Abstract
Purpose
This study aimed to determine the prevalence of immediate-type food allergy (FA) among schoolchildren in Korea.
Methods
A nationwide, cross-sectional study was performed in September 2015. A stratified random sample of 50,000 participants was selected from children and adolescents aged 6-7 years (n=17,500), 9-10 years (n=17,500), 12-13 years (n=7,500), and 15-16 years (n=7,500). Parents were asked to complete a questionnaire on the detailed history of immediate-type FA.
Results
A total of 32,001 (64.0%) responded to the questionnaire survey, and 29,842 children (59.7%) were analyzed after adjusting for missing data. The number of the cases in each age group was 9,671 (6-7 years), 9,756 (9-10 years), 5,169 (12-13 years), and 5,246 (15-16 years). The prevalence of lifetime perceived FA was 15.82%. The prevalence of current immediate-type FA was 4.06% in total, with 3.15% in 6- to 7-year-olds, 4.51% in 9- to 10-year-olds, 4.01% in 12- to 13-year-olds, and 4.49% in 15- to 16-year-olds. Among individual food items, peanut (0.22%) was the most frequent causative food, followed by hen's egg (0.21%), cow's milk (0.18%), and buckwheat (0.13%). Among the food groups, fruits (1.41%), crustaceans (0.84%), tree nuts (0.32%), and fish (0.32%) were the most common offending foods. The prevalence of food-induced anaphylaxis was 0.97%. The most frequent causative food of anaphylaxis was peanut (0.08%), followed by cow's milk (0.07%), buckwheat (0.06%), and hen's egg (0.06%), while fruits (0.28%), crustaceans (0.18%), tree nuts (0.12%), and fish (0.09%) were the most commonly responsible food groups.
Conclusions
The prevalence of current immediate-type FA and food-induced anaphylaxis in Korean schoolchildren in 2015 was 4.06% and 0.97%, respectively. Peanuts, cow's milk, hen's egg, fruits, crustaceans, and tree nuts are common allergens.
Keywords: Immediate type, children, food allergy
INTRODUCTION
The prevalence of food allergy (FA) varies in different countries, as estimates are affected by many factors, such as age, ethnicity, frequency of dietary exposure, and cooking method.1,2 In addition, methodology, type of FA, and the size of the study population also influence the results of the epidemiologic studies. 3 Among US children, the prevalence of self-reported FA in 2007-2010 was 6.53%, while 7.1% of Canadian children had FA in a telephone survey conducted between 2008 and 2009.4,5 The pooled prevalence of self-reported FA in Europe was 6.9% in children aged 0-17 years.6 In Taiwanese children aged 4-18 years, the overall prevalence of FA was 7.65%, while the prevalence of FA among 0-14-year-old Hong Kong children in 2005-2006 was 4.8%.7,8 In Japan, the FA prevalence was estimated as 5%-10% in 0- to 6-year-old children and 1%-2% in 6- to 15-year-old children.9 These data demonstrate geographical differences in FA prevalence.
In Korea, nationwide epidemiologic studies on FA in the general population were performed in 1995 and 2000, as part of the International study of Asthma and Allergies in Childhood (ISAAC). In the ISAAC, the prevalence of children ever diagnosed with FA in 6- to 12-year-olds was 4.2% in 1995 and 4.7% in 2000, while the prevalence of FA in 13- to 15-year-olds was 3.8% in 1995, increasing to 5.1% in 2000.10 Despite the large sample size of over 40,000, the diagnostic accuracy was limited because the diagnosis relied on parental report. Since then, the prevalence of immediate-type FA in the general population has been calculated using an algorithm involving detailed history, such as symptoms, time elapsed after specific ingestion, and repetitiveness. In a 2006 birth cohort study evaluating 1,177 infants, the prevalence of immediate-type FA was 5.3%, and the leading causes were hen's egg, cow's milk, peanut, and tree nuts.11 In a 2010 nationwide survey, the prevalence of immediate-type FA was estimated as 2.0% in 6- to 7-year-olds and 3.6% in 12- to 13-year-olds.12 Among 16,749 children aged 0-6 years, the prevalence of immediate-type FA was 3.7%.13
Avoidance of the offending food is important in the management of FA, especially in schoolchildren, because they are served meals through a school lunch program. Although several guidelines have been proposed for FA management in schools, minor allergic reactions and food-induced anaphylaxis have still occured.14 FA also has a negative influence on quality of life.15 In particular, schoolchildren are in a critical time for growth spurts and emotional changes. In daily nutrition intakes, patients with FA have a fear of allergic reactions and experience social isolation and depression.16,17 As a result, FA can affect a child's development and can be a potential problem among family members and friends.15
In the present study, we investigated the prevalence of immediate-type FA and common causes among Korean schoolchildren in a nationwide, cross-sectional, questionnaire survey conducted in 2015. This study provides information about one of the important health problems in schoolchildren and also helps expand our understanding of the current status of FA in Korean children and adolescents.
MATERIALS AND METHODS
Study population
This nationwide, cross-sectional, population-based epidemiologic study was conducted in Korea, in September 2015. Our study population included schoolchildren in 4 age groups of 6-7, 9-10, 12-13, and 15-16 years. Participants in this survey were selected by a 2-stage stratified random sampling design. For the first stage, schools across the country were stratified by geographic region and school type. Geographic regions were classified into 17 cities and provinces according to administrative district. School types were divided into elementary schools, middle schools, and high schools. From each stratum, sample schools were selected by the systematic probability proportional to size (PPS) sampling method. For the second stage of sampling, one class was selected randomly from each sample school. Across the nation, a total of 50,000 children were randomly chosen. In this study, 35,000 children in the first and fourth grades of 500 elementary schools (6- to 7 and 9- to 10 years-olds), 7,500 children in the first grade of 250 middle schools (12- to 13 years-olds), and 7,500 children in the first grade of 250 high schools (15- to 16 years-olds) were selected. This study was approved by the Institutional Review Board at Samsung Medical Center, Seoul, Korea (IRB No. 2015-07-204-002).
Definition of FA and anaphylaxis
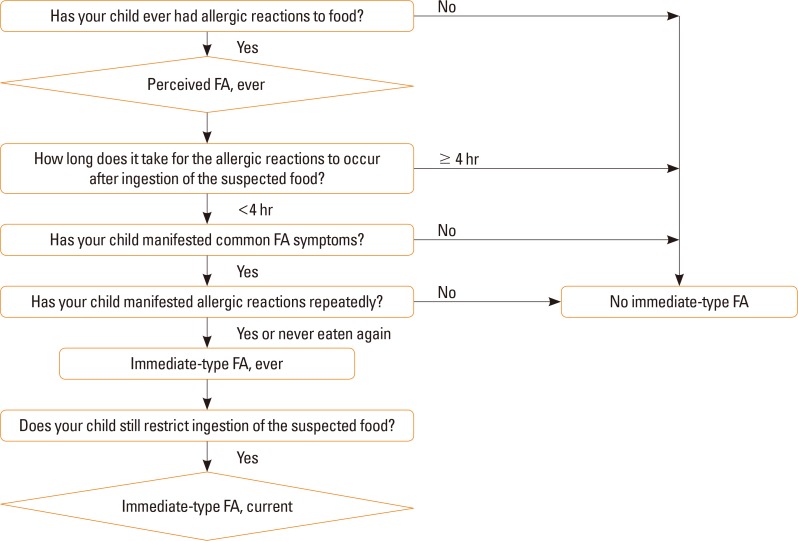
Current immediate-type FA was defined according to an algorithm that included a detailed history of immediate-type FA (Fig. 1). When the parents answered “yes” to the question “Has your child ever had allergic reactions to foods,” their children were considered as having “perceived FA, ever.” Among those who have “perceived FA, ever,” children with common allergic reactions within 4 hours after ingestion of the suspected food were chosen. Common allergic symptoms included skin or mucosal symptoms (urticaria, itching, aggravated eczema, facial edema, eyelid edema, and lip edema), respiratory symptoms (cough, rhinorrhea, wheezing, dyspnea, and cyanosis), gastrointestinal symptoms (vomiting, diarrhea, and abdominal pain), and cardiovascular symptoms (hypotension, altered mentality). Children with only 1 vague symptom, such as vomiting, diarrhea, or itching, were excluded. If children repeatedly manifested allergic reactions or never ate that food again, we determined that they had “immediate-type FA, ever.” If children still restricted ingestion of the suspected food at the time of the survey, they were regarded as having “immediate-type FA, current.” The diagnosis of anaphylaxis was defined according to the criteria proposed in the second symposium on the definition and management of anaphylaxis.18
Fig. 1. Algorithm for identifying immediate-type FA. FA, food allergy.
In the questionnaires, parents were asked to choose suspected foods among individual food items (hen's egg, cow's milk, soy, peanut, wheat, buckwheat, beef, chicken, pork, sesame, and others) and food groups (tree nuts, fruits, vegetables, fishes, and crustaceans).
Statistical analysis
Because the population for this study was selected using a stratified 2-stage random sampling design, we calculated the sampling weights to consider differential selection probabilities, non-response, and post-stratification adjustments. Data were analyzed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Questionnaires from 32,001 of 50,000 participants (64.0%) were completed. After adjustment for missing data, data from 29,842 children (59.7%) were included in the final analyses. This survey included 9,671 children aged 6-7 years, 9,756 children aged 9-10 years, 5,169 children aged 12-13 years, and 5,246 children aged 15-16 years. The demographic data are shown in Table 1.
Table 1. Characteristics of participants (n=29,842).
| Characteristics | No. (%) |
|---|---|
| Age (yr) | |
| 6–7 | 9,671 (32.4) |
| 9–10 | 9,756 (32.7) |
| 12–13 | 5,169 (17.3) |
| 15–16 | 5,246 (17.6) |
| Sex (male) | 15,239 (51.2) |
| Past history of allergic diseases | |
| Allergic rhinitis | 10,589 (36.7) |
| Atopic dermatitis | 7,214 (25.0) |
| Asthma | 1,352 (4.7) |
| Response rate by area | |
| Seoul | 3,626/6,500 (55.8) |
| Gyeonggi* | 6,018/12,620 (47.7) |
| Gangwon | 1,349/2,000 (67.5) |
| Chungcheong† | 5,491/7,660 (71.7) |
| Jeolla‡ | 3,213/6,520 (49.3) |
| Gyeongsang§ | 9,306/13,230 (70.3) |
| Jeju | 839/1,470 (57.1) |
*Gyeonggi includes Gyeonggi-do and Incheon; †Chungcheong includes Chungcheongnam-do, Chungcheongbuk-do, Daejeon, and Sejong; ‡Jeolla includes Jeollanam-do, Jeollabuk-do, and Gwangju; §Gyeongsang includes Gyeongsangnam-do, Gyeongsangbuk-do, Daegu, Busan, and Ulsan.
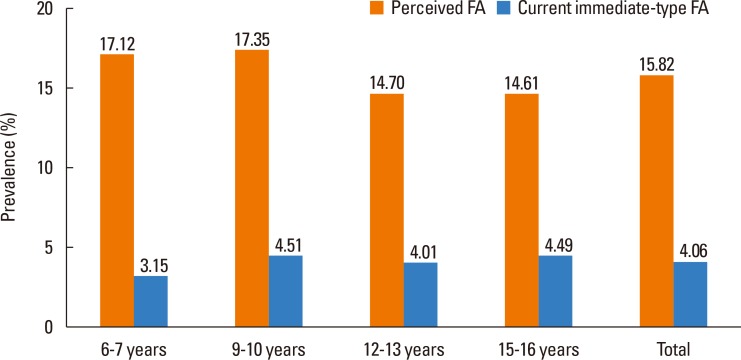
The overall prevalence of perceived FA was 15.82%, and that of current immediate-type FA was 4.06% (Fig. 2). According to age, the prevalence of immediate-type FA was 3.15% in 6- to 7-year-olds, 4.51% in 9- to 10-year-olds, 4.01% in 12- to 13-year-olds, and 4.49% in 15- to 16-year-olds.
Fig. 2. Prevalence of perceived FA and current immediate-type FA in Korean schoolchildren in 2015. FA, food allergy.
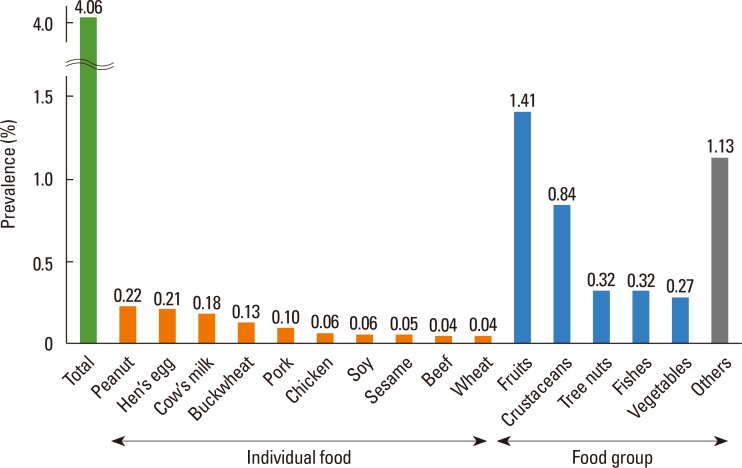
Peanut was the most frequent causative food of current immediate-type FA, with a prevalence of 0.22%, followed by hen's egg (0.21%), cow's milk (0.18%), and buckwheat (0.13%) (Fig. 3). Causative foods differed according to age (Table 2). Hen's egg was the most common cause in 6- to 7-year-olds, whereas peanut, cow's milk, and buckwheat were reported to be the most frequent cause in 9-to 10-year-olds, 12-to 13-year-olds, and 15- to 16-year-olds, respectively. Among the food groups, fruits (1.41%) were the most frequent causes of allergy, followed by crustaceans (0.84%), tree nuts (0.32%), and fish (0.32%) (Fig. 3). These frequencies were similar among different age groups (Table 2).
Fig. 3. Prevalence of current immediate-type FA in Korean schoolchildren in 2015, according to allergen. FA, food allergy.
Table 2. Prevalence of current immediate-type FA in each age group, according to allergen.
| 6- to 7-year-olds | 9- to 10-year-olds | 12- to 13-year-olds | 15- to 16-year-olds |
|---|---|---|---|
| Individual food (prevalence, %) | |||
| Hen's egg (0.25) | Peanut (0.34) | Cow's milk (0.26) | Buckwheat (0.18) |
| Peanut (0.22) | Hen's egg (0.32) | Peanut (0.23) | Pork (0.17) |
| Cow's milk (0.16) | Cow's milk (0.24) | Hen's egg (0.19) | Hen's egg (0.13) |
| Sesame (0.15) | Buckwheat (0.10) | Buckwheat (0.17) | Peanut (0.13) |
| Chicken (0.08) | Soy (0.09) | Pork (0.10) | Chicken (0.11) |
| Wheat (0.06) | Wheat (0.07) | Beef (0.07) | Cow's milk (0.08) |
| Soy (0.06) | Sesame (0.06) | Soy (0.03) | Soy (0.07) |
| Beef (0.04) | Pork (0.06) | Chicken (0.01) | Beef (0.04) |
| Buckwheat (0.03) | Chicken (0.04) | - | Wheat (0.03) |
| Pork (0.03) | Beef (0.01) | - | Sesame (0.01) |
| Food group (prevalence, %) | |||
| Fruits (0.75) | Fruits (1.52) | Fruits (1.92) | Fruits (1.39) |
| Tree nuts (0.36) | Crustaceans (0.89) | Crustaceans (0.77) | Crustaceans (1.25) |
| Crustaceans (0.34) | Tree nuts (0.47) | Fish (0.44) | Vegetables (0.47) |
| Vegetables (0.25) | Fish (0.28) | Tree nuts (0.23) | Fish (0.33) |
| Fish (0.21) | Vegetables (0.24) | Vegetables (0.11) | Tree nuts (0.26) |
FA, food allergy.
Skin or mucosal reaction was reported as the most common symptom (94.3%) of current immediate-type FA in schoolchildren, followed by respiratory symptoms (16.3%) and gastrointestinal symptoms (13.3%). Anaphylaxis was found in 22.5% of children with current immediate-type FA.
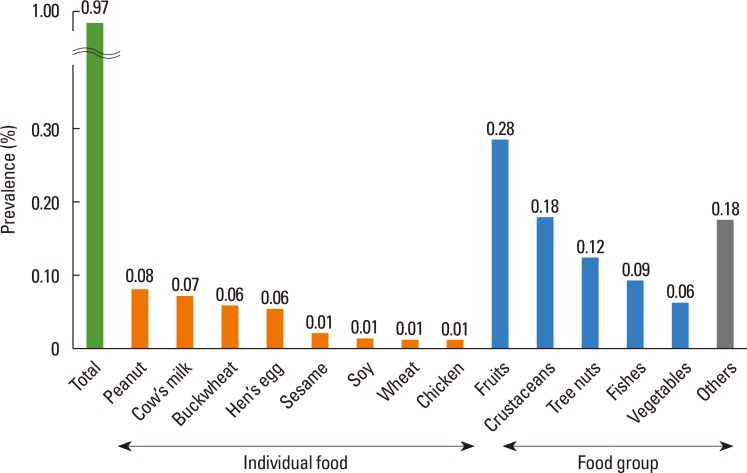
The overall prevalence of food-induced anaphylaxis was 0.97% in Korean schoolchildren. The most common causative food was peanut, with a prevalence of 0.08%, followed by cow's milk (0.07%), buckwheat (0.06%), and hen's egg (0.06%) (Fig. 4). Among the food groups, fruits (0.28%), crustaceans (0.18%), tree nuts (0.12%), and fish (0.09%) were frequent causes of food-induced anaphylaxis (Fig. 4).
Fig. 4. Prevalence of food-induced anaphylaxis in Korean schoolchildren in 2015, according to allergen.
DISCUSSION
The strength of our study is its large, nationwide probability sample; 29,842 schoolchildren participated in the present study, larger than most of the previous FA prevalence studies.2,4,19 In addition, we used a 2-stage stratified random sampling design and selected study participants who represent all Korean schoolchildren in order to avoid selection bias. Instead of using parental report for the diagnosis of FA, we overcame this limitation of diagnostic accuracy by defining immediate-type FA through an algorithm of detailed history. Consequently, we obtained more objective FA data from a large study population representative of schoolchildren nationwide.
Although peanut and tree nuts have been considered common causes of FA in the Western world, the prevalence of peanut and tree nut allergy is relatively low among children in Asia. The prevalence rates of peanut allergy in schoolchildren were 0.22% in the present study, 0.43% in Philippines, and 0.47% in Singapore, while they were reported as 1.03%, 1.50%, and 1.40%, respectively in Canada, the United Kingdom, and the United States.20,21,22 Similarly, the prevalence rates of tree nut allergy were 0.32% in the current study and 0.30% in Singapore, whereas they were 1.10% in the US and 1.67% in Australia.20,22,23 Although direct comparisons between the countries are not possible due to the differences in the size of the study population and methodologies for diagnosing FA, the prevalence appears to vary between Asian and Western countries. Different prevalence rates of filaggrin loss-of-function mutation and atopic dermatitis might make the differences in the prevalence of peanut and tree nut allergies.24,25 Another possible reason for the higher prevalence of peanut allergy in Western countries might be the exposure to peanuts with higher allergenicity, strict avoidance of peanut ingestion during infancy, or higher environmental peanut exposure.26,27 Even though the exact mechanism is unknown, further investigation is required to prevent the development of peanut or tree nut allergy, which is increasing in Western countries.22,28
Hen's egg and cow' milk are well-known causative foods of FA in many countries.1,2,12,13 Most cow's milk and hen's egg allergies develop in the first year of life and are outgrown by early to late childhood.29,30 However, recent studies have demonstrated that tolerance induction of hen's egg or cow's milk allergy is slower than previously described.31,32,33 For example, approximately half of patients with hens' egg allergy reached tolerance by 12 years of age and the allergy persisted into late adolescence in 42%.31 Twenty-one percent of patients with a cow's milk allergy had not developed tolerance by the age of 16.32 In the current study, cow's milk and hen's egg were found to be the second and third most common food allergens in schoolchildren, which appear to reflect the late acquisition of oral tolerance in both allergies.
Among the food groups, fruit was the most frequent food allergen in the present study. Fruit allergy is generally caused by a variety of proteins cross-reacting with pollens, particularly from birch, ragweed, or mugwort. Because the pollen sensitization rate in Korean schoolchildren is 0.3%-10.1% and might be increasing due to climate change and air pollution, it is noteworthy to monitor whether fruit allergy in Korean children increases in the future.34,35 Crustaceans are also common allergens. The prevalence of shellfish allergy among 14- to 16-year-old children was 5.12% in the Philippines and 5.23% in Singapore,20 whereas it was 1.3% in the US.36 Although its prevalence was only 0.84% in the current study, crustaceans were ranked at the second most common food allergen, indicating that shrimp and crab are important food allergens in Korean children.
A meta-analysis of 10 studies on anaphylaxis showed an incidence of 4.93 per 100 person-years in children aged 0-19 years.37 In our study, the overall prevalence of food-induced anaphylaxis in Korean schoolchildren was 0.97%. This high prevalence might be because we used a broad definition of anaphylaxis that reflects its potential severity, even if the actual reaction is not always life-threatening.18 Another reason for the high prevalence of food-induced anaphylaxis in the present study might be because we could not confirm the natural regression in this questionnaire survey. If a child never again ate the offending food after the anaphylactic reaction occurred, he or she was still regarded as having current food-induced anaphylaxis, leading to an overestimation of the prevalence. In the present study, we demonstrated that peanut, cow's milk, buckwheat, and hen's egg were commonly responsible for food-induced anaphylaxis. Among the food groups, fruits, crustaceans, tree nuts, and fish were frequent causes of anaphylaxis. Our results about major causes were similar to those in other studies. 38,39,40 Of interest, buckwheat is an important causative food of anaphylaxis in Korea as well as in Japan.14 In contrast, anaphylaxis triggered by fruit ingestion might be overestimated, because mild subjective symptoms were included in determining the diagnosis of anaphylaxis.18
In conclusion, the prevalence of current immediate-type FA and food-induced anaphylaxis in Korean schoolchildren in 2015 was 4.06% and 0.97%, respectively. Peanut, cow's milk, hen's egg, fruits, crustaceans, and tree nuts are common allergens.
ACKNOWLEDGMENTS
This study was funded by the Ministry of Education, Republic of Korea.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 4.McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;132:1216–1219.e5. doi: 10.1016/j.jaci.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St Pierre Y, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986–988. doi: 10.1016/j.jaci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 7.Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC, Huang IF, et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42:1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 8.Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac J Allergy Immunol. 2012;30:275–284. [PubMed] [Google Scholar]
- 9.Ebisawa M, Nishima S, Ohnishi H, Kondo N. Pediatric allergy and immunology in Japan. Pediatr Allergy Immunol. 2013;24:704–714. doi: 10.1111/pai.12117. [DOI] [PubMed] [Google Scholar]
- 10.Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004;19:716–723. doi: 10.3346/jkms.2004.19.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol. 2011;22:715–719. doi: 10.1111/j.1399-3038.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahn K, Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, et al. Prevalence of immediate-type food allergy in Korean schoolchildren: a population-based study. Allergy Asthma Proc. 2012;33:481–487. doi: 10.2500/aap.2012.33.3598. [DOI] [PubMed] [Google Scholar]
- 13.Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in Seoul. Allergy Asthma Immunol Res. 2014;6:131–136. doi: 10.4168/aair.2014.6.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urisu A, Ebisawa M, Mukoyama T, Morikawa A, Kondo N Japanese Society of Allergology. Japanese guideline for food allergy. Allergol Int. 2011;60:221–236. doi: 10.2332/allergolint.11-rai-0329. [DOI] [PubMed] [Google Scholar]
- 15.Antolín-Amérigo D, Manso L, Caminati M, de la Hoz Caballer B, Cerecedo I, Muriel A, et al. Quality of life in patients with food allergy. Clin Mol Allergy. 2016;14:4. doi: 10.1186/s12948-016-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65:933–945. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie H, Roberts G, van Laar D, Dean T. Teenagers' experiences of living with food hypersensitivity: a qualitative study. Pediatr Allergy Immunol. 2010;21:595–602. doi: 10.1111/j.1399-3038.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 19.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 20.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Venter C, Maslin K, Patil V, Kurukulaaratchy R, Grundy J, Glasbey G, et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol. 2016;27:804–811. doi: 10.1111/pai.12616. [DOI] [PubMed] [Google Scholar]
- 22.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Panjari M, Koplin JJ, Dharmage SC, Peters RL, Gurrin LC, Sawyer SM, et al. Nut allergy prevalence and differences between Asianborn children and Australian-born children of Asian descent: a state-wide survey of children at primary school entry in Victoria, Australia. Clin Exp Allergy. 2016;46:602–609. doi: 10.1111/cea.12699. [DOI] [PubMed] [Google Scholar]
- 24.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135:164–170. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkataraman D, Soto-Ramírez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol. 2014;134:876–882.e4. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134:867–875.e1. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 28.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 29.Savage J, Sicherer S, Wood R. The natural history of food allergy. J Allergy Clin Immunol Pract. 2016;4:196–203. doi: 10.1016/j.jaip.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013;131:805–812. doi: 10.1016/j.jaci.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413–1417. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007;120:1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133:492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, et al. Sensitization to aeroallergens in Korean children: a population-based study in 2010. J Korean Med Sci. 2011;26:1165–1172. doi: 10.3346/jkms.2011.26.9.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Amato G, Pawankar R, Vitale C, Lanza M, Molino A, Stanziola A, et al. Climate change and air pollution: effects on respiratory allergy. Allergy Asthma Immunol Res. 2016;8:391–395. doi: 10.4168/aair.2016.8.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau CH, Springston EE, Smith B, Pongracic J, Holl JL, Gupta RS. Parent report of childhood shellfish allergy in the United States. Allergy Asthma Proc. 2012;33:474–480. doi: 10.2500/aap.2012.33.3610. [DOI] [PubMed] [Google Scholar]
- 37.Umasunthar T, Leonardi-Bee J, Turner PJ, Hodes M, Gore C, Warner JO, et al. Incidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1621–1636. doi: 10.1111/cea.12477. [DOI] [PubMed] [Google Scholar]
- 38.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Ahn K, Kim J, Jang GC, Min TK, Yang HJ, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res. 2016;8:535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang N, Yin J, Wen L, Li H. Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy Asthma Immunol Res. 2016;8:353–361. doi: 10.4168/aair.2016.8.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]