Abstract
Purpose
Buckwheat is a major cause of anaphylaxis, and Fag e 3 is the key major allergen in buckwheat. However, an immunoassay system for the quantification of Fag e 3 has yet to be developed.
Methods
We developed a 2-site enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (mAbs) produced against recombinant Fag e 3. We applied this ELISA to quantify native Fag e 3 in total buckwheat extract.
Results
Four clones of mAbs were produced, and all recognized vicilin allergens not only from buckwheat, but also from peanut and walnut. However, the ELISA using these antibodies was only able to quantify Fag e 3 in the total extract after addition of 1% sodium dodecyl sulphate (SDS) and heating, which facilitated dissociation of the allergen. The detection limit of the developed 2-site ELISA was 0.8 µg/mL. The measurement of Fag e 3 in the total extract of buckwheat showed that approximately 12% of protein in total buckwheat extract was Fag e 3.
Conclusions
We have developed an ELISA system for the quantification of the group 3 buckwheat allergen, Fag e 3, specifically. This assay will be useful for standardization of buckwheat allergens and monitoring of buckwheat contamination in foods.
Keywords: Buckwheat, 2-site ELISA, Fag e 3, vicilin
INTRODUCTION
Buckwheat is one of favorite foods in Asian countries. Buckwheat is a potent major allergic food, especially the most important causes of allergy among grains commonly consumed in Korea.1,2 Moreover, it is one of leading causes of anaphylaxis.3 The consumption of buckwheat in Western countries has also increased recently due to its gluten-free characteristics,4 and it has been described as a hidden allergen in Italian cuisine, such as pizza and pasta.5
Labeling of allergens in food merchandise is crucial, and most developed countries legally require labeling of common food allergens. Laws for the labeling vary among countries, despite the recommendations by the Codex Alimentarius Commission.6 Buckwheat is a major cause of food allergy in Korea, and Korean Food and Drug Administration (FDA) has designated labeling of buckwheat in foods as mandatory (KFDA official announcement 2015-77). However, labeling does not provide detailed information on the content of specific allergens, and it would be helpful to know the concentration of these allergenic molecules in food products.
Various 9-, 16-, 19-, and 24-kDa protein allergens have been described as the major allergens from buckwheat.7 A 19-kDa allergen homologous to vicilin was more specifically recognized by allergic patients than by asymptomatic subjects. Specific immunoglobulin E (IgE) measurements against the recombinant 19-kDa allergen had better diagnostic value than those of whole buckwheat extracts.8,9 The 19-kDa allergen was designated as Fag e 3, according to the guidelines of the International Union of Immunological Societies Allergen Nomenclature Subcommittee.
Quantification of major allergen in extracts may be essential for standardization of allergen, pathogensis, and epidemiologic studies. Various methods have been used for the quantification of major allergens, such as radial immunodiffusion using polyclonal antibodies or 2-site enzyme-linked immunosorbent assay (ELISA) kit. Mass spectrometry techniques could be also applied to detect and quantify the allergens in foods.10
In this study, we produced monoclonal antibodies (mAbs) to recombinant Fag e 3 and developed a method to quantify Fag e 3 allergen concentrations.
MATERIALS AND METHODS
Allergen extracts
Buckwheat and walnut extracts were prepared as described previously.7 In brief, buckwheat flour was extracted with phosphate buffered saline for 72 hours at 4℃ after defatting with ethyl ether. The supernatant was dialyzed against distilled water for 48 hours after centrifugation at 50,000 g for one hour at 4℃. Subsequently, the extract was lyophilized and stored at -20℃ until use. The protein concentration of the reconstituted extract in phosphate buffered saline was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Peanut extract was kindly provided by Dr. Kyung Eun Lee (Department of Pediatrics, Yonsei University College of Medicine).
mAbs to recombinant Fag e 3
Recombinant Fag e 3 was expressed in Escherichia coli and purified using a Ni-column from inclusion bodies, as described previously.8 Mouse mAbs to Fag e 3 were produced by the fusion of myeloma cells (Sp 2.0-Ag 14) and spleen cells from BALB/c mice immunized with recombinant Fag e 3 3 times at 2-week intervals. The hybridomas that produced antibodies against recombinant Fag e 3 were screened by ELISA and cloned by limiting dilution. mAbs from the expanded clones were purified with protein G (Sigma-Aldrich, St. Louis, MO, USA). All of the antibodies were IgG1 according to mouse mAb isotyping reagents (Sigma-Aldrich).
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis
Recombinant protein or buckwheat extract was run on a 15% acrylamide gel containing SDS under reducing or non-reducing conditions. The gel was stained with Coomassie blue or transferred to a polyvinylidine difluoride membrane (0.45 µm; Millipore, Bedford, MA, USA). The membrane was blocked overnight with 3% skim milk in Tris-buffered saline containing 0.05% Tween 20. Subsequently, membranes were reacted with hybridoma culture supernatant and then incubated with 1:1,000-diluted goat anti-mouse IgE conjugated with alkaline phosphatase (Sigma-Aldrich). Color was developed with 3-bromo-4-chloro-5-indolyl-phosphate, and nitro blue tetrazolium as a substrate (Promega, Madison, WI, USA).
Biotinylation of antibody
mAbs purified with a protein G column were biotinylated with EZ-Link® Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Waltham, MA, USA). In brief, 2 mg/mL of antibodies were incubated with NHS-LC-Biotin on ice for 4 hours, and unreacted NHS-LC-Biotin was removed by extensive dialysis against phosphate buffered saline.
Measurement of Fag e 3 using a 2-site ELISA
The optimal antibody combination was established by comparing titration curves of recombinant Fag e 3 using the 3 different antibodies. The combination of 3D1 (10 µg/mL) as a capture antibody and biotinylated 4H8 (1:1,000-diluted) as a detection antibody gave optimal results. Antigens were diluted in buffers containing 1% of various detergents (Triton X, Nonidet P40, or SDS) to facilitate the dissociation of Fag e 3 in the extract. Some of the each sample was heated at 100℃ for 5 minutes after the sample was diluted in detergent buffer, and compared the results with samples without heat treatment.
In brief, the capture antibody was diluted in carbonate buffer (pH 9.6) and was coated overnight at 4℃. Serially diluted antigens (recombinant Fag e 3 or allergen extracts) were added to the wells and incubated for one hour at room temperature after blocking with 3% skim milk. This was then incubated for one more hour after adding the detection antibody, and incubated again with streptavidin-conjugated peroxidase (Sigma-Aldrich) for 30 minutes. Microtiter plates were washed at least 3 times with phosphate-buffered saline containing 0.05% Tween 20 between each step. Color was developed with 2,2′-azino-bis (3-ethylbenzothiazoline-6-suphonic acid) (Thermo Fisher Scientific) or 3.3′, 5,5′-tetramethylbenzidine (Kirkegaard Perry Laboratories, Gaithersburg, MD, USA).
For comparison, the concentration of Ara h 1, a vicilin-like allergen from the peanut, was also determined with the Ara h 1 ELISA kit (Indoor Biotechnologies Inc., Charlottesville, VA, USA).
RESULTS
Specificity of mAbs
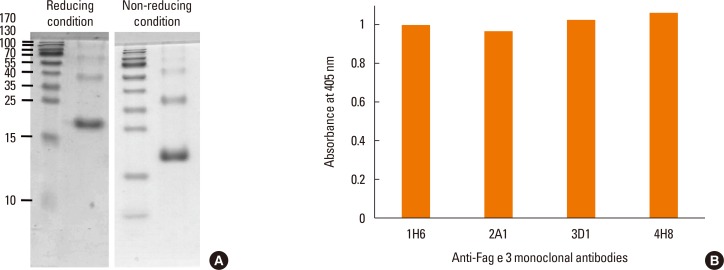
Reactivity of the mAbs to total buckwheat extract containing native allergens was examined by immunoblotting with mAbs, which were produced against recombinant protein. In the extract, Fag e 3 separated under reducing conditions was barely recognized by mAbs, despite mAbs showing good reactivity to the extract on ELISA (Fig. 1). This observation motivated us to perform the immunoblot again, while separating the extract under non-reducing conditions. Interestingly, all of the mAbs preferentially recognized a 38-kDa protein, putative dimerized Fag e 3. We also evaluated whether the mAbs could detect vicilin family major allergens of peanut (Ara h 1) and walnut (Jug r 2). Putative Ara h 1 (64 kDa) and Jug r 2 (44 kDa) under nonreducing condition only were also recognized by the all mAbs, respectively (Fig. 2).
Fig. 1. SDS-PAGE analysis of recombinant Fag e 3 (5 µg) and antibody responses to buckwheat extract. Recombinant protein was separated onto a 15% polyacrylamide gel under reducing and non-reducing conditions (A). We examined reactivity of mAbs produced against recombinant Fag e 3 to buckwheat extract by ELISA using hybridoma culture supernatants (B). SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; mAbs, monoclonal antibodies; ELISA, enzyme-linked immunosorbent assay.
Fig. 2. Recognition of native vicilin-like allergens by mAbs. We probed Fag e 3 and its homologous allergens with mAbs from extracts (10 µg) that were transferred onto a PVDF membrane after running on 15% gels that were run under reducing (A) and non-reducing (B) conditions. mAbs used to detect Fag e 3 are denoted beneath each membrane. M, molecular mass standard; B, buckwheat extract; P, peanut extract; W, walnut extract; mAbs, monoclonal antibodies; PVDF, polyvinylidene difluoride.
Sensitivity of 2-site ELISA
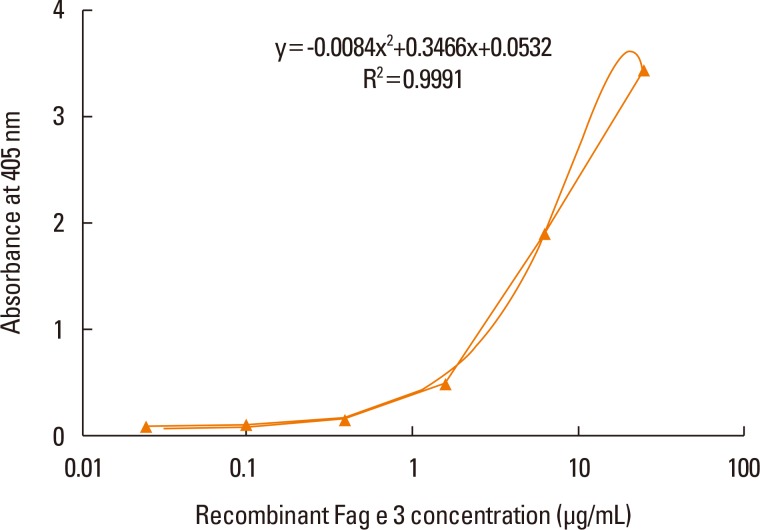
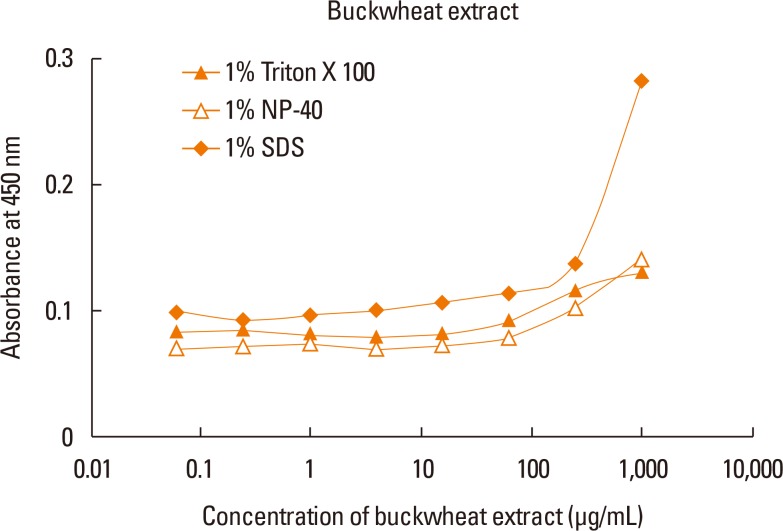
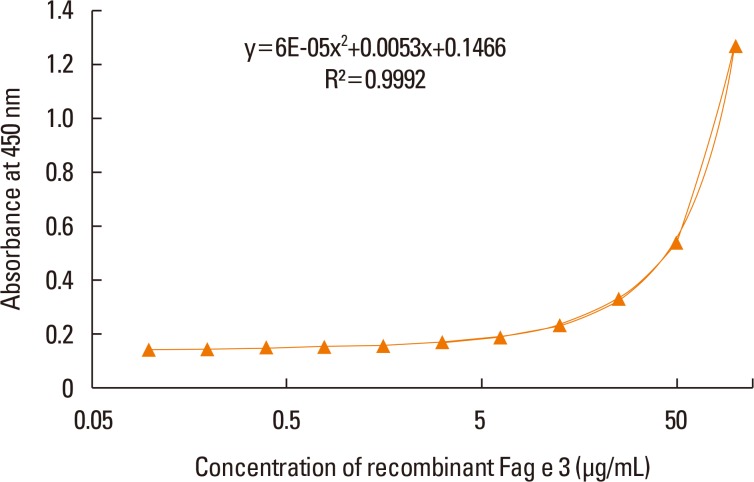
ELISA with selected antibodies was able to detect less than 1 µg/mL of recombinant Fag e 3 (Fig. 3). However, it could not detect native Fag e 3 in the total extract of buckwheat. Therefore, we added various detergents to facilitate the dissociation of Fag e 3 in the total extract (Fig. 4). An ELISA with SDS showed better detection, but had lowered overall detection capability. The 2-site ELISA system developed in this study was able to detect up to 6 µg/mL of the vicilin allergen when tested with recombinant Fag e 3. More than 100 µg/mL of total buckwheat protein is needed for efficient detection of Fag e 3 (Fig. 5).
Fig. 3. Dose-dependent curve of recombinant Fag e 3 by a 2-site ELISA. The 2-site ELISA was quantified with 4-fold dilution of recombinant Fag e 3 from 25 µg/mL. The detection limit was 0.1 µg/mL. ELISA, enzyme-linked immunosorbent assay.
Fig. 4. Application of detergents in total buckwheat extract to facilitate native Fag e 3 quantification. One percent detergent was added to the extract (A) and to recombinant Fag e 3.
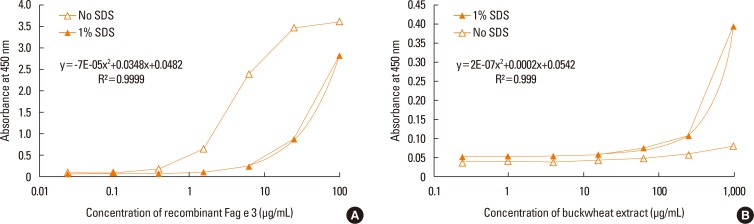
Fig. 5. Quantification of native Fag e 3 in total buckwheat extract. Addition of SDS decreased the sensitivity to recombinant Fag e 3 (A), but increased the sensitivity to native Fag e 3 in total buckwheat extract (B). The detection limit for recombinant Fag e 3 was 6 µg/mL (A). We created a dose-dependent curve of with 4-fold dilution of buckwheat extract from 1 mg/mL (B). SDS, sodium dodecyl sulphate.
However, the sensitivity of ELISA was improved by heating the antigen mixture at 100℃ for 5 minutes after the addition of 1% SDS. The detection limit of the 2-site ELISA was 0.8 µg/mL of recombinant Fag e 3 (Fig. 6). The detection limit of total buckwheat extract was also lowered to 60 µg/mL by heating the samples with the detergent. Vicilin-like allergens were not detected from peanut and walnut total extracts by the developed 2-site ELISA.
Fig. 6. Heating after adding SDS to the buckwheat. Heating improves the sensitivity of the 2-site ELISA. The detection limit was 0.8 µg/mL of recombinant Fag e 3. SDS, sodium dodecyl sulphate; ELISA, enzyme-linked immunosorbent assay.
Measurement of Fag e 3 in the allergen extracts
We estimate that 120 µg of Fag e 3 was found in 1 mg of total buckwheat extract. Fag e 3 was not also detected by the Ara h 1 ELISA kit (Indoor Biotechnologies Inc.), although 143.75 µg of Ara h 1 was detected from 1 mg of peanut extract.
DISCUSSION
Immunoassays for the quantification of allergens in food are useful for allergen avoidance and standardization of allergen extracts.11 We have developed a 2-site ELISA to quantify the buckwheat allergen Fag e 3 using mAbs raised against recombinant Fag e 3.
All mAbs showed good reactivity to total buckwheat extract. However, they were not able to readily detect native Fag e 3 in the extract under reducing condition. All the mAbs produced against recombinant Fag e 3 seem to recognize the conformational epitopes of vicilin-like allergens. Ara h 1 (64 kDa) and Jug r 2 (44 kDa) were also recognized by the mAbs under non-reducing condition.
Interestingly, individual mAbs showed good response to the extract in ELISA experiment, suggesting good affinity to native Fag e 3. However, native Fag e 3 in the extract was not detected by the combination of 2 mAbs, although recombinant Fag e 3 was well quantified. mAbs to recombinant Fag e 3 may recognize epitopes very close to each other. Binding of one mAb to an epitope region may decrease the accessibility of another mAb to a close epitope, possibly due to steric hindrance. Partial denaturation of Fag e 3 by detergents may increase the accessibility of another mAb. Epitope mapping should be performed to explain this hypothesis. In addition, the binding of mAbs to Fag e 3 may be hampered by other proteins which associated with vicilins or by interactions between vicilin molecules, as they are known to form multimers. Typically, vicilins are disk-shaped trimeric proteins with various subunit compositions due to post-translational proteolytic processing and glycosylation.12 Glycosylation may also influence antibody recognition.13 IgE reactivity to native Jug r 2 without clinical significance was described due to cross-reactive carbohydrate epitopes.14 Structural similarity of vicilin-like proteins from peanut (Ara h 1), walnut (Jug r 1), hazelnut (Cor a 11), and cashew nut (Ana o 1) was described, indicating its potential cross-reactivity,15 and all mAbs produced by our team also have affinity to Ara h 1 and Jug r 1 under non-reducing condition.
Recombinant Fag e 3 had good IgE reactivity, indicating that it closely resembles its native counterpart.8 We did not observe structural differences in recombinant Fag e 3 based on reducing and non-reducing gels. However, reducing conditions may cause native Fag e 3 to undergo a slight change in its configuration, as it showed different reactivity to mAbs under those conditions. However, the structurally change under reducing conditions is believed to retain most of its IgE epitopes, as it displayed good IgE reactivity based on IgE immunoblot analysis.7 Thermal treatment (30-50 minutes) in the presence of polysaccharides can cause structural change to vicilin-like allergens. This leads to markedly reduced immunogenicity through the Maillard reaction. However Fag t 3, a vicilin-like allergen from tartary buckwheat (Fagopyrum tataricum), was stable even after incubation at 90℃ for 30 minutes.16 Therefore, we suspect that the Maillard reaction did not occur in this study during heat treatment with 1% SDS for 5 minutes at 100℃.
We used the detergent SDS to improve the detection of Fag e 3 in the dilution buffer. Approximately 12% of buckwheat protein was found to be vicilin. Detecting allergenic components from food that is mixed with various seasonings, additives, and other foods can be challenging. In the case of the peanut, improved detection of Ara h 1 by 2-site ELISA were obtained by heating the sample and adding nonfat dry milk to the extraction buffer (up to 400-fold).17 Therefore, heating the antigens and using detergent prior to serial dilution could improve the extraction and detection of buckwheat major allergen from various foods and environments. The sensitivity of our 2-site ELISA improved approximately 7.5 times by heating before serially diluting the antigens.
Ara h 1 is a well-characterized vicilin allergen. It is a heat stable protein with a stable trimeric form (64 kDa) that is mediated through hydrophobic interactions. This protein comprises 12%-16% of the total protein in peanut extracts.18,19,20 Ara h 1 was measured not only in peanut and food extracts, but also in various environments.17,20,21 Buckwheat could be sensitized by inhalation in occupational and domestic exposure, mainly due to buckwheat chaff-stuffed pillows contaminated with buckwheat flour,22 or buckwheat flour in buckwheat noodle factory.23 It is likely that Fag e 3 can be measured in various environmental dusts such as restaurants that serve buckwheat, factories that produce buckwheat noodles, in addition to meals that contain buckwheat.
The mAbs produced against recombinant Fag e 3 also recognize vicilin-like allergens from tree nuts. Vicilin-like allergens are also described in black walnut (Jug n 2), lentil (Len c 1), pea (Pis s 1), cashew (Ana o 1), hazel (Cor a 1), and sesame (Ses i 3). Clinically relevant cross-reactivity between the pea and the peanut was reported due to vicilin-homologous allergens, Pis s 1, and Ara h 1.24 However, there is a low rate of clinical cross-reactivity among these vicilin belonged allergens.
Buckwheat 2-site ELISA kits are already produced by Crystal Chem Inc. (Downers Grove, IL, USA) and ELISA systems (Windsor, Queensland, Australia). These kits utilize buckwheat extract for the surveillance of buckwheat allergen contamination in foods. The food industry and official food control agency laboratories in Japan employ the ALLERGENEYE ELISA kit (Justia Legal Resources, Tokyo, Japan), which detects a 24-kDa allergen (a putative Fag e 1), and the FASTKIT ELISA (COSMO Bio Co., Ltd., Tokyo, Japan) (Notification No. 1106001, 2002).25 A buckwheat DNA detection system (Shimadzu Cor., Kyoto, Japan) is also available. Employing better detection systems for buckwheat allergens to complement the current food allergy labeling system is an important goal. The detection of clinically relevant allergens such as Fag e 2 or Fag e 3 shows better diagnostic value than that of the total extract.8,26 Furthermore, the importance of prevention of food allergy is being emphasized in a public health problem.27
We hope that the Fag e 3 ELISA developed in this study is helpful for the standardization of buckwheat extract as well as the detection of buckwheat allergens in foods and environments.
ACKNOWLEDGMENTS
This research was supported by a fund (2014-ER5601-00) from the Research of Korea Centers for Disease Control & Prevention. This research was also supported by a grant from the Korea Healthcare Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), Funded by the Ministry of Health, Welfare and Family Affairs, Republic of Korea (HI14C1324).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Han SM, Heo YR. Changes of prevalence of food allergy in elementary school student and perception of it in school nutritionist in Korea, 1995–2015. J Nutr Health. 2016;49:8–17. [Google Scholar]
- 2.Kim SR, Park HJ, Park KH, Lee JH, Park JW. IgE sensitization patterns to commonly consumed foods determined by skin prick test in Korean adults. J Korean Med Sci. 2016;31:1197–1201. doi: 10.3346/jkms.2016.31.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SY, Ahn K, Kim J, Jang GC, Min TK, Yang HJ, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res. 2016;8:535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrupatla CV, Kundu RV, Aronson IK. Buckwheat allergy and atopic dermatitis. J Am Acad Dermatol. 2005;53:356–357. doi: 10.1016/j.jaad.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Heffler E, Nebiolo F, Asero R, Guida G, Badiu I, Pizzimenti S, et al. Clinical manifestations, co-sensitizations, and immunoblotting profiles of buckwheat-allergic patients. Allergy. 2011;66:264–270. doi: 10.1111/j.1398-9995.2010.02469.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SL, Baumert JL. Worldwide food allergy labeling and detection of allergens in processed foods. Chem Immunol Allergy. 2015;101:227–234. doi: 10.1159/000373910. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Kang DB, Kim CW, Ko SH, Yum HY, Kim KE, et al. Identification and characterization of the major allergens of buckwheat. Allergy. 2000;55:1035–1041. doi: 10.1034/j.1398-9995.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Choi SY, Sohn JH, Lee YW, Lee EK, Hong CS, Park JW. Characterization of buckwheat 19-kD allergen and its application for diagnosing clinical reactivity. Int Arch Allergy Immunol. 2007;144:267–274. doi: 10.1159/000106315. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama N, Sato S, Yanagida N, Cabanos C, Ito K, Borres MP, et al. Clinical utility of recombinant allergen components in diagnosing buckwheat allergy. J Allergy Clin Immunol Pract. 2016;4:322–323.e3. doi: 10.1016/j.jaip.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Koeberl M, Clarke D, Lopata AL. Next generation of food allergen quantification using mass spectrometric systems. J Proteome Res. 2014;13:3499–3509. doi: 10.1021/pr500247r. [DOI] [PubMed] [Google Scholar]
- 11.Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011;52:393–400. doi: 10.3349/ymj.2011.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shewry PR, Napier JA, Tatham AS. Seed storage proteins: structures and biosynthesis. Plant Cell. 1995;7:945–956. doi: 10.1105/tpc.7.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villalta D, Conte M, Asero R, Da Re M, Stella S, Martelli P. Isolated IgE reactivity to native walnut vicilin-like protein (nJug r 2) on ISACTM microarray is due to cross-reactive carbohydrate epitopes. Clin Chem Lab Med. 2013;51:1991–1995. doi: 10.1515/cclm-2013-0027. [DOI] [PubMed] [Google Scholar]
- 14.Barre A, Sordet C, Culerrier R, Rancé F, Didier A, Rougé P. Vicilin allergens of peanut and tree nuts (walnut, hazelnut and cashew nut) share structurally related IgE-binding epitopes. Mol Immunol. 2008;45:1231–1240. doi: 10.1016/j.molimm.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Badenoch-Jones J, Spencer D, Higgins TJ, Millerd A. The role of glycosylation in storage-protein synthesis in developing pea seeds. Planta. 1981;153:201–209. doi: 10.1007/BF00383888. [DOI] [PubMed] [Google Scholar]
- 16.Yang ZH, Li C, Li YY, Wang ZH. Effects of Maillard reaction on allergenicity of buckwheat allergen Fag t 3 during thermal processing. J Sci Food Agric. 2013;93:1510–1515. doi: 10.1002/jsfa.5928. [DOI] [PubMed] [Google Scholar]
- 17.Pomés A, Vinton R, Chapman MD. Peanut allergen (Ara h 1) detection in foods containing chocolate. J Food Prot. 2004;67:793–798. doi: 10.4315/0362-028x-67.4.793. [DOI] [PubMed] [Google Scholar]
- 18.Shin DS, Compadre CM, Maleki SJ, Kopper RA, Sampson H, Huang SK, et al. Biochemical and structural analysis of the IgE binding sites on Ara h 1, an abundant and highly allergenic peanut protein. J Biol Chem. 1998;273:13753–13759. doi: 10.1074/jbc.273.22.13753. [DOI] [PubMed] [Google Scholar]
- 19.de Jong EC, Van Zijverden M, Spanhaak S, Koppelman SJ, Pellegrom H, Penninks AH. Identification and partial characterization of multiple major allergens in peanut proteins. Clin Exp Allergy. 1998;28:743–751. doi: 10.1046/j.1365-2222.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 20.Pomés A, Helm RM, Bannon GA, Burks AW, Tsay A, Chapman MD. Monitoring peanut allergen in food products by measuring Ara h 1. J Allergy Clin Immunol. 2003;111:640–645. doi: 10.1067/mai.2003.118. [DOI] [PubMed] [Google Scholar]
- 21.Perry TT, Conover-Walker MK, Pomés A, Chapman MD, Wood RA. Distribution of peanut allergen in the environment. J Allergy Clin Immunol. 2004;113:973–976. doi: 10.1016/j.jaci.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Lee KS, Hong CH, Lee KY. Three cases of childhood nocturnal asthma due to buckwheat allergy. Allergy. 2001;56:763–766. doi: 10.1034/j.1398-9995.2001.056008763.x. [DOI] [PubMed] [Google Scholar]
- 23.Park HS, Nahm DH. Buckwheat flour hypersensitivity: an occupational asthma in a noodle maker. Clin Exp Allergy. 1996;26:423–427. [PubMed] [Google Scholar]
- 24.Wensing M, Knulst AC, Piersma S, O'Kane F, Knol EF, Koppelman SJ. Patients with anaphylaxis to pea can have peanut allergy caused by cross-reactive IgE to vicilin (Ara h 1) J Allergy Clin Immunol. 2003;111:420–424. doi: 10.1067/mai.2003.61. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda R, Yoshioka Y, Akiyama H, Aburatani K, Watanabe Y, Matsumoto T, et al. Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the detection of egg, milk, wheat, buckwheat, and peanut in foods. J AOAC Int. 2006;89:1600–1608. [PubMed] [Google Scholar]
- 26.Choi SY, Sohn JH, Lee YW, Lee EK, Hong CS, Park JW. Application of the 16-kDa buckwheat 2 S storage albumin protein for diagnosis of clinical reactivity. Ann Allergy Asthma Immunol. 2007;99:254–260. doi: 10.1016/S1081-1206(10)60661-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee SE, Kim H. Update on early nutrition and food allergy in children. Yonsei Med J. 2016;57:542–548. doi: 10.3349/ymj.2016.57.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]