Abstract
Background
Filaggrin (FLG) is the major component of the epidermal granular layer and binds to and condenses the keratin cytoskeleton. FLG thus contributes to cell compaction and serves as a natural moisturizing factor by promoting unfolding and degradation into hygroscopic amino acids. Loss or downregulation of FLG has been shown to result in a weak stratum corneum, which causes water loss and increases the possibility of skin barrier-related seizure. Adiponectin (Acrp30) contributes to the functional recovery of somatic cells, including human normal epidermal keratinocytes (NHEKs).
Objective
To investigate the effect of Acrp30 in FLG expression and identifying its signal transduction mechanism.
Methods
Normal human keratinocytes were treated with Acrp30 and the levels of FLG were examined. Silent mating type information regulation 2 homolog (SIRT)-targeting siRNA and aryl hydrocarbon receptor nuclear translocator (ARNT)-targeting siRNA were used to identify the role of various signal transduction pathway components.
Results
Acrp30 upregulated SIRT1 and ARNT expression in NHEKs, resulting in increased FLG expression. Treatment with both SIRT1-targeting siRNA and ARNT-targeting siRNA blocked Acrp30 stimulation and silenced FLG expression.
Conclusion
Adiponectin upregulates FLG expression through a SIRT1-mediated pathway. Our results suggest that Acrp30 is a promising agent for skin barrier permeability improvement.
Keywords: Adiponectin, ARNT, Filaggrin, Keratinocytes, SIRT1
INTRODUCTION
Filaggrin (FLG) is a filament-associated protein that associates with keratins in epithelial cells and keratinocytes. FLG has also been designated as a natural moisturizing factor protein that contributes to the permeable barrier as an aggregated particle comprised of pro-FLG. FLG is expressed as a large precursor protein, pro-FLG. Pro-FLG is predominantly found in the granular layer of the epidermis. After dephosphorylation of pro-FLG and its proteolysis into several single FLG units, the units bind to intermediate keratin filaments and form a highly insoluble keratin matrix. This binding allows the attachment of cornified envelope proteins and lipids, which together form the stratum corneum that functions as the skin barrier. Therefore, pro-FLG and FLG expression levels have been established as keratinocyte differentiation markers; moreover, the expression of these proteins is essential for permeable barrier formation. Pro-FLG regulation is modulated by the transcription factors AP1 and peroxisome proliferator-activated receptor γ (PPARγ). Activation and binding of both transcription factors to the FLG promotor region initiates and increases the expression of pro-FLG. However, the mechanism by which FLG expression is regulated has not yet been fully elucidated1,2,3,4.
Silent mating type information regulation 2 homolog (SIRT1) is one of the best-studied members of the class III histone deacetylase family. Members of this family are involved in many cellular process regulations, including apoptosis, differentiation, cellular senescence, endocrine signaling, glucose homeostasis, aging, and longevity. SIRT1 has various targets such as acetylated p53, p300, Ku70, forkhead box O transcription factors, PPARα, PPARγ, and PPARγ coactivator-1α5,6,7. SIRT1 promotes keratinocyte differentiation through the transcription factors PPARα, PPARγ, and CCAAT/enhancer-binding protein alpha8. Also, SIRT1 upregulates hypoxia inducible factor-1 (HIF-1) in a proportional relationship9.
Adiponectin ameliorates insulin resistance, thereby improving glucose uptake and leading to more efficient energy expenditure. Adiponectin-mediated stimulation upregulates AMP-activated protein kinase (AMPK) activity, which plays a key role in the regulation of energy homeostasis and metabolic stress. The induction of AMPK stimulation suppresses essential enzymes involved in adenosine triphosphate (ATP)-consuming anabolic pathways, such as NAD+/NADPH, and increases the cellular ATP supply10,11,12. This regulation of energy expenditure is enhanced as SIRT1 deacetylation activity increases13,14. With respect to the modulation of these signal transduction pathways, adiponectin is a well-studied endocrinal agent that has been reported to show multiple beneficial effects in various tissues and organs12,15,16. However, few reports have focused on skin and/or keratinocytes, except for a few that have focused on proliferation and wound healing. In the context of the general signal transduction pathway of adiponectin stimulation, we hypothesized that adiponectin enhances FLG expression and/or regulation, thereby improving barrier permeability. A further aim of this study was to investigate the possibility of using adiponectin to ameliorate skin barrier-related diseases.
MATERIALS AND METHODS
Chemicals and reagents
Full-length recombinant human adiponectin was obtained from Biobud (Seoungnam, Korea). Hydrogen peroxide (H2O2) was purchased from Junsei Chemicals (Tokyo, Japan). H2O2 was diluted with DDW to the designated concentration and aseptically filtered using a Millex GV 0.22 µm pore disk filter unit (Millipore, Carrigtwohill, Ireland). Anti-SIRT1 (Cat# 8469) and anti-aryl hydrocarbon receptor nuclear translocator (ARNT, Cat# 5537) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA), anti-FLG antibodies (Cat# ab24584) were purchased from Abcam (Cambridge, UK), and anti-β-actin antibodies (Cat# sc-47778) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Human normal epidermal keratinocytes (NHEKs) were obtained from Life Technologies (Carlsbad, CA, USA). Keratinocytes were maintained in EpiLife growth medium (Life Technologies) at 37℃ in a humidified atmosphere containing 5% CO2/95% air. EpiLife growth medium was refreshed every two days until cells were subconfluent or the desired cell population was obtained. All experiments were performed using cells from passages 3 to 4.
Immunoblot analysis
Keratinocytes treated with the indicated compounds were washed twice in ice-cold phosphate-buffered saline (PBS). Cells were then lysed in lysis buffer and protein concentrations were determined using a bicinchoninic acid solution kit (Sigma-Aldrich, St. Louis, MO, USA). Proteins were separated on SDS-polyacrylamide/bis-acrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore Co., Bedford, MA, USA). The membranes were blocked for 1 h in skim milk (BD Bioscience, Franklin lakes, NJ, USA). After blocking the membranes, they were rinsed with TBS-Tween 20 (TBS-T, 0.1% Tween 20). Next, the membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4℃. The membranes were then washed with TBS-T and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies. After extensive washing, immunoreactive bands were detected using Enhanced Chemi-Luminescence reagents (ATTO Corporation, Tokyo, Japan).
Cell viability
Cells were seeded in 48-well plates. After treatment with the indicated compounds, the medium was discarded. Cells were washed twice with PBS and then incubated with serum-free DMEM containing 1% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Korea, Seoul, Korea). After termination of the reactions, the medium was discarded and 120 µl dimethyl sulfoxide (DMSO; Amresco, Cleveland, OH, USA) was added. The stained MTT was eluted and 100 µl was transferred to 96-well plates. The absorbance of each well at 570 nm was determined using a colorimetric plate reader.
Transfection with ARNT-targeting or SIRT1-targeting specific small interfering RNA
siRNA targeting ARNT (s1615), siRNA targeting SIRT1 (HSS177403), and siRNA consisting of a scrambled sequence predicted not to induce specific degradation of any cellular message were purchased from Genolution (Seoul, Korea). For siRNA transfection, NHEKs were cultured in 60 mm2 dishes and incubated with G-fectin transfection reagent mix (Genolution) containing 20 nM siRNA. After a 48 h incubation period, the siRNA-transfected NHEKs were treated with Acrp30 for the indicated times. siRNA transfection did not affect cell viability, as demonstrated by visual inspection under a microscope (data not shown).
Statistical analysis
Data are expressed as means±standard deviations. ANOVA with Bonferroni's correction was used to compare the means of two numeric values. Data analyses were performed with commercially available Prism 5 software (Graphpad, La Jolla, CA, USA). p-values <0.05 were considered statistically significant.
RESULTS
Cytotoxicity of adiponectin in NHEKs
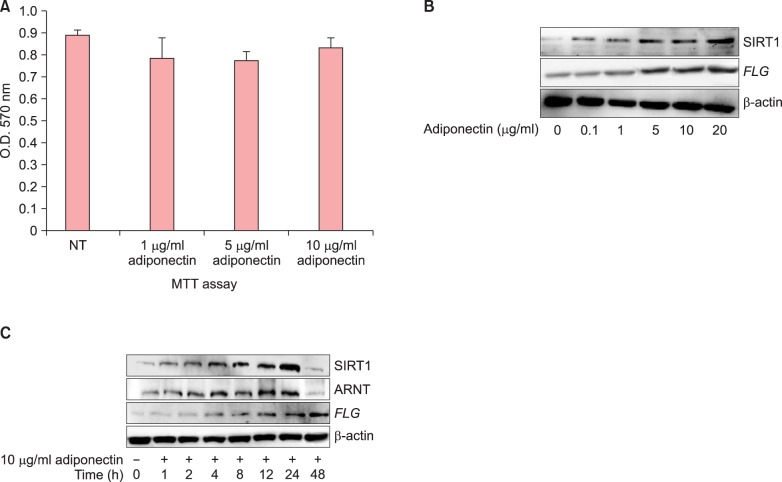
Human normal keratinocytes were treated with various concentrations of recombinant adiponectin for 24 h. Keratinocyte viability was determined by the MTT assay. Adiponectin-treated cells did not show any significant reduction in viability compared to control cells (Fig. 1A).
Fig. 1. Adiponectin stimulation increases filaggrin (FLG) expression in a time-dependent and dose-dependent manner without affecting the viability. Human normal epidermal keratinocytes (NHEKs) were treated with Acrp30 for 24 h at the indicated concentrations or with 10 µg/ml Acrp30 at the indicated time lines. (A) The MTT assay was performed to determine NHEK viability after Acrp30 treatment. (B, C) Cell lysates were generated with described lysis buffer and silent mating type information regulation 2 homolog (SIRT1), aryl hydrocarbon receptor nuclear translocator (ARNT), and FLG expression level was analyzed by immunoblotting. Representative results are shown from 3 independent experiments. NT: no treatment, O.D: optical density.
Adiponectin stimulation increases FLG expression in a time-dependent and dose-dependent manner
To test our hypothesis regarding the effect of Acrp30 on FLG expression, we treated keratinocytes with various concentrations of recombinant Acrp30 (0 to 20 µg/ml) for 24 h. Acrp30 treatment increased FLG and SIRT1 expression levels in a dose-dependent manner (Fig. 1B). The optimal concentration of Acrp30 was found to be 10 µg/ml. In addition, keratinocytes were treated with 10 µg/ml of Acrp30 for various periods of time ranging from 0 to 48 h. Acrp30 stimulation upregulated the expression of SIRT1, ARNT, and FLG in a time-dependent manner. In particular, FLG exhibited a significant increase beginning at 12 h of Acrp30 treatment. SIRT1 exhibited increased expression from 4 h of Acrp30 stimulation, whereas ARNT exhibited increased expression from 12 h of Acrp30 stimulation (Fig. 1C). Interestingly, the expression levels of SIRT1 and ARNT significantly decreased at 48 h of adiponectin stimulation. These data provide evidence that Acrp30 promotes FLG expression and possibly also SIRT1 and ARNT expression. These proteins could thus be involved in the signal transduction pathway by which FLG expression is regulated.
SIRT1 mediates Acrp30-induced FLG upregulation
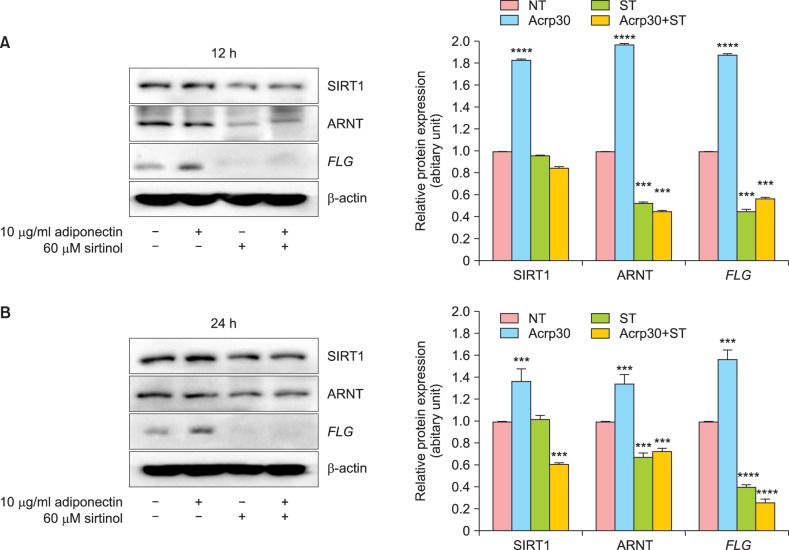
NHEKs were pretreated with the SIRT1-specific inhibitor sirtinol for 4 h to suppress the deacetylation activity of SIRT1, after which cells were treated with Acrp30 for 12 h or 24 h. Sirtinol treatment decreased the expression of FLG and suppressed Acrp30-mediated upregulation of FLG. Additionally, sirtinol downregulated ARNT expression and prevented the increase of SIRT1 expression induced by 12 h or 24 h of Acrp30 stimulation (Fig. 2).
Fig. 2. Silent mating type information regulation 2 homolog (SIRT1) inhibition blocks Acrp30-induced filaggrin (FLG) upregulation. Human normal epidermal keratinocytes were pretreated with 60 µM sirtinol for 4 h, after which they were treated with 10 µg/ml Acrp30 for 12 h (A) or 24 h (B). Cell lysates were generated with described lysis buffer and analyzed by immunoblotting. The protein amounts were quantified by comparison to β-actin. Immunoblotting was used to analyze the levels of SIRT1, aryl hydrocarbon receptor nuclear translocator (ARNT), and FLG. All blots shown are representative of 3 independent experiments. ***p<0.001 compared to the untreated group, ****p<0.0005 compared to the untreated group. NT: no treatment, ST: sirtinol.
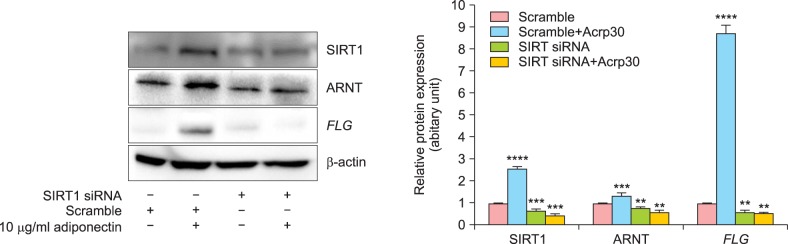
We next utilized SIRT1-targeting siRNA to investigate the role of SIRT1 in FLG expression. NHEKs were transfected with SIRT1-targeting siRNA or scrambled siRNA and incubated for 48 h, after which the cells were treated with Acrp30 for 24 h. Importantly, SIRT1 expression was successfully knocked down by SIRT1-targeting siRNA. Acrp30 stimulation did not alter FLG expression levels in SIRT1 siRNA-treated cells (Fig. 3).
Fig. 3. siRNA-mediated silencing of silent mating type information regulation 2 homolog (SIRT1) blocks Acrp30 stimulation and filaggrin (FLG) regulation. Human normal epidermal keratinocytes were transfected with 20 nM of SIRT1-targeting siRNA or scrambled siRNA and incubated for 48 h. Next, the transfected cells were treated with 10 µg/ml Acrp30 for 24 h. Cell lysates were generated with described lysis buffer and analyzed by immunoblotting. The protein amounts were quantified by comparison to β-actin. Immunoblotting was used to analyze the levels of SIRT1, aryl hydrocarbon receptor nuclear translocator (ARNT), and FLG. All blots shown are representative of 3 independent experiments. **p<0.05 compared to the untreated group,. ***p<0.001 compared to the untreated group, ****p <0.0005 compared to the untreated group.
These results support our hypothesis that SIRT1 and ARNT play a role in the regulation of FLG expression.
Adiponectin upregulates ARNT in a SIRT1-dependent manner and regulates FLG expression
We next addressed whether Acrp30 affects ARNT and/or FLG expression in NHEKs, based on our finding that treatment of NHEKs with Acrp30 for 12 h resulted in a significant increase in ARNT expression (Fig. 1B, C). We found that siRNA-mediated knockdown of SIRT1 significantly downregulated ARNT and suppressed FLG expression (Fig. 3). siRNA-mediated knockdown of SIRT1 also blocked Acrp30-induced upregulation of ARNT.
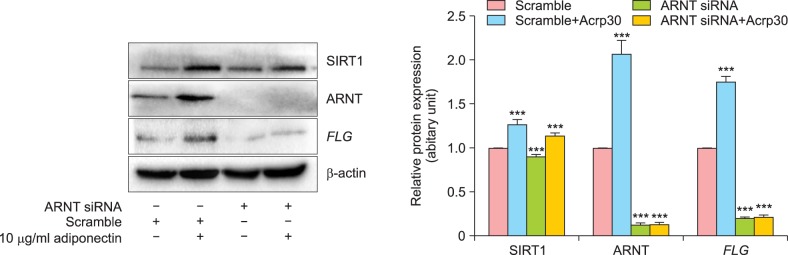
Next, we silenced ARNT expression using ARNT-targeting siRNA. NHEKs were transfected with ARNT-targeting siRNA and then treated with 10 µg/ml Acrp30 for 24 h. ARNT expression was then analyzed by immunoblotting (Fig. 4). Importantly, ARNT-targeting siRNA successfully silenced ARTN expression. ARNT siRNA-treated cells did not show any significant difference in FLG expression and did not exhibit Acrp30-mediated stimulation of FLG expression.
Fig. 4. siRNA-mediated knockdown of aryl hydrocarbon receptor nuclear translocator (ARNT) blocks Acrp30 stimulation and filaggrin (FLG) regulation. Human normal epidermal keratinocytes were transfected with 20 nM of ARNT-targeting siRNA or scrambled siRNA and incubated for 48 h. Next, the transfected cells were treated with 10 µg/ml Acrp30 for 24 h. Cell lysates were generated with described lysis buffer and analyzed by immunoblotting. The protein amounts were quantified by comparison to β-actin. Immunoblotting was used to analyze the levels of silent mating type information regulation 2 homolog (SIRT1), ARNT, and FLG. All blots shown are representative results of 3 independent experiments. ***p<0.001 compared to the untreated group.
These data support our hypothesis regarding Acrp30 stimulation and SIRT1 signaling in FLG expression.
DISCUSSION
Acrp30 is a well-established adipokine that is secreted from adipocytes and sebocytes and has beneficial effects on the whole somatic system11,17,18,19. Although Acrp30 has been predicted to positively affect skin function, few studies have tested this hypothesis. Here we tested the hypothesis that Acrp30 affects permeability of the skin barrier due to its stimulatory and signal transduction aspects.
The effect of Acrp30 on FLG expression in NHEKs has not previously been reported. We found that Acrp30 treatment upregulated FLG expression in a dose-dependent and time-dependent manner (Fig. 1B, C) without significantly affecting NHEK viability (Fig. 1A). We also observed dose-dependent and time-dependent increases in SIRT1 expression (Fig. 1). Acrp30-mediated upregulation of SIRT1 has been reported in numerous studies and has become a well-established aspect of Acrp30 stimulation. We observed an increase of SIRT1 expression in NHEKs, which is consistent with previous reports. Additional studies have indicated that the expression and activation of SIRT1 in NHEKs promotes their differentiation. The expression and development of FLG in NHEKs is an essential part of differentiation, which is important in forming the permeability barrier. Therefore, the data presented above provide strong evidence in support of our hypothesis that FLG regulation is related to Acrp30 stimulation.
Recent studies have provided some evidence that FLG regulation is related to SIRT1. Specifically, the upregulation of SIRT1 has been shown to promote FLG expression and the silencing of SIRT1 has been shown to suppress FLG4. On the other hand, ARNT (also referred to as HIF-1β) has been identified as an FLG expression promotor related to the aryl hydrocarbon receptor20,21. Some studies have indicated that SIRT1 promotes HIF-1α expression, but none of these have investigated the relationship with ARNT9.
Acrp30 stimulation induced ARNT upregulation, which follows the trend of SIRT1 expression (Fig. 1). To investigate the relationship between Acrp30, SIRT1, and FLG regulation, NHEKs were treated with a SIRT1-specific inhibitor, sirtinol, for 12 h or 24 h. Pharmacological inhibition of SIRT1 suppressed FLG expression and also ARNT expression. Moreover, after pretreatment with sirtinol, the effects of Acrp30 stimulation were blocked, e.g., Acrp30 did not affect ARNT expression or FLG expression (Fig. 2). This finding provides partial evidence for the role of SIRT1 in the regulation of FLG expression.
siRNA studies provided strong evidence for our hypothesis. In particular, NHEKs were treated with SIRT1-targeting siRNA for 48 h to knockdown SIRT1 expression. siRNA-mediated silencing of SIRT1 suppressed ARNT and FLG expression and blocked Acrp30 stimulation, whereas Acrp30 treatment of scrambled siRNA-treated cells resulted in upregulation of SIRT1, ARNT, and FLG (Fig. 3). In addition, ARNT-targeting siRNA-treated cells exhibited reduced FLG expression and blocked Acrp30 stimulation, which is consistent with our SIRT1 siRNA data (Fig. 4). Therefore, these results provide strong evidence that Acrp30-mediated upregulation of FLG is SIRT1-dependent. ARNT has been shown to initiate signal transduction by forming a complex with AhR22; however, we found that AhR-independent ARNT signaling is related to SIRT1 and triggers FLG regulation.
The data presented here support a relationship between SIRT1 and ARNT regulation. The novel SIRT1 activator resveratrol has been shown to upregulate SIRT1 and ARNT23. Although we did not notice any correlation with FLG, other studies have established relationships between ARNT and FLG3,21.
SIRT1 has been shown to promote keratinocyte differentiation in vitro8. Other studies have established that genes related to keratinocyte differentiation, such as Filaggrin and involucrin, are regulated by ARNT21,23,24. In the present study, we showed that ARNT expression is also SIRT1-dependent.
In conclusion, our results support the hypothesis that Acrp30 regulates FLG expression through a SIRT1-dependent signal transduction pathway in human keratinocytes, thereby making a critical contribution to the permeability of the skin barrier. The mutation or silence of FLG had shown essential relationships with skin barrier disease25. Therefore, Acrp30 may thus be a useful adjunct in the treatment of skin barrier-related diseases such as atopic dermatitis or contact dermatitis.
ACKNOWLEDGMENT
This study was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2687).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kezic S, Kammeyer A, Calkoen F, Fluhr JW, Bos JD. Natural moisturizing factor components in the stratum corneum as biomarkers of Filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol. 2009;161:1098–1104. doi: 10.1111/j.1365-2133.2009.09342.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong WJ, Richardson T, Seykora JT, Cotsarelis G, Simon MC. Hypoxia-inducible factors regulate Filaggrin expression and epidermal barrier function. J Invest Dermatol. 2015;135:454–461. doi: 10.1038/jid.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ming M, Zhao B, Shea CR, Shah P, Qiang L, White SR, et al. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J Allergy Clin Immunol. 2015;135:936–945.e4. doi: 10.1016/j.jaci.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Kesimer M, Tolun G, Zheng X, Xu Q, Lu J, et al. The NAD(+)-dependent protein deacetylase activity of SIRT1 is regulated by its oligomeric status. Sci Rep. 2012;2:640. doi: 10.1038/srep00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- 8.Blander G, Bhimavarapu A, Mammone T, Maes D, Elliston K, Reich C, et al. SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol. 2009;129:41–49. doi: 10.1038/jid.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS One. 2012;7:e33433. doi: 10.1371/journal.pone.0033433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Jin T, Kim OY, Shin MJ, Choi EY, Lee SS, Han YS, et al. Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs) J Agric Food Chem. 2014;62:10468–10474. doi: 10.1021/jf502849j. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Gauthier MS, Sun L, Ruderman N, Lodish H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 2010;24:4229–4239. doi: 10.1096/fj.10-159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010;59:551–553. doi: 10.2337/db09-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 17.Kovács D, Lovászi M, Póliska S, Oláh A, Bíró T, Veres I, et al. Sebocytes differentially express and secrete adipokines. Exp Dermatol. 2016;25:194–199. doi: 10.1111/exd.12879. [DOI] [PubMed] [Google Scholar]
- 18.Costa Cdos S, Rohden F, Hammes TO, Margis R, Bortolotto JW, Padoin AV, et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1-3 mRNA expression in human visceral adipocytes. Obes Surg. 2011;21:356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- 19.Naowaboot J, Chung CH, Choi R. Rutin stimulates adipocyte differentiation and adiponectin secretion in 3T3-L1 adipocytes. J Med Assoc Thai. 2015;98(Suppl 3):S1–S6. [PubMed] [Google Scholar]
- 20.Furue M, Takahara M, Nakahara T, Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res. 2014;306:769–779. doi: 10.1007/s00403-014-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917–927. doi: 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J Dermatol Sci. 2014;75:16–23. doi: 10.1016/j.jdermsci.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer M, Farwanah H, Willrodt AH, Huebner AJ, Sandhoff K, Roop D, et al. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol Med. 2012;4:364–379. doi: 10.1002/emmm.201200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EJ, Jeong MS, Li K, Park MK, Lee MK, Yoon Y, et al. Genetic polymorphism of FLG in Korean ichthyosis vulgaris patients. Ann Dermatol. 2011;23:170–176. doi: 10.5021/ad.2011.23.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]