Abstract
Background
Problems of skin aging and its prevention currently attract increasing attention with the growth of human life expectancy. The morphology of the stratum corneum (SC) is well known, but investigation of age-related changes of its structure is difficult in the absence of non-invasive sampling methods. The residual skin surface components (RSSC) that overlay the SC can be easily collected non-invasively.
Objective
The aim of this study was to examine morphology of RSSC samples collected from the surface of facial skin of healthy female volunteers of different age.
Methods
RSSC samples were non-invasively collected from 53 adult female volunteers (22 aged in the range 18∼25 years and 31 aged in the range 50∼73 years). The samples were analysed microscopically.
Results
Distinct age-related changes were determined for lipid droplet size, corneocyte desquamation level and lipid crystal count. There was a significant (p=0.0006) decrease in lipid droplet size among older women. Similarly, significantly (p=0.0401) lower lipid crystal numbers were present in the older group. Conversely, corneocyte desquamation was significantly higher (p=0.0007) in older women. No age-related difference in microbial presence in the RSSC could be detected. Result patterns were generally similar to those previously found in male volunteers; however gender-related differences in the absolute values were revealed.
Conclusion
Non-invasively collected RSSC samples allow identifying age-related changes on facial skin surface. The results of this study highlight gender-dependence of distinct elements of age-associated impairment of epidermal barrier and can be employed for developing new approaches to prevent changes associated with skin aging.
Keywords: Aging, Corneocyte desquamation, Lipids, Skin surface, Women
INTRODUCTION
It is well proven that life expectancy all over the world is rapidly growing with the majority of babies born since the beginning of the 21st century expected to reach the age of 100 years1. Due to this demographic phenomenon the health and wellbeing of older people become increasingly important2. As women are known to have longer average life expectancies, it appears that problems of post-menopausal period will attract more close attention of health-care professionals. Amongst these problems skin aging becomes a prominent global challenge3,4.
Natural skin aging process results in the loss of elasticity, dryness, fine wrinkling, atrophy and laxity5, and two major mechanisms contributing to the development of these changes are: (a) true biological (intrinsic) aging and (b) environment-dependent extrinsic aging caused by exposures to sunlight-related ultraviolet radiation (often referred to as photoaging), smoking and air pollution5,6,7. Skin aging in women starts after the age of 30 years8 and is further accelerated in the menopause, apparently due to decreasing levels of estrogens9,10. Age-related skin changes cause not only visible signs of aging, but also structural and functional impairments that may negatively affect quality of life. For this reason skin aging prevention and aged skin rejuvenation are becoming areas of active research5. Such treatments as hormone replacement therapy9,10 and local application of retinoids5 were shown to be efficient, and it is becoming clear that a number of dietary ingredients can prevent or even reverse age-related skin changes11,12.
Although it is important to understand physiological mechanisms of these beneficial effects, the use of invasive methods of skin sampling is hardly possible, especially for facial skin investigation. It should also be taken into account that the skin is a multilayered structure, the epidermis being its superficial layer, and the outermost component of the epidermis, the stratum corneum (SC), exerts most of the key barrier functions affected by aging13,14. The SC is known to be covered with a thin layer of semi-liquid substance presenting a mixture of lipid-rich sebum secreted by the sebaceous glands, lipids produced by the epidermal cells, desquamated corneocytes and sweat15. This substance generally corresponds to the “acid mantle”, which is believed to be one of the key factors in supporting skin barrier homeostasis, SC integrity and its antimicrobial defence16,17. Given obviously complex composition of this material, it may; however, be more correct to use the term residual skin surface components (RSSC) as suggested by Shetage et al.15.
We have recently devised a simple method for non-invasively collecting samples of the RSSC and characterized morphological features of this material collected in a pilot study involving male volunteers of different age18. The successful use of the new collection method encouraged us to extend its use to testing female volunteers as well. The present paper describes this work and compares its results to our earlier observations made in male subjects.
MATERIALS AND METHODS
Study protocol
This study continued a pilot project conducted jointly by Lycotec Ltd. (Cambridge, UK) and the Institute of Cardiology of the Ministry of Health of the Russian Federation (Saratov, Russian Federation). The enrolment of study participants was carried out in Saratov, from the existing pool of healthy volunteers. Study protocol was approved by the local Ethics Committee (FGBU SarNIIK 18.02.2014) guaranteeing that the study conformed to the European Medicines Agency Guidelines for Good Clinical Practice. Written informed informed consent was provided by all volunteers who agreed to participate in the study. All samples collected by study participants were analyzed in the laboratory of Lycotec Ltd. in Cambridge, UK.
Inclusion and exclusion criteria
The following inclusion criteria were applied to all individuals volunteering to take part in the present study:
Only healthy adult Caucasian females were considered for recruitment. The study included two distinct age groups: Group Y (young adult women, 18∼25 years of age; mean=21.6; standard deviation [SD]=2.4) and Group O (older [postmenopausal] women, 50∼73 years of age; mean=62.4; SD=6.7). Only volunteers who did not have systemic chronic diseases and disorders involving skin (e.g., psoriasis, pronounced acne, allergic skin conditions etc.) were enrolled in the study. Volunteers receiving on-going medication potentially affecting skin (e.g., hormonal treatments or hormone replacement therapy) were not included in the study. Only postmenopausal women were recruited in the Group O. The total number of recruited volunteers was 53 (22 women in the Group Y and 31 women in the Group O).
Sample collection and preparation
All participants of the study were requested to avoid facial hygienic manipulations for 24 hours before material collection, which was carried out in the morning. Volunteers of the younger age group (Group Y) were instructed to collect samples in the middle of the menstrual cycle (day 13∼15).
Sample collection and preparation was performed according to the procedures described in our previous paper18. Briefly, samples of the RSSC were collected using polyester swabs from the surface of the facial skin (the sides of the nose). During the procedure two samples were taken (one swab per side). Following collection each sample was placed on the surface of a microscope slide. A second microscope slide was pressed against the surface of the first one, thus providing a pair of identical smears. No fixation was applied to the smears. All slides with collected samples (i.e., four slides per study participant) were coded to provide sample anonymity for blinded analysis. Once material collections were completed, all samples were sent to the laboratory of Lycotec Ltd. for further processing and eventual microscopic examination.
Sample analysis
Once in the laboratory, one slide of the first pair was stained with H&E in order to identify any cells or cell remnants that could be present. The second slide was stained with Oil Red O (lipid stain, ab150678; Abcam, Cambridge, UK) for lipid visualization and lipid droplet size evaluation. One slide from the other pair was stained with crystal violet solution (gram staining) to assess the level of microorganism presence, while the remaining slide was kept unstained for possible future use.
All stained smears were subjected to microscopic examination by an experienced cytopathologist. Microscopy was performed using Olympus BX41 laboratory microscope (Olympus, Tokyo, Japan) at ×1,000 magnification. The analysis included examining 40 fields of view within one smear. Dark field microscopy was applied for lipid crystal visualization. Photomicrographs were prepared using Olympus DP71 camera.
Typical structural elements of the RSSC comprising lipid droplets, characteristic crystals, desquamated corneocytes and bacterial presence were analysed as previously described18. Measured parameters comprised lipid droplet size, numbers of desquamated corneocytes and lipid crystals and bacterial presence estimates (using Bacterial Presence Assessment Scale as described before18).
The indicated micro objects/structures were quantified using CellB imaging software (Digital Imaging Solutions; Olympus).
All microscopic examinations were performed blindly.
Data analysis
Results of all quantitative measurements (or counting) obtained during microscopy of the smears (lipid droplet size, numbers of desquamated corneocytes and lipid crystals, bacterial presence degree) were compared between the Groups Y and O using descriptive statistics. Mean, SD, median, and range values as well as 95% confidence intervals were determined. T-test (two-sided p-values calculated) was applied to determine statistical significance of the differences between the groups. Scatter diagrams were employed for presenting individual results for all measurements. Bar charts were used for presenting comparisons of group means. In addition, the outcome of this study was compared to the results of our previous study that produced similar parameters for male volunteers of comparable age groups18. All data handling and statistical analyses were carried out using IBM SPSS ver. 19.0 statistical package (IBM Co., Armonk, NY, USA).
RESULTS
We have provided a general outline of RSSC morphology in our previous paper focused on male subjects 18, and examination of samples collected from female volunteers has not revealed any qualitative differences deserving special attention. Briefly, lipid droplets of varying size detectable by Oil Red staining were usually abundant in these samples. Desquamated corneocytes (single or clusters) were commonly found as well. Similarly to our findings in males18, we could observe undissolvable crystals (likely to be lipid-derived) in most, but not all samples. Likewise, microorganism presence was evident in all samples. Quantitative analysis of these structures allowed detecting a number of age- and sex-dependent features that are considered in more detail below.
Lipid droplet size
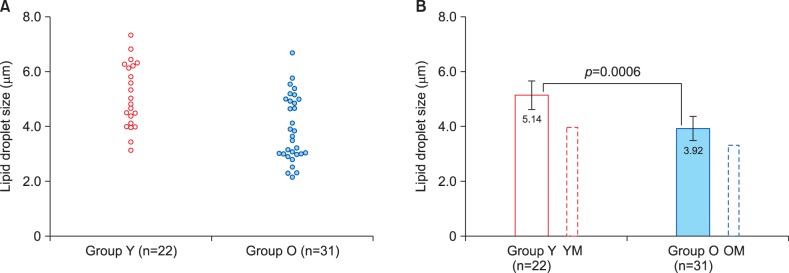
Fig. 1 presents results of lipid droplet size measurements in the two groups of female volunteers. Individual result distributions within the groups (Fig. 1A) demonstrate that lipid droplets tended to be larger in younger women (Group Y). Comparison of mean lipid droplet size values (Fig. 1B) has confirmed this trend demonstrating a highly significant difference between the age groups (p=0.0006). Fig. 1 also provides a comparison of mean lipid droplet size values in women with our findings in men published earlier18. It is interesting to note that, whereas the same age-related trend was present in the both genders, lipid droplets in female subjects tended to be larger than in men (Table 1, 2).
Fig. 1. Lipid droplet size measurement results in the two study groups. Group Y: young adult women, 18∼25 years of age, Group O: older (postmenopausal) women, 50∼73 years of age. (A) Scatter diagram showing individual result distribution. (B) Bar chart demonstrating group means and their comparison using t-test. Additional bars outlined by dotted lines show results for relevant male groups obtained in our previous study18. Error bars present 95% confidence intervals. YM: young adult men, OM: older men.
Table 1. Comparison of morphological parameters of residual skin surface components in young female and male volunteers.
| Variable | Female (n=22) | Male (n=34) | t-test | p-value |
|---|---|---|---|---|
| Lipid droplet size (µm) | 5.14±1.16 | 3.96±0.97 | 4.0967 | 0.0001 |
| Desquamated corneocyte (n) | 71.59±21.51 | 138.79±77.97 | −3.9354 | 0.0002 |
| Lipid crystal count (n) | 7.59±6.45 | 5.03±5.13 | 1.6491 | 0.1049 |
| Microbial presence (BPAS) (°) | 1.87±0.52 | 1.94±0.52 | −0.4951 | 0.6226 |
Values are presented as mean±standard deviation.
Table 2. Comparison of morphological parameters of residual skin surface components in older female and male volunteers.
| Variable | Female (n=31) | Male (n=26) | t-test | p-value |
|---|---|---|---|---|
| Lipid droplet size (µm) | 3.92±1.20 | 3.24±0.92 | 2.3098 | 0.0247 |
| Desquamated corneocyte (n) | 119.03±58.43 | 179.15±86.83 | −3.1085 | 0.0030 |
| Lipid crystal count (n) | 4.48±4.30 | 2.46±2.83 | 2.0536 | 0.0448 |
| Microbial presence (BPAS) (o) | 1.98±0.43 | 2.54±0.90 | −3.0765 | 0.0033 |
Values are presented as mean±standard deviation.
Corneocyte desquamation
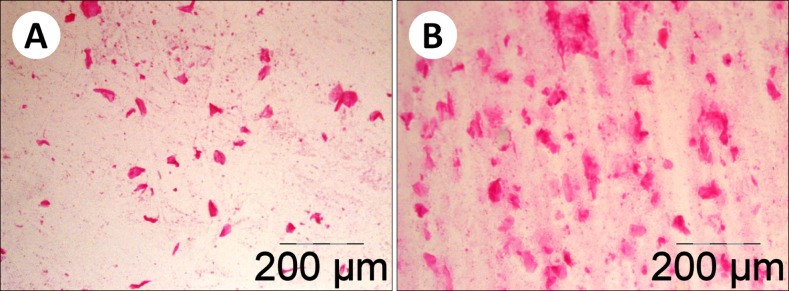
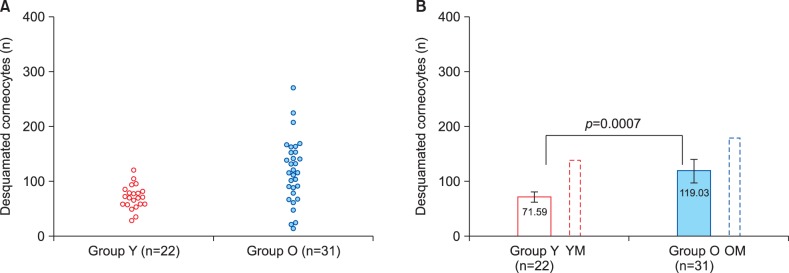
Microscopic assessment of corneocyte desquamation showed that it levels in most samples obtained from postmenopausal women (Group O) were visibly higher than in the younger group (Group Y). This difference is demonstrated by Fig. 2 that shows smears obtained from a young volunteer (Fig. 2A) and an older woman (Fig. 2B). Quantification of desquamated corneocyte numbers convincingly confirmed this initial impression. Fig. 3 demonstrates quantitative results of this part of the study. Although inter-individual variation was relatively high among postmenopausal women (Group O), result distribution in this group was clearly shifted upwards compared to the Group Y (Fig. 3A). Comparison of the mean numbers of desquamated corneocytes (Fig. 3B) has indicated that the mean level of desquamation in postmenopausal women was by 66% higher than in the younger group. T-test has revealed a highly significant difference between the age groups (p=0.0007). Again, the general pattern of age-dependent differences was similar in female and previously investigated male volunteers18, but absolute desquamation levels tended to be considerably higher in men (Table 1, 2). Nevertheless, the difference between older and younger men18 appeared to be less pronounced than between older and younger women (Fig. 3B).
Fig. 2. Desquamated corneocytes observed in Group Y (young adult women, 18∼25 years of age) (A) and Group O (older [postmenopausal] women, 50∼73 years of age) (B) volunteers (H&E).
Fig. 3. Desquamated corneocyte counts in the two study groups. Group Y: young adult women, 18∼25 years of age, Group O: older (postmenopausal) women, 50∼73 years of age. (A) Scatter diagram showing individual result distribution. (B) Bar chart demonstrating group means and their comparison using t-test. Additional bars outlined by dotted lines show results for relevant male groups obtained in our previous study18. Error bars present 95% confidence intervals. YM: young adult men, OM: older men.
Lipid crystal numbers
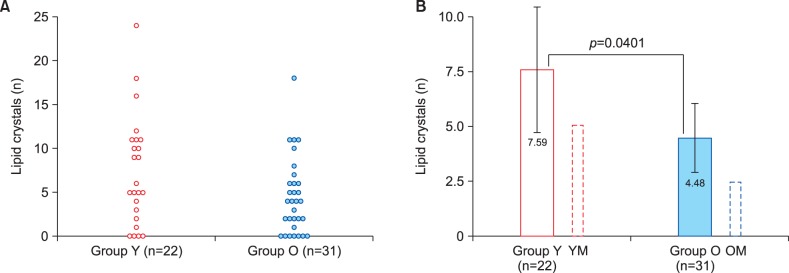
As in our previous study in male volunteers18, crystal structures could be identified in RSSC samples from the majority of female volunteers. Fig. 4A shows that crystals could not be found in 4 subjects of the Group Y and 7 subjects of the Group O. While amongst younger women there was a wide variation of lipid crystal numbers, only one subject of the Group O had more than 11 crystals found (Fig. 4A). Mean lipid crystal number was higher in the Group Y (Fig. 4B), but the difference between the groups was only marginally significant (p=0.0401) because of high inter-individual variability. Age-related pattern of lipid crystal presence was, again, similar between males and females, but absolute values tended to be higher in female volunteers (Fig. 4B, Table 1, 2).
Fig. 4. Lipid crystal counts in the two study groups. Group Y: young adult women, 18∼25 years of age, Group O: older (postmenopausal) women, 50∼73 years of age. (A) Scatter diagram showing individual result distribution. (B) Bar chart demonstrating group means and their comparison using t-test. Additional bars outlined by dotted lines show results for relevant male groups obtained in our previous study18. Error bars present 95% confidence intervals. YM: young adult men, OM: older men.
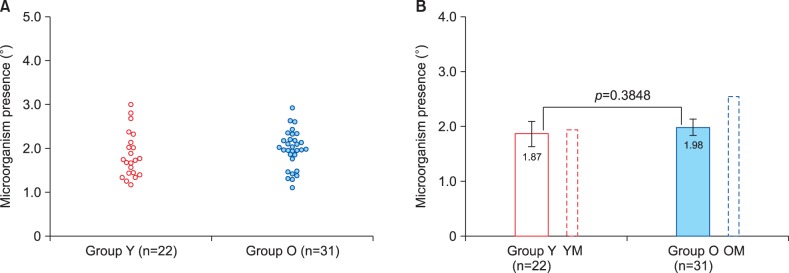
Microbial presence in the residual skin surface components
The outcome of semi-quantitative analysis of the microbial presence in RSSC samples from female volunteers is presented in Fig. 5. It is obvious that both result distributions and mean values of microorganism presence degree were remarkably similar in the two age groups. However, comparison of these results with the outcome of our previous study in male volunteers18 has shown that estimated microorganism presence was at the same level in young men, but significantly higher in older men (Fig. 5B, Table 2).
Fig. 5. Microbial presence estimates (according to BPAS) in the two study groups. Group Y: young adult women, 18∼25 years of age, Group O: older (postmenopausal) women, 50∼73 years of age. (A) Scatter diagram showing individual result distribution. (B) Bar chart showing group means and their comparison using t-test. Additional bars outlined by dotted lines show results for relevant male groups obtained in our previous study18. Error bars present 95% confidence intervals. YM: young adult men, OM: older men.
Comparison of residual skin surface components characteristics in female and male volunteers
Although results obtained in male volunteers were reported in our previous publication18, the sample collection technique used in the present study was identical compared with the previous one. Given that age characteristics of the two compared groups were also similar to those investigated in males, it was logical to compare gender-related differences in the outcomes of the two studies. Table 1 and 2 compare results obtained in younger and older subjects respectively.
Table 1 demonstrates that lipid droplet size in young women was significantly (p=0.0001) higher than in young men. In contrast, young men had a much higher level of corneocyte desquamation (p=0.0002) compared to young women. Lipid crystal count appeared to be higher in young women, but the difference was marginally insignificant. At the same time microbial presence level in young subjects was not gender-dependent.
Comparison of female and male subjects of older age (Table 2) revealed a generally similar pattern for lipid droplet size (significantly higher in women) and corneocyte desquamation (significantly higher in men). However, lipid crystal count was significantly higher (p=0.0448) in older female volunteers, whereas microbial presence was significantly higher (p=0.0033) in older men.
DISCUSSION
The results described in this paper complement our previous study performed in male volunteers18 and further confirm that material collected non-invasively by swabbing the surface of human facial skin may be successfully used for investigating morphological characteristics of the RSSC. It is also important to note that the outcome of this study further demonstrates obvious parallelism of age-related changes in RSSC components in male and female subjects.
Our previous study18 has demonstrated that the RSSC is a substance very rich in lipids (most probably originating from the sebum) that often contains crystals formerly described only in extracts from acne comedones19. This lipid film overlaying the SC apparently contributes to protective functions of the skin barrier, such as water retention and resistance to oxidation20,21.The observed age-dependent differences in lipid droplet size and lipid crystal numbers described in our previous paper18 and corroborated by the present study appear to indicate either changes in physicochemical properties of skin surface lipids or more active lipid production by the sebaceous glands of young individuals, both women and men. These findings confirm reports of other authors on more pronounced lipid presence on the surface of the epidermis of younger people22,23,24. This abundance is inevitably followed by an age-dependent decline described for individuals of the both genders22,23,24. It is, however, likely that mechanisms of this phenomenon are sex-dependent are. In young women high estrogen level presents a major anti-ageing factor9,25 that disappears in the postmenopausal period unless hormone replacement therapy is applied26. Indeed, skin is a hormone-sensitive organ. The presence of estrogen receptors in the human skin, in particular keratinocytes and sebocytes, is a well proven fact9,25,27, and experimental studies showed that age-related decrease in the amount of estrogen receptor β is likely to be one of the key factors in photoaging28. Likewise, in male individuals androgens are known to stimulate sebum production29, and facial skin has the highest level of androgen receptors30.
Desquamated keratinocytes that are permanently released from the surface of the SC constitute another informative component of the RSSC. Results of this study alongside with our previous observations18 clearly indicate that desquamation levels increase with age in individuals of both genders. Our findings indirectly confirm earlier results of Chu and Kollias31 who concluded that the adherence of corneocytes to the SC weakens with age resulting in desquamation facilitation. However, significantly higher desquamation levels observed in men suggest that sex-specific hormonal influences are likely to affect this physiological phenomenon as well. One of possible mechanisms limiting corneocyte desquamation in women may be related to the intracrine production of estrogens in keratinocytes27,32, which is known to decline with age33, but may be at least partially preserved in postmenopausal women.
The determination of microbial presence in the RSSC undertaken in this study has not revealed any age-associated differences in women of different age groups. In contrast, our previous work indicated that older men had significantly higher microbial presence on the surface of their facial skin. We earlier suggested that it might happen in older people due to age-related impairment of the immune system34, but the absence of this phenomenon in older female volunteers suggests that this explanation may not be entirely applicable to this group, and other factors, especially sex-specific hormonal influences, are likely to influence this component of skin homeostasis as well.
Although some of our findings do not seem to entirely agree with earlier observations of Luebberding et al.35 who described essentially dissimilar ageing-dependent changes in SC hydration and sebum production in men and women, it should be noted that different analytical methods and measurable parameters applied in the two studies could be behind these discrepancies.
It can be concluded that this study has confirmed our previous findings18 that indicated that morphological investigation of RSSC samples presents a useful tool for studying age-related changes of human skin. Age-specific alteration patterns revealed in female volunteers were in general similar to those formerly observed in men; however quantitative levels of lipid-related features and corneocyte exfoliation significantly differed between females and males, most probably suggesting strong hormonal influences modulating skin ageing in individuals of different genders.
The results of this study highlight gender-dependence of distinct elements of age-associated decline in the efficiency of the epidermal barrier. We believe that our findings can be employed for developing new efficient approaches to skin ageing prevention.
ACKNOWLEDGMENT
Volunteers who took part in the study are thanked for their cooperation. Dr Alexandre Loktionov (DiagNodus Ltd., Cambridge, UK) is thanked for his critical advice and help in manuscript preparation.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP. Investing in health to create a third demographic dividend. Gerontologist. 2016;56(Suppl 2):S167–S177. doi: 10.1093/geront/gnw035. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Peytavi U, Kottner J, Sterry W, Hodin MW, Griffiths TW, Watson RE, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016;56(Suppl 2):S230–S242. doi: 10.1093/geront/gnw003. [DOI] [PubMed] [Google Scholar]
- 4.Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care (New Rochelle) 2013;2:5–10. doi: 10.1089/wound.2011.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Nuaimi Y, Sherratt MJ, Griffiths CE. Skin health in older age. Maturitas. 2014;79:256–264. doi: 10.1016/j.maturitas.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 7.Vierkötter A, Krutmann J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinol. 2012;4:227–231. doi: 10.4161/derm.19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwazuru O, Miyamoto K, Yoshikawa N, Imayama S. Skin wrinkling morphology changes suddenly in the early 30s. Skin Res Technol. 2012;18:495–503. doi: 10.1111/j.1600-0846.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson S, Thornton J. Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging. 2007;2:283–297. doi: 10.2147/cia.s798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmerson E, Hardman MJ. The role of estrogen deficiency in skin ageing and wound healing. Biogerontology. 2012;13:3–20. doi: 10.1007/s10522-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 11.Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51–54. doi: 10.1111/j.1473-2165.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins G, Wainwright LJ, Holland R, Barrett KE, Casey J. Wrinkle reduction in post-menopausal women consuming a novel oral supplement: a double-blind placebo-controlled randomized study. Int J Cosmet Sci. 2014;36:22–31. doi: 10.1111/ics.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 14.Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm. 2012;435:3–9. doi: 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Shetage SS, Traynor MJ, Brown MB, Raji M, Graham-Kalio D, Chilcott RP. Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Skin Res Technol. 2014;20:97–107. doi: 10.1111/srt.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 17.Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93:261–267. doi: 10.2340/00015555-1531. [DOI] [PubMed] [Google Scholar]
- 18.Chalyk NE, Bandaletova TY, Kyle NH, Petyaev IM. Age-related differences in morphological characteristics of residual skin surface components collected from the surface of facial skin of healthy male volunteers. Skin Res Technol. 2017;23:212–220. doi: 10.1111/srt.12321. [DOI] [PubMed] [Google Scholar]
- 19.González-Serva A, Kroumpouzos G. Demonstration of polarizable crystals in fresh comedonal extracts: sebum crystallizes. Acta Derm Venereol. 2004;84:418–421. doi: 10.1080/00015550410018370. [DOI] [PubMed] [Google Scholar]
- 20.Tóth BI, Oláh A, Szöllosi AG, Czifra G, Bíró T. “Sebocytes' makeup”: novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflugers Arch. 2011;461:593–606. doi: 10.1007/s00424-011-0941-6. [DOI] [PubMed] [Google Scholar]
- 21.Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L. The antioxidant network of the stratum corneum. Curr Probl Dermatol. 2001;29:26–42. doi: 10.1159/000060651. [DOI] [PubMed] [Google Scholar]
- 22.Passi S, De Pità O, Puddu P, Littarru GP. Lipophilic antioxidants in human sebum and aging. Free Radic Res. 2002;36:471–477. doi: 10.1080/10715760290021342. [DOI] [PubMed] [Google Scholar]
- 23.Luebberding S, Krueger N, Kerscher M. Age-related changes in skin barrier function - quantitative evaluation of 150 female subjects. Int J Cosmet Sci. 2013;35:183–190. doi: 10.1111/ics.12024. [DOI] [PubMed] [Google Scholar]
- 24.Boireau-Adamezyk E, Baillet-Guffroy A, Stamatas GN. Age-dependent changes in stratum corneum barrier function. Skin Res Technol. 2014;20:409–415. doi: 10.1111/srt.12132. [DOI] [PubMed] [Google Scholar]
- 25.Shah MG, Maibach HI. Estrogen and skin. An overview. Am J Clin Dermatol. 2001;2:143–150. doi: 10.2165/00128071-200102030-00003. [DOI] [PubMed] [Google Scholar]
- 26.Piérard GE, Humbert P, Berardesca E, Gaspard U, Hermanns-Lê T, Piérard-Franchimont C. Revisiting the cutaneous impact of oral hormone replacement therapy. Biomed Res Int. 2013;2013:971760. doi: 10.1155/2013/971760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KC, Wang Y, Oh IG, Jenkins S, Freedman LP, Thompson CC, et al. Estrogen receptor beta is a novel therapeutic target for photoaging. Mol Pharmacol. 2010;77:744–750. doi: 10.1124/mol.109.062877. [DOI] [PubMed] [Google Scholar]
- 29.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 30.Seo YJ, Li ZJ, Choi DK, Sohn KC, Kim HR, Lee Y, et al. Regional difference in sebum production by androgen susceptibility in human facial skin. Exp Dermatol. 2014;23:70–72. doi: 10.1111/exd.12291. [DOI] [PubMed] [Google Scholar]
- 31.Chu M, Kollias N. Documentation of normal stratum corneum scaling in an average population: features of differences among age, ethnicity and body site. Br J Dermatol. 2011;164:497–507. doi: 10.1111/j.1365-2133.2010.10120.x. [DOI] [PubMed] [Google Scholar]
- 32.Pomari E, Dalla Valle L, Pertile P, Colombo L, Thornton MJ. Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts. FASEB J. 2015;29:508–524. doi: 10.1096/fj.14-251363. [DOI] [PubMed] [Google Scholar]
- 33.Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, et al. The role of estrogen-metabolizing enzymes and estrogen receptors in human epidermis. Mol Cell Endocrinol. 2011;344:35–40. doi: 10.1016/j.mce.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63:776–781. doi: 10.1111/jgs.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013;35:477–483. doi: 10.1111/ics.12068. [DOI] [PubMed] [Google Scholar]