Abstract
Hepatic fibrosis occurs during the progression of primary sclerosing cholangitis (PSC) and is characterized by accumulation of extracellular matrix proteins. Proliferating cholangiocytes and activated hepatic stellate cells (HSCs) participate in the promotion of liver fibrosis during cholestasis. Gonadotropin-releasing hormone (GnRH) is a trophic peptide hormone synthesized by hypothalamic neurons and the biliary epithelium and exerts its biological effects on cholangiocytes by interaction with the receptor subtype (GnRHR1) expressed by cholangiocytes and HSCs. Previously, we demonstrated that administration of GnRH to normal rats increased intrahepatic biliary mass (IBDM) and hepatic fibrosis. Also, miR-200b is associated with the progression of hepatic fibrosis; however, the role of the GnRH/GnRHR1/miR-200b axis in the development of hepatic fibrosis in PSC is unknown. Herein, using the mouse model of PSC (multidrug resistance gene 2 knockout), the hepatic knockdown of GnRH decreased IBDM and liver fibrosis. In vivo and in vitro administration of GnRH increased the expression of miR-200b and fibrosis markers. The GnRH/GnRHR1 axis and miR-200b were up-regulated in human PSC samples. Cetrorelix, a GnRHR1 antagonist, inhibited the expression of fibrotic genes in vitro and decreased IBDM and hepatic fibrosis in vivo. Inhibition of miR-200b decreased the expression of fibrosis genes in vitro in cholangiocyte and HSC lines. Targeting the GnRH/GnRHR1/miR-200b axis may be key for the management of hepatic fibrosis during the progression of PSC.
In addition to secretion of water and electrolytes to modify the composition of ductal bile before it reaches the duodenum, cholangiocytes are the target cells of a number of cholangiopathies, including primary sclerosing cholangitis (PSC) and primary biliary cholangitis.1, 2 A number of neuroendocrine factors regulate the homeostasis of the biliary epithelium through changes in the balance between biliary proliferation and apoptosis (hallmarks of cholangiopathies), leading to changes in liver fibrosis.3, 4, 5, 6, 7 PSC is a chronic disease that affects the biliary epithelium, causing biliary proliferation, inflammation, fibrosis, liver cirrhosis, and ultimately death.1, 2, 7 Therapy for the management of PSC is currently lacking because of the poor knowledge of the pathogenesis of this disease; therefore, studies aimed to better understand the molecular mechanisms underlying PSC pathogenesis are necessary. The multidrug resistance gene 2 knockout (Mdr2−/−; official gene name Abcb4) mouse model is widely used for studying the mechanisms of the pathogenesis and management of PSC.4, 8, 9, 10 The Mdr2−/− mouse serves as a genetic model of progressive PSC coupled with biliary fibrosis.4, 8, 9
The peptide gonadotropin-releasing hormone (GnRH; ie, synthesized and released from neurons within the hypothalamus)11 exerts its effects by interaction with both receptor subtypes, GnRHR1 and GnRHR2, that are also expressed in peripheral organs, such as pancreas, colon, and liver.12, 13 We have previously shown that GnRH induces biliary hyperplasia in normal and cholestatic bile duct–ligated rats by both paracrine/autocrine mechanisms by a specific interaction with the receptor subtype, GnRHR1.14 Supporting the selective role of GnRHR1 on biliary functions, in mammals GnRHR2 transcription occurs but it does not produce a functional C-terminal multitransmembrane protein.15 No data exist regarding the role of the GnRH/GnRHR1 axis in models of PSC and human PSC samples.
miRNAs are small noncoding RNA molecules that regulate a number of pathophysiological processes, including cell proliferation/apoptosis, stem cell differentiation, and the progression of cholestatic liver disease.16, 17 miR-200 is a highly conserved family of miRNAs that regulate cellular proliferation and remodeling during liver injury, fibrosis, and hepatocellular carcinoma.18, 19, 20, 21 The miR-200 family includes miR-200a, miR-200b, miR-200c, miR-141, and miR-429. A number of studies have demonstrated the role of miR-200b in the management of liver diseases, including cholangiopathies and hepatic fibrosis.22, 23, 24 For example, one study has shown that miR-200b is overexpressed in malignant cholangiocytes and its inhibition increases sensitivity to chemotherapeutic agents, such as gemcitabine.22 Furthermore, miR-200b is up-regulated in patients with biliary atresia and accelerates the activation and migration of hepatic stellate cells (HSCs).24 Moreover, another study has demonstrated that the progression of liver fibrosis in humans and mice positively correlates with overexpression of the miR-200b.23 No information exists regarding the role of miR-200b in the modulation of biliary damage and liver fibrosis in PSC. On the basis of this background, we hypothesized that GnRH/GnRHR1 stimulates biliary proliferation and liver fibrosis by both autocrine/paracrine pathways (by coordinately activating cholangiocytes and HSCs) in Mdr2−/− mice and human PSC through changes in miR-200b.
Materials and Methods
Reagents
Reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise indicated. The rat antibody against cytokeratin-19 (CK-19) was obtained from Developmental Studies Hybridoma Bank (Iowa City, IA). The rabbit synaptophysin, the goat monoclonal antibody against hepatocyte nuclear factor-4α, and the anti-desmin antibody (Y66; Alexa Fluor 488) were purchased from Abcam (Cambridge, MA). The rabbit antibodies for GnRHR1 and GnRH were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). The Nova Ultra Sirius Red Stain kit to detect interstitial collagen deposition was purchased from IHC World (Woodstock, MD). The enzyme immunoassay kits to measure GnRH levels in supernatants from isolated cholangiocytes (after incubation for 4 hours at 37°C)6 as well as serum from healthy control and early- and late-stage male PSC patients were purchased from Phoenix Pharmaceutical Inc. (Burlingame, CA). All real-time PCR primers and the RNeasy kit for the purification of total RNA and reagents for real-time PCRs were obtained from Qiagen (Valencia, CA). We used the following mouse primers: GnRHR1 (PPM04852B); GnRH (PPM57676A); proliferating cell nuclear antigen (PCNA; PPM03456F); Ki-67 (PPM03457B); transforming growth factor-β1 (TGF-β1; PPM02991B); TGF-β1 receptor (TGF-β1R; PPM03072C); Serpine1 (PPM03093C); glyceraldehyde-3-phosphate dehydrogenase (PPM02946E); and miRNA primers [miR-200b (4427975: assay002251) and U6 snRNA (4427975: assay 001973, housekeeping for miR-200b)]. For real-time PCR analysis in human hepatic stellate cell lines (HHSteCs), we used the following primers: TGF-β1 (PPH00508A), TGF-β1R (PPH00237C), matrix metalloproteinase 9 (PPH00152E), Serpine1 (PPH00215F), and glyceraldehyde-3-phosphate dehydrogenase (PPH00150F). GnRH and mismatched Vivo-Morpholinos were obtained from Gene Tools, LLC (Philomath, OR). The GnRHR1 antagonist, cetrorelix acetate (referred to as cetrorelix),14, 25 was purchased from R&D Systems (Minneapolis, MN). Control and miR-200b inhibitors were purchased from Ambion Inc. (Austin, TX).
Animal Models
FVB/NJ wild-type (WT) and Mdr2−/− mice (25 to 30 g, 12 weeks old) were purchased from Jackson Laboratories (Sacramento, CA), were housed in a temperature-controlled environment (22°C), were fed standard mice chow, and had access to drinking water ad libitum. The studies were performed in WT mice treated with saline or GnRH (250 ng/kg body weight)26, 27 by i.p. implanted Alzet osmotic minipumps for 1 week. In separate experiments, Mdr2−/− mice were treated with the following: Vivo-Morpholino sequences against GnRH (5′-GATCGTTTCCATTCTGTTTGGATGT-3′, 1 mg/kg body weight/day to reduce the hepatic expression of GnRH) or mismatch Vivo-Morpholino sequences (5′-GAACCTTTCGATTCTCTTTCGATGT-3′) administrated by two tail vein injections for 1 week14; or cetrorelix (10 mg/kg body weight/day)28 by i.p. implanted Alzet osmotic minipumps for 1 week. Before each experimental procedure, animals were treated with 200 to 250 mg/kg body weight euthasol following the regulations of the panel on euthanasia of the American Veterinary Medical Association. All animal experiments were performed in accordance with protocols approved by the Baylor Scott & White Health Institutional Animal Care and Use Committee.
Isolated Cholangiocytes and HSCs, IMCLs, and HHSteCs
Cholangiocytes were isolated by immunoaffinity separation5, 6 using a monoclonal antibody (IgM; a gift from Dr. Ronald A. Faris, Brown University, Providence, RI). Cell number and viability were assessed by trypan blue exclusion. HSCs from mouse liver were isolated by laser capture microdissection (LCM) as follows: briefly, frozen liver sections (n = 3, 10 μm thick) were incubated overnight with a desmin (marker of HSCs)29 antibody. After washes, sections were incubated with a fluorescent secondary antibody. Next, desmin-positive cells were captured from slides by the LCM system Leica LMD7 (Leica Microsystems, Buffalo Grove, IL) and collected. RNA was extracted with the Arcturus PicroPure RNA isolation kit (Thermo Fischer Scientific, Mountain View, CA). The in vitro studies were performed in immortalized murine cholangiocyte lines (IMCLs), which display morphological, phenotypic, and functional characteristics similar to those of freshly isolated mouse cholangiocytes,5, 6, 30 and in HHSteCs that were purchased from Sciencell (Carlsbad, CA).
Expression of GnRHR1 in Liver Sections, Cholangiocytes, and LCM-Isolated HSCs
The expression of GnRHR1 (the only GnRH receptor subtype that mediates GnRH effects on cholangiocytes)14 was evaluated by the following: immunofluorescence in frozen liver sections (4 to 5 μm thick) costained with CK-19 (a cholangiocyte-specific marker),31 synaptophysin (a marker of HSCs),4 or hepatocyte nuclear factor-4α (a hepatocyte marker)32; and semiquantitative immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick, 10 different fields analyzed from three samples from three different animals). We evaluated the expression of GnRHR1 by real-time PCR in total RNA (1 μg) from isolated cholangiocytes and LCM-isolated HSCs. After immunohistochemistry, sections were then observed with Leica Microsystems DM 4500 B Microscopy (Weltzlar, Germany) equipped with a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). Observations were processed with an Image Analysis System (Delta Sistemi, Rome, Italy). After immunofluorescence, sections were stained with DAPI (ThermoFisher Scientific) and analyzed by an Olympus Fluoview 300 confocal microscope (Olympus, Center Valley, PA).
Measurement of GnRH Expression in Liver Sections, Cholangiocytes, LCM-Isolated HSCs, and GnRH Levels in Cholangiocyte Supernatant and Human Serum Samples
We measured the expression of GnRH by the following: i) immunofluorescence in frozen liver sections (4 to 5 μm thick) costained with CK-19, hepatocyte nuclear factor-4α, or synaptophysin; ii) semiquantitative immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick, 10 different fields analyzed from three samples from three different animals); and iii) real-time PCR in total RNA (1 μg) from isolated cholangiocytes and LCM-isolated HSCs. To demonstrate the specificity of the immunoreactions, negative controls (the primary antibody was replaced with the same dilution with normal serum from the same species) were performed for all immunoreactions. Sections were analyzed in a coded manner using Leica Microsystems DM 4500 B Microscopy equipped with a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). Observations were processed with an Image Analysis System (Delta Sistemi). We measured the levels of GnRH in the supernatant of purified cholangiocytes as well as serum from the selected human samples using commercially available enzyme-linked immunosorbent assay assay kits following the manufacturer's instructions (IBL-America, Minneapolis, MN).
Measurement of IBDM and Liver Fibrosis
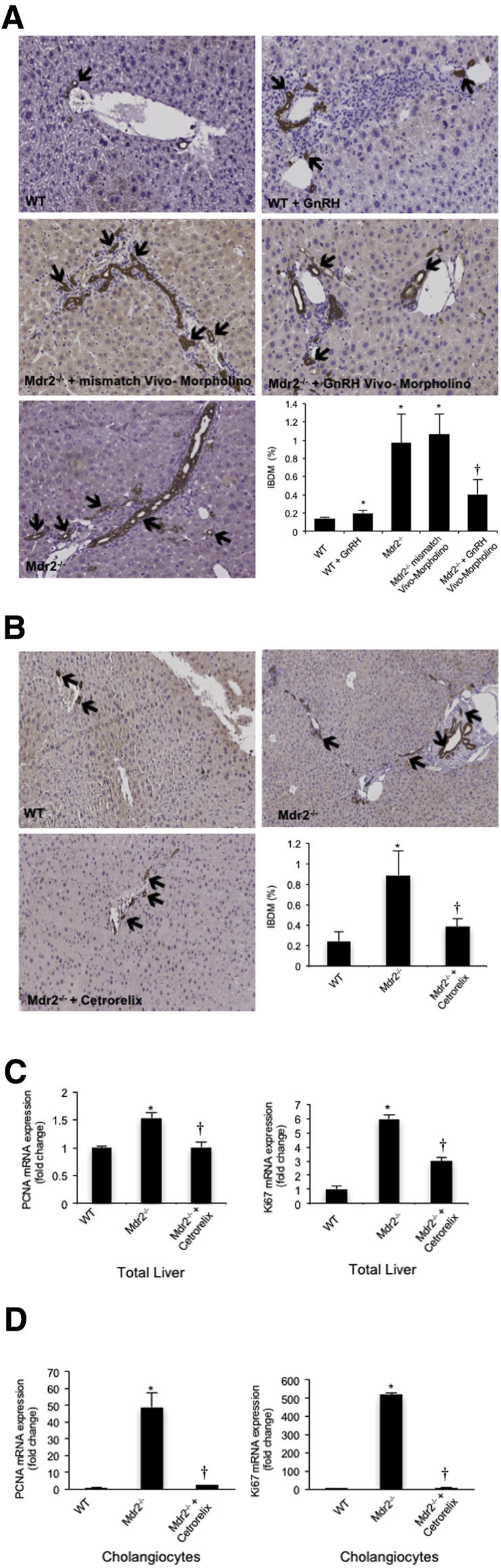
Intrahepatic bile duct mass (IBDM) in liver sections (4 to 5 μm thick, 10 different fields analyzed from three samples from three different animals) was measured by semiquantitative immunohistochemistry as the area occupied by CK-19–positive bile ducts/total area × 1005. Sections were examined using the Olympus Image Pro-Analyzer software version 7.0 (Olympus, Tokyo, Japan). We also evaluated by real-time PCR the mRNA expression of PCNA and Ki-67 in total liver and isolated cholangiocytes from WT and Mdr2−/− mice and Mdr2−/− mice treated with cetrorelix.
Hepatic fibrosis was evaluated by Sirius Red staining in paraffin-embedded liver sections (4 to 5 μm thick, 10 different fields analyzed from three samples from three different animals). Slides were scanned by a digital scanner (SCN400; Leica Microsystems, Buffalo Grove, IL) and quantified using Image-Pro Premier 9.1 (Media Cybernetics, Rockville, MD). We evaluated in isolated cholangiocytes the mRNA expression of the fibrosis genes, TGFB1, TGFB1R, and Serpine1, by real-time PCR.
Expression of miR-200b in Total Liver and Cholangiocytes
To demonstrate that the effects of GnRH on biliary proliferation and liver fibrosis are mediated by changes in biliary miR-200b expression, we evaluated by real-time PCR the expression of miR-200b in total liver and isolated cholangiocytes from WT mice treated with saline or GnRH as well as Mdr2−/− mice. The rationale for evaluating the role of miR-200b in these models is based on findings showing that the expression of miR-200b markedly increased in Mdr2−/− mice compared to WT mice as well as human PSC samples compared to healthy controls (N. Wu and G. Alpini, unpublished data).
Effect of GnRH on the Expression of miR-200b and Fibrosis Genes in Vitro
The in vitro experiments were performed in IMCLs and HHSteCs that were stimulated with 0.2% bovine serum albumin (basal) or GnRH (100 nmol/L)14 for 48 hours at 37°C in the absence or presence of 10 nmol/L cetrorelix; after stimulation, we measured the expression of miR-200b and/or PCNA, Ki-67, and the fibrosis genes, TGFB1, TGFB1R, and Serpine1, by real time-time PCR in IMCLs, and TGFB1, TGFB1R, MMP9, and Serpine1 in HHSteCs.
We evaluated the expression of GnRH in IMCLs and GnRHR1 in IMCL and HHSteC smears by immunofluorescence. In separate experiments, control (stable transfected with the empty vector) or GnRH stable-transfected IMCLs were cultured until confluency before evaluating the following: GnRH levels in cholangiocyte supernatant (after incubation for 4 hours at 37°C)6 by enzyme immunoassay kits; and the expression of miR-200b and selected fibrosis genes by real-time PCR. In separate experiments, HHSteCs were incubated for 12 hours with the supernatant of Neg- or shGnRH-transfected IMCLs (containing different levels of GnRH) before evaluating the expression of miR-200b and selected fibrosis gene factors by real-time PCR in these cells. Control IMCLs (stable transfected with the empty vector) or IMCLs lacking GnRH were established using SureSilencing shRNA (Super-Array, Frederick, MD) plasmid for mouse GnRH containing a marker for neomycin resistance for the selection of stably transfected cells, according to the instructions provided by the vendor.14 We have previously used this approach to establish IMCLs lacking the gene for secretin and its receptor.5, 6
To demonstrate a link between GnRH, miR-200b, and liver fibrosis, IMCLs and HHSteCs were treated with miR-200b/control precursor and/or antisense inhibitors and controls (Ambion, Austin, TX) in the absence or presence of 100 nmol/L GnRH for 24 hours before evaluating the expression of selected fibrosis genes by real-time PCR assay.
Measurement of GnRH and GnRHR1 Immunoreactivity and Expression of GnRH and GnRH Serum Levels in Normal and PSC Patients
Unidentified human samples collected by needle biopsies (Tables 1 and 2) were obtained from Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy) under a protocol approved by the Ethics Committee by the Humanitas Research Hospital and also reviewed by the Central Texas Veteran's Health Care System Institutional Review Board and Research & Development Committee. The protocol was also approved by the Texas A&M HSC College of Medicine Institutional Review Board.
Table 1.
Characteristics of Healthy Controls and PSC Patients
| Groups | Patient no. | Diagnosis | Sex | Cirrhosis | Therapy | Origin |
|---|---|---|---|---|---|---|
| Control | 1 | Normal liver | Male | Untreated | BioChain (Newark, CA) | |
| 2 | Normal liver | Male | Untreated | BioChain | ||
| 3 | Normal liver | Male | Untreated | BioChain | ||
| 4 | Normal liver | Male | Untreated | BioChain | ||
| PSC | 1 | Late-stage PSC |
Male | Yes | Untreated | Humanitas Research Hospital (Milan, Italy) |
| 2 | Late-stage PSC |
Male | No | Untreated | Humanitas Research Hospital | |
| 3 | Late-stage PSC |
Male | No | Untreated | Humanitas Research Hospital |
Unidentified human samples were obtained from Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy).
PSC, primary sclerosing cholangitis.
Table 2.
Characteristics of Healthy Controls and Early- and Late-Stage PSC Patients
| Groups | Patient no. | Diagnosis | Sex | Cirrhosis | Therapy | Origin |
|---|---|---|---|---|---|---|
| Control | 1 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | |
| 2 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| 3 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| 4 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| 5 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| 6 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| 7 | Serum from normal patient | Male | Untreated | Humanitas Research Hospital | ||
| Early-stage PSC | 1 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital |
| 2 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 3 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 4 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 5 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 6 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 7 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 8 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| Late-stage PSC | 1 | Serum from PSC late stage | Male | No | Untreated | Humanitas Research Hospital |
| 2 | Serum from PSC early stage | Male | No | Untreated | Humanitas Research Hospital | |
| 3 | Serum from PSC late stage | Male | No | Untreated | Humanitas Research Hospital | |
| 4 | Serum from PSC late stage | Male | No | Untreated | Humanitas Research Hospital |
Unidentified human samples were obtained from Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy).
PSC, primary sclerosing cholangitis.
We evaluated the immunoreactivity of GnRHR1 and GnRH in one liver section from a healthy male and a late-stage male PSC patient by immunohistochemistry. After staining, sections were then observed with Leica Microsystems DM 4500 B Microscopy equipped with a Jenoptik Prog Res C10 Plus Videocam. Observations were processed with an Image Analysis System (Delta Sistemi). For real-time PCR analysis, total RNA was extracted from paraffin-embedded sections from samples obtained from four normal control and three late-stage PSC patients using the RNeasy FFPE kit (73504; Qiagen, Valencia, CA). From these samples, the mRNA expression (from cDNA samples) for human GnRHR1 (NM_001012763), GnRH (NM_000825), and glyceraldehyde-3-phosphate dehydrogenase (NM_002046) was evaluated by real-time PCR using human primers purchased from Qiagen. GnRH serum levels in control (n = 7), early-stage (n = 8), and late-stage (n = 4) male PSC samples were evaluated by enzyme immunoassay.
Statistical Analysis
All data are expressed as means ± SEM. Differences between groups were analyzed by unpaired t-test when two groups were analyzed and analysis of variance when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Expression of GnRHR1
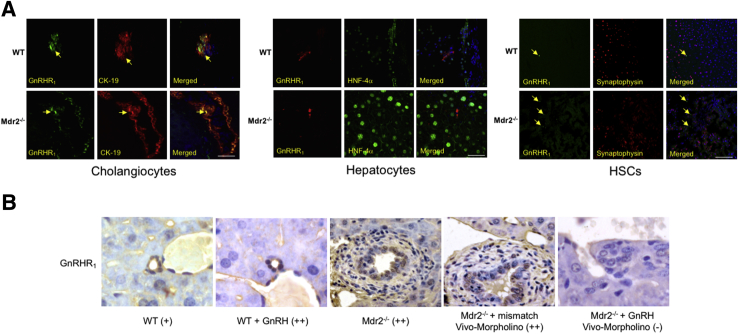
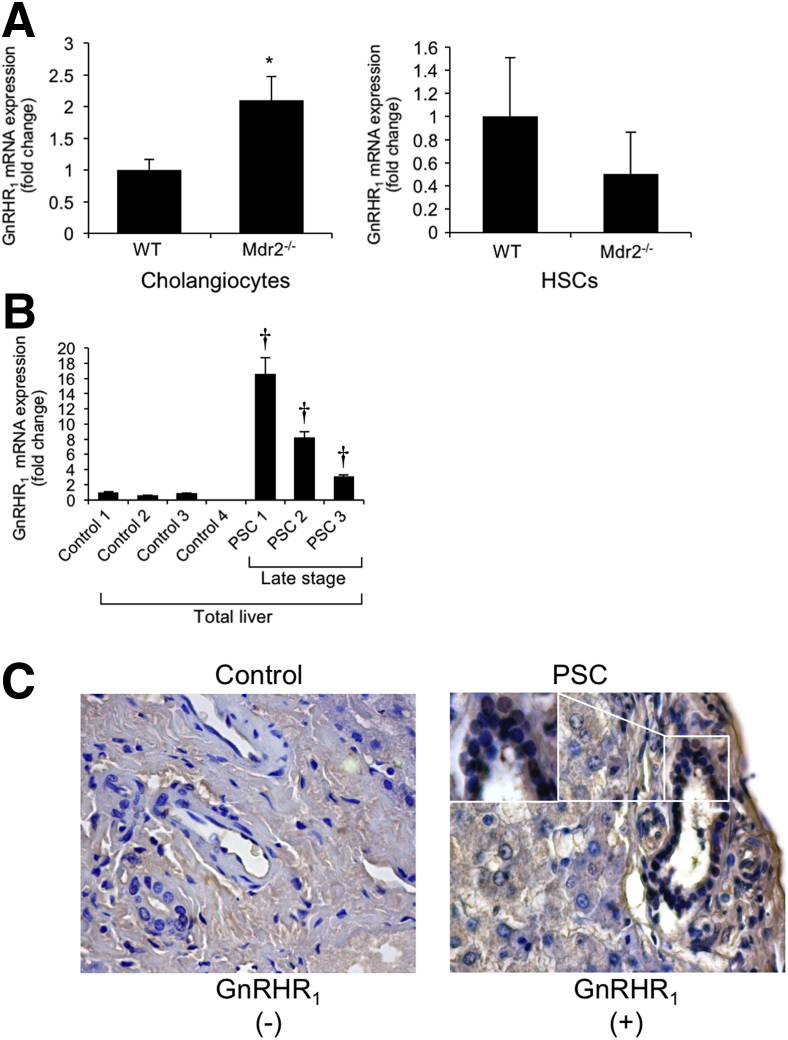
By immunofluorescence in liver sections from WT and Mdr2−/− mice, we demonstrated immunoreactivity for GnRHR1 in intrahepatic bile ducts and HSCs (costained with CK-19 and synaptophysin, respectively) (Figure 1A); the immunoreactivity of GnRHR1 was virtually absent in hepatocytes costained with hepatocyte nuclear factor-4α (Figure 1A). By semiquantitative immunohistochemistry in liver sections, we demonstrated the following: cholangiocytes express GnRHR1; and there was enhanced immunoreactivity for GnRHR1 in cholangiocytes from WT mice treated with GnRH and Mdr2−/− mice and Mdr2−/− mice treated with mismatch Vivo-Morpholino compared to the corresponding control mice (Figure 1B). The expression of GnRHR1 decreased in cholangiocytes from Mdr2−/− mice treated with GnRH Vivo-Morpholino compared to Mdr2−/− mice treated with mismatch Vivo-Morpholino (Figure 1B). There was enhanced mRNA expression of GnRHR1 in cholangiocytes (but not LCM-isolated HSCs) from Mdr2−/− mice compared to WT mice (Figure 2A) and increased mRNA expression of GnRHR1 in total liver samples from late-stage male PSC patients (n = 3) compared to their healthy controls (n = 4) (Figure 2B). By immunohistochemistry in a human liver section, we did not detect expression for GnRHR1 in normal bile ducts, but we demonstrated immunoreactivity for the receptor in bile ducts from a late-stage male PSC sample (a lower and higher magnification are shown); the immunoreactivity for GnRHR1 was absent in hepatocytes from the control and PSC human liver section (Figure 2C).
Figure 1.
A: Intrahepatic cholangiocytes and HSCs express GnRHR1. Nuclei were stained with DAPI (blue). The immunoreactivity of GnRHR1 is absent in hepatocytes. Localization of GnRHR1 and CK-19 in bile ducts is indicated by arrows. B: There is enhanced immunoreactivity for GnRHR1 in cholangiocytes from WT mice treated with GnRH and Mdr2−/− mice compared to WT mice. The expression of GnRHR1 decreases in cholangiocytes from Mdr2−/− mice treated with GnRH Vivo-Morpholino compared to Mdr2−/− treated with mismatch Vivo-Morpholino. Scale bars = 25 μm (A). Original magnification, ×40 (B). HNF-4α, hepatocyte nuclear factor-4α.
Figure 2.
A and B: mRNA expression of GnRHR1 in isolated cholangiocytes is enhanced but not in LCM-isolated stellate cells from Mdr2−/− compared to WT mice (A) and in total liver samples from late-stage human PSC samples compared to healthy controls (B). A: For isolated cholangiocytes, data are from eight PCRs from three cumulative preparations of cholangiocytes from four mice. For LMC-isolated HSCs, data are from seven real-time PCRs from one preparation of LCM-isolated HSCs from one mouse. B: Data are from three PCRs from three different samples from four normal healthy control and three PSC patients. C: There is immunoreactivity for GnRHR1 in bile ducts from PSC samples but not in control human samples. Data are expressed as means ± SEM (A and B). n = 12 isolated cholangiocytes (A); n = 7 LMC-isolated HSCs (A); n = 3 PSC patients (B); n = 4 normal healthy controls (B); n =1 control human samples (C); n =1 PSC patient (C). ∗P < 0.05 versus WT mice; †P < 0.05 versus healthy human samples. Original magnification, ×40 (C).
Measurement of GnRH Expression
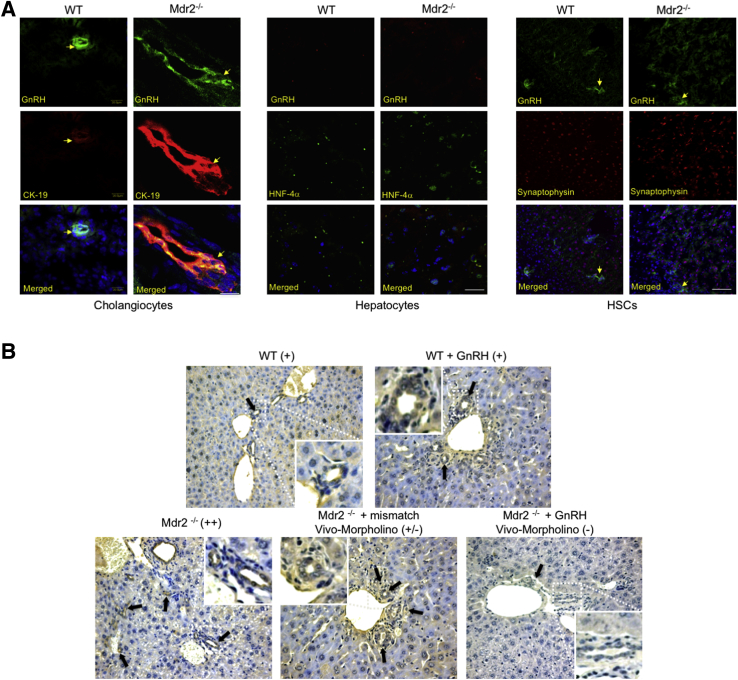
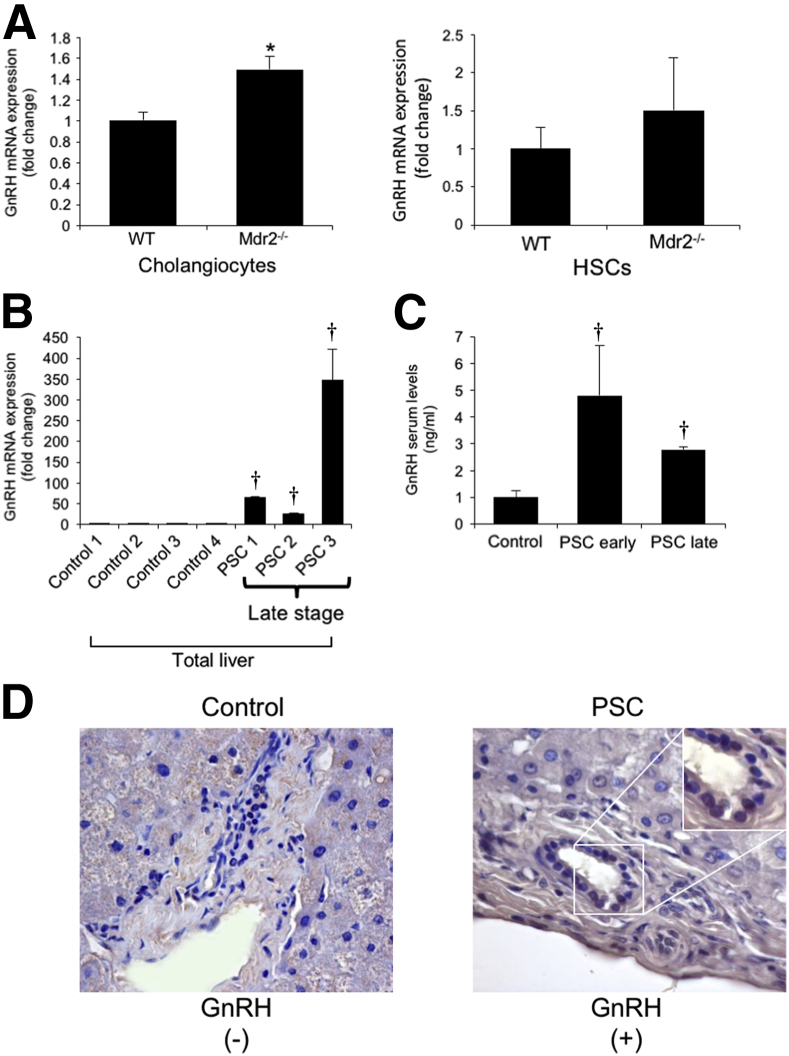
We demonstrated that cholangiocytes and HSCs (but not hepatocytes) are immunoreactive for GnRH by immunofluorescence in liver sections (Figure 3A). By immunohistochemistry, we demonstrated that GnRH expression increased in liver sections from Mdr2−/− compared to WT mice (Figure 3B) and decreased in Mdr2−/− mice treated with GnRH Vivo-Morpholino compared to both Mdr2−/− mice and Mdr2−/− mice treated with mismatch Vivo-Morpholino (Figure 3B). There was enhanced mRNA expression of GnRH in isolated cholangiocytes (but not LCM-isolated HSCs) from Mdr2−/− compared to WT mice (Figure 4A) and enhanced mRNA expression of GnRH in total liver from late-stage human PSC samples (n = 3) compared to healthy control samples (n = 4) (Figure 4B). We observed enhanced GnRH levels in the serum of early- and late-stage male PSC patients compared to healthy controls (Figure 4C). By immunohistochemistry, we did not detect expression for GnRH in bile ducts from a liver section from a healthy control patient, but we demonstrated immunoreactivity for GnRH in bile ducts from a late-stage male PSC sample; the immunoreactivity for GnRH was absent in hepatocytes from both the control and PSC human liver section (a lower and higher magnification are shown) (Figure 4D). The immunoreactivity of GnRH was absent in hepatocytes from control and PSC human liver sections (Figure 4D). There was an increase in GnRH levels in cholangiocyte supernatant from WT mice treated with GnRH as well as in Mdr2−/− mice compared to WT mice (Table 3).
Figure 3.
A: Bile ducts and HSCs (but not hepatocytes) display immunoreactivity for GnRH. Nuclei were stained with DAPI (blue). Localization of GnRH and CK-19 in bile ducts is indicated by yellow arrows. B: By immunohistochemistry, the immunoreactivity of GnRH (black arrows) increases in Mdr2−/− mice compared to WT mice; and decreases in Mdr2−/− mice treated with GnRH Vivo-Morpholino compared to Mdr2−/− mice and Mdr2−/− mice treated with mismatch Vivo-Morpholino. Scale bars = 25 μm (A). Original magnification, ×20 (B). HNF-4α, hepatocyte nuclear factor-4α.
Figure 4.
A and B: There is enhanced mRNA expression of GnRH in isolated cholangiocytes but not LMC-isolated stellate cells from Mdr2−/− compared to WT mice (A) and increased expression of GnRH in total liver from late-stage human PSC compared to healthy control samples (B). A: For isolated cholangiocytes, data are from eight PCRs from three cumulative preparations of cholangiocytes from four mice. For LMC-isolated HSCs, data are from seven real-time PCRs from one preparation of LCM-isolated HSCs from one mouse. B: Data are from three PCRs from three different samples from four healthy controls and three late-stage PSC patients. C: There are enhanced GnRH levels in the serum of early- and late-stage human PSC patients compared to healthy controls. D: By immunohistochemistry, there is immunoreactivity for GnRH in cholangiocytes from late-stage PSC sample (a lower and higher magnification are shown) but not in human control liver sample; there is no immunoreactivity for GnRH in hepatocytes from control and PSC liver sections. Data are expressed as means ± SEM (A and B). n = 12 isolated cholangiocytes (A); n =7 LMC-isolated HSCs (A); n = 8 early-stage PSC (C); n = 4 late-stage PSC (C); n = 7 healthy controls (C); n =1 control human samples (D); n =1 PSC patient (D). ∗P < 0.05 versus WT mice; †P < 0.05 versus control human samples. Original magnification, ×40 (D).
Table 3.
Measurement of GnRH Levels in Cholangiocyte Supernatant
| Treatment | Normal WT mice | Normal WT mice + GnRH | Mdr2−/− mice |
|---|---|---|---|
| Body weight, g | 25.3 ± 0.8 (23) | 28.1 ± 0.4 (10) | 26.4 ± 0.4 (29) |
| Cholangiocyte GnRH supernatant, ng/mL | 0.5 ± 0.2 (20) | 7.95 ± 1.0 (13)∗ | 11.46 ± 5.4 (21)∗ |
Data are expressed as means ± SEM (n).
∗P < 0.05 versus normal WT mice.
GnRH, gonadotropin-releasing hormone; Mdr2−/−, multidrug resistance gene 2 knockout; WT, wild type.
Measurement of IBDM and Liver Fibrosis
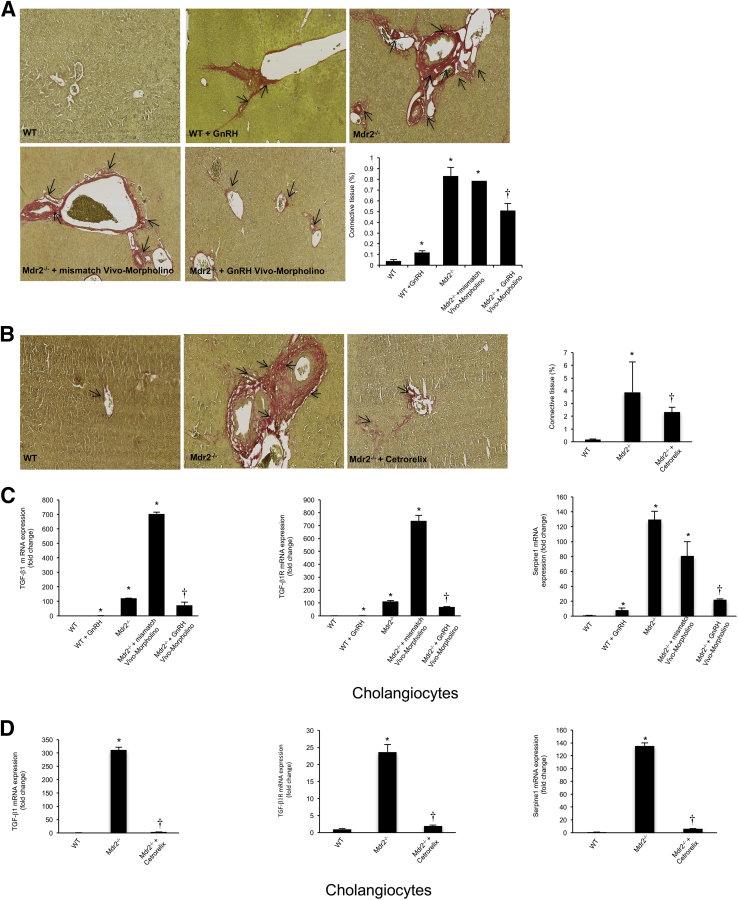
There was enhanced IBDM and liver fibrosis in WT mice treated with GnRH compared to WT mice (Figure 5A and Figure 6A). Increased IBDM and liver fibrosis (observed in Mdr2−/− mice and Mdr2−/− mice treated with mismatch Vivo-Morpholino) were reduced by the administration of GnRH Vivo-Morpholino (Figure 5A and Figure 6A) or cetrorelix (Figure 5B and Figure 6B). Cetrorelix was well tolerated by the mice, with no changes in body weight and liver morphology (data not shown); in support of our finding, another study has shown that cetrorelix induces only mild damage to the liver.33 Cetrorelix reduces the mRNA expression of PCNA and Ki-67 (markers of proliferation) in total liver from Mdr2−/− mice (Figure 5C) as well as isolated cholangiocytes from Mdr2−/− mice (Figure 5D). By real-time PCR, there was increased mRNA expression of TGF-β1, TGF-β1R, and Serpine1 in isolated cholangiocytes from WT mice treated with GnRH compared to WT mice (Figure 6C). The increase in fibrosis gene expression (observed in Mdr2−/− mice and Mdr2−/− mice treated with mismatch Vivo-Morpholino) was reduced by treatment with GnRH Vivo-Morpholino and cetrorelix (Figure 6, C and D).
Figure 5.
There is enhanced intrahepatic biliary mass (IBDM) in WT mice treated with GnRH compared to WT mice. A and B: IBDM increases in Mdr2−/− mice as well as mismatched-treated Mdr2−/− mice compared to WT mice, and returns to values similar to that of WT mice in Mdr2−/− mice treated with GnRH Vivo-Morpholino (A) or cetrorelix (B). Data are from 10 cumulative values obtained from three slides from each group. Arrows indicate CK-19–positive bile ducts. C and D: Cetrorelix reduces the mRNA expression of PCNA and Ki-67 in total liver (C) and isolated cholangiocytes (D) from Mdr2−/− mice. Data are from three real-time PCRs from three different total liver samples (C) and four real-time PCRs from three cumulative preparations of cholangiocytes from four mice (D). Data are expressed as means ± SEM. n = 12 (C and D). ∗P < 0.05 versus WT mice; †P < 0.05 versus Mdr2−/− mice. Original magnification, ×20 (A and B).
Figure 6.
A–C: Liver fibrosis and fibrosis gene expression increases in isolated cholangiocytes from WT mice treated with GnRH compared to WT mice. B and D: The increase in liver fibrosis and fibrosis gene expression (observed in Mdr2−/− mice as well as Mdr2−/− mice treated with mismatch Vivo-Morpholino) is reduced by the administration of GnRH Vivo-Morpholino or cetrorelix, respectively. A and B: Data are from 10 cumulative values obtained from three different slides from three different mice. Arrows indicate the collagen deposition around bile ducts. C: Data are from four real-time PCRs from three cumulative preparations of cholangiocytes from four mice. Values are as follows: WT, 1.0 ± 0.08, and WT + GnRH, 1.8 ± 0.1 for TGF-β1; WT, 1.0 ± 0.04, and WT + GnRH, 1.5 ± 0.2 for TGF-β1R. Data are expressed as means ± SEM (A–D). n = 12 (C). ∗P < 0.05 versus WT mice; †P < 0.05 versus Mdr2−/− mice. Original magnification, ×40 (A and B).
Modulation of the Expression of miR-200b and Fibrosis by the GnRH/GnRHR1 Axis
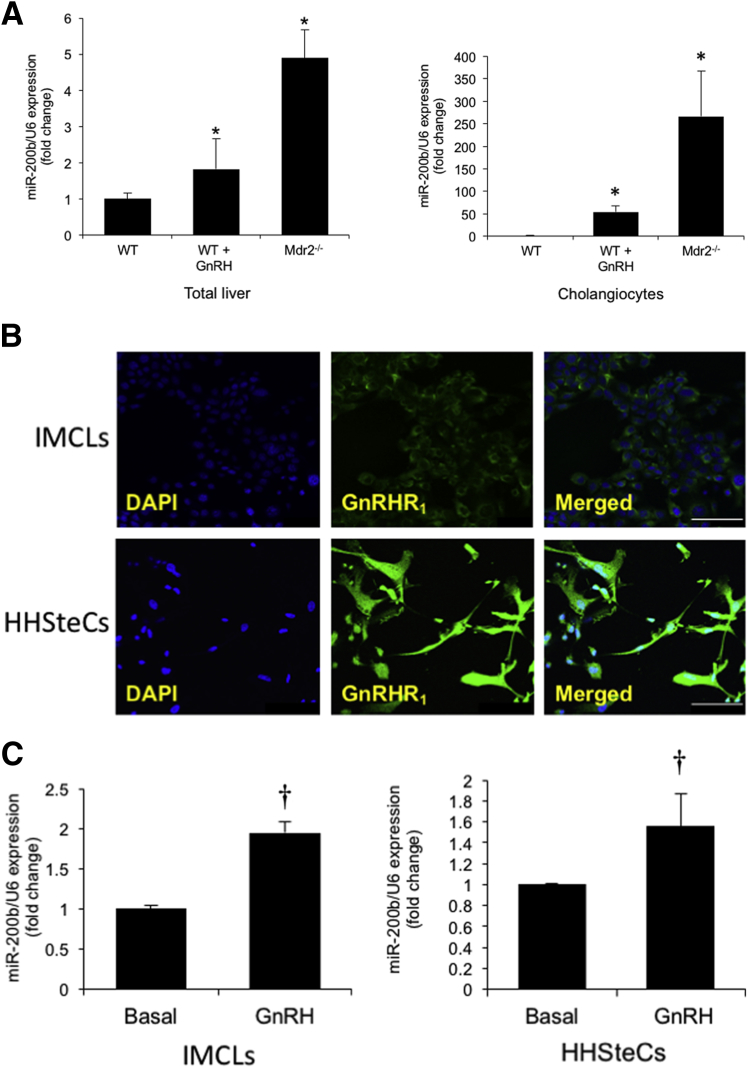
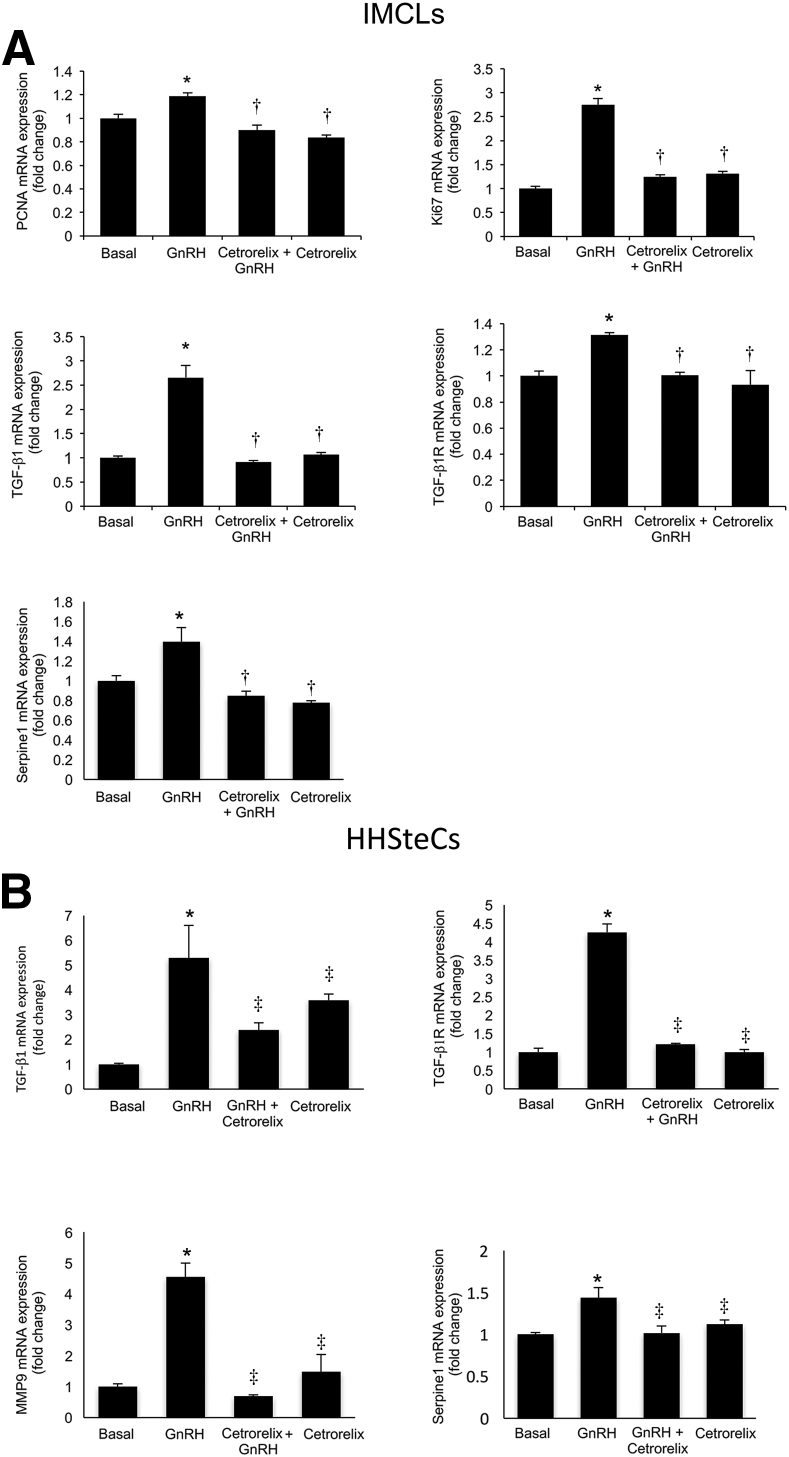
There was enhanced expression of miR-200b in total liver and isolated cholangiocytes from WT mice treated with GnRH as well as Mdr2−/− mice compared to the corresponding control mice (Figure 7A). By immunofluorescence, we demonstrated that IMCLs and HHSteCs express GnRHR1 (Figure 7B). We also demonstrated that GnRH increased the expression of miR-200b in IMCLs and HHSteCs (Figure 7C). We also observed an increase in the mRNA expression of PCNA and Ki-67 (markers of cell proliferation), TGF-β1, TGF-β1R, and Serpine1 in IMCLs treated in vitro with GnRH, which was reduced by preincubation with cetrorelix (Figure 8A). Similarly, there was an increase in the expression of TGF-β1, TGF-β1R, matrix metalloproteinase 9, and Serpine1 in HHSteCs treated in vitro with GnRH; this increase was reduced after preincubation with cetrorelix (Figure 8B).
Figure 7.
A: The expression of miR-200b increases in total liver and isolated cholangiocytes from normal WT mice treated with GnRH compared to WT mice as well as Mdr2−/− mice compared to WT mice. Data are from 16 real-time PCRs from three cumulative preparations of cholangiocytes from four mice. GnRH increases the expression of miR-200b in IMCLs and HHSteCs (C) that are immunoreactive for GnRHR1 (B). Nuclei were stained with DAPI (blue). C: Data are from four real-time PCRs from four individual preparations of IMCLs and HHSteCs. Data are expressed as means ± SEM. n = 12 (A); n = 4 (C). ∗P < 0.05 versus WT mice; †P < 0.05 versus basal. Scale bars = 25 μm (B).
Figure 8.
A:In vitro, GnRH increases the expression of miR-200b, proliferation, and selected fibrosis genes in IMCLs, which is reduced by preincubation with cetrorelix. Data are from four real-time PCRs from four individual preparations of IMCLs. B: GnRH increases the expression of selected fibrosis genes in HHSteCs compared to basal that is reduced by preincubation with cetrorelix. Data are from four PCRs from four individual preparations of HHSteCs. Data are expressed as means ± SEM. ∗P < 0.05 versus basal; †P < 0.05 versus IMCLs treated with GnRH; and ‡P < 0.05 versus HHSteCs treated with GnRH.
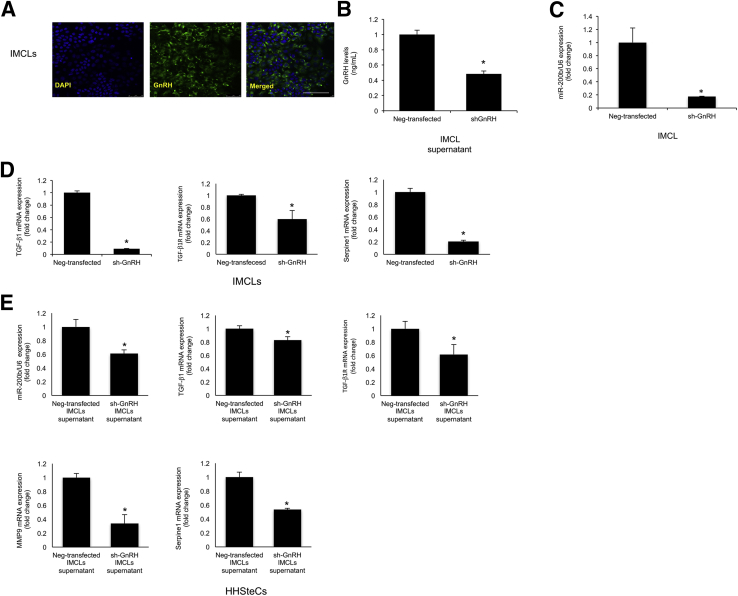
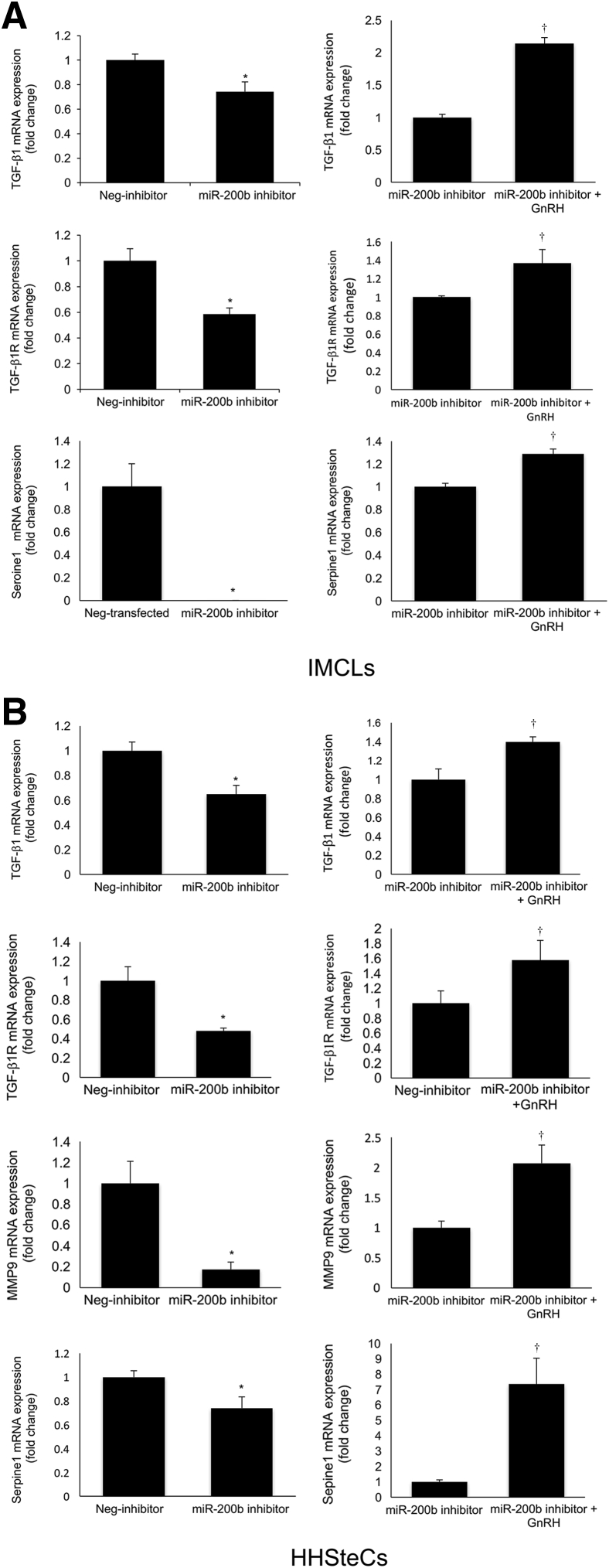
By immunofluorescence, we demonstrated that IMCLs express immunoreactivity for GnRH (Figure 9A). In shGnRH IMCLs (expressing lower levels of GnRH) (Figure 9B) there was reduced expression of miR-200b (Figure 9C) and TGF-β1, TGF-β1R, and Serpine1 (Figure 9D). When HHSteCs were incubated with the supernatant of shGnRH IMCLs, there was a decrease in the expression of miR-200b and TGF-β1, TGF-β1R, matrix metalloproteinase 9, and Serpine1 compared to HHSteCs treated with the supernatant of Neg-transfected IMCLs (Figure 9E). In both IMCLs and HHSteCs treated with an miR-200b antisense inhibitor, there was reduced expression of selected fibrosis genes (Figure 10). In IMCLs and HHSteCs pretreated with an miR-200b antisense inhibitor and subsequently with GnRH, there was increased expression of selected fibrosis genes (Figure 10); these data demonstrated a direct link between GnRH, miR-200b, and the expression of fibrosis genes.
Figure 9.
A: Immunofluorescence shows that IMCLs express immunoreactivity for GnRH. Nuclei were stained with DAPI (blue). B: In shGnRH IMCLs (expressing lower levels of GnRH), there is reduced expression of miR-200b (C) and selected fibrosis genes (D). C and D: Data are from three PCR real-time reactions from three different preparations of IMCL. E: When HHSteCs were incubated with the supernatant of shGnRH IMCLs, there was a decrease in the expression of miR-200b and selected fibrosis genes compared to HHSteCs treated with the supernatant of Neg-transfected IMCLs. Data are from four real-time PCRs from three different preparations of HHSteCs. Data are expressed as means ± SEM. n = 4 (B); n = 3 (C and D). ∗P < 0.05 versus Neg-transfected IMCLs. Scale bar = 25 μm (A).
Figure 10.
A and B: In both IMCLs and HHSteCs treated with an miR-200b antisense inhibitor, there is a reduced expression of selected fibrosis genes; the simultaneous treatment of IMCLs and HHSteCs with an miR-200b antisense inhibitor plus GnRH results in increased expression of fibrosis genes. Data are expressed as means ± SEM of four real-time PCRs from three different preparations of IMCLs (A) and HHSteCs (B). ∗P < 0.05 versus Neg-inhibitor; †P < 0.05 versus miR-200b inhibitor.
Discussion
In the current study, we provide novel evidence that the selective up-regulation of the GnRH/GnRHR1 axis in cholangiocytes in the Mdr2−/− mouse model of PSC and human PSC samples may be a key factor contributing to enhanced autocrine biliary damage and paracrine activation of HSCs, resulting in enhanced liver fibrosis. These findings are also supported by our previous study showing that GnRH is up-regulated in cholestatic bile duct–ligated rats14; however, this study had limitations because we did not observe the up-regulation of GnRHR1.14 Other studies have demonstrated the expression of GnRH and its receptors in liver, pancreas, and intestine.12, 34, 35 In another cell system, GnRH stimulates the proliferation of other epithelial cells, such as human ovarian cancer cells, by interaction with GnRHR1.36 Our studies provide the first evidence regarding the role of the GnRH/GnRHR1 axis in the progression of liver fibrosis in cholestatic liver diseases, including PSC.
A number of studies have shown that the homeostasis of the biliary epithelium is coordinately regulated by a number of neuroendocrine factors, which modulate cholangiocyte proliferation by autocrine/paracrine mechanisms.1, 3, 4, 5, 6, 14, 32, 37, 38 There is also growing evidence regarding the role of autocrine/paracrine factors in the modulation of biliary damage and liver fibrosis in PSC.4, 10, 39, 40, 41 For example, inhibition of histamine from mast cells decreases biliary hyperplasia and liver fibrosis in Mdr2−/− mice.10 A recent study has demonstrated up-regulation of the secretin/secretin receptor/TGF-β1 axis in Mdr2−/− mice and human PSC samples, and inhibition of the secretin/secretin receptor/TGF-β1 axis reduces biliary hyperplasia and liver fibrosis in Mdr2−/− mice.4 Also, platelet-derived growth factor-β has been shown to activate HSCs and induces biliary fibrosis in Mdr2−/− mice.40 Furthermore, blockage of β-adrenoceptor signaling has been shown to ameliorate liver fibrosis in Mdr2−/− mice.39 Moreover, absence of the intestinal microbiota has been shown to exacerbate hepatobiliary damage in Mdr2−/− mice.41 In this context, our study provides novel evidence that the GnRH/GnRHR1 axis may be a new and important target for modulating biliary damage and liver fibrosis in PSC.
To further support the key role of GnRHR1 in modulating GnRH effects on the biliary epithelium, we demonstrated that the type 1 receptor antagonist, cetrorelix, inhibits biliary hyperplasia and liver fibrosis in Mdr2−/− mice. Although a study has shown that cetrorelix exerts minimal toxicity in the liver,33 no studies exist regarding the inhibitory effects of this receptor antagonist on biliary functions and liver damage in PSC. Supporting the antiproliferative properties of cetrorelix, a study has demonstrated that this receptor antagonist inhibits the proliferation of primary cell cultures from human prostate carcinoma.42 One clinical study has shown that cetrorelix inhibits the growth of ovarian or mullerian carcinoma in patients refractory to platinum chemotherapy.43
Because we demonstrated that cholangiocytes from Mdr2−/− mice express and secrete more GnRH compared to WT mice, we performed experiments aimed to reduce the hepatic expression of GnRH as well as biliary proliferation and liver fibrosis. To validate our experimental approach, we first measured the mRNA expression of GnRH in cholangiocytes and HSCs and GnRH levels in cholangiocyte supernatant from the selected groups of animals. The selective increase in both GnRH expression and levels in cholangiocytes as well as serum and total liver samples from PSC patients support the key role of cholangiocytes in the activation of HSCs during the progression of PSC. This finding is supported by our previous study showing enhanced secretion of GnRH at both the basolateral and apical domain of cholangiocytes,14 suggesting that proliferating cholangiocytes contribute to liver fibrosis (by an autocrine loop) and a paracrine mechanism by activation of HSCs.4
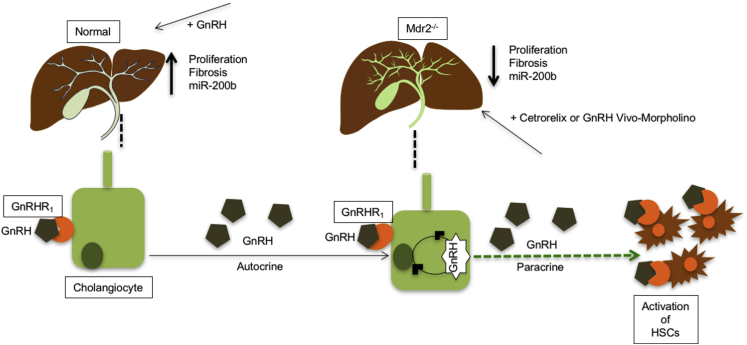
Similar to a previous study showing that intravenous administration of pulsatile GnRH in patients with hypothalamic amenorrhea ameliorates the GnRH secretion from hypothalamus,44 we observed a marked increase in GnRH expression/secretion in cholangiocytes, supporting the autocrine role of cholangiocytes in GnRH stimulation of liver fibrosis during PSC. The marginal role of the hypothalamus in regulating biliary damage and liver fibrosis in PSC through secretion of GnRH is also supported by a study demonstrating that hypothalamic-pituitary function is impaired in end-stage nonalcoholic liver diseases.45 Furthermore, disturbance in gonadotrophin secretion with inappropriately low levels of luteinizing hormone and follicle-stimulating hormone (whose secretion is regulated by GnRH) has been observed in amenorrheic women with alcoholic and nonalcoholic cirrhosis.46 In the last sets of experiments, we performed in vitro studies in IMCLs and HHSteCs to demonstrate that the effects of GnRH on biliary proliferation and liver fibrosis are directly mediated by changes in the expression of miR-200b, an miRNA that regulates fibrosis in various tissues, such as liver and kidney.47, 48 A potential shortcoming of our study is that we did not evaluate the role of the GnRH/GnRHR1 axis in biliary damage and liver fibrosis in female Mdr2−/− and female PSC samples. However, this is part of another ongoing project. Our findings are summarized in a cartoon (Figure 11) depicting the role of the GnRH/GnRHR1/miR-200b axis in the progression of biliary proliferation and liver fibrosis. Administration of GnRH increases biliary proliferation and liver fibrosis through up-regulation of miR-200b. Inhibition of the hepatic expression of the GnRH/GnRHR1 axis by Vivo-Morpholino or cetrorelix reduces biliary proliferation and liver fibrosis through decreased miR-200b expression. In conclusion, our study provides novel insights that the modulation of the GnRH/GnRHR1 axis and biliary GnRH secretion regulates the progression of liver fibrosis in PSC.
Figure 11.
Schematic diagram related to the role of the GnRH/GnRHR1/miR-200b axis in the progression of liver proliferation and fibrosis. Administration of GnRH increases biliary proliferation and liver fibrosis through up-regulation of miR-200b. Inhibition of the hepatic expression of the GnRH/GnRHR1 axis by Vivo-Morpholino or cetrorelix reduces biliary proliferation and liver fibrosis through decreased miR-200b expression.
Acknowledgment
We thank Dr. Ronald A. Faris (Brown University, Providence, RI) for providing the IgM antibody.
Footnotes
Supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Senior Career Scientist Award, Veterans Affairs Merit awards 5I01BX000574 (G.F.), 5I01BX002192 (S.G.) 1I01BX001724 (F.M.) and 1I01BX003031 (H.F.), University of Rome La Sapienza, Funds for Investment in Basic Research Accordi di Programma 2010 RBAP10Z7FS (E.G.), and NIH grants DK054811, DK076898, and DK062975 (G.A., F.M., and S.G.) and DK108959 (H.F.). This material is the result of work supported by resources at the Central Texas Veterans Health Care System.
K.K. and F.M. contributed equally to this work.
E.G., G.A., and S.G. contributed equally to this work as senior authors.
Disclosures: None declared.
The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the US Government.
Current address of F.B. and P.I., Program for Autoimmune Liver Diseases, Department of Medicine and Surgery, University of Milan-Bicocca, Milan, Italy.
Contributor Information
Gianfranco Alpini, Email: galpini@tamu.edu.
Shannon Glaser, Email: sglaser@medicine.tamhsc.edu.
References
- 1.Maroni L., Haibo B., Ray D., Zhou T., Wan Y., Meng F., Marzioni M., Alpini G. Functional and structural features of cholangiocytes in health and disease. Cell Mol Gastroenterol Hepatol. 2015;1:368–380. doi: 10.1016/j.jcmgh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton J.E., Talwalkar J.A., Lazaridis K.N., Gores G.J., Lindor K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvaro D., Mancino M.G., Glaser S., Gaudio E., Marzioni M., Francis H., Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., Onori P., Marzioni M., Alvaro D., Franchitto A., Gaudio E., Glaser S., Alpini G. The secretin receptor axis modulates liver fibrosis through changes in TGF-β1 biliary secretion. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaser S., Lam I.P., Franchitto A., Gaudio E., Onori P., Chow B.K., Wise C., Kopriva S., Venter J., White M., Ueno Y., Dostal D., Carpino G., Mancinelli R., Butler W., Chiasson V., DeMorrow S., Francis H., Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser S., Meng F., Han Y., Onori P., Chow B.K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J.M., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazaridis K.N., LaRusso N.F. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fickert P., Fuchsbichler A., Wagner M., Zollner G., Kaser A., Tilg H., Krause R., Lammert F., Langner C., Zatloukal K., Marschall H.U., Denk H., Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Popov Y., Patsenker E., Fickert P., Trauner M., Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Jones H., Hargrove L., Kennedy L., Meng F., Graf-Eaton A., Owens J., Alpini G., Johnson C., Bernuzzi F., Demieville J., DeMorrow S., Invernizzi P., Francis H. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2-/- mice. Hepatology. 2016;64:1202–1216. doi: 10.1002/hep.28704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wierman M.E., Bruder J.M., Kepa J.K. Regulation of gonadotropin-releasing hormone (GnRH) gene expression in hypothalamic neuronal cells. Cell Mol Neurobiol. 1995;15:79–88. doi: 10.1007/BF02069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakar S.S., Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98:57–62. [PubMed] [Google Scholar]
- 13.Aguilar-Rojas A., Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (Review) Oncol Rep. 2009;22:981–990. doi: 10.3892/or_00000525. [DOI] [PubMed] [Google Scholar]
- 14.Ray D., Han Y., Franchitto A., DeMorrow S., Meng F., Venter J., McMillin M., Kennedy L., Francis H., Onori P., Mancinelli R., Gaudio E., Alpini G., Glaser S.S. Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms. Am J Pathol. 2015;185:1061–1072. doi: 10.1016/j.ajpath.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart A.J., Katz A.A., Millar R.P., Morgan K. Retention and silencing of prepro-GnRH-II and type II GnRH receptor genes in mammals. Neuroendocrinology. 2009;90:416–432. doi: 10.1159/000233303. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y., Yu X., Hu S., Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y., Shen X.J., Zou Q., Zhao Q.L. Biological functions of microRNAs. Bioorg Khim. 2010;36:747–752. doi: 10.1134/s1068162010060026. [DOI] [PubMed] [Google Scholar]
- 18.Korpal M., Lee E.S., Hu G., Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim Y.Y., Wright J.A., Attema J.L., Gregory P.A., Bert A.G., Smith E., Thomas D., Lopez A.F., Drew P.A., Khew-Goodall Y., Goodall G.J. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci. 2013;126:2256–2266. doi: 10.1242/jcs.122275. [DOI] [PubMed] [Google Scholar]
- 20.Sossey-Alaoui K., Bialkowska K., Plow E.F. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem. 2009;284:33019–33029. doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue X., Zhang Y., Zhi Q., Tu M., Xu Y., Sun J., Wei J., Lu Z., Miao Y., Gao W. MiR200-upregulated Vasohibin 2 promotes the malignant transformation of tumors by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. Cell Commun Signal. 2014;12:62. doi: 10.1186/s12964-014-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng F., Henson R., Lang M., Wehbe H., Maheshwari S., Mendell J.T., Jiang J., Schmittgen T.D., Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y., Toyoda H., Tanaka M., Kuroda M., Harada Y., Matsuda F., Tajima A., Kosaka N., Ochiya T., Shimotohno K. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One. 2011;6:e16081. doi: 10.1371/journal.pone.0016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Y., Wang J., Chen Y., Zhou K., Wen J., Wang Y., Zhou Y., Pan W., Cai W. Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stallate cells by activating PI3K/Akt signaling. Cell Signal. 2014;26:925–932. doi: 10.1016/j.cellsig.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Britten J.L., Malik M., Levy G., Mendoza M., Catherino W.H. Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production. Fertil Steril. 2012;98:1299–1307. doi: 10.1016/j.fertnstert.2012.07.1123. [DOI] [PubMed] [Google Scholar]
- 26.Glanowska K.M., Burger L.L., Moenter S.M. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34:15060–15069. doi: 10.1523/JNEUROSCI.2200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson M.J., Kasowski H., Dobrjansky A. Continuous gonadotropin-releasing hormone infusion stimulates dramatic gonadal development in hypogonadal female mice. Biol Reprod. 1994;50:680–685. doi: 10.1095/biolreprod50.3.680. [DOI] [PubMed] [Google Scholar]
- 28.Shetty G., Wilson G., Huhtaniemi I., Boettger-Tong H., Meistrich M.L. Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology. 2001;142:2789–2795. doi: 10.1210/endo.142.7.8237. [DOI] [PubMed] [Google Scholar]
- 29.Puche J.E., Lee Y.A., Jiao J., Aloman C., Fiel M.I., Munoz U., Kraus T., Lee T., Yee H.F., Jr., Friedman S.L. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology. 2013;57:339–350. doi: 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno Y., Alpini G., Yahagi K., Kanno N., Moritoki Y., Fukushima K., Glaser S., LeSage G., Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 31.Glaser S., Benedetti A., Marucci L., Alvaro D., Baiocchi L., Kanno N., Caligiuri A., Phinizy J.L., Chowdury U., Papa E., LeSage G., Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Ning G., Duncan S.A. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- 33.Suszka-Switek A., Czekaj P., Pajak J., Skowronek R., Wrona-Bogus K., Plewka D., Kozlowska-Rup D., Wiaderkiewicz R., Jankowski A. Morphological and enzymatic changes caused by a long-term treatment of female rats with a low dose of gonadoliberin agonist and antagonist. Med Sci Monit. 2012;18:BR315–BR330. doi: 10.12659/MSM.883264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Huang G., Huang W. [Gonadotropin releasing hormone and its receptor in the tissue of human hepatocellular carcinoma] Zhonghua Yi Zue Za Zhi. 1998;78:343–346. Chinese. [PubMed] [Google Scholar]
- 35.Sand E., Bergvall M., Ekblad E., D'Amato M., Ohlsson B. Expression and distribution of GnRH, LH, and FSH and their receptors in gastrointestinal tract of man and rat. Regul Pept. 2013;187:24–28. doi: 10.1016/j.regpep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Grundker C., Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. Reprod Biol Endocrinol. 2003;1:65. doi: 10.1186/1477-7827-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alpini G., Glaser S., Ueno Y., Pham L., Podila P.V., Caligiuri A., LeSage G., LaRusso N.F. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 38.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y., Meininger C.J., Franchitto A., Onori P., Marzioni M., Taffetani S., Fava G., Stoica G., Venter J., Reichenbach R., DeMorrow S., Summers R., Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Strack I., Schulte S., Varnholt H., Schievenbusch S., Tox U., Wendland K., Steffen H.M., Drebber U., Dienes H.P., Odenthal M. beta-Adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest. 2011;91:252–261. doi: 10.1038/labinvest.2010.162. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida S., Ikenaga N., Liu S.B., Peng Z.W., Chung J., Sverdlov D.Y., Miyamoto M., Kim Y.O., Ogawa S., Arch R.H., Schuppan D., Popov Y. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014;147:1378–1392. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Tabibian J.H., O'Hara S.P., Trussoni C.E., Tietz P.S., Splinter P.L., Mounajjed T., Hagey L.R., LaRusso N.F. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185–196. doi: 10.1002/hep.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castellon E., Clementi M., Hitschfeld C., Sanchez C., Benitez D., Saenz L., Contreras H., Huidobro C. Effect of leuprolide and cetrorelix on cell growth, apoptosis, and GnRH receptor expression in primary cell cultures from human prostate carcinoma. Cancer Invest. 2006;24:261–268. doi: 10.1080/07357900600629591. [DOI] [PubMed] [Google Scholar]
- 43.Emons G., Grundker C., Gunthert A.R., Westphalen S., Kavanagh J., Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer. 2003;10:291–299. doi: 10.1677/erc.0.0100291. [DOI] [PubMed] [Google Scholar]
- 44.Santoro N., Wierman M.E., Filicori M., Waldstreicher J., Crowley W.F., Jr. Intravenous administration of pulsatile gonadotropin-releasing hormone in hypothalamic amenorrhea: effects of dosage. J Clin Endocrinol Metab. 1986;62:109–116. doi: 10.1210/jcem-62-1-109. [DOI] [PubMed] [Google Scholar]
- 45.Handelsman D.J., Strasser S., McDonald J.A., Conway A.J., McCaughan G.W. Hypothalamic-pituitary-testicular function in end-stage non-alcoholic liver disease before and after liver transplantation. Clin Endocrinol (Oxf) 1995;43:331–337. doi: 10.1111/j.1365-2265.1995.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 46.Bell H., Raknerud N., Falch J.A., Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol. 1995;132:444–449. doi: 10.1530/eje.0.1320444. [DOI] [PubMed] [Google Scholar]
- 47.Noetel A., Kwiecinski M., Elfimova N., Huang J., Odenthal M. microRNA are central players in anti- and profibrotic gene regulation during liver fibrosis. Front Physiol. 2012;3:49. doi: 10.3389/fphys.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vettori S., Gay S., Distler O. Role of microRNAs in fibrosis. Open Rheumatol J. 2012;6:130–139. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]