Abstract
Purpose
Nasal cytology is important in the diagnosis and treatment of nasal inflammatory diseases. Treatment of allergic rhinitis (AR) according to nasal cytology has not been fully studied. We plan to explore the individualized treatment of AR according to nasal cytology.
Methods
Nasal cytology from 468 AR patients was examined for inflammatory cell quantity (grade 0-5) and the percentage of neutrophils and eosinophils. Results were subdivided into the following categories: AR(Eos), eosinophil ≥50% of the whole inflammatory cells; AR(Neu), neutrophils ≥90%; AR(Eos/Neu), 10%≤ eosinophil <50%; AR(Low), grade 0/1 inflammatory cell quantity. Nasal cytology-guided treatment was implemented: all AR(Eos) patients (n=22) and half of the AR(Neu) patients (AR[Neu1], n=22) were treated with mometasone furoate spray and oral loratadine. Another half of the AR(Neu) patients (AR[Neu2], n=22) were treated with oral clarithromycin. Visual analog scale (VAS), symptom scores, and nasal cytology were evaluated 2 weeks before and after treatment.
Results
There were 224/468 (47.86%) AR(Eos), 67/468 (14.32%) AR(Neu), 112/468 (23.93%) AR(Eos/Neu), and 65/468 (13.89%) AR(Low) of the AR patients studied. There were no significant differences in clinical characteristics among these subgroups, except that the nasal blockage score was higher in AR(Eos) patients than in AR(Neu) patients (1.99 vs 1.50, P=0.02). Comparing AR(Eos) patients with AR(Neu1) patients 2 weeks after treatment, nasal symptoms and VAS were significantly lower in AR(Eos) patients, except for nasal blockage symptoms (P<0.05 of nasal itching and sneezing; P<0.01 for nasal secretion, total scores, and VAS). Comparing AR(Neu1) with AR(Neu2) patients, nasal symptoms, and VAS were significantly lower in AR(Neu2), except for nasal blockage and nasal itching symptoms (P<0.05 for nasal secretions, sneezing, total score, and VAS).
Conclusions
Nasal cytology may have important value in subtyping AR and optimizing AR treatment. Treating neutrophils is very important in AR patients with locally predominant neutrophils.
Keywords: Nasal cytology, neutrophils, allergic rhinitis
INTRODUCTION
Nasal cytology directly reflects nasal inflammation and is an important tool for the diagnosis and treatment of rhinitis.1 There are several possible collection methods for nasal cytology, including nasal smear, swab, scraping, and irrigation. Each sampling method may reflect inflammation of different layers of the nasal mucosa: smear or lavage—surface secretion, scraping—epithelium, brush—between secretion and epithelium, biopsy—all layers.2 However, the use of nasal cytology in clinic is limited by the complicated nature of the procedure, variable results, and lack of a standardized grading system.1,3
In most allergic rhinitis (AR) guidelines, the diagnostic criteria for AR include typical atopic history and nasal symptoms (nasal itching, sneezing, watery secretions, and nasal obstruction) as well as documented sensitivities to aeroallergens by skin prick or serum specific immunoglobulin E (IgE) testing. Treatment plans are according to the subjective evaluation of symptoms. Objective indices of local inflammation are not commonly incorporated to guide treatment.4
Eosinophils are an important marker of allergic inflammation. Most drugs (corticosteroids, anti-leukotrienes, and anti-histamines) are focused on controlling eosinophilic inflammation in the treatment of AR.4,5 Recently, researchers have explored the role of neutrophils in allergic inflammation and disease, especially in severe asthma patients.6 The high-affinity receptors for immunoglobulin E (FcɛRI) are expressed on human neutrophils in asthma, which may be enhanced by stimulation with specific allergens.7,8 These studies provide a possible new mechanism by which neutrophils contribute to allergic diseases. Since neutrophils are relatively steroid-resistant, local or oral corticosteroids are minimally effective in the treatment of this neutrophilic inflammation.9,10
The expression of neutrophils in AR patients and the influence of neutrophilic inflammation in the current treatment of AR are not well understood. In this study, we observed the nasal cytology of house dust mite (HDM)-sensitive AR patients in clinic. We also investigate the utility of nasal cytology in guiding the optimal treatment of AR, especially for AR patients with predominantly neutrophilic infiltrates.
MATERIALS AND METHODS
Study subjects
Patients who had at least 2 perennial nasal symptoms (nasal obstruction, secretion, nasal itching, and sneezing) for at least 1 year were considered for inclusion. Patients with a history of recent “cold” or other respiratory infection, or with symptoms of thick, yellow, or green nasal secretions were excluded. Anterior rhinoscopy and nasal endoscopic examination were used to exclude infection in each patient. Computerized tomography (CT) scan was taken to exclude the inflammation of the nasal sinuses if necessary. All patients were sensitized to HDM, diagnosed by either positive skin prick tests or positive serum specific IgE (sIgE) to HDMs (≥0.7 kUA/L). The allergen extract used in skin prick testing for HDM was manufactured by ALK (Hørsholm, Denmark), and serum sIgE test products were from the Thermo Fisher Scientific (Waltham, MA, USA). Patients with atypical symptoms were examined by 2 experienced physicians to confirm the diagnosis.
Visual analog scale (VAS) scores were assessed by each patient, with “0” indicating no troublesome nasal symptoms, and “100” indicating the worst thinkable troublesome nasal symptoms. The severity of nasal symptoms (nasal blockage, nasal itching, secretions, and sneezing) were recorded as follows: 0=sysmptomless, 1=mild, 2=moderate, and 3=severe.
Informed consent was obtained from all patients or the guardians of patients less than 18 years old. This study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (register No. ChiCTR-OPN-15006592).
Methods of nasal cytology
All patients denied the use of nasal or systemic corticosteroids, antihistamines, nasal cromoglycate, anti-leukotrienes, or anti-biotics for at least 14 days prior to initiation of the study. None of the patients had ever received immunotherapy. We compared different methods of nasal cytology, and selected nasal smear with glass stick (NSGS) as the sampling method in this study (see Supplementary Table 1). The procedure of NSGS is as below: First, asking the patients to blow their nose, even if they have no obvious secretions. Then, place a thin glass stick into the nasal vestibule or the anterior part of the nasal cavity if necessary, gently rotate the stick, and let the secretions attach. Avoid rubbing the surface of the nasal mucosa as much as possible. Take the stick out and smear a thin layer on a slide, covering 2/3 of it. If the secretion is not enough to cover the demanded area, sampling can be repeated. If the patient has no secretions at all, the smear should be done another time. The slides were air dried, stained with Wright-Giemsa, and examined with a light microscope. Cells were counted and categorized as epithelial cells, neutrophils, eosinophils, or other types of inflammatory cells. Since other types of inflammatory cells except neutrophils and eosinophils were very low (less than 3% of the whole inflammatory cells on average), we focused on neutrophils and eosinophils in this study.
Grading system for nasal cytology using NSGS
The grading system for subtyping different groups was developed in accordance with other reports and the expertise of the investigators.11,12
Inflammatory cell quantity: the whole slide was browsed. The average number of inflammatory cells (neutrophils and eosinophils) per field (×100) was graded as: 1) grade 0, inflammatory cells <10 per field; 2) grade 1, inflammatory cells 10-49 per field; 3) grade 2, inflammatory cells 50-149 per field; grade 3, inflammatory cells 150-299 per field; and 4) grade 4, inflammatory cells ≥300 per field.
Eosinophil vs. neutrophil cell counts: the percentage of neutrophils and eosinophils per 100 inflammatory cells were calculated if the quantity grade was ≥2. The exact percentage was not calculated if the quantity grade was <2. Since the current study was focused on AR, patients were subdivided according to the percentage of inflammatory cells: 1) AR(Eos): eosinophils ≥50% of whole inflammatory cells; 2) AR(Neu): neutrophils ≥90% of the whole inflammatory cells; 3) AR(Eos/Neu): 10%≤eosinophils <50% of the whole inflammatory cells; and 4) AR(Low), grade 0 or grade 1 inflammatory cell quantity (only a rough impression was given).
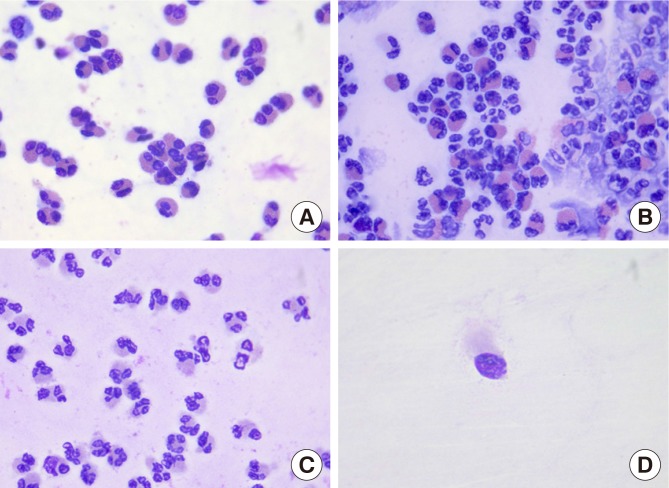
The typical slides of different cell distributions are shown in Fig. 1.
Fig. 1. The typical slides of different cell distributions in AR. (A) AR(Eos): eosinophils ≥50% of whole inflammatory cells. (B) AR(Eos/Neu): 10%≤ eosinophils <50% of whole inflammatory cells. (C) AR(Neu): eosinophils <10% of the whole inflammatory cells. (D) AR(Low): grade 0 or grade 1 inflammatory cell quantity.
AR, allergic rhinitis.
Nasal cytology of AR patients in clinic
AR patients that met the inclusion criteria above were recruited for 1 year. Basic characteristics, symptoms, and nasal cytology were recorded. The AR patients were subdivided as AR(Eos), AR(Eos/Neu), AR(Neu), and AR(Low) as defined above.
Nasal cytology results as a guide for the treatment of AR
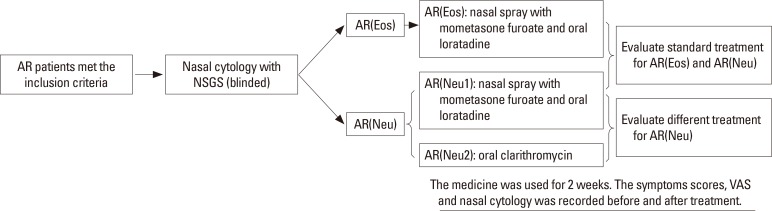
We focused on the treatment of AR(Neu) patients in this study. We recruited AR(Neu) patients (≥6 years old) to investigate the role of nasal cytology results in guiding treatment. Part of AR(Eos) patients (≥6 years old) were also recruited. The slides were observed by a skillful technician and completely blinded. If the patient was diagnosed as AR(Neu), he/she was randomly distributed in the AR(Neu1) or AR(Neu2) group. For patients in the AR(Neu1) group, nasal spray with mometasone furoate once daily (<12 years: 1 spray each nostril, once daily; ≥12 years: 2 sprays each nostril, once daily) and oral loratadine once daily (<12 years: 5 mg, once daily; ≥12 years: 10 mg, once daily) were given. For patients in AR(Neu2) group, oral clarithromycin twice daily (<12 years: 125 mg, twice daily; ≥12 years: 250 mg, twice daily) were given. If the patient was diagnosed as AR(Eos), he/she was distributed in the AR(Eos) group and use the same treatment as the AR(Neu1) group. Other treatments than above ones were not used during the course of the observation. All patients were treated for 2 weeks. The symptoms scores and VAS were recorded before the treatment, and 1 and 2 weeks after the treatment. Nasal cytology was taken before the treatment, and 2 weeks after the treatment. The flow diagram is shown in Fig. 2.
Fig. 2. The flow diagram of treatment of AR according to nasal cytology. AR, allergic rhinitis.
We evaluated the effectiveness of AR(Neu) treatment with different treatment schedules through comparing the AR(Neu1) and AR(Neu2) groups. We evaluated the effectiveness of the classic treatment on AR(Neu) through comparing the AR(Neu1) and AR(Eos) groups.
Statistical analysis
Descriptive parameters are expressed as mean±standard deviation (SD). An analysis of variance (ANOVA) test was used to compare clinical characteristics (nasal symptoms, VAS, etc.) between individual groups: the AR(Eos), AR(Eos/Neu), and AR(Neu), AR(Low) groups. Paired test was used to compare changes in symptoms and cell numbers before and after treatment. A P value of P<0.05 was considered statistically significant. SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
RESULTS
Nasal cytology in AR patients
There were 493 AR patients met the inclusion criteria of the current study. Nasal cytology samples were successfully taken from 468 AR patients. Twenty-five patients had unsuccessful samples all of whom were not so cooperative children. Clinical characteristics and nasal cytology results are shown in Table 1.
Table 1. AR subgroups according to nasal cytology.
| Characteristics | AR(Eos) | AR(Eos/Neu) | AR(Neu) | AR(Low) |
|---|---|---|---|---|
| No. (%) | 224/468 (47.86) | 112/468 (23.93) | 67/468 (14.32) | 65/468 (13.89) |
| Gender (male) | 132 (58.93) | 70 (62.50) | 49 (73.13) | 34 (52.31) |
| Age (year) | 18.76±14.60 | 15.64±14.23 | 15.06±14.03 | 18.59±12.39 |
| History (year) | 3.24±2.95 | 3.21±2.30 | 2.95±2.54 | 3.86±2.40 |
| Asthma | 24 (10.71) | 10 (8.93) | 6 (8.96) | 4 (6.15) |
| VAS | 61.85±20.56 | 59.38±22.23 | 56.73±23.66 | 55.25±22.03 |
| Nasal blockage | 1.99±0.79* | 1.71±1.00 | 1.50±0.80 | 1.60±0.68 |
| Nasal itching | 1.53±0.77 | 1.42±0.58 | 1.50±0.74 | 1.65±0.59 |
| Nasal secretion | 1.70±0.76 | 1.79±0.51 | 1.73±0.77 | 1.75±0.79 |
| Sneezing | 1.77±0.61 | 1.63±0.71 | 1.59±0.73 | 1.60±0.88 |
| Total score | 6.99±1.92 | 6.54±2.21 | 6.32±2.42 | 6.60±1.93 |
| Eos % | 86.70±15.00 | 28.77±11.61 | 4.00±4.17 | - |
| Neu % | 13.30±15.00 | 71.23±11.61 | 96.00±4.17 | - |
| Inflammatory cell grade and distribution | 2.85±0.52 (grade 2: 49, grade 3: 159, grade 4: 16) |
2.79±0.54 (grade 2: 31, grade 3: 74, grade 4: 7) |
3.20±0.59† (grade 2: 6, grade 3: 41, grade 4: 20) |
- (grade 0: 5, grade 1: 60) |
Data are shown as mean±SD or number (%).
AR, allergic rhinitis; VAS, visual analog scale; SD, standard deviation.
*Compared to AR(Neu), P=0.02; †Compared to AR(Eos) and AR(Eos/Neu), P<0.01.
The AR(Eos) group comprised 224/468 (47.86%) of all AR patients, in which the percentage of eosinophils was 86.7%±15.0%. This indicates that approximately half of all AR patients had a predominance of eosinophils in their nasal secretions. However, the AR(Neu) group comprised 67/468 (14.32%), in which the percentage of neutrophils was 96.00%±4.17%. Also, 112/468 (23.93%) patients belonged to the AR(Eos/Neu) group, in which the percentages of neutrophils and eosinophils were 77.23% and 28.77%, respectively. The AR(Low) group comprised 65/468 (13.89%) of all AR patients, which had very few inflammatory cells in nasal secretions. There were no significant differences in clinical characteristics between the subgroups except that the nasal blockage score was higher in the AR(Eos) group than in the AR(Neu) group (1.99 vs 1.50, P=0.02) and that the inflammatory cell grade was higher in the AR(Neu) group than in the other subgroups (P<0.01). The average percentage of neutrophils and eosinophils were 36.95% and 48.95%, respectively, in all AR patients. The distribution of inflammatory cell quantity grades in all AR patients were as follows: grade 0=5; grade 1=60; grade 2=86; grade 3=274; and grade 4=43.
Treatment of AR based upon nasal cytology results
There were 66 patients recruited for treatment observation: 22 in the AR(Eos) group, 22 in the AR(Neu1) group, and 22 in the AR(Neu2) group). There were no significant differences in clinical characteristics among the subgroups, except that the nasal blockage score was higher in the AR(Eos) group than in the AR (Neu1) group (P=0.014) and in the AR(Neu2) group (P=0.048) (Table 2).
Table 2. General characteristics of the AR(Eos), AR(Neu1), and AR(Neu2) groups.
| Characteristics | AR(Eos) | AR(Neu1) | AR(Neu2) |
|---|---|---|---|
| No. | 22 | 22 | 22 |
| Gender (male) | 12 (54.55) | 16 (72.73) | 17 (77.27) |
| Age (year) | 15.59±11.53 | 15.86±13.31 | 15.82±12.24 |
| History (year) | 2.83±2.09 | 3.08±2.29 | 3.13±2.45 |
| Asthma (%) | 2 (9.10) | 3 (13.64) | 2 (9.10) |
| VAS | 59.72±20.68 | 58.32±26.64 | 59.91±24.10 |
| Nasal blockage* | 2.22±0.73 | 1.64±0.85 | 1.59±0.80 |
| Nasal itching | 1.67±0.84 | 1.59±0.80 | 1.55±0.60 |
| Nasal secretion | 1.72±0.75 | 1.73±0.83 | 1.77±0.75 |
| Sneezing | 1.78±0.65 | 1.68±0.72 | 1.68±0.84 |
| Total score | 7.39±2.03 | 6.64±2.17 | 6.59±2.04 |
| Eos % | 91.01±13.36 | 4.73±4.15 | 3.86±4.08 |
| Neu % | 8.99±13.36 | 95.27±4.15 | 96.14±4.08 |
| Inflammation cell grades | 3.05±0.58 | 3.14±0.77 | 3.23±0.75 |
Data are shown as mean±SD or number (%).
AR, allergic rhinitis; VAS, visual analog scale; SD, standard deviation.
*The score of AR(Eos) was higher than that of AR(Neu1) (P=0.014) and AR(Neu2) (P=0.048).
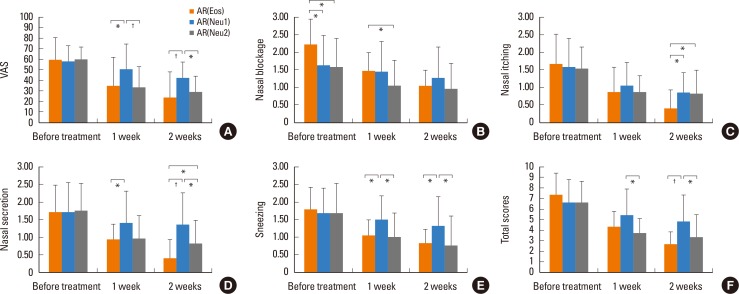
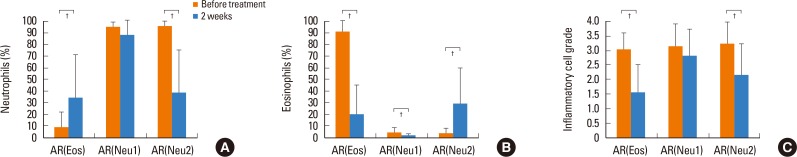
Comparing the AR(Eos) and AR(Neu1) groups 2 weeks after treatment, nasal symptoms and VAS were significantly lower in the AR(Eos), except for nasal blockage symptoms (P<0.05 for nasal itching and sneezing; P<0.01 each for nasal secretion, total scores, and VAS) (Fig. 3). The inflammatory cell quantity grade decreased significantly after treatment in the AR(Eos) group (P<0.01), but not in the AR(Neu1) group. The percentage of eosinophils decreased significantly in both groups (P<0.01). The percentage of neutrophils increased significantly in the AR(Eos) group (P<0.01), but not in the AR(Neu1) group (Fig. 4).
Fig. 3. Changes in nasal symptoms in the AR(Eos), AR(Neu1), and AR(Neu2) groups before and after treatment. (A) VAS. (B) Nasal blockage. (C) Nasal itching. (D) Nasal secretion. (E) Sneezing. (F) Total scores. AR, allergic rhinitis; VAS, visual analog scale. *P<0.05; †P<0.01.
Fig. 4. Changes in nasal cytology in the AR(Eos), AR(Neu1), and AR(Neu2) groups before and after treatment. (A) Neutrophils. (B) Eosinophils. (C) Inflammatory cells grade. AR, allergic rhinitis. *P<0.05; †P<0.01.
Comparing the AR(Neu1) and AR(Neu2) groups 2 weeks after treatment, nasal symptoms and VAS were significantly lower in the AR(Neu2) group, except for nasal blockage and nasal itching symptoms (P<0.05 each for nasal secretions, sneezing, total score, and VAS) (Fig. 3). The inflammatory cell quantity grade decreased significantly in the AR(Neu2) group (P<0.01). The percentage of eosinophils increased significantly in the AR(Neu2) group (P<0.01). The percentage of neutrophils decreased significantly in the AR(Neu2) group (P<0.01) (Fig. 4). The nasal cytology in the AR(Neu1) group was described above.
DISCUSSION
The optimal method of collecting nasal cytology specimens should be minimally invasive, reliable, well tolerated by most patients, easy to perform in clinic, and produce enough inflammatory cells for analysis.13 We compared several different nasal cytology collection methods and found that NSGS was easy to take and had good reliability (see Supplementary).
In this study, we examined nasal cytology results in HDM-sensitive patients with perennial AR using NSGS. We found that the mean percentages of neutrophils and eosinophils in nasal smears of AR patients were 36.95% and 48.95%, respectively. Although eosinophils consisted of the majority of inflammatory cells in the nasal secretion of most AR patients, neutrophils were found to be increased in some patients, especially for 14.32% of the AR(Neu) patients in the current study who had airway inflammation with predominantly neutrophilic infiltrates. There have been many studies about nasal cytology of AR patients. However, all the aforementioned studies have not reported the characteristics of AR patients who possess different local nasal inflammatory cells.12,14,15
Some studies also showed the increasing participation of neutrophils during allergic inflammation. Ciprandi et al.16 found a larger number of neutrophils as compared to eosinophils in nasal mucosa after continuous, low exposure to HDM. A peak in the number of neutrophils was found during the period of maximal HDM-allergic inflammation.14 It is reported that Der p has anti-apoptotic effects on neutrophils through the toll-like receptor 4 (TLR4)/protein kinase C (PKC)δ/extracellular signal-regulated kinase (ERK)/nuclear factor-kappa B (NF-κB) pathway, which may prolong the survival time of local neutrophils.17 In addition, interleukin (IL)-17 is increased in AR patients, which could recruit a large number of neutrophils.18 Fransson et al.19 showed a positive correlation between increased neutrophil quantity in nasal secretions and nasal secretion symptom scores. They also stated that both the total nasal symptom scores and secretion scores correlated with the number of neutrophils in lavage fluids after allergen provocation, whose potential mechanism may be the release of proteolytic enzymes (histamine, cathepsin G, and elastase) from neutrophil granules.
Medications recommended by current guidelines for the treatment of AR may not reduce the inflammation caused by neutrophils. Benson et al.20 found that there was a significant decrease in IL-4, IL-6, eosinophilic cationic protein (ECP), and IgE, after topical corticosteroid treatment in AR patients, while no significant changes in the levels of IFN-gamma, IL-1beta, TNF-alpha, or neutrophils. Other studies have shown similar results.21,22,23,24
Since AR(Neu) and AR(Eos) patients had similar clinical characteristics, it would be difficult to differentiate these patients solely by history and physical examination. Similar treatments would be given to these patients in accordance with current guidelines. In this study, we treated the AR(Eos) and AR(Neu1) groups with the same nasal corticosteroid and antihistamine. After treatment, the nasal symptoms scores and VAS decreased significantly in the AR(Eos) group as compared to the AR(Neu1) group, except for nasal blockage (This may be explained by the higher baseline score of nasal blockage in the AR[Eos] group as compared to the AR[Neu1] group). Although the eosinophils decreased significantly in both groups, the symptoms did not relieve much in the AR(Neu1) group. The reason for this may be that there were a large amount of neutrophils locally in the AR(Neu1) group and not influenced by the treatments. This suggests that standard treatments would be much less effective in AR(Neu) patients than AR(Eos) patients.
We explored the treatment of AR(Neu) patients with a different schedule in this study. Clarithromycin was selected due to its anti-inflammatory and immunomodulatory properties, especially for neutrophilic inflammation.25 Although it is not recommended in the guideline of AR, we use it according to its mechanism. Two weeks after treatment, VAS, total scores, nasal secretions, and sneezing symptoms were much more improved in the AR(Neu2) group, which received clarithromycin solely, as opposed to the AR(Neu1) group. Although the percentage of eosinophils increased in the AR(Neu2) group after treatment, VAS and nasal symptoms were still more relieved in the AR(Neu2) group than in the AR(Neu1) group. This might be due to a tremendous decrease in neutrophils and neutrophilic inflammation in the nasal membrane. This implies the importance of treating neutrophilic inflammation in AR(Neu) patients.
Since neutrophils in AR was not fully studied in clinic, it was also quite important to differentiate AR(Neu) and “AR with transient infection.” First, in AR(Neu) patients, there were no history of recent “cold” or other respiratory infection. Meanwhile, AR(Neu) patients often had hypersensitive symptoms (sneezing and itching nose), very similar to those of other AR patients. However, in “AR with transient infection” patients, a history of recent “cold” or other respiratory infection could usually be found, and their symptoms were more like infectious rhinosinusitis (nasal obstruction and purulent secretion). Secondly, the symptoms of AR(Neu) patients lasted for months, even for years. Although they used the classic medicines (such as anti-histamine, nasal steroid, and anti-leukotriene, etc.) for a long time, their symptoms relieved limitedly and were recurrent. In “AR patients with transient infection,” nasal obstruction and purulent secretion were transient, which shifted to nasal hypersensitive symptoms after antibiotic use. The symptoms were changeable even with-out treatment. Nevertheless, AR(Neu) and “AR with transient infection” could not be completely differentiated without objective indicators, which need further studies in the future.
In conclusion, NSGS is a non-invasive, reliable, simple method for nasal cytology. Subtyping of AR patients using nasal cytology may play an important role in optimizing and individualizing AR treatment. Treating neutrophils is very important in AR patients with predominantly neutrophilic infiltrates. Further studies are needed to elucidate the exact mechanism of neutrophils in AR.
ACKNOWLEDGMENTS
The authors are grateful to Qiumei Shi and Huifang Tan for their assistance in data collection from allergic rhinitis (AR) patients. This study is supported by the State Natural Sciences Fund of China (No. 81570898) and the 12th 5-year science and technology support program (No. 2014BAI07B04).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTS
References
- 1.Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. NASAL cytology: practical aspects and clinical relevance. Clin Exp Allergy. 2016;46:785–792. doi: 10.1111/cea.12730. [DOI] [PubMed] [Google Scholar]
- 2.Pipkorn U, Karlsson G. Methods for obtaining specimens from the nasal mucosa for morphological and biochemical analysis. Eur Respir J. 1988;1:856–862. [PubMed] [Google Scholar]
- 3.Mygind N. Essential allergy. Oxford: Blackwell Scientific Publications; 1986. [Google Scholar]
- 4.Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, Bachert C, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 5.Howarth PH. Eosinophils and rhinitis. Clin Exp Allergy Rev. 2005;5:55–63. [Google Scholar]
- 6.Foley SC, Hamid Q. Images in allergy and immunology: neutrophils in asthma. J Allergy Clin Immunol. 2007;119:1282–1286. doi: 10.1016/j.jaci.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Gounni AS, Lamkhioued B, Koussih L, Ra C, Renzi PM, Hamid Q. Human neutrophils express the high-affinity receptor for immunoglobulin E (Fc epsilon RI): role in asthma. FASEB J. 2001;15:940–949. doi: 10.1096/fj.00-0378com. [DOI] [PubMed] [Google Scholar]
- 8.Alphonse MP, Saffar AS, Shan L, HayGlass KT, Simons FE, Gounni AS. Regulation of the high affinity IgE receptor (Fc epsilonRI) in human neutrophils: role of seasonal allergen exposure and Th-2 cytokines. PLoS One. 2008;3:e1921. doi: 10.1371/journal.pone.0001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauber HP, Gotfried M, Newman K, Danda R, Servi RJ, Christodoulopoulos P, et al. Effect of HFA-flunisolide on peripheral lung inflammation in asthma. J Allergy Clin Immunol. 2003;112:58–63. doi: 10.1067/mai.2003.1612. [DOI] [PubMed] [Google Scholar]
- 10.Fukakusa M, Bergeron C, Tulic MK, Fiset PO, Al Dewachi O, Laviolette M, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115:280–286. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Benson M, Strannegård IL, Wennergren G, Strannegård O. Interleukin-5 and interleukin-8 in relation to eosinophils and neutrophils in nasal fluids from school children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 1999;10:178–185. doi: 10.1034/j.1399-3038.1999.00036.x. [DOI] [PubMed] [Google Scholar]
- 12.Canakcioglu S, Tahamiler R, Saritzali G, Alimoglu Y, Isildak H, Guvenc MG, et al. Evaluation of nasal cytology in subjects with chronic rhinitis: a 7-year study. Am J Otolaryngol. 2009;30:312–317. doi: 10.1016/j.amjoto.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Piacentini GL, Kaulbach H, Scott T, Kaliner MA. Evaluation of nasal cytology: a comparison between methods. Allergy. 1998;53:326–328. doi: 10.1111/j.1398-9995.1998.tb03898.x. [DOI] [PubMed] [Google Scholar]
- 14.Gelardi M, Peroni DG, Incorvaia C, Quaranta N, De Luca C, Barberi S, et al. Seasonal changes in nasal cytology in mite-allergic patients. J Inflamm Res. 2014;7:39–44. doi: 10.2147/JIR.S54581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Özgür A, Arslanoğlu S, Etıt D, Demıray U, Önal HK. Comparison of nasal cytology and symptom scores in patients with seasonal allergic rhinitis, before and after treatment. J Laryngol Otol. 2011;125:1028–1032. doi: 10.1017/S0022215111001721. [DOI] [PubMed] [Google Scholar]
- 16.Ciprandi G, Buscaglia S, Pesce G, Pronzato C, Ricca V, Parmiani S, et al. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995;96:971–979. doi: 10.1016/s0091-6749(95)70235-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim EH, Lee JS, Lee NR, Baek SY, Kim EJ, Lee SJ, et al. Regulation of constitutive neutrophil apoptosis due to house dust mite allergen in normal and allergic rhinitis subjects. PLoS One. 2014;9:e105814. doi: 10.1371/journal.pone.0105814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuekun H, Qintai Y, Yulian C, Gehua Z. Correlation of gammadelta-T-cells, Th17 cells and IL-17 in peripheral blood of patients with allergic rhinitis. Asian Pac J Allergy Immunol. 2014;32:235–239. doi: 10.12932/AP0432.32.3.2014. [DOI] [PubMed] [Google Scholar]
- 19.Fransson M, Benson M, Wennergren G, Cardell LO. A role for neutrophils in intermittent allergic rhinitis. Acta Otolaryngol. 2004;124:616–620. doi: 10.1080/00016480310015173. [DOI] [PubMed] [Google Scholar]
- 20.Benson M, Strannegård IL, Strannegård O, Wennergren G. Topical steroid treatment of allergic rhinitis decreases nasal fluid TH2 cytokines, eosinophils, eosinophil cationic protein, and IgE but has no significant effect on IFN-gamma, IL-1beta, TNF-alpha, or neutrophils. J Allergy Clin Immunol. 2000;106:307–312. doi: 10.1067/mai.2000.108111. [DOI] [PubMed] [Google Scholar]
- 21.Pelucchi A, Chiapparino A, Mastropasqua B, Marazzini L, Hernandez A, Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to methacholine in allergic rhinitis in response to grass pollens. J Allergy Clin Immunol. 1995;95:515–523. doi: 10.1016/s0091-6749(95)70313-6. [DOI] [PubMed] [Google Scholar]
- 22.Djukanović R, Homeyard S, Gratziou C, Madden J, Walls A, Montefort S, et al. The effect of treatment with oral corticosteroids on asthma symptoms and airway inflammation. Am J Respir Crit Care Med. 1997;155:826–832. doi: 10.1164/ajrccm.155.3.9117012. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer EO, Orgel HA, Rogenes PR, Field EA. Nasal cytology in patients with allergic rhinitis: effects of intranasal fluticasone propionate. J Allergy Clin Immunol. 1994;94:708–715. doi: 10.1016/0091-6749(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 24.Rak S, Jacobson MR, Sudderick RM, Masuyama K, Juliusson S, Kay AB, et al. Influence of prolonged treatment with topical corticosteroid (fluticasone propionate) on early and late phase nasal responses and cellular infiltration in the nasal mucosa after allergen challenge. Clin Exp Allergy. 1994;24:930–939. doi: 10.1111/j.1365-2222.1994.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 25.Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136:531–545. doi: 10.1016/j.jaci.2015.05.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.