Abstract
Background
High doses of human recombinant erythropoietin (rHuEPO) to achieve hemoglobin levels above 13 g/dL in chronic kidney disease appear associated with elevated mortality.
Study Design
We conducted logistic regression and survival analyses in a retrospective cohort of maintenance hemodialysis (MHD) patients to examine the hypothesis that the induced iron depletion with resultant relative thrombocytosis may be a possible contributor to the link between the high rHuEPO dose associated hemoglobin ≥13 g/dL and mortality.
Setting & Participants
The national database of a large dialysis organization (DaVita) with 40,787 MHD patients during July to December 2001 and their survival up to July 2004 were examined.
Predictors
Hemoglobin level, platelet count and administered rHuEPO dose during each calendar quarter.
Outcomes & other Measurements
Case-mix adjusted 3-year all-cause mortality; and measures of iron stores including serum ferritin and iron saturation ratio (ISAT).
Results
Higher platelet count was associated with lower iron stores and higher prescribed rHuEPO dose. Compared to hemoglobin of 12-13 g/dL, hemoglobin ≥13 g/dL was associated with increased mortality in the presence of relative thrombocytosis, i.e., platelet count ≥300,000/μl, (case-mix adjusted death-rate ratio [RR]: 1.21, 95% confidence limits [CL]: 1.02–1.44, P=0.03) as opposed to the absence of relative thrombocytosis (RR: 1.04, 95% CL: 0.98–1.08, P=0.13). Prescribed rHuEPO dose >20,000 units/week was associated with higher likelihood of iron depletion (ISAT<20%) and relative thrombocytosis (case-mix adjusted odds ratio: 2.53 [CL: 2.37–2.69] and 1.36 [CL: 1.30–1.42], respectively, p<0.001) and increased mortality over 3 years (death-rate ratio of 1.59, CL: 1.54, 1.65, p<0.001).
Limitations
Our results may incorporate uncontrolled confounding. Achieved hemoglobin may have different mortality-predictability than targeted hemoglobin.
Conclusions
Iron depletion and associated relative thrombocytosis might contribute to increased mortality when administering high rHuEPO doses to achieve hemoglobin ≥13 g/dL in MHD patients. Randomized trials are needed to test these observational associations.
Keywords: Anemia, thrombocytosis, iron stores, hemodialysis population, erythropoiesis stimulating agent (ESA), malnutrition-inflammation-cachexia syndrome (MICS)
Introduction
Among patients with terminal (stage 5) CKD undergoing maintenance dialysis treatment, anemia has been associated with increased mortality.[1–3] As a result, most guidelines recommend treating CKD associated anemia with erythropoiesis stimulating agents (ESA) to improve hemoglobin levels to at least 10 or 11 g/dL levels.[1, 2, 4] While higher hemoglobin levels are incrementally associated with greater survival in most epidemiologic studies, a randomized controlled trial (RCT) designed to normalize hemoglobin to levels at or above 14 g/dL in dialysis patients showed a paradoxical increase in cardiovascular events and a trend towards higher mortality.[5] Two recent RCTs with similar objectives of normalizing hemoglobin but in earlier CKD stages resulted in similar findings.[6, 7] A recent meta-analysis of these and other trials indicated a higher mortality in CKD patients in whom a target hemoglobin consistent with the normal range of the general population, (≥13 g/dL) was attempted.[8, 9] Consistent with these RCT findings, a recent observational study in 58,058 MHD patients showed that a hemoglobin concentration in the range of 12 to 13 g/dL was associated with the greatest survival, whereas hemoglobin levels above 13 or below 11.5 g/dL was associated with higher mortality when compared to the 11–11.5 g/dL group.[3] Despite the apparent consistency of these findings, it is not clear why hemoglobin and survival exhibit such a U-shaped association.
Recent studies have indicated a potential link between the relative state of iron depletion and higher mortality in patients with advanced CKD who undergo maintenance hemodialysis (MHD).[10, 11] Treatment of anemia with ESA but without adequate iron supplementation may deplete iron stores.[12] Iron depletion is known to be associated with relative thrombocytosis (increased platelet count),[13] which might lead to higher risk of thromboembolic events.[14, 15] Given the high risk of cardiovascular and other thrombotic events in patients with CKD, we hypothesized that CKD patients who received higher ESA doses and achieve higher hemoglobin levels tend to have lower iron stores and higher blood platelet counts, which may predispose them to increased thrombotic events and the increased death risk.
Methods
Study Design
We conducted a retrospective cohort study in maintenance hemodialysis (MHD) patients of a 3-year national database of a large dialysis organization in the United States (DaVita, Inc) and followed the subjects for up to 3 years.
Settings and Participants
We extracted, refined, and examined data from all individuals with CKD stage 5, who underwent MHD treatment from July 1 to December 31, 2001, in any one of the 560 outpatient dialysis facilities of DaVita, Inc, and followed them until June 30, 2004. The study was approved by the Institutional Review Committees of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research; because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, the requirement for informed consent was waived.
Clinical and Demographic Measures
The study cohort has been described previously.[11, 16, 17] To minimize measurement variability, all repeated measures for each patient during any of the two baseline calendar quarters (Summer and Fall 2001) were averaged and the summary estimate was used in all models. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. MHD patients qualified for this study were 18 years or older and required to have a dialysis vintage of 90 days or longer during at least half of the baseline calendar quarter.
Thirteen-week averaged post-dialysis weight and baseline height were used to calculate the body mass index (BMI=weight[kg]/height squared[m2]). The dose of administered recombinant human erythropoietin (rHuEPO, EPOGEN™, Amgen, Inc, Thousand Oaks, CA) was also calculated for each baseline calendar quarter.[17–20] The dates of death or other censoring events such as kidney transplantation or leaving the country were obtained for all patients who did not survive or were lost up to June 30, 2004.
History of diabetes mellitus was available in the database, while histories of tobacco smoking and preexisting comorbid conditions were obtained by linking the DaVita database to Medical Evidence Form 2728.[21] The latter were categorized into ten comorbid conditions: (1) ischemic heart disease, (2) congestive heart failure, (3) status post (s/p) cardiac arrest, (4) s/p myocardial infarction, (5) pericarditis, (6) cardiac dysrhythmia, (7) cerebrovascular events, (8) peripheral vascular disease (9) chronic obstructive pulmonary disease, and (10) cancer.
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 hrs. All laboratory values were measured by automated and standardized methods in the DaVita Laboratory. Most laboratory values, including complete blood cell counts and serum levels of urea nitrogen, creatinine, albumin, calcium, phosphorus, bicarbonate, iron and total iron binding capacity (TIBC), were measured monthly. Serum ferritin was measured quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to bi-weekly in most patients. Serum iron saturation ratio (ISAT) and ferritin values serves as markers of iron stores in this study. Kt/V was used to estimate dialysis dose and normalized protein equivalent of total nitrogen appearance (nPNA), also known as normalized protein catabolic rate (nPCR), an estimation of daily protein intake, was assessed monthly as a measure of protein intake. Most blood samples were collected pre-dialysis with the exception of the post-dialysis serum urea nitrogen that was obtained to calculate urea kinetics.
Analytical Methods
We used Cox proportional-hazards and logistic regression with three levels of regression adjustment: (I) A minimally adjusted model that included mortality as the outcome measure, the predictors and the entry calendar quarter; (II) Case-mix adjusted models that included all of the above plus diabetes mellitus and 10 pre-existing comorbid states, history of tobacco smoking, categories of dialysis vintage (<6 mos, 6 mos to 2 yrs, 2–5 yrs and ≥5 yrs), primary insurance (Medicare, Medicaid, private and others), marital status (married, single, divorced, widowed and other or unknown), the standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a dialysis catheter, and residual renal function during the entry quarter, i.e. urinary urea clearance; and (III) Malnutrition-inflammation-complex syndrome (MICS) adjusted models which included all of the covariates in the case-mix model as well as 13 surrogates of nutritional status and inflammation, including BMI, the average dose of rHuEPO (if not modeled as a predictor), and 11 laboratory variables as surrogates of the nutritional state or inflammation, together also known as MICS, with known association with clinical outcomes in MHD patients [11, 22, 23]: (1) nPNA as an indicator of daily protein intake, (2) serum albumin, (3) serum TIBC, (4) serum ferritin; (5) serum creatinine, (6) serum phosphorus, (7) serum calcium, (8) serum bicarbonate, (9) peripheral white blood cell count (WBC), (10) lymphocyte percentage, and (11) iron saturation ratio. In our view results from the minimally adjusted models are likely to be underadjusted due to omission of potential confounders, while results from the MICS adjusted models may be overadjusted due to possible inclusion of biological intermediates. We thus prefer to base inferences on the case-mix adjusted models. Because we cannot be certain of the best model, however, we have included all three levels of adjustments in some of the presented analyses to provide the full spectrum of results.
Plots of log [-log (survival rate)] against log (survival time) were used to check the proportionality assumption. Missing covariate data (under 2% for most laboratory and demographic variables and under 3% for any of the 10 comorbid conditions) were imputed by the mean of the existing values (except for ferritin and PTH where median was used). All analyses were carried out with the SAS, version 9.1, SAS Institute, Inc., Cary, North Carolina, and Stata version 9.0, Stata Corporation, College Station, Texas.
Results
The original 6-month (July to Dec, 2001) national database of all DaVita MHD patients included 47,156 subjects. After deleting those patients who did not continue hemodialysis treatment for more than 45 days, 41,093 MHD patients remained for analysis, of whom 306 patients had missing core data including platelet count or hemoglobin values. The final cohort included 40,787 MHD patients, of whom patients 33,024 patients originated from the first calendar quarter dataset and the rest from the subsequent calendar quarter. Analysis follow-up time started from the first day of the calendar quarter that the patient met the above criteria. To compare patient characteristics in high vs. normal to low platelet count groups, we defined “relative” thrombocytosis as a platelet count equal or greater than 300,000/μl based on several previous studies [24–29] and prior to the start of data analysis as a pre-determined definition, whereas “absolute” thrombocytosis is defined as a platelet count equal or greater than 450,000/μl. In this study, we exclusively examined the relative thrombocytosis, which based on our pre-determined definition was present in 15% of the MHD patients at any given time, whereas absolute thrombocytosis was present in only one percent of the entire cohort. In our cohort, the mean platelet count was 226,328/μl (SD: 75,678/μl, median: 215,667/μl inter-quartile range: 173,500/μl to 268,333/μl).
Table 1 shows baseline demographic, clinical and laboratory characteristics of the studied MHD patients. Those with relative thrombocytosis averaged 2 years younger and were more likely to be women and diabetic. Even though about 60% of patients in both groups received IV iron during the baseline calendar quarter, the thrombocytotic patients had been prescribed 30% higher weekly doses of rHuEPO of ~18,000 units/wk and had lower serum ISAT level (24±12%) compared to their non-thrombocytotic counterparts, who received lower weekly rHuEPO doses of ~13,500 units/wk and who had a higher ISAT (30±13%). Serum Ferritin was lower in thrombocytotic MHD patients. Examining bivariate associations between platelet count and relevant clinical and laboratory variables showed that higher platelet count was associated with lower ISAT, lower hemoglobin and higher WBC count. Figure 1 shows that ISAT decreases monotonically across 25,000/μl increments of blood platelet counts.
Table 1.
Baseline (first calendar quarter) data of 40,787 MHD patients, including 33,024 patients from the first quarter (Jul–Aug–Sep 2001) and 7,763 patients from the subsequent quarter (Oct–Nov–Dec 2001)a
| Variable a | Platelet count <300,000/μl n= 34,573 | Platelet count ≥300,000/μl n=6,214 |
|---|---|---|
| Age (years) | 61±15 | 59±15 |
| Sex (% women) | 45 | 53 |
| Diabetes mellitus (%) | 45 | 52 |
| Race/ethnicity (%):* | ||
| Non-Hispanic Whites | 35 | 35 |
| Blacks | 34 | 37 |
| Hispanics | 16 | 14 |
| Vintage (time on dialysis,%):* | ||
| <6 months | 11 | 15 |
| >=5 years | 21 | 17 |
| Primary insurance (%):* | ||
| Medicare | 68 | 65 |
| Medicaid | 5 | 7 |
| Marital Status (%):* | ||
| Married | 43 | 40 |
| Single | 24 | 26 |
| Kt/V (dialysis dose) | 1.3 | 1.2 |
| Comorbidity (%):* | ||
| Heart Failure c | 27 | 26 |
| PVD b | 11 | 12 |
| Ischemic Heart Disease b | 18 | 17 |
| Myocardial Infarct c | 6 | 6 |
| Protein Catabolic Rate (g/kg/day) | 1.00±0.24 | 0.96±0.24 |
| Laboratory tests: | ||
| Serum albumin (g/dL) | 3.8±0.4 | 3.6±0.5 |
| Creatinine (mg/dL) | 9.5±3.3 | 8.8±3.2 |
| TIBC (mg/dL) c | 202±41 | 201±49 |
| bicarbonate (mg/dL) c | 22.0±2.7 | 22.0±2.8 |
| phosphorus (mg/dL) b | 5.7±1.5 | 5.7±1.6 |
| calcium (mg/dL) | 9.2±0.8 | 9.2±0.8 |
| ferritin (ng/mL) ¶ | 580±310 | 498±342 |
| iron saturation ratio | 30±13 | 24±12 |
| Blood hemoglobin (g/dL) | 11.9±1.2 | 11.2±1.4 |
| Platelet count (×103/υl) | 205±51 | 363±67 |
| WBC (×103/υl) | 7.0±2.1 | 9.1±2.9 |
| lymphocyte (% of total WBC) | 21±8 | 19±8 |
| rHuEPO dose (units/week) ¶ | 13,418±8,518 | 17,795±10,987 |
| Use of any IV iron (%) | 60 | 61 |
P-value <0.001 for the difference between the two groups, unless otherwise specified:
0.01 ≤ p < 0.05
p≥0.05
Categorical variables with more than two strata have a global statistical test across all categories (p<0.001).
Count data are in percentage, and continuous values are in mean±SD if normally distributed or median (IQR/2) if skewed.
Figure 1. Association between platelet count and ISAT.
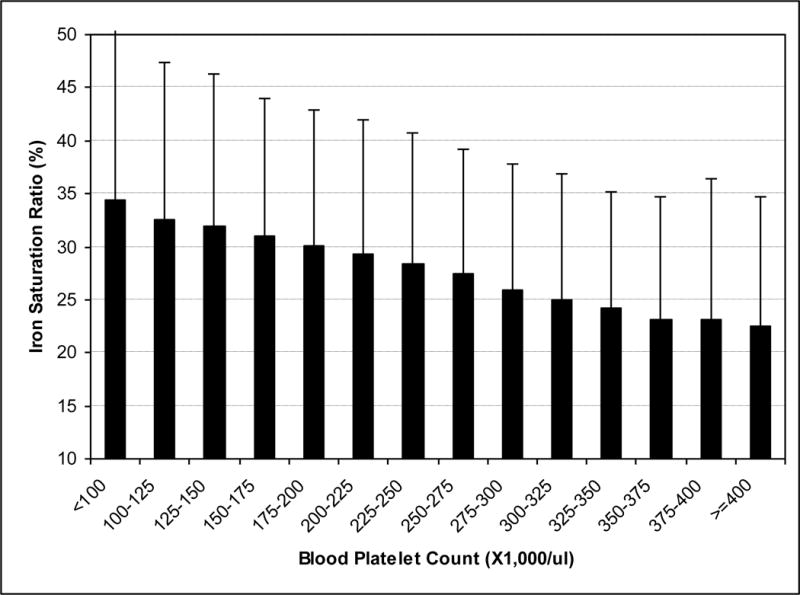
Serum ISAT across 25,000/mcl increments of blood platelet counts in 40,787 MHD patients (error bars represent standard deviations in each group).
To study the association between platelet counts and iron stores and their potential link to the anemia management, we stratified the MHD patients with a hemoglobin above 10 g/dL and increments of 1 g/dL into two mutually exclusive groups of non-thrombocytotic (platelet <300,000/μl) and thrombocytotic (platelet≥300,000/μl) patients. As shown in Table 2 and Figure 2, thrombocytotic MHD patients had lower iron stores than non-thrombocytotic patients independent of the level of hemoglobin. In each hemoglobin group, mortality rate was higher in thrombocytotic patients than non-thrombocytotic ones (Table 3).
Table 2.
Four categories of hemoglobin > 10 g/dL in 40,787 MHD patients, stratified according to absence or presence of relative thrombocytosis (platelet count < or ≥300 ×103/υl). * The continuous values are in mean (±SD).
| A. Blood platelet count <300,000 (n= 34,573) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hemoglobin categories (g/dL) | Number of patients (%) | All cause death (%) | CV death (%) | ISAT (%)* | Ferritin (ng/mL)* | Platelet (×103/υl)* | WBC (×103/υl)* | EPO dose (units/week) |
| 10–10.99 | 4,497 (11) | 1,869 [42] | 602 [14] | 25.7 ± 13.3 | 653 ± 517 | 212 ± 53 | 7.1 ± 2.3 | 25,894 ± 23,885 |
| 11–11.99 | 10,570 (26) | 3,948 [38] | 1,342 [13] | 28.0 ± 13.5 | 675 ± 456 | 206 ± 50 | 6.9 ± 2.1 | 17,755 ± 16,038 |
| 12–12.99 | 11,380 (28) | 3,893 [35] | 1,246 [11] | 29.7 ± 14.0 | 665 ± 468 | 202 ± 49 | 7.0 ± 2.0 | 14,949 ± 13,234 |
| ≥13 | 5,343 (13) | 1,843 [35] | 606 [12] | 30.3 ± 14.3 | 600 ± 483 | 199 ± 49 | 7.0 ± 2.0 | 13,837 ± 14,065 |
| B. Blood platelet count ≥300,000 (n=6,214) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hemoglobin categories (g/dL) | Number of patients (%) | All cause death (%) | CV death (%) | ISAT (%)* | Ferritin (ng/mL)* | Platelet (×103/υl)* | WBC (×103/υl)* | EPO dose (units/week) |
| 10–10.99 | 1,357 (3) | 619 [46] | 222 [17] | 21.3 ± 11.7 | 613 ± 564 | 367 ± 66 | 9.1 ± 2.7 | 28,429 ± 33,374 |
| 11–11.99 | 1,844 (5) | 762 [42] | 267 [15] | 23.3 ± 11.5 | 637 ± 499 | 357 ± 61 | 8.9 ± 2.8 | 20,647 ± 18,273 |
| 12–12.99 | 1,289 (3) | 471 [37] | 167 [13] | 25.0 ± 12.0 | 633 ± 487 | 352 ± 60 | 9.0 ± 2.9 | 17,170 ± 33,983 |
| ≥13 | 457 (1) | 176 [39] | 61 [14] | 25.3 ± 13.0 | 531± 441 | 351 ± 60 | 9.2 ± 3.0 | 17,965 ± 47,958 |
Values in parentheses represent the proportion of the MHD patients in each category (for count data). Values in brackets are the crude death rate in the indicated group during the 3 years of observation.
Figure 2. Association between ISAT, platelet count and hemoglobin.
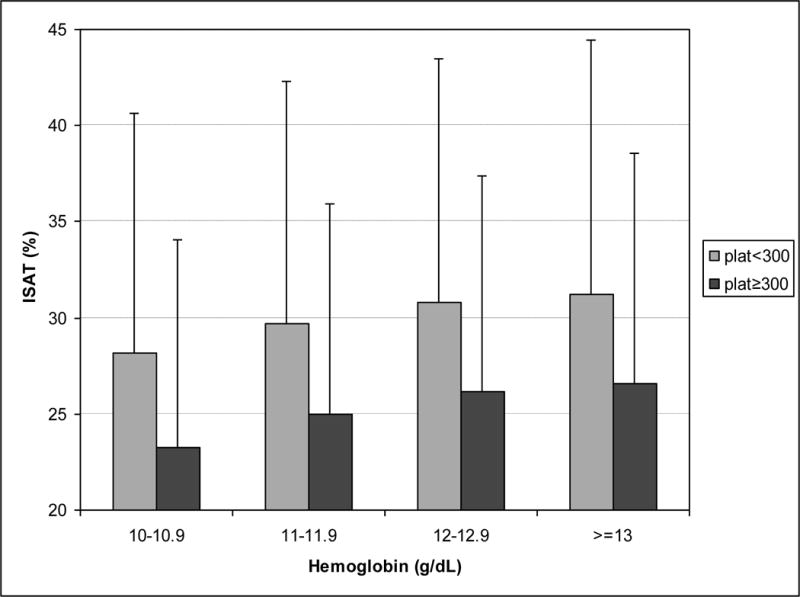
Association between serum ISAT and blood hemoglobin across the two strata of absence or presence of relative thrombocytosis (platelet count < or ≥300,000/μl) in 40,787 MHD patients. Error bars represent standard deviations in each group.
Table 3.
Death rate ratios (RR) and 95% confidence level (CL) across hemoglobin categories >10 g/dL stratified according to absence or presence of relative thrombocytosis (platelet count < or ≥300 ×103/υl).
| A. Blood platelet count <300,000 (n= 34,573)
|
|
|||
|---|---|---|---|---|
| Hemoglobin categories (g/dL) | Minimally adjusted | Case-mix adjusted | ||
| RR (95% CL) | P-value | RR (95% CL) | P-value | |
|
|
|
|||
| 10–10.99 | 1.43 (1.36–1.52) | <0.001 | 1.46 (1.38–1.55) | <0.001 |
| 11–11.99 | 1.12 (1.07–1.17) | <0.001 | 1.12 (1.07–1.17) | <0.001 |
| 12–12.99 (reference) | 1.0 | n/a | 1.0 | n/a |
| ≥13 | 1.03 (0.97–1.09) | 0.4 | 1.04 (0.99–1.10) | 0.1 |
| B. Blood platelet count ≥300,000 (n=6,214)
|
|
|||
|---|---|---|---|---|
| Hemoglobin categories (g/dL) | Minimally adjusted | Case-mix adjusted* | ||
| RR (95% CL) | P-value | RR (95% CL) | P-value | |
|
|
|
|||
| 10–10.99 | 1.55 (1.37–1.75) | <0.001 | 1.62 (1.43–1.84) | <0.001 |
| 11–11.99 | 1.25 (1.11–1.40) | <0.001 | 1.31 (1.17–1.47) | <0.001 |
| 12–12.99 (reference) | 1.0 | n/a | 1.0 | n/a |
| ≥13 | 1.11 (0.94–1.32) | 0.2 | 1.21 (1.02–1.44) | 0.03 |
case-mix adjusted models include adjustment for age, sex, diabetes mellitus, standardized mortality ratio, race, vintage, primary insurance, marital status, dialysis dose, dialysis catheter, and baseline comorbid states
To examine whether the risk of death was higher in the setting of relative thrombocytosis after case-mix adjustment, proportional-hazards models were employed, as shown in Table 3 and Figure 3. Using a hemoglobin level of 12 to 13 g/dL as the reference group, the case-mix adjusted 3-year death rates in MHD patients with hemoglobin ≥13 g/dL was 21% higher in thrombocytotic patients (p=0.03), whereas it was not different from the reference group in non-thrombocytotic patients. Compared to non-thrombocytotic patients, thrombocytotic MHD patients had higher death rates at all commensurate hemoglobin levels (Figure 3, right panel). This difference in predictive ability was confirmed by inclusion of the product of hemoglobin level and platelet count in the model (results not shown).
Figure 3. Case-mix adjusted hemoglobin-mortality ratios stratified according to absence or presence of relative thrombocytosis (platelet count < or ≥300 ×103υ1).
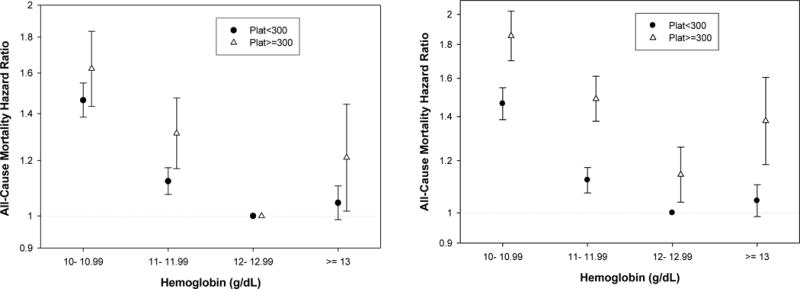
Case-mix adjusted hemoglobin-death rate ratios stratified according to absence or presence of relative thrombocytosis (platelet count < or ≥300,000/μl) in 40,787 MHD patients. (A) Left panel: Hazard ratios originate from two distinct survival regression models (based on platelet cutoff value of 300,000/μl). Hemoglobin 12 to 13 serves as the reference group in each of the two regression models. (B) Right panel: Hazard ratios are calculated using one survival regression model, where hemoglobin 12 to 13 in the normal platelet count group (<300,000/μl) serves as the only reference group for all other hemoglobin categories.
We further examined the impact of anemia management by rHuEPO on the platelet count, iron stores and survival. In 32,418 patients who received any dose of rHuEPO in the first 3 months of the cohort, the 13-week averaged rHuEPO dose was divided into 6 increments of 5,000 units/wk as shown in Table 4. Patients who received higher rHuEPO dose had higher crude mortality rates, lower ISAT and ferritin levels, and higher platelet counts. In proportional hazard models, administration of rHuEPO≥20,000 units/week was associated with case-mix and additional MICS adjusted mortality ratios of 1.59 (95% CL: 1.54, 1.65) and 1.31 (1.26, 1.37), respectively (P<0.001). Figure 4 shows that, over 3 years, the mortality associated with administered rHuEPO dose was higher with weekly doses in the range of 15,000 to 20,000 units/wk or more.
Table 4.
Six categories of the averaged administered rHuEPO over 3 months in 32,418 MHD patients. * The continuous values are means (±SD).
| rHuEPO categories (u/wk) | Number of patients (%) | All cause death (%) | CV death (%) | ISAT (%)* | Ferritin (ng/mL)* | Platelet (×103/υl)* | WBC (×103/υl)* |
|---|---|---|---|---|---|---|---|
| 1–4,999 | 3,711 (12) | 1,162 [32] | 422 [12] | 35 ± 13 | 784 ± 471 | 214 ± 60 | 7.3 ± 2.2 |
| 5,000–9,999 | 6,563 (20) | 2,203 [34] | 722 [12] | 33 ± 12 | 752 ± 488 | 219 ± 63 | 7.2 ± 2.1 |
| 10,000–14,999 | 5,941 (18) | 2,108 [36] | 702 [13] | 31 ± 12 | 702 ± 450 | 221 ± 68 | 7.1 ± 2.2 |
| 15,000–19,999 | 4,491 (14) | 1,751 [39] | 569 [14] | 29 ± 12 | 686 ± 480 | 222 ± 71 | 7.1 ± 2.2 |
| 20,000–24,999 | 3,183 (10) | 1,269 [40] | 428 [15] | 28 ± 12 | 667 ± 484 | 228 ± 78 | 7.2 ± 2.3 |
| ≥25,000 | 8,529 (26) | 3,986 [47] | 1,354 [18] | 27 ± 12 | 660 ± 574 | 232 ± 87 | 7.2 ± 2.7 |
Values in parentheses represent the proportion of the MHD patients in each category (for count data) or standard deviation (for continuous data). Values in brackets are the crude death rates in the indicated group during the 3 years of observation.
Figure 4. Baseline EPO and death rate ratios in the 3 year cohort.
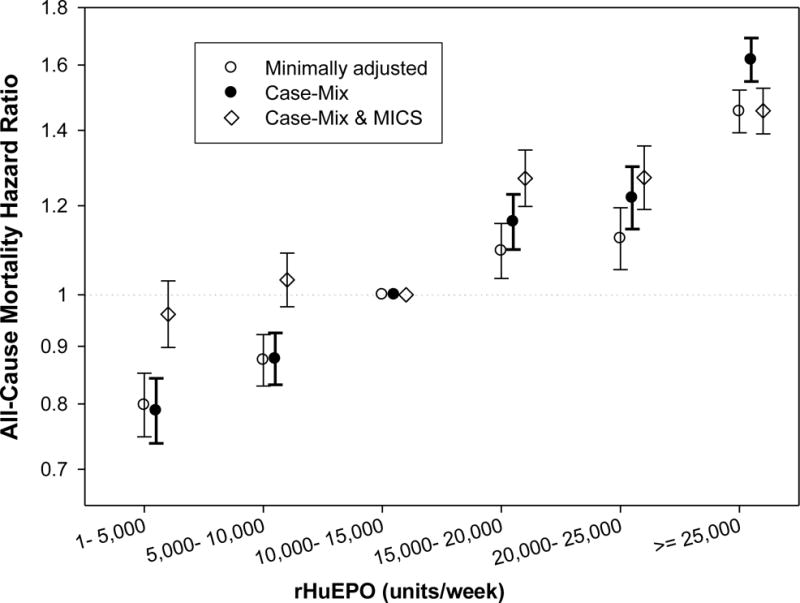
The association between the 3-month (13 weeks) averaged administered rHuEPO dose over each calendar quarter all-cause mortality in the 3 year cohort in 40,787 MHD patients. The minimally adjusted model is controlled for the entry calendar quarters
In order to identify the potential risk factors that may link high rHuEPO dose to mortality, we performed logistic regression analyses.Table 5 shows that, after adjustment, relative iron depletion (ISAT<20%, present in 16% of all patients) was more frequent in patients who received rHuEPO>20,000 units/wk. Both rHuEPO dose >20,000 units/wk and iron depletion (both ISAT <20% and incremental ISAT drops by 10%) were associated with thromocytosis.
Table 5.
Odds ratios for relative iron depletion, i.e. ISAT<20%, and for relative thrombocytosis, i.e., platelet count ≥300 ×103/υl
|
|
|
|||
|---|---|---|---|---|
| Minimally adjusted | Case-mix adjusted** | |||
| Odds ratio of iron depletion | OR (95% CL) | P-value | OR (95% CL) | P-value |
|
|
|
|||
| EPO (every 5,000 u/wk increase) | 1.10 (1.09–1.11) | <0.001 | 1.10 (1.09–1.11) | <0.001 |
| EPO≥20,000 u/wk (vs. <20,000/wk)* | 2.56 (2.41–2.72) | <0.001 | 2.53 (2.37–2.69) | <0.001 |
|
|
|
|||
|
|
|
|||
|---|---|---|---|---|
| Minimally adjusted | Case-mix adjusted** | |||
| Odds ratio for relative thromobocytosis | OR (95% CL) | P-value | OR (95% CL) | P-value |
| EPO (every 5,000 u/wk increase) | 1.07 (1.06–1.08) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| ISAT (every 10% decrease) | 1.44 (1.41–1.48) | <0.001 | 1.40 (1.36–1.43) | <0.001 |
| ISAT <20% (vs. ≥20%) | 2.50 (2.36–2.66) | <0.001 | 2.29 (2.15–2.43) | <0.001 |
|
|
|
|||
The models include only those MHD patients who received at least 1,000 units of EPO over the baseline 3 months.
case-mix adjusted models include adjustment for age, sex, diabetes mellitus, standardized mortality ratio, race, vintage, primary insurance, marital status, dialysis dose, dialysis catheter, and baseline comorbid states
Discussion
We found that relatively higher platelet counts are associated with higher prescribed rHuEPO dose and lower iron stores in 40,787 MHD patients from a large dialysis organization. In patients with relative thrombocytosis, i.e., platelet count ≥ 300,000/μl, a hemoglobin level above 13g/dL was associated with higher mortality compared to hemoglobin in the 12 to 13 g/dL range. Higher prescribed rHuEPO doses were associated with increased risk of relative iron depletion and relative thrombocytosis and increased mortality. These findings suggest that the observed association between higher prescribed rHuEPO dose to achieve hemoglobin levels above 13 g/dL may be linked to iron store depletion and subsequent thrombocytosis, which may in turn increase risks of adverse cardiovascular events and death.
The reactivity of platelets plays a central role in the genesis of thrombosis, especially in the setting of known atherosclerotic cardiovascular disease (as is often present in CKD patients). To this end, anti-platelet therapy is employed to reduce the occurrence of thrombotic events.[15] In diabetic peritoneal dialysis patients thrombocytosis is associated with the severity of cardiovascular disease.[28] High ex vivo platelet reactivity appears to be associated with ischemic events.[14] Because the platelet is a fundamental component in the generation of an arterial thrombus, we expect patients with more active platelets or higher platelet counts to have worse outcomes with respect to ischemic events. We found that a U-shaped hemoglobin-survival association described in a recent study [3] is more prominent in the setting of relative thrombocytosis (Figure 3). Our platelet link hypothesis is a possible explanation of why targeting or achieving hemoglobin levels above 13 g/dl by administering high rHuEPO doses has been associated with higher mortality in both observational [3] and interventional studies.[5–7]
Iron depletion is associated with an increased platelet count known as “reactive” thrombocytosis.[13, 30, 31]. An important factor affecting platelet counts appears to be the iron saturation ratio.[13] Platelet counts increase following phlebotomy in iron overloaded patients with liver cirrhosis.[32] Decreased iron saturation can stimulate megakaryopoiesis, possibly because iron may have an inhibitory effect on platelet maturation.[13] Moreover, iron depletion is associated with decreased antioxidant defense and increased oxidant stress, resulting in a tendency towards platelet aggregation.[33] In infants with iron deficiency anemia there is worsening of whole blood platelet aggregation that can be reversed by iron therapy.[34] Significant platelet aggregation and adhesiveness has also been reported in severe iron deficiency due to menorrhagia.[35] The activity of platelet monoamine oxidase is lowered in platelets from the blood of patients with iron-deficiency anemia.[36] A recent randomized trial to examine the effect of adding intravenous iron gluconate to high dose rHuEPO in anemic MHD patients with high ferritn>500 ng/ml [37] found a decline in platelet counts in patients receiving IV iron, while the platelet count remained unchanged in patients not given iron (mean platelet change was −29,000/mul vs. −0/mul; p = 0.02).[31] Consistent with the above studies, in our current study, we found that iron stores were lower in MHD patients with higher platelet counts (Figures 1 and 2), while mortality was higher at all levels of hemoglobin concentration in those with relative thrombocytosis (Figure 3.b.).
Treatment with rHuEPO in renal failure patients has been associated with platelet count elevation.[38–40] In animal studies, high doses of rHuPO produced an increase of platelet counts followed by a gradual return to normal after one to two weeks.[39, 40] Nonetheless, rHuEPO administration without adequate iron supplementation often leads to relative iron depletion. It is thus not clear whether the observed relative thrombocytosis is the result of iron depletion during rHuEpo therapy or whether direct mechanisms without iron involvement can also lead to a rHuEPO associated elevation in platelet count. A recent study showed that in rats receiving daily intravenous rHuEpo injections without iron supplementation, platelet counts increased by at least 120%.[40] Iron-supplemented rats receiving rHuEpo also showed some increase in platelet count but the duration of the rise was shorter. The authors concluded that there are both iron mediated and also direct stimulatory effects of rHuEPO on platelet production.[40]
In summary, among MHD patients who received rHuEPO doses >20,000 units/wk, we found increased frequency of relative iron depletion, relative thrombocytosis, and death. The strengths of our study include: (1) relatively recent data (2001–2004); (2) uniform laboratory measurements with all laboratory data obtained from one facility (DaVita Laboratory), (3) large sample size; and (4) 3-month averaged laboratory data (most values are the means of several measurements) to minimize measurement variability. Nonetheless, our study is observational with potential for confounding by unmeasured factors. Achieved hemoglobin may have different mortality-predictability than “targeted” hemoglobin in controlled trials[5–7] The limited comorbidity data were obtained from the dialysis initiation form (Form 2728), in which comorbid conditions are underreported [21] and which is probably more inaccurate for patients with greater dialysis duration. Our data lacked explicit laboratory markers of inflammation such as C-reactive protein, although we did use other surrogate laboratory markers of inflammation. Furthermore, our analysis is based on data from a single 3-year period of the cohort. Nonetheless, over half of dialysis patients die within 3 years, hence short-term survival of dialysis patients is of major clinical relevance. Finally, we used the arbitrary cutoff level of 300,000/μl platelet as the definition of “relative thrombocytosis”, as used in several previous studies;[24–26] This cutoff level is still within the normal platelet count range of most laboratory centers, i.e., up to 450,000/μl platelet. However, it is possible that even “relative” thrombocytosis is detrimental and predisposes to cardiovascular events, esp. in dialysis patients who have high burden of vasculopathy.
Setting aside data limitations, the associations we observed are still not necessarily causal, for the rHuEPO dose may have been raised to overcome a hyporesponsive anemia (for instance due to primary iron deficiency or inflammation). If, however, the associations represent effects of excessive rHuEPO dosing, similar associations may arise in patients without CKD who are treated for anemia. Adequate and appropriate, but not excessive, iron repletion might mitigate these problems and may be an important adjunct for erythropoietin stimulating treatments. Given the severity of the outcomes, it seems clear that randomized trials are needed to delineate relationships between anemia management, iron depletion, relative thrombocytosis, and mortality in populations eligible for such treatments. Nevertheless, it must be noted that iron overload is also associated with risk of oxidative stress and poor outcome, as well known in the literature.[41] Hence, we warn against over-interpretation of our observational data and against overuse of IV iron in the CKD or other populations, which may be associated with worse untoward effects. The findings of our current epidemiologic study should be taken with great caution and considered only as a first step towards generating new hypotheses that should first be tested in additional studies before considered as a valid explanation.
Acknowledgments
The abstract of this paper was presented orally at the American Society of Nephrology (ASN) annual conference, November 1–4, 2007, in San Francisco, CA. The authors thank Dr. Naomi V. Dahl from Watson Laboratories (Morristown, NJ) for her important inputs in advancing the hypothesis and reviewing relevant cancer literature.
Funding Source:
KKZ is the principal investigator of the supporting research grants for this manuscript including from the National Institutes of Health (R01DK078106), American Heart Association (0655776Y) and DaVita Clinical Research, and a philanthropist grant by Mr. Harold Simmons.
Footnotes
Coauthors’ contribution:
KKZ contributed to the design and funding of the study, collation and analysis of data, and writing of the manuscript and its revisions. ES contributed to statistical analyses and writing of the manuscript and its revision. CPK and SG contributed to the analysis of the data and reviewed and approved the final manuscript. CJM contributed to the design of the study, provision of data, and final review and approval of the manuscript. JDK and ARN contributed to the study design and manuscript preparation.
Potential Conflict of Interest:
KKZ has served as a paid consultant for AMAG (manufacturer of ferumoxytol), Amgen (manufacturer of epogen and aranesp), Watson (manufacturer of infed and ferrlecit), Vifor (manufacturer of venofer), Roche (manufacturer of mircera), Ortho-Biotech (manufacturer of procrit) and DaVita (provider of dialysis treatment). ARN has served as a paid consultant for Amgen, Watson, Roche, Ortho-Biotech and DaVita. CPK has served as a paid consultant for AMAG and Amgen. JDK has served as a paid consultant for Amgen, Roche and DaVita. Other authors have not declared conflicts of interest.
References
- 1.Van Wyck DB, Bailie G, Aronoff G. Just the FAQs: frequently asked questions about iron and anemia in patients with chronic kidney disease. Am J Kidney Dis. 2002;39:426–432. doi: 10.1053/ajkd.2002.30566. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Pisoni RL, Akizawa T, Cruz JM, DeOreo PB, Lameire NH, Held PJ. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns Study (DOPPS) findings. Am J Kidney Dis. 2004;44:27–33. doi: 10.1053/j.ajkd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation I. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kid Dis. 2006;47(suppl S3):146–146. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 7.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 8.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 9.Strippoli GF, Tognoni G, Navaneethan SD, Nicolucci A, Craig JC. Haemoglobin targets: we were wrong, time to move on. Lancet. 2007;369:346–350. doi: 10.1016/S0140-6736(07)60165-2. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Liu E, Kopple JD. A low serum iron level is a predictor of poor outcome in hemodialysis patients. Am J Kidney Dis. 2004;43:671–684. doi: 10.1053/j.ajkd.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG. Time-Dependent Associations between Iron and Mortality in Hemodialysis Patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 12.Coyne D. Challenging the boundaries of anemia management: a balanced approach to i.v. iron and EPO therapy. Kidney Int Suppl. 2006:S1–3. doi: 10.1038/sj.ki.5000402. [DOI] [PubMed] [Google Scholar]
- 13.Kadikoylu G, Yavasoglu I, Bolaman Z, Senturk T. Platelet parameters in women with iron deficiency anemia. J Natl Med Assoc. 2006;98:398–402. [PMC free article] [PubMed] [Google Scholar]
- 14.Gurbel PA. The relationship of platelet reactivity to the occurrence of post-stenting ischemic events: emergence of a new cardiovascular risk factor. Rev Cardiovasc Med. 2006;7(Suppl 4):S20–28. [PubMed] [Google Scholar]
- 15.Aljaroudi WA, Halabi AR, Harrington RA. Platelet inhibitor therapy for patients with cardiovascular disease: looking toward the future. Curr Hematol Rep. 2005;4:397–404. [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006;69:560–564. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 18.Barany P, Divino Filho JC, Bergstrom J. High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis. 1997;29:565–568. doi: 10.1016/s0272-6386(97)90339-5. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 20.Gunnell J, Yeun JY, Depner TA, Kaysen GA. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1999;33:63–72. doi: 10.1016/s0272-6386(99)70259-3. [DOI] [PubMed] [Google Scholar]
- 21.Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr, Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20:1880–1889. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 24.Nather A, Mayerhofer K, Grimm C, Hefler L, Leodolter S, Obermair A, Joura EA. Thrombocytosis and anaemia in women with recurrent ovarian cancer prior to a second-line chemotherapy. Anticancer Res. 2003;23:2991–2994. [PubMed] [Google Scholar]
- 25.Hefler L, Mayerhofer K, Leibman B, Obermair A, Reinthaller A, Kainz C, Tempfer C. Tumor anemia and thrombocytosis in patients with vulvar cancer. Tumour Biol. 2000;21:309–314. doi: 10.1159/000030136. [DOI] [PubMed] [Google Scholar]
- 26.Valentini G, Chianese U, Tirri G, Giordano M. Thrombocytosis in progressive generalized sclerosis (scleroderma) and in other rheumatic diseases. Z Rheumatol. 1978;37:233–241. [PubMed] [Google Scholar]
- 27.Rodriguez GC, Clarke-Pearson DL, Soper JT, Berchuck A, Synan I, Dodge RK. The negative prognostic implications of thrombocytosis in women with stage IB cervical cancer. Obstet Gynecol. 1994;83:445–448. [PubMed] [Google Scholar]
- 28.Sokunbi DO, Wadhwa NK, Suh H. Vascular disease outcome and thrombocytosis in diabetic and nondiabetic end-stage renal disease patients on peritoneal dialysis. Adv Perit Dial. 1994;10:77–80. [PubMed] [Google Scholar]
- 29.Dominguez I, Crippa S, Thayer SP, Hung YP, Ferrone CR, Warshaw AL, Fernandez-Del Castillo C. Preoperative Platelet Count and Survival Prognosis in Resected Pancreatic Ductal Adenocarcinoma. World J Surg. 2008 doi: 10.1007/s00268-007-9423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross S, Keefer V, Newman AJ. The Platelets in Iron-Deficiency Anemia. I. the Response to Oral and Parenteral Iron. Pediatrics. 1964;34:315–323. [PubMed] [Google Scholar]
- 31.Dahl NV, Henry DH, Coyne DW. Thrombosis with Erythropoietic Stimulating Agents-Does Iron-Deficient Erythropoiesis Play a Role? Semin Dial. 2008 doi: 10.1111/j.1525-139X.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 32.Franchini M. Platelet count increase following phlebotomy in iron overloaded patients with liver cirrhosis. Hematology. 2003;8:259–262. doi: 10.1080/1024533031000153649. [DOI] [PubMed] [Google Scholar]
- 33.Tekin D, Yavuzer S, Tekin M, Akar N, Cin S. Possible effects of antioxidant status on increased platelet aggregation in childhood iron-deficiency anemia. Pediatr Int. 2001;43:74–77. doi: 10.1046/j.1442-200x.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 34.Kurekci AE, Atay AA, Sarici SU, Zeybek C, Koseoglu V, Ozcan O. Effect of iron therapy on the whole blood platelet aggregation in infants with iron deficiency anemia. Thromb Res. 2000;97:281–285. doi: 10.1016/s0049-3848(99)00150-4. [DOI] [PubMed] [Google Scholar]
- 35.Sidi Y, Douer D, Krugliac J, Pinkhas J. Platelet aggregation and adhesiveness in severe iron deficiency due to menorrhage. New Istanbul Contrib Clin Sci. 1978;12:161–165. [PubMed] [Google Scholar]
- 36.Youdim MB, Woods HF, Mitchell B, Grahame-Smith DG, Callender S. Human platelet monoamine oxidase activity in iron-deficiency anaemia. Clin Sci Mol Med. 1975;48:289–295. doi: 10.1042/cs0480289. [DOI] [PubMed] [Google Scholar]
- 37.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 38.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, E RW, Friedman EA, G SE, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 39.Beguin Y, Loo M, R’Zik S, Sautois B, Lejeune F, Rorive G, Fillet G. Effect of recombinant human erythropoietin on platelets in patients with anemia of renal failure: correlation of platelet count with erythropoietic activity and iron parameters. Eur J Haematol. 1994;53:265–270. doi: 10.1111/j.1600-0609.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 40.Loo M, Beguin Y. The effect of recombinant human erythropoietin on platelet counts is strongly modulated by the adequacy of iron supply. Blood. 1999;93:3286–3293. [PubMed] [Google Scholar]
- 41.Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. Br Med J. 1978;2:1113–1115. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]


