 significantly reduced the observed LAO activity. Depending on the enzyme, the amount loaded and its substrate preference, LAO activity was observed after as little as 10 min of incubation at room temperature; however, incubation times of 16–24 h were used routinely in screening for LAO activity. In-gel detection was successful in the range of pH 4–11. 0.1 M Tris–HCl, pH 8.0, and 0.1 M bis–Tris, pH 5.5, buffers were used routinely for analyzing LAO activities in mushroom extracts. With a single l-amino acid at 5 mM as substrate, OPD concentrations of 0.2–5 mM were analyzed and those below 1 mM resulted in apparently lower LAO activity, while there was no difference in signals for those above 1 mM OPD. Using HRP concentrations of 0.1–0.8 U/ml, LAO activity staining was always observed; however, lower HRP concentrations required longer incubation times for similar results. Therefore, HRP at 0.5 U/ml and OPD at 1 mM were used routinely.
Different types of substrates were assessed for in-gel detection of LAO activity. The use of l-amino acid concentrations of 0.5–10 mM was assessed; LAO activity bands of similar intensities were observed in the range of 2–5 mM, while higher concentrations resulted in partial substrate inhibition. CSM containing adenine, uracil, and 12 l-amino acids (Arg, Asp, His, Ile, Leu, Lys, Met, Phe, Thr, Trp, Tyr, Val, each at different concentrations) was optimized at concentrations of 0.1–0.5% in combination with 1 and 2 mM OPD. Since similar LAO activity was observed for all the CSM–OPD combinations, the simplest mixture, with 0.1% CSM and 1 mM OPD, was used. Complex mixtures of l-amino acids and peptides, tryptone (a pancreatic digest of casein), and NZ-Case-Plus (bovine milk casein enzymatic hydrolysate) at 1% were better substrates than CSM but poorer than the single l-amino acid preferred by the enzyme. In contrast, peptone (Type I from meat) at 1% was a poor substrate.
In-gel LAO activity was inhibited by the addition of catalase (100–12,000 U/ml) to the reaction mixture, however, only very weak inhibition was observed at concentrations above 1000 U/ml and the inhibition was not complete at the highest concentration assessed (not shown).
The addition of FAD (0.025–1 mM) had no effect on in-gel LAO activities (not shown), indicating that the co-factor is tightly bound to these enzymes or there is enough of the co-factor present in the extracts. Similarly, no increase of activity upon addition of FAD was observed for an LAO from Trichoderma harzianum (Lukasheva and Berezov 2002). Alternatively, some of these enzymes may not depend on FAD but contain a quinone co-factor (Campillo-Brocal et al. 2015).
For better assessment of approximate molecular masses, as well as of sample purity, the highly sensitive imidazole–zinc negative staining (Fernandez-Patron et al. 1992) can be performed prior to the detection of LAO activity. No effect on the detected in-gel LAO activity was observed when gels were first negatively stained and de-stained by EDTA.
LAO activities in fungal fruiting bodies
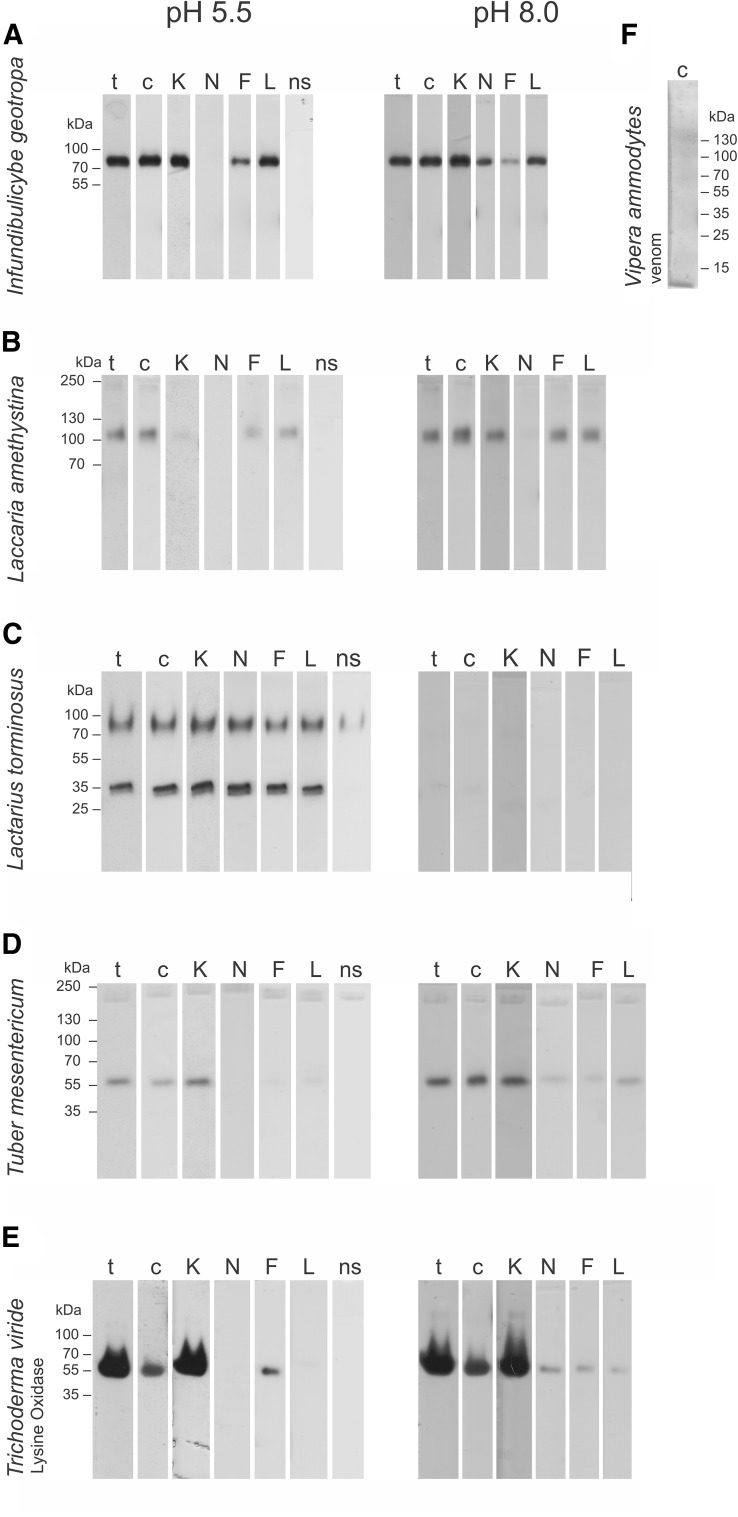
Fungal fruiting bodies contain a variety of l-amino acid oxidases exhibiting different substrate specificities, pH dependence, and molecular masses (Table 1; Fig. 1).
Fig. 1.
In-gel LAO activity at pH 5.5 and pH 8.0 with various substrates for selected species of basidiomycetes (A, B, C), ascomycete (D), commercially available lysine oxidase from T. viride (E) and V. ammodytes ammodytes venom (F). Following SDS-PAGE, LAO activity was detected by staining for H2O2 production using the OPD-HRP system and various LAO substrates: 1% tryptone (t), 0.1% CSM (c), 5 mM l-Lys (K), 5 mM l-Asn (N), 5 mM l-Phe (F), 5 mM l-Leu (L) in 0.1 M bis–Tris, pH 5.5, and 0.1 M Tris–HCl, pH 8.0 and control without substrate (ns) was performed at pH 5.5
Fungal LAOs exhibit broad substrate specificities in accordance with previously reported properties (Antonyuk et al. 2010; Nuutinen et al. 2012; Nuutinen and Timonen 2008; Stasyk et al. 2010). Complex substrates (tryptone and CSM) revealed the most variability in LAO activities in different fungal species. However, based on the Macrolepiota procera sample, which was positive only on tryptone and not on the substrate exclusively composed of l-amino acids, it is possible that its tryptone-oxidizing activity is not mediated by LAO but by some other type of oxidase. Defined mixtures of substrates such as CSM are, therefore, a better choice of substrate for screening LAO activities. For l-Lys oxidase from T. viride, included as a positive control, strong activity toward l-Lys was confirmed and the use of complex substrates in screening was validated, even for an LAO with narrow substrate specificity (Fig. 1E). Furthermore, the approximate molecular mass of T. viride l-Lys oxidase, determined by the in-gel method for LAO activity, corresponds well to the molecular mass of the monomer (56 kDa) determined by various independent methods (Kusakabe et al. 1980; Lukasheva and Berezov 2002). CSM revealed LAO activities in extracts of H. fasciculare and I. badia that were not detected by the selected single l-amino acids used in the assays. An assessment of substrate specificity is possible using this method with different l-amino acids as substrates; for example, positively charged and hydrophobic substrates were oxidized by L. amethystina LAO while the uncharged l-Asn was not at pH 5.5 and very weakly at pH 8 (Table 1).
In general, pH 5.5 proved better than pH 8.0 for broad screening of LAO activities in fungal fruiting bodies. Many LAO activities are higher at pH 5.5, especially those from C. comatus, L. nuda, and L. torminosus, while others are active over a broad pH range and as an exception LAO activity from T. mesentericum was higher at pH 8.0 (Fig. 1; Table 1). The few LAO activities reported from fungi exhibit broad pH profiles but pH optima were slightly basic (pH 7.0–8.0) (Furuya et al. 2000; Nuutinen et al. 2012; Nuutinen and Timonen 2008; Pollegioni et al. 2013) and the highest activity of snake venom LAOs was observed between pH 7.5 and 9 (Izidoro et al. 2014).
Several different molecular mass proteins with LAO activity were observed in some fungal species, similarly as reported previously for Hebeloma and Laccaria (Nuutinen and Timonen 2008). These correspond to either different oligomeric forms, LAO isoforms with different glycosylation patterns, different LAO enzymes or enzymes other than LAO that oxidize OPD. Only the latter are present in extracts of P. ostreatus and C. nebularis. Different LAO activities from C. comatus and L. amethystina gave similar intensities in different conditions and the activity of the band with higher molecular mass was always lower. On the other hand, the 30 kDa LAO activity from I. badia showed higher activity at pH 8 while the 25 kDa LAO activity was higher at pH 5.5.
The taxonomic relatedness, ecological growth mode, and edibility of the fungal species analyzed do not correlate to the presence of LAO activity (Table 1). In some species no LAO activity could be detected; however, the absence of LAO activities is not proof of the absence of LAO enzymes in these species. Enzymes could have denatured during the procedures of detection or the amount of enzyme in the samples was below the limit of detection. Further, the content of proteins in fungal fruiting bodies varies according to the season and growth conditions and enzymes could be expressed only in response to specific developmental or environmental signals. Since LAOs are important enzymes of basal amino acid metabolism they are probably ubiquitous but present in low concentrations. It is possible that higher concentrations are present in those organisms that have recruited these enzymes for defensive or offensive purposes.
The adapted method for in-gel detection of LAO activity is simple, sensitive, reproducible, and convenient for analysis of large numbers of complex samples with the added value of assessing the number and approximate molecular masses of LAOs in samples. Coupled with assessment of sample purity using negative staining, it provides an invaluable tool for LAO purification from novel sources. Furthermore, choice of the substrate used in the reaction mixture allows for general or specific LAO activities to be screened in terms of amino acid preference and pH optimum. While spectrophotometric measurement of LAO activity enables more samples to be processed simultaneously in a microplate format, it is less suitable for screening complex samples that often give false positive results. LAO activity signals can be masked by the amino acids present in the crude extract and similar signals are obtained with and without the addition of the substrate (JS, personal observation). The in-gel method is much more robust, however, some enzymes may escape detection due to their low stability. LAO in Vipera ammodytes ammodytes venom showed much lower activity than enzymes from fungi and a much larger number of isoforms (Fig. 1F). A robust method for screening enzymes to be used in biotechnological applications is beneficial, since stability is an important factor for their industrial applications.
Conclusion
The riches of LAO activities in fungal fruiting bodies are demonstrated by the development of a simple but reproducible method for in-gel detection. Crude aqueous extracts of mushrooms exhibit a number of LAO activities with different substrate specificities, pH optima, and apparent molecular masses that could find use in a number of medical and biotechnological applications.
Acknowledgements
We are grateful to Dr. Adrijana Leonardi for a sample of horned viper venom, to Dr. Jože Brzin for collecting mushroom samples, and to Prof. Roger H. Pain for critical reading of the manuscript and language editing. This work was supported by the Slovenian Research Agency (Grant Number P4-0127 to J.K.).
Abbreviations
- CSM
Complete supplement mixture
- FAD
Flavin adenine dinucleotide
- HRP
Horseradish peroxidase
- LAO
l-Amino acid oxidase
- OPD
o-Phenylenediamine
Compliance with ethical standards
Conflict of interest
Authors declare no conflicts of interest.
References
- Antonyuk VO, Klyuchivska OY, Stoika RS. Cytotoxic proteins of Amanita virosa Secr. mushroom: purification, characteristics and action towards mammalian cells. Toxicon. 2010;55(7):1297–1305. doi: 10.1016/j.toxicon.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Campillo-Brocal JC, Chacon-Verdu MD, Lucas-Elio P, Sanchez-Amat A. Distribution in microbial genomes of genes similar to lodA and goxA which encode a novel family of quinoproteins with amino acid oxidase activity. BMC Genom. 2015;16:231. doi: 10.1186/s12864-015-1455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec J, Kos J, Ravnikar M, Dreo T, Sabotič J. Proteins of higher fungi—from forest to application. Trends Biotechnol. 2012;30(5):259–273. doi: 10.1016/j.tibtech.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Castellanos-Serra L, Rodriguez P. Reverse staining of sodium dodecyl sulfate polyacrylamide gels by imidazole-zinc salts: sensitive detection of unmodified proteins. Biotechniques. 1992;12(4):564–573. [PubMed] [Google Scholar]
- Furuya Y, Sawada H, Hirahara T, Ito K, Ohshiro T, Izumi Y. A novel enzyme, l-tryptophan oxidase, from a basidiomycete, Coprinus sp. SF-1: purification and characterization. Biosci Biotechnol Biochem. 2000;64(7):1486–1493. doi: 10.1271/bbb.64.1486. [DOI] [PubMed] [Google Scholar]
- Hossain GS, Li J, Shin HD, Du G, Liu L, Chen J. l-Amino acid oxidases from microbial sources: types, properties, functions, and applications. Appl Microbiol Biotechnol. 2014;98(4):1507–1515. doi: 10.1007/s00253-013-5444-2. [DOI] [PubMed] [Google Scholar]
- Izidoro LF, Sobrinho JC, Mendes MM, Costa TR, Grabner AN, Rodrigues VM, da Silva SL, Zanchi FB, Zuliani JP, Fernandes CF, Calderon LA, Stabeli RG, Soares AM. Snake venom l-amino acid oxidases: trends in pharmacology and biochemistry. Biomed Res Int. 2014;2014:196754. doi: 10.1155/2014/196754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto M, Takahashi T. A spectrophotometric microplate assay for l-amino acid oxidase. Anal Biochem. 2001;298(1):136–139. doi: 10.1006/abio.2001.5381. [DOI] [PubMed] [Google Scholar]
- Kusakabe H, Kodama K, Kuninaka A, Yoshino H, Misono H, Soda K. A new antitumor enzyme, l-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem. 1980;255(3):976–981. [PubMed] [Google Scholar]
- Lukasheva EV, Berezov TT. l-lysine alpha-oxidase: physicochemical and biological properties. Biochemistry (Mosc) 2002;67(10):1152–1158. doi: 10.1023/A:1020967408229. [DOI] [PubMed] [Google Scholar]
- Nuutinen JT, Timonen S. Identification of nitrogen mineralization enzymes, l-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol Res. 2008;112(12):1453–1464. doi: 10.1016/j.mycres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Nuutinen JT, Marttinen E, Soliymani R, Hilden K, Timonen S. l-Amino acid oxidase of the fungus Hebeloma cylindrosporum displays substrate preference towards glutamate. Microbiology. 2012;158(Pt 1):272–283. doi: 10.1099/mic.0.054486-0. [DOI] [PubMed] [Google Scholar]
- Pišlar A, Sabotič J, Šlenc J, Brzin J, Kos J. Cytotoxic l-amino-acid oxidases from Amanita phalloides and Clitocybe geotropa induce caspase-dependent apoptosis. Cell Death Discov. 2016;2:16021. doi: 10.1038/cddiscovery.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L, Motta P, Molla G. l-Amino acid oxidase as biocatalyst: a dream too far? Appl Microbiol Biotechnol. 2013;97(21):9323–9341. doi: 10.1007/s00253-013-5230-1. [DOI] [PubMed] [Google Scholar]
- Rau JE, Fischer U. In-gel detection of l-amino acid oxidases based on the visualisation of hydrogen peroxide production. J Microbiol Methods. 2011;85(3):228–229. doi: 10.1016/j.mimet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Sabotič J, Trček T, Popovič T, Brzin J. Basidiomycetes harbour a hidden treasure of proteolytic diversity. J Biotechnol. 2007;128(2):297–307. doi: 10.1016/j.jbiotec.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Stasyk T, Lutsik-Kordovsky M, Wernstedt C, Antonyuk V, Klyuchivska O, Souchelnytskyi S, Hellman U, Stoika R. A new highly toxic protein isolated from the death cap Amanita phalloides is an l-amino acid oxidase. FEBS J. 2010;277(5):1260–1269. doi: 10.1111/j.1742-4658.2010.07557.x. [DOI] [PubMed] [Google Scholar]
- Tan N-H, Fung S-Y. Snake venom l-amino acid oxidases and their potential biomedical applications. Malays J Biochem Mol Biol. 2008;16(1):1–10. [Google Scholar]
- Yu Z, Wang Y, Zhou N, Zhao M, Qiu J, Lin J. Advances in detection methods of l-amino acid oxidase activity. Appl Biochem Biotechnol. 2014;174(1):13–27. doi: 10.1007/s12010-014-1005-0. [DOI] [PubMed] [Google Scholar]