Abstract
Background
COPD affects over 13 million Americans, and accounts for over half a million hospitalizations annually. The Hospital Readmission Reduction Program, established by the Affordable Care Act requires the Centers for Medicare and Medicaid Services to reduce payments to hospitals with excess readmissions for COPD as of 2015. This study sought to develop a predictive readmission scale to identify COPD patients at higher readmission risk.
Methods
Demographic and clinical data on 339,389 patients from New York and California (derivation cohort) and 258,113 patients from Washington and Florida (validation cohort) were abstracted from the State Inpatient Database (2006–2011), and the Readmission After COPD Exacerbation (RACE) Scale was developed to predict 30-day readmission risk.
Results
Thirty-day COPD readmission rates were 7.54% for the derivation cohort and 6.70% for the validation cohort. Factors including age 40–65 years (odds ratio [OR] 1.17; 95% CI, 1.12–1.21), male gender (OR 1.16; 95% CI, 1.13–1.19), African American (OR 1.11; 95% CI, 1.06–1.16), 1st income quartile (OR 1.10; 95% CI, 1.06–1.15), 2nd income quartile (OR 1.06; 95% CI, 1.02–1.10), Medicaid insured (OR 1.83; 95% CI, 1.73–1.93), Medicare insured (OR 1.45; 95% CI, 1.38–1.52), anemia (OR 1.05; 95% CI, 1.02–1.09), congestive heart failure (OR 1.06; 95% CI, 1.02–1.09), depression (OR 1.18; 95% CI, 1.14–1.23), drug abuse (OR 1.17; 95% CI, 1.09–1.25), and psychoses (OR 1.19; 95% CI, 1.13–1.25) were independently associated with increased readmission rates, P<0.01. When the devised RACE scale was applied to both cohorts together, it explained 92.3% of readmission variability.
Conclusion
The RACE Scale reliably predicts an individual patient’s 30-day COPD readmission risk based on specific factors present at initial admission. By identifying these patients at high risk of readmission with the RACE Scale, patient-specific readmission-reduction strategies can be implemented to improve patient care as well as reduce readmissions and health care expenditures.
Keywords: chronic obstructive pulmonary disease, readmission, risk factors, risk assessment
Introduction
COPD affects approximately 13.7 million people in the US, accounting for 6.5% of the population. In the US, COPD is the primary cause of over 10 million physician office visits, 1.5 million emergency department visits, and nearly 700,000 hospitalizations annually.1
US health care costs rose substantially from $260 billion (9.18% of the gross domestic product [GDP]) in 1980 to $3.0 trillion (17.5% of the GDP) in 2014.2 It is estimated that by 2040, health care spending could reach 30% of the GDP.3 In 2014, Medicare paid a total of $173 billion on inpatient and outpatient services to 4,700 hospitals, an increase of 4% from the previous year.4 The Centers for Disease Control and Prevention estimates that the total national medical costs for treating COPD will rise from $59.3 billion in 2010 to $90.6 billion in 2020.5
Hospital readmissions are a substantial drain on resources from the US health care delivery system. In 2005, 6.2% of all Medicare hospitalizations resulted in readmissions within 7 days and 17.6% within 30 days. Of these, 84% of 7-day readmissions and 76% of 30-day readmissions were considered preventable.6 Of COPD afflicted Medicare beneficiaries 10.7% were readmitted within 15 days costing approximately $350 million.6 The Medicare Payment Advisory Commission (MedPAC) postulated that the rising cost of Medicare reimbursements for preventable readmissions, if unchecked, will result in higher health care premiums, higher taxes, and will significantly reduce the resources available for other federal priorities.6
To address the increasing cost of hospital readmissions, the federal Affordable Care Act (ACA) was established in March 2010.7 The ACA later added the Hospital Readmissions Reduction Program (HRRP), which effective as of October 2012, required the Centers for Medicare and Medicaid Services (CMS) to reduce payments and reimbursement to hospitals with excess 30-day readmissions (from time of hospital discharge to admission to the same or different hospital).6,7
The HRRP program was initially aimed at reducing readmissions for congestive heart failure (CHF), pneumonia, and acute myocardial infarction; however, other procedures and conditions have subsequently been added.6 In an attempt to reduce inpatient care costs, hospitals failing to reduce readmission rates by predefined amounts are penalized by a withholding of CMS funding/reimbursements.6,8 When the HRRP was first initiated, the CMS estimated that over two-thirds of hospitals would receive penalties comprising approximately 1% of their reimbursement for Medicare patients with high 30-day readmission rates.8 These penalties increased to 3% in 2015, resulting in a total forfeiture of approximately $280 million in Medicare funds from 2,217 hospitals.8 The significant penalties levied on hospitals were imposed to provide incentives for both hospitals and health care providers to improve patient outcomes and reduce readmissions. President Obama reported a reduction in 30-day readmission from 19.1% in 2010 to 17.8% in 2015, an equivalent of 565,000 fewer readmissions.9 Since the inception of the HRRP program, the Healthcare Cost and Utilization Project (HCUP) (2015) reported a reduction in all-cause 30-day COPD readmission rates from 21.2% in 2009 to 20.0% in 2013, leading to a saving of $188 million.10 In 2016, MedPAC reported a reduction in potentially preventable readmissions among COPD patients from 16.8% in 2010 to 14.7% in 2014.4
Given the high prevalence of COPD, and the reimbursement penalties for excessive readmissions, it is crucial that physicians be able to identify factors associated with increased readmission risk and develop strategies aimed at reducing this risk. This study sought to evaluate a large cohort of COPD patients to identify specific demographic and clinical factors present at initial admission that are associated with readmissions and to develop a predictive readmission scale that could reliably identify COPD patients at higher risk for 30-day readmission. The scale was then assessed for its accuracy in predicting an individual’s risk for readmission by validating it on a distinct separate cohort of patients.
Methods
Data for the current study was extracted from the State Inpatient Database (SID), which is a part of the HCUP of the Agency for Healthcare Research and Quality. Four states were included (New York, California, Florida, and Washington) over a 6-year period (2006–2011). The HCUP SID consists of individual discharge data files from organizations in 47 participating states, encompassing approximately 90% of the hospital inpatient discharge records from that state. The SID database is a publicly accessible database. Ethics approval for the following study was obtained from Saint Barnabas Medical Center. No patient consent was required as the study is a retrospective study utilizing the SID database and no specific patient identifiable information was utilized. There were 339,389 patients from California and New York and 258,113 patients from Florida and Washington over the age of 40 years with index admissions meeting the criteria for COPD with International Classification of Diseases (ICD)-9 codes for emphysema (492.0, 492.20, 492.21, 492.22, 492.8), chronic bronchitis (491.0, 491.1, 491.2, 491.8, 491.9), and chronic airway obstruction, not otherwise specified (NOS) (496) identified. Demographic and clinical data including age, gender, race, median income quartile, primary payer, length of stay, discharge disposition, the presence of comorbidities, and COPD readmission were extracted. Readmissions for reasons other than COPD were excluded, and readmission time for COPD was limited to 30 days (Figure 1). Endpoints examined included 30-day readmission for COPD and overall inpatient mortality.
Figure 1.
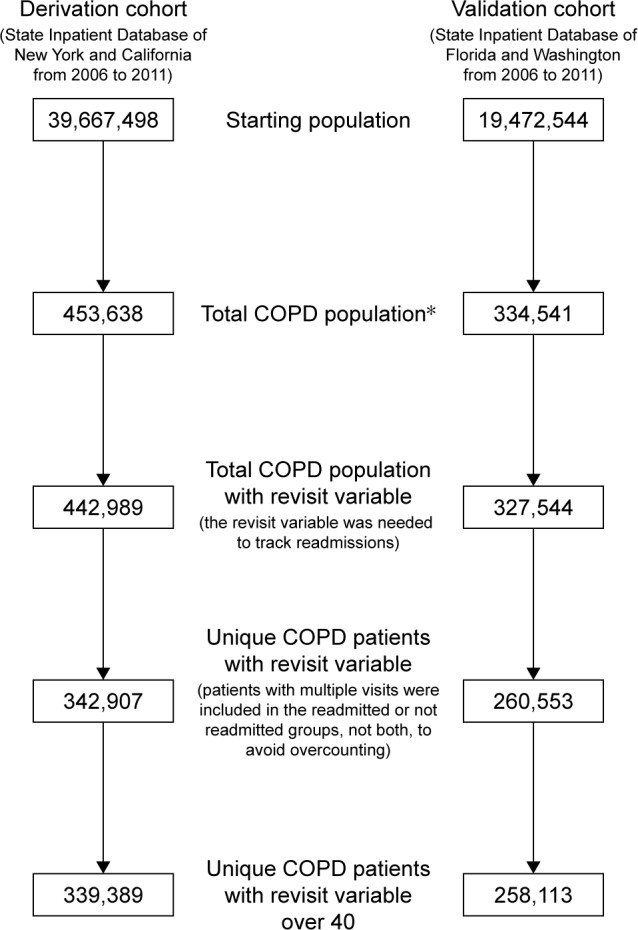
Flowchart of data exclusions used to create the Readmission After COPD Exacerbation risk Scale.
Note: *International Classification of Diseases (ICD)-9 codes for COPD: 491, 491.0, 191.1, 491.2, 491.20, 491.21, 491.22, 491.8, 491.9, 492, 492.0, 492.8, 496.
Categorical data were analyzed using chi square, and univariate analysis was performed using binary logistic regression. Statistical significance was accepted at P<0.01. Factors that were statistically significant in univariate analysis were included in the multivariate logistic regression, and odds ratios (OR) and 95% confidence intervals (CI) were calculated. All data analysis was performed using SAS statistical software, version 9.3. Values less than ten were not included, according to SID guidelines.
Factors that were statistically significant from the multivariate analysis (P<0.01) from the derivation cohort (New York and California) were used to construct the Readmission After COPD Exacerbation (RACE) Scale. Scores for each OR were calculated using the below formula:
To assess the accuracy of the scale, the RACE Scale was validated by generating a RACE score for all patients in both the derivation and validation cohorts. Logistic regression was performed of the percent of patients readmitted with each RACE score. The RACE Scale was only applied to individuals without missing variables.
Results
Demographics and clinicopathological data
A total of 339,389 patients from the derivation cohort and 258,113 patients from the validation cohort were identified in the SID (2006 and 2011) to have been admitted for COPD. Demographic and clinicopathological distributions for both the derivation and validation cohorts were similar (Tables 1 and 2). Most patients were Caucasian (74.5% derivation cohort and 81.4% validation cohort), >65 years (70.2% derivation cohort and 67.6% validation cohort). Male-to-female ratio was 1.25:1 in the derivation and 1.24:1 in the validation cohort. The most common comorbidities were hypertension (63.2% derivation cohort and 64.4% validation cohort), diabetes mellitus (28.3% derivation and 28.8% validation cohort), and CHF (26.9% derivation and 22.9% validation cohort). Mortality rates during the index admission were 2.19% in the derivation cohort and 1.60% in the validation cohort.
Table 1.
Demographic and comorbidities data involving 339,389 COPD patients of derivation cohort
| Characteristic | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Total (%) |
|---|---|---|---|---|---|---|---|
| N | 50,679 | 50,487 | 58,786 | 60,344 | 59,316 | 59,777 | 339,389 |
| Age, years | |||||||
| 40–64 | 14,558 (28.7) | 14,900 (29.5) | 16,985 (28.9) | 18,346 (30.4) | 18,080 (30.5) | 18,374 (30.7) | 101,243 (29.8) |
| 65+ | 36,121 (71.3) | 35,587 (70.5) | 41,801 (71.1) | 41,998 (69.6) | 41,236 (69.5) | 41,403 (69.3) | 238,246 (70.2) |
| Gender | |||||||
| Male | 22,356 (44.3) | 22,564 (44.8) | 26,156 (44.6) | 26,755 (44.4) | 26,356 (44.5) | 26,555 (44.5) | 150,742 (44.5) |
| Female | 28,157 (55.7) | 27,757 (55.2) | 32,476 (55.4) | 33,442 (55.6) | 32,821 (55.5) | 33,097 (55.5) | 187,750 (55.5) |
| Race | |||||||
| Caucasian | 37,607 (76.5) | 36,704 (74.8) | 43,215 (75.2) | 44,086 (74.7) | 42,791 (73.7) | 42,553 (72.6) | 246,956 (74.5) |
| African American | 4,864 (9.89) | 5,028 (10.2) | 5,686 (9.90) | 6,169 (10.5) | 6,195 (10.7) | 6,355 (10.8) | 34,297 (10.4) |
| Other | 6,693 (13.6) | 7,370 (15.0) | 8,555 (14.9) | 8,804 (14.9) | 9,087 (15.7) | 9,692 (16.5) | 50,201 (15.2) |
| Income quartile | |||||||
| 1st | 13,997 (28.5) | 14,282 (29.2) | 16,494 (28.9) | 17,094 (29.2) | 17,305 (30.1) | 17,073 (29.5) | 96,245 (29.3) |
| 2nd | 13,446 (27.4) | 13,397 (27.4) | 15,773 (27.7) | 16,475 (28.2) | 15,578 (27.1) | 16,153 (27.9) | 90,822 (27.6) |
| 3rd | 12,170 (24.8) | 11,975 (24.5) | 13,907 (24.9) | 13,879 (23.7) | 13,691 (23.8) | 14,018 (24.2) | 79,640 (24.2) |
| 4th | 9,479 (19.3) | 9,211 (18.9) | 10,860 (19.0) | 11,030 (18.9) | 10,874 (18.9) | 10,694 (18.5) | 62,148 (18.9) |
| Primary payer | |||||||
| Medicaid | 5,506 (10.9) | 5,895 (11.7) | 6,664 (11.3) | 7,400 (12.3) | 7,469 (12.6) | 7,552 (12.6) | 40,486 (11.9) |
| Medicare | 36,935 (72.8) | 36,065 (71.4) | 42,395 (72.1) | 43,042 (71.3) | 42,762 (72.1) | 43,265 (72.4) | 244,464 (72.0) |
| Private insurance | 6,513 (12.9) | 6,540 (13.0) | 7,351 (12.5) | 7,360 (12.2) | 6,436 (10.9) | 6,402 (10.7) | 40,602 (12.0) |
| Self pay | 855 (1.69) | 1,018 (2.02) | 1,177 (2.00) | 1,359 (2.25) | 1,314 (2.22) | 1,226 (2.05) | 6,949 (2.05) |
| Length of stay, days | |||||||
| ≤2 | 13,718 (27.1) | 13,779 (27.3) | 15,446 (26.3) | 16,835 (27.9) | 17,508 (29.5) | 17,579 (29.4) | 94,865 (28.0) |
| 3 | 8,790 (17.3) | 8,859 (17.6) | 10,466 (17.8) | 10,857 (18.0) | 11,061 (18.7) | 11,139 (18.6) | 61,172 (18.0) |
| 4 | 7,259 (14.3) | 7,178 (14.2) | 8,714 (14.8) | 8,943 (14.8) | 8,654 (14.6) | 8,695 (14.6) | 49,443 (14.6) |
| 5 | 5,330 (10.5) | 5,330 (10.6) | 6,397 (10.9) | 6,452 (10.7) | 6,100 (10.3) | 6,311 (10.6) | 35,920 (10.6) |
| 6 | 3,913 (7.68) | 3,882 (7.69) | 4,537 (7.72) | 4,576 (7.58) | 4,409 (7.43) | 4,402 (7.36) | 25,719 (7.58) |
| ≥7 | 11,669 (27.1) | 11,459 (22.7) | 13,226 (22.5) | 12,681 (21.0) | 11,584 (19.5) | 11,651 (19.5) | 72,270 (21.3) |
| Discharge location | |||||||
| Home | 30,900 (61.0) | 30,831 (61.1) | 35,841 (61.0) | 36,844 (61.1) | 36,148 (60.9) | 36,507 (61.1) | 207,071 (61.0) |
| Home health care | 8,596 (17.0) | 8,452 (16.7) | 10,013 (17.0) | 10,361 (17.2) | 10,766 (18.2) | 10,663 (17.8) | 58,851 (17.3) |
| Rehabilitation center/SNF | 8,259 (16.3) | 8,260 (16.4) | 9,673 (16.5) | 9,870 (16.4) | 9,393 (15.8) | 9,582 (16.0) | 55,037 (16.2) |
| Short term hospital | 690 (1.36) | 696 (1.38) | 824 (1.40) | 848 (1.41) | 720 (1.21) | 739 (1.24) | 4,517 (1.33) |
| Other | 2,232 (4.40) | 2,247 (4.45) | 2,415 (4.11) | 2,419 (4.01) | 2,289 (3.86) | 2,283 (3.82) | 13,885 (4.09) |
| Mortalities during admission | 1,334 (2.63) | 1,305 (2.58) | 1,350 (2.30) | 1,267 (2.10) | 1,083 (1.83) | 1,103 (1.85) | 7,442 (2.19) |
| Comorbidities | |||||||
| AIDS | 71 (0.14) | 77 (0.15) | 82 (0.14) | 96 (0.16) | 81 (0.14) | 110 (0.18) | 517 (0.15) |
| Alcohol abuse | 1,974 (3.90) | 2,012 (3.99) | 2,426 (4.13) | 2,631 (4.36) | 2,728 (4.60) | 2,691 (4.50) | 14,462 (4.26) |
| Anemia | 7,254 (14.3) | 7,814 (15.5) | 9,856 (16.8) | 10,526 (17.4) | 10,379 (17.5) | 10,825 (18.1) | 56,654 (16.7) |
| Coagulopathy | 800 (1.58) | 922 (1.83) | 1,226 (2.09) | 1,471 (2.44) | 1,667 (2.81) | 1,869 (3.13) | 7,955 (2.34) |
| Congestive heart failure | 12,934 (25.5) | 13,249 (26.2) | 15,657 (26.6) | 16,582 (27.5) | 16,235 (27.4) | 16,536 (27.7) | 91,193 (26.9) |
| Depression | 5,966 (11.8) | 6,338 (12.6) | 7,143 (12.2) | 7,775 (12.9) | 7,768 (13.1) | 8,195 (13.7) | 43,185 (12.7) |
| Diabetes | 12,919 (25.5) | 13,520 (26.8) | 16,062 (27.3) | 17,805 (29.5) | 17,729 (29.9) | 18,117 (30.3) | 96,152 (28.3) |
| Drug abuse | 1,351 (2.67) | 1,548 (3.07) | 1,636 (2.78) | 1,884 (3.12) | 2,134 (3.60) | 2,353 (3.94) | 10,906 (3.21) |
| Fluid and electrolyte disorder | 10,512 (20.7) | 10,963 (21.7) | 13,521 (23.0) | 14,374 (23.8) | 14,550 (24.5) | 15,503 (25.9) | 79,423 (23.4) |
| Hypertension | 29,634 (58.5) | 30,491 (60.4) | 36,828 (62.7) | 38,478 (63.8) | 38,955 (65.7) | 40,101 (67.1) | 214,487 (63.2) |
| Hypothyroidism | 5,835 (11.5) | 6,078 (12.0) | 7,201 (12.3) | 7,852 (13.0) | 7,940 (13.4) | 8,245 (13.8) | 43,151 (12.7) |
| Liver disease | 897 (1.77) | 1,022 (2.02) | 1,333 (2.27) | 1,455 (2.41) | 1,569 (2.65) | 1,740 (2.91) | 8,016 (2.36) |
| Lymphoma | 272 (0.54) | 280 (0.55) | 377 (0.64) | 385 (0.63) | 386 (0.65) | 400 (0.67) | 2,098 (0.62) |
| Metastatic cancer | 708 (1.40) | 755 (1.50) | 889 (1.51) | 943 (1.56) | 918 (1.55) | 938 (1.57) | 5,151 (1.52) |
| Neurological disorder | 3,260 (6.43) | 3,612 (7.15) | 4,428 (7.53) | 4,734 (7.85) | 4,685 (7.90) | 4,852 (8.12) | 25,571 (7.53) |
| Obesity | 4,645 (9.17) | 5,012 (9.93) | 6,514 (11.1) | 7,376 (12.2) | 7,490 (12.6) | 8,469 (14.2) | 39,506 (11.6) |
| Paralysis | 824 (1.63) | 766 (1.52) | 1,051 (1.79) | 996 (1.65) | 1,003 (1.69) | 958 (1.60) | 5,598 (1.65) |
| Peptic ulcer disease | 52 (0.10) | 44 (0.09) | 43 (0.07) | 33 (0.05) | 30 (0.05) | 32 (0.05) | 234 (0.07) |
| Peripheral vascular disease | 3,189 (6.29) | 3,386 (6.71) | 4,170 (7.09) | 4,522 (7.49) | 4,797 (8.09) | 5,045 (8.44) | 25,109 (7.40) |
| Psychoses | 2,792 (5.51) | 3,041 (6.02) | 3,719 (6.33) | 3,949 (6.54) | 4,234 (7.14) | 4,493 (7.52) | 22,228 (6.55) |
| Pulmonary circulation disorder | 234 (0.46) | 237 (0.47) | 291 (0.50) | 326 (0.54) | 343 (0.58) | 317 (0.53) | 1,748 (0.52) |
| Renal failure | 4,221 (8.33) | 4,899 (9.70) | 6,475 (11.0) | 7,429 (12.3) | 7,925 (13.4) | 8,600 (14.4) | 39,549 (11.7) |
| Rheumatoid arthritis | 1,231 (2.43) | 1,295 (2.57) | 1,619 (2.75) | 1,701 (2.82) | 1,757 (2.96) | 1,842 (3.08) | 9,445 (2.78) |
| Solid tumor without metastasis | 1,465 (2.89) | 1,506 (2.98) | 1,712 (2.91) | 1,823 (3.02) | 1,852 (3.12) | 1,780 (2.98) | 10,138 (2.99) |
| Valvular disease | 3,070 (6.06) | 3,095 (6.13) | 3,684 (6.27) | 3,931 (6.51) | 3,787 (6.38) | 4,143 (6.93) | 21,710 (6.40) |
| Weight loss | 1,259 (2.48) | 1,268 (2.51) | 1,882 (3.20) | 2,383 (3.95) | 2,629 (4.43) | 2,902 (4.85) | 12,323 (3.63) |
Notes: Anemia combines chronic blood loss anemia and iron deficiency anemia. Diabetes combines complicated and uncomplicated diabetes. Data presented as N (%).
Abbreviations: N, number of patients; SNF, skilled nursing facility.
Table 2.
Demographic and comorbidities data involving 258,113 COPD patients of validation cohort
| Characteristic | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Total (%) |
|---|---|---|---|---|---|---|---|
| N | 36,638 | 33,249 | 44,741 | 46,873 | 47,837 | 48,775 | 258,113 |
| Age, years | |||||||
| 40–64 | 11,091 (30.3) | 10,542 (31.7) | 14,079 (31.5) | 15,462 (33.0) | 16,048 (33.6) | 16,372 (33.6) | 83,594 (32.4) |
| 65+ | 25,547 (69.7) | 22,707 (68.3) | 30,662 (68.5) | 31,411 (67.0) | 31,789 (66.5) | 32,403 (66.4) | 174,519 (67.6) |
| Gender | |||||||
| Male | 16,270 (44.4) | 14,839 (44.6) | 20,088 (44.9) | 21,010 (44.8) | 21,253 (44.4) | 21,718 (44.5) | 115,178 (44.6) |
| Female | 20,368 (55.6) | 18,410 (55.4) | 24,653 (55.1) | 25,863 (55.2) | 26,584 (55.6) | 27,057 (55.5) | 142,935 (55.4) |
| Race | |||||||
| Caucasian | 25,638 (81.7) | 25,605 (81.4) | 33,185 (82.9) | 37,358 (81.5) | 37,842 (80.9) | 38,407 (80.4) | 198,035 (81.4) |
| African American | 2,480 (7.90) | 2,591 (8.23) | 3,127 (7.81) | 3,535 (7.71) | 3,682 (7.87) | 3,785 (7.92) | 19,200 (7.89) |
| Other | 3,257 (10.4) | 3,275 (10.4) | 3,731 (9.32) | 4,972 (10.8) | 5,236 (11.2) | 5,580 (11.7) | 26,051 (10.7) |
| Income quartile | |||||||
| 1st | 12,355 (34.5) | 11,105 (33.2) | 14,584 (33.4) | 15,584 (34.0) | 15,845 (33.9) | 16,333 (34.2) | 85,806 (34.0) |
| 2nd | 10,300 (28.8) | 9,120 (28.1) | 12,756 (29.2) | 12,984 (28.4) | 13,379 (28.6) | 13,456 (28.2) | 71,995 (28.5) |
| 3rd | 7,910 (22.1) | 7,491 (23.1) | 10,055 (23.0) | 10,484 (22.9) | 10,745 (23.0) | 10,928 (22.9) | 57,613 (22.8) |
| 4th | 5,248 (14.7) | 4,764 (14.7) | 6,331 (14.5) | 6,746 (14.7) | 6,826 (14.6) | 7,056 (14.8) | 36,971 (14.7) |
| Primary payer | |||||||
| Medicaid | 2,581 (7.04) | 2,338 (7.03) | 3,313 (7.40) | 3,855 (8.22) | 4,356 (9.11) | 4,482 (9.19) | 20,925 (8.11) |
| Medicare | 27,031 (73.8) | 24,356 (73.3) | 32,420 (72.5) | 33,917 (72.4) | 34,658 (72.5) | 35,702 (73.2) | 188,084 (72.9) |
| Private insurance | 4,428 (12.1) | 4,004 (12.0) | 5,678 (12.7) | 5,448 (11.6) | 5,071 (10.6) | 4,803 (9.85) | 29,432 (11.4) |
| Self pay | 1,228 (3.35) | 1,084 (3.26) | 1,578 (3.53) | 1,743 (3.72) | 1,775 (3.71) | 1,849 (3.79) | 9,257 (3.59) |
| Length of stay (days) | |||||||
| ≤2 | 9,632 (26.3) | 8,831 (26.6) | 11,858 (26.5) | 12,815 (27.3) | 13,801 (28.9) | 14,107 (28.9) | 71,044 (27.5) |
| 3 | 6,896 (18.8) | 6,326 (19.0) | 8,537 (19.1) | 9,150 (19.5) | 9,285 (19.4) | 9,458 (19.4) | 49,652 (19.2) |
| 4 | 5,511 (15.0) | 5,110 (15.4) | 6,809 (15.2) | 7,200 (15.4) | 7,255 (15.2) | 7,586 (15.6) | 39,471 (15.3) |
| 5 | 4,015 (11.0) | 3,558 (10.7) | 5,061 (11.3) | 5,381 (11.5) | 5,295 (11.1) | 5,224 (10.7) | 28,534 (11.1) |
| 6 | 2,886 (7.88) | 2,615 (7.86) | 3,489 (7.80) | 3,554 (7.58) | 3,518 (7.35) | 3,577 (7.33) | 19,639 (7.61) |
| ≥7 | 7,698 (21.0) | 6,809 (20.5) | 8,987 (20.1) | 8,773 (18.7) | 8,683 (18.2) | 8,823 (18.1) | 49,773 (19.3) |
| Discharge location | |||||||
| Home | 24,095 (65.8) | 21,721 (65.3) | 29,737 (66.5) | 31,300 (66.8) | 31,800 (66.5) | 32,350 (66.3) | 171,003 (66.3) |
| Home health care | 5,588 (15.3) | 5,160 (15.5) | 6,870 (15.4) | 7,154 (15.3) | 7,555 (15.8) | 7,876 (16.2) | 40,203 (15.6) |
| Rehabilitation center/SNF | 5,308 (14.5) | 4,834 (14.5) | 6,260 (14.0) | 6,417 (13.7) | 6,509 (13.6) | 6,609 (13.6) | 35,937 (13.9) |
| Short term hospital | 321 (0.88) | 286 (0.86) | 367 (0.82) | 405 (0.86) | 435 (0.91) | 405 (0.83) | 2,219 (0.86) |
| Other | 1,326 (3.62) | 1,248 (3.75) | 1,507 (3.37) | 1,597 (3.41) | 1,538 (3.22) | 1,535 (3.15) | 8,751 (3.39) |
| Mortalities during admission | 729 (1.99) | 651 (1.96) | 736 (1.65) | 722 (1.54) | 645 (1.35) | 656 (1.34) | 4,139 (1.60) |
| Comorbidities | |||||||
| AIDS | 106 (0.29) | 128 (0.38) | 133 (0.30) | 162 (0.35) | 197 (0.41) | 179 (0.37) | 905 (0.35) |
| Alcohol abuse | 1,353 (3.69) | 1,334 (4.01) | 1,895 (4.24) | 2,096 (4.47) | 2,255 (4.71) | 2,380 (4.88) | 11,313 (4.38) |
| Anemia | 5,053 (13.8) | 4,865 (14.6) | 7,136 (16.0) | 8,174 (17.4) | 8,538 (17.9) | 8,872 (18.2) | 42,638 (16.5) |
| Coagulopathy | 784 (2.14) | 801 (2.41) | 1,129 (2.52) | 1,396 (2.98) | 1,636 (3.42) | 1,864 (3.82) | 7,610 (2.95) |
| Congestive heart failure | 8,386 (22.9) | 7,383 (22.2) | 10,203 (22.8) | 10,829 (23.1) | 11,195 (23.4) | 11,062 (22.7) | 59,058 (22.9) |
| Depression | 4,309 (11.8) | 4,079 (12.3) | 5,702 (12.7) | 6,607 (14.1) | 7,176 (15.0) | 7,388 (15.2) | 35,261 (13.7) |
| Diabetes | 9,474 (25.9) | 9,104 (27.4) | 12,478 (27.9) | 13,787 (29.4) | 14,576 (30.5) | 14,969 (30.7) | 74,388 (28.8) |
| Drug abuse | 582 (1.59) | 613 (1.84) | 895 (2.00) | 997 (2.13) | 1,165 (2.44) | 1,289 (2.64) | 5,541 (2.15) |
| Fluid and electrolyte disorder | 7,793 (21.3) | 7,113 (21.4) | 10,504 (23.5) | 11,309 (24.1) | 11,617 (24.3) | 12,270 (25.2) | 60,606 (23.5) |
| Hypertension | 21,793 (59.5) | 20,713 (62.3) | 28,307 (63.3) | 30,625 (65.3) | 31,945 (66.8) | 32,872 (67.4) | 166,255 (64.4) |
| Hypothyroidism | 4,092 (11.2) | 3,980 (12.0) | 5,554 (12.4) | 6,262 (13.4) | 6,665 (13.9) | 6,821 (14.0) | 33,374 (12.9) |
| Liver disease | 618 (1.69) | 599 (1.80) | 860 (1.92) | 1,029 (2.20) | 1,084 (2.27) | 1,105 (2.27) | 5,295 (2.05) |
| Lymphoma | 200 (0.55) | 178 (0.54) | 247 (0.55) | 273 (0.58) | 277 (0.58) | 332 (0.68) | 1,507 (0.58) |
| Metastatic cancer | 491 (1.34) | 442 (1.33) | 620 (1.39) | 705 (1.50) | 727 (1.52) | 702 (1.44) | 3,687 (1.43) |
| Neurological disorder | 2,209 (6.03) | 2,510 (7.55) | 3,397 (7.59) | 3,703 (7.90) | 3,921 (8.20) | 3,864 (7.92) | 19,604 (7.60) |
| Obesity | 3,903 (10.7) | 3,935 (11.8) | 5,675 (12.7) | 6,614 (14.1) | 7,081 (14.8) | 7,510 (15.4) | 34,718 (13.5) |
| Paralysis | 393 (1.07) | 384 (1.15) | 509 (1.14) | 516 (1.10) | 560 (1.17) | 561 (1.15) | 2,923 (1.13) |
| Peptic ulcer disease | 17 (0.05) | 13 (0.04) | 11 (0.02) | 13 (0.03) | 17 (0.04) | 14 (0.03) | 85 (0.03) |
| Peripheral vascular disease | 2,853 (7.79) | 2,779 (8.36) | 3,770 (8.43) | 4,304 (9.18) | 4,391 (9.18) | 4,441 (9.11) | 22,538 (8.73) |
| Psychoses | 1,700 (4.64) | 1,681 (5.06) | 2,244 (5.02) | 2,514 (5.36) | 2,751 (5.75) | 2,855 (5.85) | 13,745 (5.33) |
| Pulmonary circulation disorder | 152 (0.41) | 168 (0.51) | 203 (0.45) | 281 (0.60) | 252 (0.53) | 283 (0.58) | 1,339 (0.52) |
| Renal failure | 2,878 (7.86) | 2,860 (8.60) | 4,345 (9.71) | 5,226 (11.2) | 5,717 (12.0) | 6,376 (13.1) | 27,402 (10.6) |
| Rheumatoid arthritis | 983 (2.68) | 914 (2.75) | 1,222 (2.73) | 1,395 (2.98) | 1,503 (3.14) | 1,710 (3.51) | 7,727 (2.99) |
| Solid tumor without metastasis | 1,002 (2.73) | 870 (2.62) | 1,268 (2.83) | 1,345 (2.87) | 1,278 (2.67) | 1,384 (2.84) | 7,147 (2.77) |
| Valvular disease | 2,735 (7.46) | 2,495 (7.50) | 3,418 (7.64) | 3,558 (7.59) | 3,626 (7.58) | 3,696 (7.58) | 19,528 (7.57) |
| Weight loss | 866 (2.36) | 809 (2.43) | 1,184 (2.65) | 1,513 (3.23) | 1,594 (3.33) | 1,724 (3.53) | 7,690 (2.98) |
Notes: Anemia combines chronic blood loss anemia and iron deficiency anemia. Diabetes combines complicated and uncomplicated diabetes. Data presented as N (%).
Abbreviations: N, number of patients; SNF, skilled nursing facility.
Readmissions
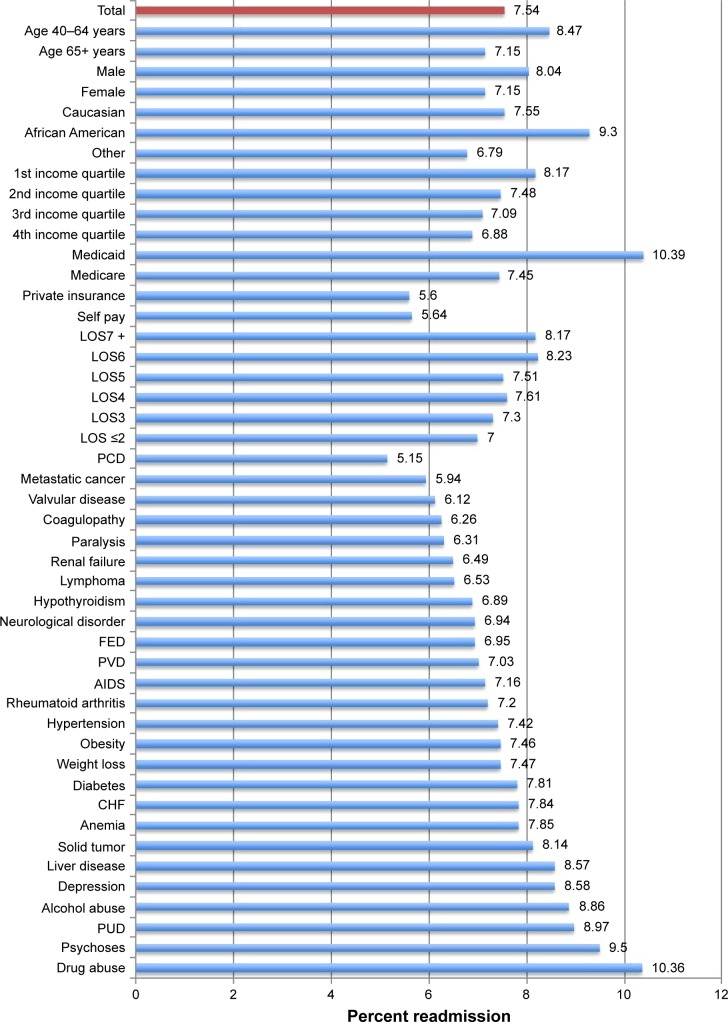
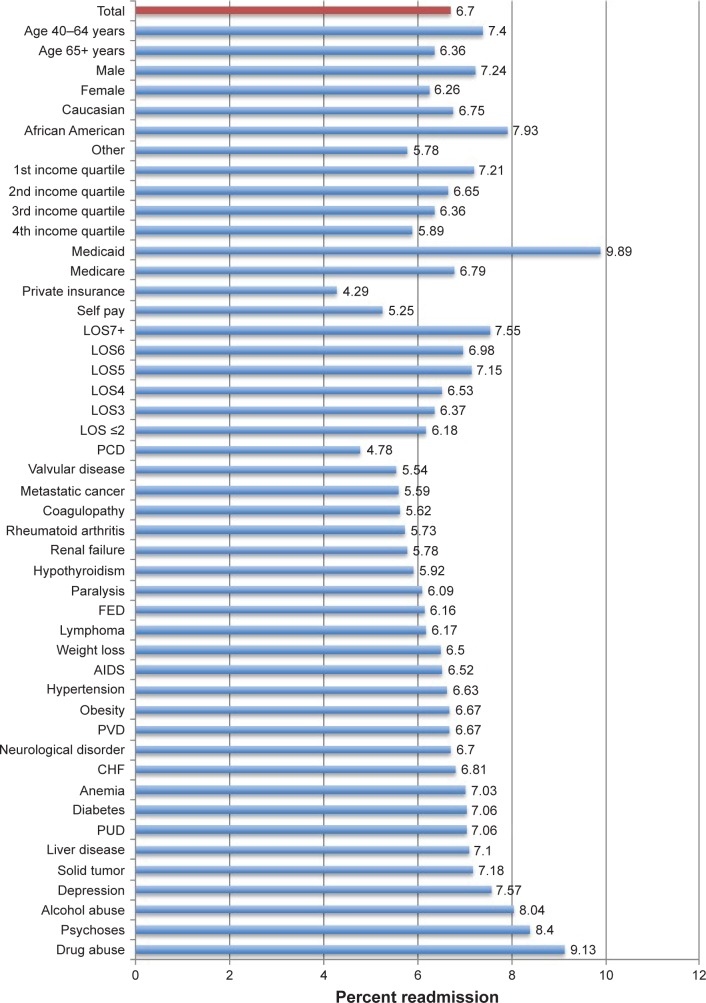
The overall readmission rate was 7.54% in the derivation cohort, with readmission rates being the highest among African Americans (9.30%), patients aged 40–64 years (8.47%), patients with Medicaid Insurance (10.39%) and patients with a history of drug abuse (10.36%) and psychoses (9.50%) (Figure 2). Similarly, the overall readmission rate was 6.70% in the validation cohort, with readmission rates being highest among African Americans (7.93%), patients aged 40–64 (7.40%), patients with Medicaid Insurance (9.89%), and patients with a history of drug abuse (9.13%) and psychoses (8.40%) (Figure 3).
Figure 2.
Readmission rates by demographic and clinicopathological characteristic among COPD patients of derivation cohort.
Abbreviations: LOS, length of stay (days); CHF, congestive heart failure; PCD, pulmonary circulation disorder; FED, fluid and electrolyte disorder; PVD, peripheral vascular disease; PUD, peptic ulcer disease.
Figure 3.
Readmission rates by demographic and clinicopathological characteristic among COPD patients of validation cohort.
Abbreviations: LOS, length of stay (days); CHF, congestive heart failure; PCD, pulmonary circulation disorder; FED, fluid and electrolyte disorder; PVD, peripheral vascular disease; PUD, peptic ulcer disease.
Univariate analysis
On univariate analysis of the derivation cohort, age, gender, race, income quartile, primary payer, alcohol abuse, anemia, CHF, depression, diabetes, drug abuse, liver disease, psychoses, and solid tumors without metastasis significantly affected readmission rates, P<0.01 (Table 3). Similarly, all of these variables except for congestive heart failure, liver disease, and solid tumor without metastasis, significantly affected readmission rates in the validation cohort, P<0.01 (Table 4).
Table 3.
Univariate and multivariate analysis of factors predicting readmission after COPD of derivation cohort
| Characteristic | Univariate odds ratio (95% confidence intervals) | Multivariate odds ratio (95% confidence intervals) |
|---|---|---|
| Age, years | ||
| 40–64 | 1.20 (1.17–1.24)* | 1.17 (1.12–1.21)* |
| 65+ | Reference | Reference |
| Gender | ||
| Male | 1.14 (1.11–1.17)* | 1.16 (1.13–1.19)* |
| Female | Reference | Reference |
| Race | ||
| Caucasian | Reference | Reference |
| African American | 1.26 (1.21–1.31)* | 1.11 (1.06–1.16)* |
| Other | 0.89 (0.86–0.93) | 0.82 (0.78–0.85) |
| Income quartile | ||
| 1st | 1.21 (1.16–1.25)* | 1.10 (1.06–1.15)* |
| 2nd | 1.10 (1.05–1.14)* | 1.06 (1.02–1.10)* |
| 3rd | 1.03 (0.99–1.08) | 1.01 (0.97–1.05) |
| 4th | Reference | Reference |
| Primary payer | ||
| Medicaid | 1.95 (1.85–2.06)* | 1.83 (1.73–1.93)* |
| Medicare | 1.36 (1.30–1.42)* | 1.45 (1.38–1.52)* |
| Private insurance | Reference | Reference |
| Self pay | 1.01 (0.90–1.13) | 0.89 (0.79–1.00) |
| Comorbidities | ||
| AIDS | 0.95 (0.68–1.32) | – |
| Alcohol abuse | 1.20 (1.13–1.28)* | 1.06 (0.99–1.13) |
| Anemia | 1.05 (1.02–1.09)* | 1.05 (1.02–1.09)* |
| Coagulopathy | 0.82 (0.74–0.89) | – |
| Congestive heart failure | 1.06 (1.03–1.09)* | 1.06 (1.02–1.09)* |
| Depression | 1.18 (1.13–1.22)* | 1.18 (1.14–1.23)* |
| Diabetes | 1.06 (1.03–1.09)* | 1.01 (0.98–1.04) |
| Drug abuse | 1.44 (1.35–1.53)* | 1.17 (1.09–1.25)* |
| Fluid and electrolyte disorder | 0.89 (0.86–0.93) | – |
| Hypertension | 0.95 (0.93–0.98) | – |
| Hypothyroidism | 0.90 (0.87–0.94) | – |
| Liver disease | 1.15 (1.07–1.25)* | 0.98 (0.90–1.06) |
| Lymphoma | 0.86 (0.72–1.02) | – |
| Metastatic cancer | 0.77 (0.69–0.87) | – |
| Neurological disorder | 0.91 (0.86–0.96) | – |
| Obesity | 0.99 (0.95–1.03) | – |
| Paralysis | 0.82 (0.74–0.92) | – |
| Peptic ulcer disease | 1.21 (0.77–1.89) | – |
| Peripheral vascular disorders | 0.92 (0.88–0.97) | – |
| Psychoses | 1.31 (1.25–0.38)* | 1.19 (1.13–1.25)* |
| Pulmonary circulation disorder | 0.67 (0.54–0.82) | – |
| Rheumatoid arthritis | 0.84 (0.80–0.88) | – |
| Renal failure | 0.83 (0.80–0.87) | – |
| Solid tumor without metastasis | 1.09 (1.01–1.17)* | 1.07 (1.00–1.16) |
| Valvular disease | 0.79 (0.74–0.83) | – |
| Weight loss | 0.99 (0.92–1.06) | – |
Notes:
Statistically significant at P-value <0.01. Anemia combines chronic blood loss anemia and iron deficiency anemia. Diabetes combines complicated and uncomplicated diabetes. “–” indicates that the factor was not significant in univariate analysis and therefore not included in the multivariate analysis.
Table 4.
Univariate and multivariate analysis of factors predicting readmission after COPD of validation cohort
| Characteristic | Univariate odds ratio (95% confidence intervals) | Multivariate odds ratio (95% confidence intervals) |
|---|---|---|
| Age, years | ||
| 40–64 | 1.18 (1.14–1.21)* | 1.22 (1.17–1.28)* |
| 65+ | Reference | Reference |
| Gender | ||
| Male | 1.17 (1.13–1.21)* | 1.19 (1.15–1.23)* |
| Female | Reference | Reference |
| Race | ||
| Caucasian | Reference | Reference |
| African American | 1.19 (1.13–1.26)* | 1.08 (1.02–1.14)* |
| Other | 0.85 (0.80–0.90) | 0.82 (0.77–0.87) |
| Income quartile | ||
| 1st | 1.24 (1.18–1.31)* | 1.15 (1.09–1.21)* |
| 2nd | 1.14 (1.08–1.20)* | 1.08 (1.02–1.14)* |
| 3rd | 1.09 (1.03–1.15)* | 1.05 (0.99–1.11) |
| 4th | Reference | Reference |
| Primary payer | ||
| Medicaid | 2.45 (2.28–2.64)* | 2.24 (2.07–2.42)* |
| Medicare | 1.63 (1.53–1.72)* | 1.80 (1.68–1.93)* |
| Private insurance | Reference | Reference |
| Self pay | 1.24 (1.11–1.38)* | 1.11 (0.99–1.25) |
| Comorbidities | ||
| AIDS | 0.97 (0.75–1.27) | – |
| Alcohol abuse | 1.23 (1.15–1.32)* | 1.09 (1.01–1.17)* |
| Anemia | 1.06 (1.02–1.11)* | 1.06 (1.01–1.10)* |
| Coagulopathy | 0.83 (0.75–0.91) | – |
| Congestive heart failure | 1.02 (0.99–1.06) | – |
| Depression | 1.17 (1.12–1.22)* | 1.16 (1.11–1.22)* |
| Diabetes | 1.08 (1.05–1.12)* | 1.06 (1.02–1.09)* |
| Drug abuse | 1.41 (1.29–1.55)* | 1.13 (1.02–1.25)* |
| Fluid and electrolyte disorder | 0.89 (0.86–0.92) | – |
| Hypertension | 0.97 (0.94–1.00) | – |
| Hypothyroidism | 0.86 (0.82–0.90) | – |
| Liver disease | 1.07 (0.96–1.19) | – |
| Lymphoma | 0.92 (0.74–1.13) | – |
| Metastatic cancer | 0.82 (0.71–0.95) | – |
| Neurological disorder | 1.00 (0.94–1.06) | – |
| Obesity | 1.00 (0.95–1.04) | – |
| Paralysis | 0.90 (0.77–1.05) | – |
| Peptic ulcer disease | 1.06 (0.46–2.43) | – |
| Peripheral vascular disorders | 1.00 (0.94–1.05) | – |
| Psychoses | 1.30 (1.22–1.38)* | 1.16 (1.08–1.24)* |
| Pulmonary circulation disorder | 0.70 (0.54–0.90) | – |
| Rheumatoid arthritis | 0.84 (0.80–0.89) | – |
| Renal failure | 0.84 (0.80–0.89) | – |
| Solid tumor without metastasis | 1.08 (0.99–1.18) | – |
| Valvular disease | 0.81 (0.76–0.86) | – |
| Weight loss | 0.97 (0.88–1.06) | – |
Notes:
Statistically significant at P-value <0.01. Anemia combines chronic blood loss anemia and iron deficiency anemia. Diabetes combines complicated and uncomplicated diabetes. “–” indicates that the factor was not significant in univariate analysis and therefore not included in the multivariate analysis.
Multivariate analysis
On multivariate analysis of the derivation cohort, patients aged 40–64 years (OR 1.17; 95% CI, 1.12–1.21), male gender (OR 1.16; 95% CI, 1.13–1.19), African American (OR 1.11; 95% CI, 1.06–1.16), 1st income quartile (OR 1.10; 95% CI, 1.06–1.15), 2nd income quartile (OR 1.06; 95% CI, 1.02–1.10), Medicaid insured (OR 1.83; 95% CI, 1.73–1.93), Medicare insured (OR 1.45; 95% CI, 1.38–1.52), anemia (OR 1.05; 95% CI, 1.02–1.09), CHF (OR 1.06; 95% CI, 1.02–1.09), depression (OR 1.18; 95% CI, 1.14–1.23), drug abuse (OR 1.17; 95% CI, 1.09–1.25), and psychoses (OR 1.19; 95% CI, 1.13–1.25) were independently associated with increased readmission rates, P<0.01 (Table 3).
Multivariate analysis of the validation cohort identified aged 40–64 years (OR 1.22; 95% CI, 1.17–1.28), male gender (OR 1.19; 95% CI, 1.15–1.23), African American race (OR 1.02; 95% CI, 1.02–1.14), 1st income quartile (OR 1.15; 95% CI, 1.09–1.21), 2nd income quartile (OR 1.08; 95% CI 1.02–1.14), Medicaid insured (OR 2.24; 95% CI, 2.07–2.42), Medicare insured (OR 1.80; 95% CI, 1.68–1.93), alcohol abuse (OR 1.09; 95% CI, 1.01–1.17), anemia (OR 1.06; 95% CI, 1.01–1.10), depression (OR 1.16; 95% CI, 1.11–1.22), diabetes (OR 1.06; 95% CI, 1.02–1.09), drug abuse (OR 1.13; 95% CI, 1.02–1.25), and psychoses (OR 1.16; 95% CI, 1.08–1.24) as independently associated with increased readmission rates, P<0.01 (Table 4).
RACE Scale
Factors associated with an increased 30-day readmission risk following COPD admission, as determined from the multivariate analysis of the derivation cohort, were analyzed to create the RACE Scale (Table 5). The scale was then applied to patients in the derivation and validation cohorts to assess the accuracy of the RACE Scale in predicting a patient’s risk of readmission. The percentage of patients readmitted for each score was plotted. On logistic regression, an R2 of 0.9230 was calculated, indicating that the RACE score could explain 92.30% of readmission variability in both cohorts analyzed (Figure 4).
Table 5.
Values for components of Readmission After COPD Exacerbation (RACE) Scale
| Characteristic | Point value |
|---|---|
| Age component | |
| 40–64 years | 7 |
| 65+ years | 0 |
| Gender component | |
| Male | 6 |
| Income component | |
| 1st quartile | 4 |
| 2nd quartile | 2 |
| Race component | |
| African American race | 3 |
| Primary payer component | |
| Medicaid | 33 |
| Medicare | 18 |
| Comorbidities component | |
| Psychoses | 8 |
| Depression | 7 |
| Drug abuse | 7 |
| Anemia | 2 |
| Congestive heart failure | 2 |
|
| |
| Max score | 79 |
Note: Anemia combines chronic blood loss anemia and iron deficiency anemia.
Figure 4.
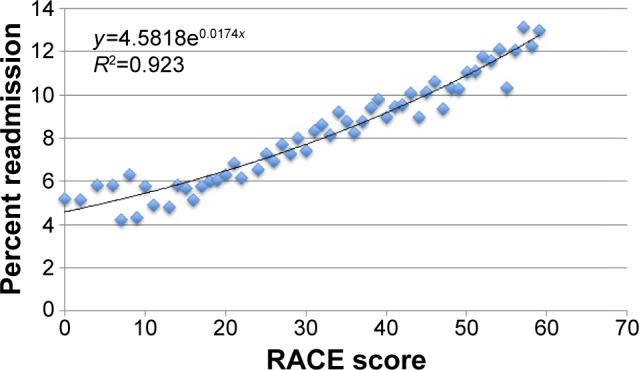
Readmission After COPD Exacerbation (RACE) scores vs percent readmission among all patients.
Discussion
COPD is a significant health and economic burden on society, affecting over 13 million Americans and resulting in half a million hospital stays annually.11 With over 10% of COPD patients being readmitted within 30-days, COPD is one of the leading causes of readmission in the US.6 A significant number of these readmissions are considered potentially preventable.6,12 In an attempt to reduce excessive federal health care expenditures, the ACA compels the CMS to develop a phased elimination of substantial overpayments to hospitals for patients on Medicare Advantage plans caused by preventable readmissions to hospitals for COPD (among other illnesses), a measure that will produce an estimated $132 billion in savings over the next 10 years.6,13 With over two-thirds of hospitals penalized for having excess readmission rates, it is crucial to identify patients at high risk for readmission to allow early preventative intervention.8
The current study observed that 30-day COPD readmission rates were highest among patients of African American race, patients <65 years of age, males, those from lower income households, and those with multiple comorbidities. Shah et al (2015) conducted a retrospective study involving 947,084 Medicare COPD patients and reported a 30-day all-cause readmission rate of 20.2%. Furthermore, the authors reported that patients age <80 (OR 1.0 vs OR 0.97 among patients >80 years, P<0.001) and African Americans (OR 1.06 vs OR 1.00 among Caucasians, P<0.001) had significantly higher odds of readmission.14 Those who were readmitted had longer index admission length of stays (5 days vs 4 days, P=0.02) and were also more likely to have required intensive care (27.91% vs 25.85%, P<0.001).15 Sharif et al (2014) reported that comorbid CHF (OR 1.2, P=0.021), anxiety (OR 1.5, P=0.003), depression (OR 1.3, P=0.016), and osteoporosis (OR 1.3, P=0.008) were independently associated with 30-day readmission.16 Patients who received physician follow-up within 30 days of discharge were significantly less likely to be readmitted (OR 0.7; P<0.001).16 Roberts et al (2016) conducted a study involving 3,612 COPD admissions, and reported that comorbidities and prior hospitalizations were the strongest predictors of early COPD-related rehospitalizations (<30 days), while respiratory-related therapies including the use of steroids (OR 1.62, P=0.007) and nebulizers (OR 1.65, P=0.007) during the index admission were the strongest predictor of late COPD-related re-hospitalizations (>30 days), but not early rehospitalizations.17 Similarly, several studies have indicated that patients prescribed steroids and antibiotics were less likely to be readmitted.18–21
Acute exacerbations of COPD have also been linked with poorer outcomes and higher mortality rates. In a study by Guerrero et al, patients readmitted within 30 days had worse dyspnea, poorer lung function, and more severe disease. Furthermore, readmission within 30 days was a significant risk factor for mortality at 1 year (HR 2.48; 95% CI, 1.10–5.59).22
Nearly all published studies have documented complications from inpatient treatment, inadequate quality of care or care coordination, and lack of patient follow-up and education as factors most closely related to hospital readmission after treatment for COPD.23,24 These same studies have implied that the single best strategy to reduce COPD and other disease specific readmissions is coordinated care.25–27 Williams reported that coordinating care among physicians with a patient-centered approach was more successful at reducing readmissions than a single physician led disease-centered approach.25 Dharmarajan et al demonstrated that most readmissions among patients with CHF, acute myocardial infarction, and pneumonia, were for reasons not related to the index admission diagnosis.26 Multimodal and multifaceted programs with interventions targeting inpatient care during the index admission and follow-up outpatient and home care are required to successfully prevent COPD readmissions. Enhancing initial risk-stratification to determine when to safely discharge patients or transfer patients to alternative health care centers to avoid hospitalization is vital to conserve health care resources. Several such models have been developed and utilized with varying efficacy.28–33 Laverty et al (2015) conducted a pre- and post- implementation study involving a care bundle program involving patient education, outpatient community services, and pulmonary rehabilitation. Prior to the implementation of the care bundle program, COPD readmission rates were rising at a rate of 2.13% per year, while following the implementation of the bundle care program, COPD readmission rates were declining at a rate of 5.32% per year (P=0.012).34 Similarly, Ko et al (2014) reported a significant decline in the number of COPD admissions per patient following the implementation of a comprehensive COPD management plan involving education sessions and telephone follow-up (2.15±1.91 vs 1.54±2.11 episodes per year per person before and after implementation, respectively, P<0.001).35
The RACE Scale is a risk-stratification model for physicians to utilize during the index COPD admission. Although many of the factors determined to increase readmission rates are not modifiable (such as age and race), and some factors are related to one another (such as income and insurance), physicians can modify treatment and follow-up to optimize patient outcomes. For example, specialized multidisciplinary programs can be implemented targeting the elderly and African American patients, optimizing medical comorbidities during the index admission, and scheduling follow-up appointments after discharge. By quickly and accurately calculating the individual’s risk of 30-day readmission, physicians can make more appropriate clinical decisions regarding treatment and management, enabling better coordination between health care professionals to ensure high-risk patients receive necessary care to prevent readmission.
This study contains several limitations inherent to the retrospective nature of a database study. The current model does not follow the exact guidelines set by the HRRP; however, given the current political climate and the continuous data being collected and analyzed on the efficacy of the law, these guidelines are likely to change at least in part. Additionally, the current study utilizes data from only four states from the SID. Given the similarities of these states, determining all-cause readmissions would limit generalizations to all patient populations and different countries around the world. Although reasons for readmissions may vary with patient populations and countries, COPD-readmissions are likely to remain similar, and therefore this model determines only 30-day COPD-readmission. Readmissions for exacerbations of COPD were 7.54% and 6.70% in the derivation and validation cohorts, respectively, while all-cause readmission rates for COPD in the literature were approximately 20%.10 Thus, COPD exacerbations make up about one third of these readmissions, making them a worthwhile target when attempting to reduce the number of readmissions. The CMS adjusts for factors such as age and comorbidities present on admission; however, factors such as socioeconomic status and income are not included. Several published studies have linked poorer patient outcomes with low socioeconomic status and low income patients, and safety-net hospitals with higher proportions of low-income patients have been shown to be negatively affected. Adjusting for socioeconomic status has been shown to change hospital rankings and should be considered by the CMS.36,37 Further limitations include coding and sampling errors, misclassification and inaccurate reporting of variables. The diagnosis of COPD and comorbidities were based on administrative codes in the SID database and were not clinically confirmed; however, it has been shown that these administrative codes are highly specific and accurate.38 The SID database also does not include emergency department or outpatient data, which may lead to underestimating the overall COPD incidence rates. There may also be some degree of selection bias, since only patients with data for all studied variables were included in the creation of this scale. These limitations however, would apply to all groups and therefore should not negate overall findings. Furthermore, only four states from the SID database was chosen a priori, but should be representative of the US, given the large number of available data and geographic locations. Despite these limitations, the large number of patients included in this study allows for an accurate understanding of the changing trends in COPD admissions and the factors influencing read-missions. Further studies examining long term follow up, late complications (>30 days after discharge), and outpatient mortality are required to further fully understand factors associated with COPD readmissions. Randomized control trials should also be conducted to fully understand the impact of various factors and interventions on readmission rates. Additional validation of this scale may also be warranted, applying it to smaller specific populations.
Conclusion
COPD affects over 13 million Americans in the US. Given rising costs for Medicare beneficiaries receiving inpatient COPD care including the costs associated with high readmission rates, statutory components of the ACA and the HRRP were implemented that require CMS to reduce payments to hospitals with excess COPD readmissions. To avoid penalties and to improve patient outcomes and reduce health care expenditures, identifying patients at greatest readmission risk has become a priority for most US hospitals. The RACE Scale is a risk stratification model that allows for a quick and accurate assessment of an individual’s risk for 30-day readmission (using demographic and clinical characteristics present at admission), to enable early intervention and precautionary measures during the index admission. Further studies including randomized control trials should be conducted to fully understand the impact of various factors and interventions on readmission rates. By identifying high-risk patients during the index admission, individualized care programs before and after hospital discharge can be developed and implemented. The RACE Scale should help reduce readmission rates and increase quality of patient care while reducing overall health care expenditures.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance-United States, 1999–2011. Chest. 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare and Medicaid Services National Health Expenditure Data: Historical. 2015. [Accessed April 21, 2016]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html.
- 3.Fuchs VR. The gross domestic product and health care spending. N Engl J Med. 2013;369:107–109. doi: 10.1056/NEJMp1305298. [DOI] [PubMed] [Google Scholar]
- 4.Medicare Payment Advisory Commission March 2016: Report to the Congress: Medicare Payment Policy (Chapter 3 Hospital inpatient and outpatient services) 2016. [Accessed March 06, 2017]. Available from: http://www.medpac.gov/docs/default-source/reports/chapter-3-hospital-inpatient-and-outpatient-services-march-2016-report-.pdf?sfvrsn=0.
- 5.Centers for Disease Control and Prevention Increase Expected in Medical Care Costs for COPD. [Accessed March 07, 2017]. Available from: https://www.cdc.gov/features/ds-copd-costs/
- 6.Medicare Payment Advisory Commission June 2007: Report to the Congress: Promoting Greater Efficiency in Medicine (Chapter 5 Payment policy for inpatient readmissions) 2007. [Accessed March 06, 2017]. Available from: http://www.medpac.gov/docs/default-source/reports/Jun07_Ch05.pdf?sfvrsn=0.
- 7.The Patient Protection and Affordable Care Act, HR 3590, 11th Cong (2010) 2010. [Accessed March 06, 2017]. Available from: https://www.congress.gov/bill/111th-congress/house-bill/3590.
- 8.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 9.Obama B. United States Health Care Reform: progress to date and next steps. JAMA. 2016;316:512–532. doi: 10.1001/jama.2016.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fingar K, Washington R. Trends in Hospital Readmissions for Four High-Volume Conditions, 2009–2013: Statistical Brief #196. 2006. [Accessed May 31, 2017]. Available from https://www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.jsp. [PubMed]
- 11.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 12.Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge Follow-up Characteristics Associated With 30-Day Readmission After Heart Failure Hospitalization. Med Care. 2016;54:365–372. doi: 10.1097/MLR.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenson RA. Implementing health care reform – why Medicare matters. N Engl J Med. 2010;363:101–103. doi: 10.1056/NEJMp1005588. [DOI] [PubMed] [Google Scholar]
- 14.Shah T, Churpek MM, Coca PM, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah CP, Weis E, Lajous M, Shields JA, Shields CL. Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis. Ophthalmology. 2005;112:1599–1607. doi: 10.1016/j.ophtha.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Sharif R, Parekh TM, Pierson KS, Kuo YF, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:685–694. doi: 10.1513/AnnalsATS.201310-358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts MH, Clerisme-Beaty E, Kozma CM, Paris A, Slaton T, Mapel DW. A retrospective analysis to identify predictors of COPD-related rehospitalization. BMC Pulm Med. 2016;16:68. doi: 10.1186/s12890-016-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354:456–460. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 19.Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 20.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 21.Stefan MS, Rothberg MB, Shieh MS, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143:82–90. doi: 10.1378/chest.12-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrero M, Crisafulli E, Liapikou A, et al. Readmission for Acute Exacerbation within 30-days of discharge is associated with a subsequent progressive increase in mortality risk in COPD patients: A long-term observational study. PLoS One. 2016;11(3):e0150737. doi: 10.1371/journal.pone.0150737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu N, Huang KC, Johnson JA. Reducing excess readmissions: promising effect of hospital readmissions reduction program in US hospitals. Int J Qual Health Care. 2016;28:53–58. doi: 10.1093/intqhc/mzv090. [DOI] [PubMed] [Google Scholar]
- 24.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306:1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 25.Williams MV. A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309:394–396. doi: 10.1001/jama.2012.233964. [DOI] [PubMed] [Google Scholar]
- 26.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott KW, Jha AK. Putting quality on the global health agenda. N Engl J Med. 2014;371:3–5. doi: 10.1056/NEJMp1402157. [DOI] [PubMed] [Google Scholar]
- 28.Basoor A, Doshi NC, Cotant JF, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013;19:200–206. doi: 10.1111/chf.12031. [DOI] [PubMed] [Google Scholar]
- 29.Collins SP, Pang PS, Fonarow GC, Yancy CW, Bonow RO, Gheorghiade M. Is hospital admission for heart failure really necessary?: the role of the emergency department and observation unit in preventing hospitalization and rehospitalization. J Am Coll Cardiol. 2013;61:121–126. doi: 10.1016/j.jacc.2012.08.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 31.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 32.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laverty AA, Elkin SL, Watt HC, et al. Impact of a COPD discharge care bundle on readmissions following admission with acute exacerbation: interrupted time series analysis. PLoS One. 2015;10:e0116187. doi: 10.1371/journal.pone.0116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko FW, Ngai JC, Ng SS, et al. COPD care programme can reduce readmissions and in-patient bed days. Respir Med. 2014;108:1771–1778. doi: 10.1016/j.rmed.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Glance LG, Kellerman AL, Osler TM, Li Y, Li W, Dick AW. Impact of Risk Adjustment for Socioeconomic Status on Risk-Adjsuted Surgical Readmission Rates. Ann Surg. 2016;263(4):698–704. doi: 10.1097/SLA.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Q, Koenig L, Faerberg J, Steinberg CR, Vaz C, Wheatley MP. The Medicare Hospital Readmission Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49(3):818–837. doi: 10.1111/1475-6773.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]