Summary
Observational studies in chronic kidney disease (CKD) populations have consistently shown the strong mortality-predictability of such markers of protein-energy wasting (PEW) as hypoalbuminemia, low serum cholesterol levels, low body mass index and reduced dietary protein intake. Even though the PEW-mortality association data are traditionally reported mostly in maintenance dialysis patients, emerging studies extend the existence of these associations to pre-dialysis stages of CKD. Both obesity and cholesterol paradoxes have recently been reported in pre-dialysis CKD, underscoring the overwhelming impact of PEW, a short-term killer, on reversing the long-term effect of conventional cardiovascular risk factors. Multiple pathophysiologic mechanisms have been suggested to explain the link between PEW and mortality in CKD, including derangements in the muscle and adipose tissues, the gastrointestinal, hematopoietic and immune systems and complications related to deficiencies involving multiple micro-nutrients, and the maladaptive activation of the inflammatory cascade. In addition to well described pathophysiologic mechanisms involved in the higher mortality seen with PEW we also discuss the potential role of novel factors such as circulating actin and pro-inflammatory HDL. Whether PEW is causally related to adverse outcomes in CKD needs to be verified in randomized controlled trials of nutritional interventions. The initiation of major clinical trials targeting nutritional interventions with the goal of improving survival in CKD offer the promise of extending the survival of this vulnerable patient population.
Keywords: protein-energy wasting, mortality, chronic kidney disease, muscle wasting, obesity paradox, hypoalbuminemia
Introduction
Individuals with chronic kidney disease (CKD) experience extremely high mortality rates,1–3 mostly secondary to complications of cardiovascular (CV) disease. Interestingly, so-called traditional CV risk factors such as hypercholesterolemia or hypertension cannot explain this high burden of CV disease and death in CKD patient populations, an apparently puzzling finding that has also been confirmed in randomized controlled trials.4 In sharp contradistinction to the general population, such surrogates of metabolic syndrome as hyperlipidemia and obesity are paradoxically associated with longevity in CKD patients. These associations are known as “bad-gone good phenomenon”, “altered-risk factor pattern” or “reverse epidemiology”.5–7 Observational studies have found a consistent and robust association between various markers of diminished nutritional status such as hypoalbuminemia, and poor clinical outcomes in CKD patients. Despite the traditional use of the term “uremic malnutrition” to describe the strong mortality-predictability of hypoalbuminemia or other measures of poor nutrition, an expert panel has recently recommended the use of the term protein-energy wasting (PEW) in order to better characterize the complex nature of the nutritional abnormalities found in patients with diseases of the kidney that are associated with wasting syndrome.8
One significant limitation of observational studies is that the associations described therein do not necessarily prove a causal link between risk factors and outcomes. In the case of PEW, it can be argued that the measures of poor nutrition used to predict adverse outcomes are a consequence, rather than the cause of these outcomes i.e., the so-called reverse causation.9 Certain unmeasured morbid conditions could also be a cause for both the poor nutritional state and the adverse outcomes, leading to residual confounding that cannot be controlled for in multivariate models. These limitations aside, observational studies are useful in uncovering novel risk factors which can later be proven to be useful markers of adverse outcomes. We will argue in favor of PEW being strongly correlated with adverse outcomes in CKD, and discuss the additional pieces of evidence needed to prove a cause-effect relationship between the two. We will emphasize recent findings from individuals with non-dialysis dependent (NDD)-CKD that could allow extending observations from studies in maintenance dialysis patients to this much larger group of individuals with a chronic disease state.
PEW AND MORTALITY IN ADVANCED CKD
The PEW can be characterized by multiple clinical, biochemical and nutritional parameters. An expert panel convened by the International Society of Renal Nutrition and Metabolism (ISRNM) recommended that the diagnosis of PEW be made by using four readily available categories of criteria:8 (1) biochemical measures (serum albumin, prealbumin and cholesterol); (2) measures of body mass (body mass index [BMI], unintentional weight loss and total body fat), (3) measures of muscle mass (muscle mass, mid-arm circumference and creatinine appearance); and (4) measures of dietary intake (dietary protein and energy intake). A series of additional markers have also been advocated,8 but the data available on these is more limited at this stage. Most, if not all of the foregoing markers are associated with mortality in dialysis patients. Both low BMI10–13 and low serum cholesterol (Figure 1)14;15 have been consistently and counter intuitively shown to be associated with increased death risk especially in maintenance hemodialysis (MHD) patients. An increased protein intake as reflected by normalized low protein nitrogen appearance (nPNA, also known as normalized protein catabolic rate [nPCR]), of up to 1.4 g/kg/day is associated with greater survival (Figure 2).16 Finally low serum albumin concentration, and especially its decline over time, remains one of the strongest predictors of mortality in MHD patients (Figure 3).17
Figure 1.
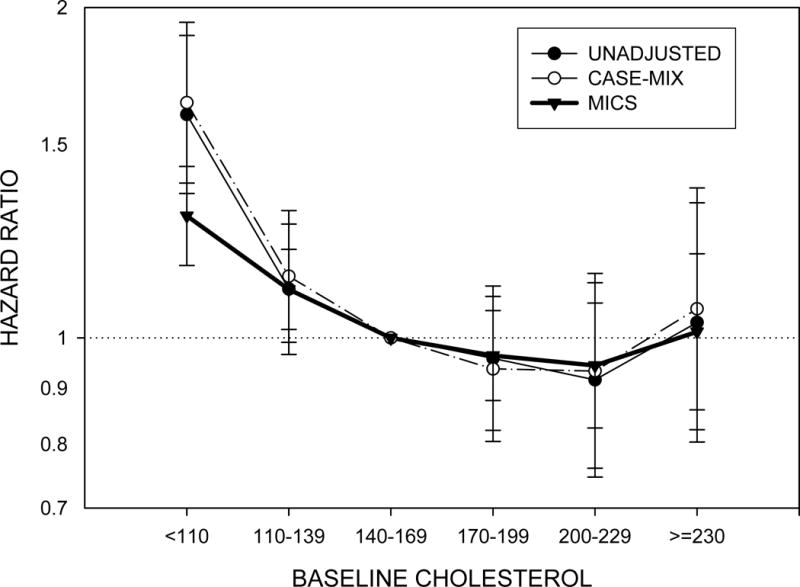
Cardiovascular mortality associated with baseline cholesterol level in 15,859 patients receiving maintenance hemodialysis. Based on data from Reference 13.
Figure 2.
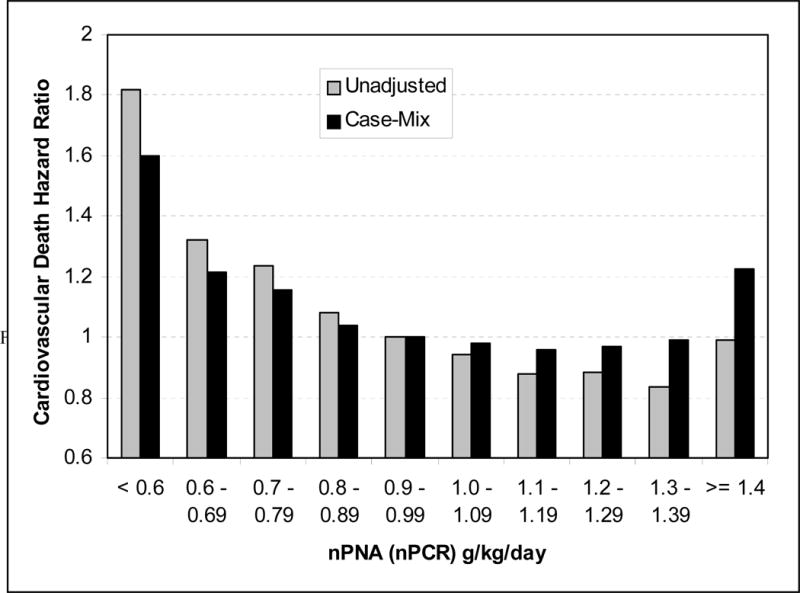
Cardiovascular mortality risk associated with various levels of normalized protein catabolic rate in 53,933 maintenance hemodialysis patients, unadjusted, and after adjustment for case-mix. Based on data from Reference 32.
Figure 3.
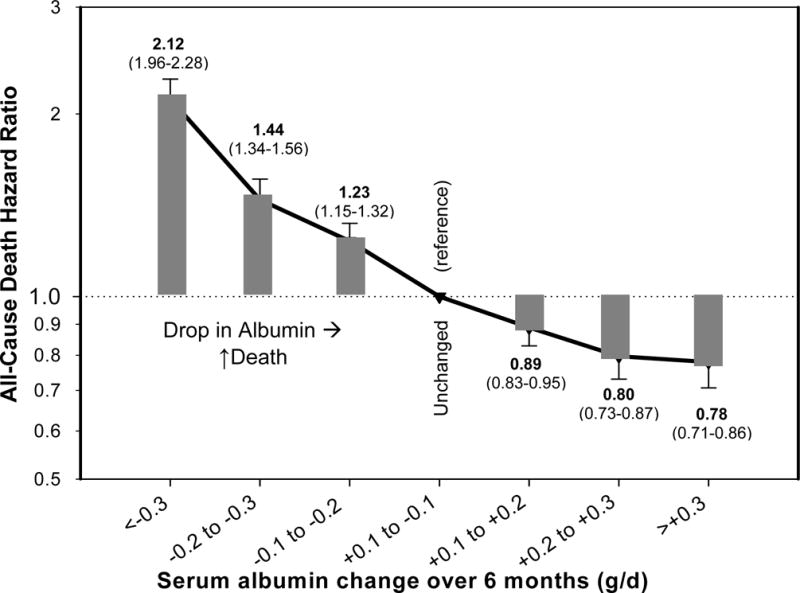
All-cause mortality risk associated with a change in serum albumin concentration over a 6 month time period in 58,058 maintenance hemodialysis patients. Based on data from Reference 16.
PEW AND MORTALITY IN NON-DIALYSIS DEPENDENT CKD
Less information is available on the association between markers of PEW and outcomes in patients with NDD-CKD. The association between lower albumin and adverse outcomes has been examined in 3 studies of patients with moderately advanced CKD.18–20 Lower albumin was independently associated with higher all cause mortality and a higher incidence of CV events in these studies (Table 1).
Table 1.
Studies examining the association between serum albumin level and outcomes in patients with non-dialysis dependent chronic kidney disease
| Study | Patients | Follow-up | Results | Comments |
|---|---|---|---|---|
| Weiner et al,20 2008 | 1678 participants in the ARIC and CHS studies; eGFR 51.1±8.5 ml/min/1.73m2. | Median 108 months. | Lower albumin level associated with cardiac events and all cause mortality. | Few patients with advanced (stage 4) CKD. |
| Muntner et al,19 2005 | 807 participants in the ARIC study with CKD | 10.5 years. | Lower albumin associated with increased risk of major cardiovascular events. | Mortality not examined. |
| Menon et al,18 2005 | 697 participants in the MDRD study; GFR 32.9±12.2 ml/min. | Median 125 months. | Low albumin associated with all cause mortality independent of CRP level. | Young non-diabetic White patients with low mortality rate; questionable external validity. |
ARIC: Atherosclerosis Risk in the Community; CHS: Cardiovascular Health Study; CKD: chronic kidney disease; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate.
Four studies have examined the association between blood lipid levels and outcomes in NDD-CKD;19–22 three of these used mortality as the outcome measure,20–22 one used cardiovascular events19 (Table 2). Only one of these studies examined patients with advanced NDD-CKD; lower total and LDL cholesterol was associated with higher all-cause and cardiovascular mortality in this study, but the association became non-significant after adjustments for case-mix characteristics; nevertheless, higher cholesterol and triglyceride levels were not associated with higher mortality, even after adjustments.22 Of the 3 studies that examined patients with less advanced NDD-CKD, one found a positive association between triglyceride level and adverse outcomes (but no association for total and HDL cholesterol),20 the second found a positive association between total cholesterol and major cardiovascular events (but no association for triglycerides and HDL cholesterol),19 and the third one found no association between lipid levels and cardiovascular death.21
Table 2.
Studies examining the association between lipid levels and outcomes in patients with non-dialysis dependent chronic kidney disease
| Study | Patients | Follow-up | Results | Comments |
|---|---|---|---|---|
| Weiner et al,20 2008 | 1678 participants in the ARIC and CHS studies; eGFR 51.1±8.5 ml/min/1.73m2. | Median 108 months. | Triglyceride level associated with the composite of myocardial infarction, stroke, and all-cause mortality. Total and HDL cholesterol not associated with the composite outcome. | Few patients with advanced (stage 4) CKD. |
| Kovesdy et al,22 2007 | 986 male patients in the US, eGFR 37.4±17.6 ml/min/1.73m2. | Median 3 years. | Lipid levels (total, LDL and HDL cholesterol and triglycerides) not associated with all-cause and cardiovascular mortality after adjustment for confounders. | Single center, males only. |
| Muntner et al,19 2005 | 807 participants in the ARIC study with CKD | 10.5 years. | Higher total cholesterol associated with increased risk of a major CHD event; HDL cholesterol and triglyceride levels not associated with CHD. | Mortality not examined. |
| Shlipak et al,21 2005 | 5808 participants in the CHS study; 1249 with eGFR <60. | 8.6 years. | Triglyceride, LDL and HDL cholesterol levels not associated with cardiovascular death. | Community-dwelling elderly individuals. |
ARIC: Atherosclerosis Risk in the Community; CHS: Cardiovascular Health Study; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate.
In MHD patients, a low body mass index (BMI) has been consistently shown to be associated with higher death rate,10 whereas obesity is associated with greater survival, even at its extremes of morbid obesity.11 In NDD-CKD patients, there are at least 8 recent studies that have examined the association between BMI and outcomes (Table 3).19–21;23–27 Four of these studies found an association between lower BMI and poor outcomes (Figure 4),23–26 and four found no association between BMI and the studied outcomes.19–21;27 None of the listed studies found adverse outcomes to be associated with higher BMI.
Table 3.
Studies examining the association between body mass index and outcomes in patients with non-dialysis dependent chronic kidney disease
| Study | Patients | Follow-up | Results | Comments |
|---|---|---|---|---|
| Weiner et al,20 2008 | 1678 participants in the ARIC and CHS studies; eGFR 51.1±8.5 ml/min/1.73m2. | Median 108 months. | BMI not associated with all cause mortality, myocardial infarction or stroke incidence. | Few patients with advanced (stage 4) CKD. |
| Kovesdy et al,25 2007 | 521 male patients in the US, eGFR 37.5±16.8 ml/min/1.73m2. | Median 2.3 years. | Higher BMI associated with lower all-cause mortality. | Inverse association of BMI with mortality was restricted to non-diabetic patients. |
| Kwan et al,26 2007 | 15,355 participants in the ARIC study; 429 with CKD stage 3 and 32 with CKD stage 4. | Mean 9.9±1.8 years. | Higher BMI associated with lower all-cause mortality in participants with CKD. | Higher BMI associated with deleterious metabolic changes in CKD. |
| Madero et al,27 2007 | 1759 patients screened for the MDRD study; GFR 39±21 ml/min/1.73m2. | Mean 10 years. | BMI not associated with mortality. | Young non-diabetic White patients with low mortality rate; questionable external validity. |
| Muntner et al,19 2005 | 807 participants in the ARIC study with CKD. | 10.5 years. | Higher BMI and obesity was not associated with incidence of CHD after adjustments. | Mortality was not examined. |
| Shlipak et al,21 2005 | 5808 participants in the CHS study; 1249 with eGFR <60. | 8.6 years. | BMI not associated with cardiovascular death. | Community-dwelling elderly individuals. |
| Evans et al,23 2005 | 920 patients in Sweden, median eGFR 17.5 ml/min/1.73m2 for men and 15.9 ml/min/1.73m2 for women. | Median 5 years. | Lower BMI associated with higher mortality. | Few patients died prior to starting dialysis. |
| Jurkovitz et al,24 2006 [abstract] | 33,474 at high risk for kidney disease. | Risk of death lower among participants with BMI >30 kg/m2. | CKD did not modify the effect of obesity on mortality. |
ARIC: Atherosclerosis Risk in the Community; BMI: body mass index; CHD: coronary heart disease; CHS: Cardiovascular Health Study; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; MDRD: Modification of Diet in Renal Disease.
Figure 4.
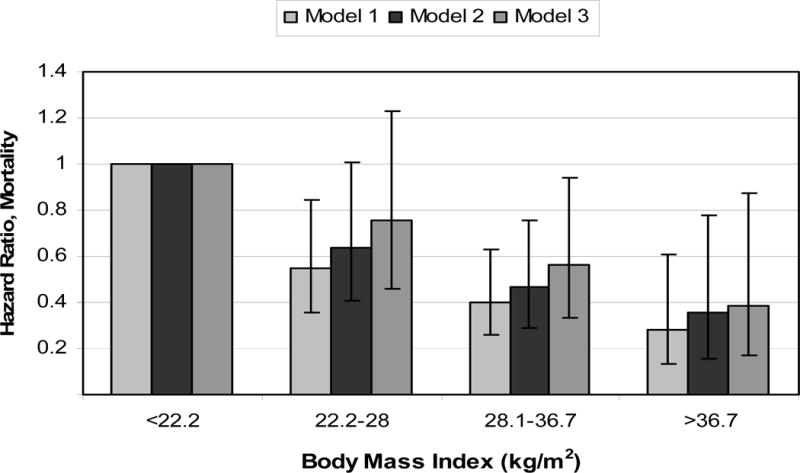
All-cause mortality risk associated with categories of body mass index in non-dialysis dependent patients with CKD stage 1–5. Models were adjusted for age and race (Model 1), age, race, co-morbidity index, smoking, blood pressure, kidney function, proteinuria, and medication use (Model 2) and age, race, co-morbidity index, smoking, blood pressure, kidney function, proteinuria, medication use, albumin, white blood cell count, hemoglobin, percent of lymphocytes in WBC, cholesterol and bicarbonate (Model 3). The group with body mass index of <22.2 kg/m2 served as reference. Based on data from Reference no. 41.
We can thus conclude that markers of PEW are consistently associated with adverse outcomes in MHD patients, and some of these markers also appear to display similar associations in patients with NDD-CKD, especially in those with more advanced disease state. Some of the associations seen in MHD patients were not observed in patients with earlier stages of NDD-CKD. This may be related to the fact that such patients may resemble more closely individuals without CKD rather than patients on dialysis, thus resulting in discrepant findings when assessing risk factors that show a reversal of the “usual” risk factor patterns in dialysis patients, such as BMI or serum cholesterol (a.k.a. “reverse epidemiology”5, “risk factor paradox”6 or “altered risk factor pattern”.7 For markers that do not display a reversal of risk factor pattern (such as serum albumin), the association with adverse outcomes appears consistent in all studies, irrespective of the stage of CKD (Table 1).
PEW AND MORTALITY: ASSOCIATION VS. CAUSALITY
There are compelling observational data linking PEW to higher mortality in CKD and other chronic disease states with wasting (such as chronic heart failure [CHF],28 rheumatoid arthritis,29 or chronic obstructive pulmonary disease [COPD]30). Nevertheless, proving the causality of the foregoing relationships requires additional evidence. First, the observed associations require plausible explanations based on known biologic processes; it is preferable if a single mechanism of action is invoked as the driving force behind the observed PEW-death associations.31 In the case of PEW, however, the underlying mechanisms appear complex, and no single, adequately inclusive pathophysiologic mechanism has been set forth to date to explain the entire observed outcomes (Table 4). It needs to be stressed, that the criteria recommended to diagnose PEW (such as low albumin, cholesterol or BMI) are meant to be used as markers of such a state, but given the complexity of malnutrition, they may not be causally responsible for the negative outcome.8
Table 4.
Putative mechanisms of action underlying the higher mortality associated with protein-energy wasting
| Affected system | Effect of PEW | Mechanism of action |
|---|---|---|
| Immune system | Immune deficiency | Increased susceptibility to bacterial or viral infections, poor wound healing. |
| Skeletal muscle | Sarcopenia Increased circulating actin | Reduced skeletal, respiratory and cardiac muscle function. Lower muscle oxidative metabolism with decreased antioxidant defense. Decrease in bioavailability of activated vitamin D and gelsolin. |
| Endocrine system | Loss of fat tissue Decreased adiponectin level Increased insulin resistance | Decreased sequestration of uremic toxins. Decreased production of anti-inflammatory cytokines and adiponectin. Increased levels of advanced glycation end products. |
| Lipoproteins | Decreased cholesterol level Pro-inflammatory conversion of HDL | Decreased ability to bind to circulating endotoxin, with activation of the cytokine cascade. Decreased anti-inflammatory effect of HDL. |
| Inflammatory Cytokines | Increased CRP and IL-6 Decreased IL-10 | Pro-inflammatory cytokines lead to endothelial dysfunction and increased atherosclerotic plaque formation. |
| Hematopoietic system | Increased platelet activation Increased myeloperoxidase Low hemoglobin; iron depletion | Accelerated atherogenesis; expansion of atherosclerotic plaques and/or unstable plaques. |
| Nutritional | Anorexia and dietary restrictions leading to decreased intake of fresh fruits and vegetables, legumes, dairy product and high-value proteins. Decreased level of anti-oxidative vitamins and trace elements Reduced levels of both nutritional and activated vitamin D | Atherogenic effects of (self) imposed diet. Decreased protein intake leads to further PEW and mortality. Enhanced oxidative stress, with consequent inflammation, endothelial dysfunction, and atherosclerosis. Increased vascular calcification. |
| Gastrointestinal | Atrophy of gut lining Decreased intestinal secretions and altered gut flora | Decreased absorption of nutrients and increased absorption of endotoxins. |
CKD-specific effect.
PEW: protein-energy wasting.
CRP: C-reactive protein
IL-6: interleukin-6
VDRA: vitamin D receptor activator
Comorbid illnesses may serve as a link between PEW and morbidity and mortality in CKD patients, especially since patients with the lowest serum albumin or BMI levels are more likely to have severe underlying diseases such as long standing diabetes mellitus, chronic lung or heart failure or peripheral vascular disease.32 It appears less likely, though, that PEW is merely a surrogate marker of increased comorbidity, since epidemiologic studies indicate that nutrient intake and PEM are independently associated with increased morbidity and mortality even in patients with less severe comorbid conditions.33;34 As detailed below, PEW could be one of the causes of such disease states and be thus at least partially responsible for the higher mortality associated with these conditions.
The reduction in muscle mass (sarcopenia) observed in PEW may be caused by uremic toxins or by other metabolic, hormonal or neuropathic derangements. Muscle wasting may lead to reduced skeletal, respiratory and cardiac muscle function, compromising the vital functions of these organ systems; it may also restrict muscle-based oxidative metabolism and thus lead to a decreased antioxidant defense.35 Widespread tissue damage may also lead to the emergence of circulating actin that can consume gelsolin (which is primarily produced by skeletal muscle), vitamin D binding protein and other circulating molecules with salutary and protective action.36–38 Preliminary data indicate that circulating actin and gelsolin may indeed be associated with survival in MHD patients (personal communication with Dr. R. Thadhani, Boston, MA).
The PEW, by virtue of its malnutrition component, may lead to impaired immune function and host resistance resulting in increased susceptibility to infections and poor wound healing.39;40 Certain nutrients such as arginine and glutamine may enhance the immune response.41–43 CKD patients may be particularly susceptible to zinc,44;45 vitamin B6 (pyridoxine), vitamin C and folic acid deficiencies,46;47 most of which can induce alterations in host defense, such as diminished antibody response, polymorphonuclear leukocyte or lymphocyte dysfunction and impaired wound healing. Levocarnitine may protect against endotoxins and also suppress elaboration of tumor necrosis factor alpha (TNF-α) from monocytes.48 Since uremia itself and associated comorbid illnesses may also compromise the immune system,49;50 it is possible that CKD patients be even more susceptible to the immune attenuating effects of the PEW. Impaired host resistance, aggravated by PEW in these individuals, may predispose to inflammatory diseases, such as hepatitis C infections, which in turn is associated with increased death in CKD.51;52 Indirect arguments also suggest that malnutrition might increase cardiac death as a consequence of decreased L-arginine availability and the ensuing diminished synthesis of nitric oxide.53
Several independent studies have shown a relationship between inflammatory processes, most commonly indicated by elevated serum C-reactive protein (CRP) and interleukin-6 (IL-6) concentrations, and risk of CV death among CKD patients54–56 and in the general population.57;58 Since inflammation may be associated with both anorexia59 and increased net protein catabolism, inflammation may indeed be the missing link between the PEW and mortality in CKD. Inflammation may induce endothelial cell damage and endothelial dysfunction predisposing to atherosclerotic plaque formation.60 It is important to note that inflammation may be both a cause and a consequences of PEW.61;62
Another theory invokes the characteristics of consumed food in the mechanism of action of PEW-induced increased mortality. An atherogenic diet is imposed upon most individuals with CKD.63;64 Due to the difficulty of maintaining adequate energy intake on low protein, low potassium diets, patients may tend to rely more on food sources containing high amounts of atherogenic fat. Moreover, a recent study based on food frequency questionnaires indicate that MHD patients consume significantly lower amounts of potassium, dietary fiber, vitamin C and certain cardioprotective carotenoids.64 Such patients appear to have a lower intake of dietary nutrients including minerals and vitamins, but a higher intake of cholesterol. Most CKD patients are exposed to traditional restrictions in potassium intake, which may result in reduced fruit and vegetable intake, leaving meat and other high fat foods as the main sources of calories.64 Furthermore, derangements of the gastrointestinal tract are characteristic of malnutrition, with atrophy of the gut lining, decreased intestinal secretions and altered gut flora leading to further reduction in gut function and the ability to absorb nutrients.65
Gradual loss of body fat content during the progression of CKD could result in decreased sequestration of uremic toxins66 and lower production of certain anti-inflammatory cytokines and adiponectine.67 Furthermore, circulating lipoproteins may serve as a defense mechanism by neutralizing endotoxins intruding through the leaky GI tract.68 This so-called “endotoxin-lipoprotein hypothesis” may explain the link between low levels of serum cholesterol and increased CV disease and death in both CKD and CHF patients; hence, higher concentration of unbound endotoxins occurring in the setting of low serum cholesterol may activate the proinflammatory cytokine cascade leading to endothelial dysfunction and atherosclerosis.68 Increased proinflammatory conversion of HDL-cholesterol may also play a role in attenuating the pool of protective lipoproteins.69
Other mechanisms that may be directly or indirectly related to adverse effects of malnutrition and PEW include vitamin D deficiency,70 refractory anemia and iron depletion, which may lead to increased platelet count and/or activation,71 and increased myeloperoxidase activity.72 It is very likely that the higher mortality associated with PEW be a result of a combination of the above mechanisms, rather than a single effect related to any of the individual components.
Proof of Concept: Nutritional Interventions to Improve Survival
A second and very important criterion needs to be satisfied in order to prove the (direct or indirect) causal link between PEW and mortality: Proof of improved survival from nutritional interventions in randomized controlled trials. Fortunately, the complex mechanism of action of PEW allows for a multitude of interventions to try improving nutritional status. Indeed, there have been several small clinical trials that showed improvement in various nutritional markers with oral or parenteral nutritional supplementation,73–76 anabolic therapies using human growth hormone77 or testosterone supplementation,78–80 or frequent (daily) dialysis.81;82 Unfortunately, the same complexity of the mechanism of action of PEW does not allow translating observations from studies using single nutritional markers as end points to improved survival. Randomized controlled trials of nutritional intervention that use hard clinical end points are few, but this will hopefully change in the near future.
The recently published French Intradialytic Nutrition Evaluation Study (FineS) compared the impact on mortality of oral nutritional supplementation with that of intradialytic parenteral nutrition in addition to the same oral intervention in 186 malnourished hemodialysis patients.83 IDPN plus oral nutritional supplementation did not improve 2-year mortality compared to oral nutritional supplementation alone, but an increase in prealbumin of >30 mg/L within 3 months predicted a 54% decrease in mortality, reduced hospitalizations and improved general well-being, independent of the method of nutritional supplementation.83 This was the first study to show a survival benefit from an intervention that improved a marker of PEW.
A much larger randomized controlled trial is presently in recruitment phase; the OPPORTUNITY study (Clinicaltrials.gov identifier: NCT00503698) is evaluating the effect of human growth hormone (compared to placebo) on survival in adult chronic hemodialysis patients. This study is planning to enroll 2500 patients, with a projected follow-up of 2 years, and is scheduled to end in 2010. If this study shows a benefit from growth hormone therapy in dialysis patients, we expect that several more studies be conducted to evaluate other potential interventions to improve PEW and survival in this patient population. After failed attempts to improve survival in MHD patients by treating traditional risk factors such as high cholesterol4 and non-traditional risk factors such as anemia84;85 and dialysis dose,86 nutritional interventions hold promise to be the first major breakthrough in the therapy of CKD patients since the introduction of dialysis.
CONCLUSIONS
PEW is one of the strongest risk factors associated with mortality in both dialysis patients and other CKD patient populations. More studies are needed to establish which markers of PEW would best predict outcomes in patients with NDD-CKD. The causal nature of the relationship between markers of PEW and mortality could be proven if clinical trials aimed at improving nutritional status in CKD patients result in a survival benefit.
References
- 1.U.S. Renal Data System. USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 2.Kovesdy CP, Trivedi BK, Anderson JE. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis. 2006;13:183–188. doi: 10.1053/j.ackd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 4.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann EH, Bower JD, Salahudeen AK. Risk factor paradox in hemodialysis: better nutrition as a partial explanation. ASAIO J. 2001;47:74–81. doi: 10.1097/00002480-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Kopple JD. How to reconcile conventional and altered risk factor patterns in dialysis patients. Semin Dial. 2007;20:602–605. doi: 10.1111/j.1525-139X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 8.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Kalantar-Zadeh K. Introduction: the reverse epidemiology controversy. Semin Dial. 2007;20:485. doi: 10.1111/j.1525-139X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt DS, Salahudeen AK. Obesity-survival paradox-still a controversy? Semin Dial. 2007;20:486–492. doi: 10.1111/j.1525-139X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C, Landray MJ, Wheeler DC. Misleading associations between cholesterol and vascular outcomes in dialysis patients: the need for randomized trials. Semin Dial. 2007;20:498–503. doi: 10.1111/j.1525-139X.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 16.Shinaberger CS, Kilpatrick RD, Regidor DL, et al. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis. 2006;48:37–49. doi: 10.1053/j.ajkd.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20:1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 18.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 20.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse Association between Lipid Levels and Mortality in Men with Chronic Kidney Disease Who Are Not Yet on Dialysis: Effects of Case Mix and the Malnutrition-Inflammation-Cachexia Syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 23.Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863–870. doi: 10.1053/j.ajkd.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 24.Jurkovitz C, Li S, Bakris G, Brown W, McCullough P, Vassalotti J, McGill J, Singh A, Norris K. Effect of obesity on mortality in patients at high risk for kidney disease: results from KEEP. Journal of the American Society of Nephrology. 2006;17:11A. [Google Scholar]
- 25.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 26.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:992–998. doi: 10.2215/CJN.04221206. [DOI] [PubMed] [Google Scholar]
- 27.Madero M, Sarnak MJ, Wang X, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411. doi: 10.1053/j.ajkd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Escalante A, Haas RW, del R I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–1629. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139:1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 31.Hill AB. THE ENVIRONMENT AND DISEASE: ASSOCIATION OR CAUSATION? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopple JD, Zhu X, Lew NL, et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 33.Kopple JD. McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr. 1997;65:1544–1557. doi: 10.1093/ajcn/65.5.1544. [DOI] [PubMed] [Google Scholar]
- 34.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 35.Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S39–S50. doi: 10.1016/j.ejon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Lee PS, Waxman AB, Cotich KL, et al. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit Care Med. 2007;35:849–855. doi: 10.1097/01.CCM.0000253815.26311.24. [DOI] [PubMed] [Google Scholar]
- 37.Rothenbach PA, Dahl B, Schwartz JJ, et al. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol. 2004;96:25–31. doi: 10.1152/japplphysiol.01074.2002. [DOI] [PubMed] [Google Scholar]
- 38.Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J Investig Med. 2002;50:54–60. doi: 10.2310/6650.2002.33518. [DOI] [PubMed] [Google Scholar]
- 39.Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2008;121:S388–S392. doi: 10.1016/j.jaci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Kalantar-Zadeh K, Kopple J. Malnutrition as a cause of morbidity and mortality in dialysis patients. In: Kopple J, Massry S, editors. Nutritional Management of Renal Disease. Philadelphia, Lipincott: Williams & Wilkins; 2004. [Google Scholar]
- 41.Hulsewe KW, van Acker BA, von Meyenfeldt MF, et al. Nutritional depletion and dietary manipulation: effects on the immune response. World J Surg. 1999;23:536–544. doi: 10.1007/pl00012344. [DOI] [PubMed] [Google Scholar]
- 42.Souba WW. Nutritional support. N Engl J Med. 1997;336:41–48. doi: 10.1056/NEJM199701023360107. [DOI] [PubMed] [Google Scholar]
- 43.Alexander JW. Immunoenhancement via enteral nutrition. Arch Surg. 1993;128:1242–1245. doi: 10.1001/archsurg.1993.01420230070011. [DOI] [PubMed] [Google Scholar]
- 44.Kimmel PL, Phillips TM, Lew SQ, et al. Zinc modulates mononuclear cellular calcitriol metabolism in peritoneal dialysis patients. Kidney Int. 1996;49:1407–1412. doi: 10.1038/ki.1996.198. [DOI] [PubMed] [Google Scholar]
- 45.Erten Y, Kayatas M, Sezer S, et al. Zinc deficiency: prevalence and causes in hemodialysis patients and effect on cellular immune response. Transplant Proc. 1998;30:850–851. doi: 10.1016/s0041-1345(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 46.Casciato DA, McAdam LP, Kopple JD, et al. Immunologic abnormalities in hemodialysis patients: improvement after pyridoxine therapy. Nephron. 1984;38:9–16. doi: 10.1159/000183270. [DOI] [PubMed] [Google Scholar]
- 47.Dobbelstein H, Korner WF, Mempel W, et al. Vitamin B6 deficiency in uremia and its implications for the depression of immune responses. Kidney Int. 1974;5:233–239. doi: 10.1038/ki.1974.28. [DOI] [PubMed] [Google Scholar]
- 48.DeSimone C, Famularo G, Tzantzoglou S, et al. Carnitine depletion in peripheral blood mononuclear cells from patients with AIDS: effect of oral L-carnitine. AIDS. 1994;8:655–660. doi: 10.1097/00002030-199405000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Vanholder R, Dell’Aquila R, Jacobs V, et al. Depressed phagocytosis in hemodialyzed patients: in vivo and in vitro mechanisms. Nephron. 1993;63:409–415. doi: 10.1159/000187244. [DOI] [PubMed] [Google Scholar]
- 50.Vanholder R, Van LA, Dhondt AM, et al. Influence of uraemia and haemodialysis on host defence and infection. Nephrol Dial Transplant. 1996;11:593–598. doi: 10.1093/oxfordjournals.ndt.a027346. [DOI] [PubMed] [Google Scholar]
- 51.Kalantar-Zadeh K, Daar ES, Eysselein VE, et al. Hepatitis C infection in dialysis patients: a link to poor clinical outcome? Int Urol Nephrol. 2007;39:247–259. doi: 10.1007/s11255-006-9075-8. [DOI] [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 53.Ritz E. Why are lipids not predictive of cardiovascular death in the dialysis patient? Miner Electrolyte Metab. 1996;22:9–12. [PubMed] [Google Scholar]
- 54.Kaysen GA. Inflammation nutritional state and outcome in end stage renal disease. Miner Electrolyte Metab. 1999;25:242–250. doi: 10.1159/000057455. [DOI] [PubMed] [Google Scholar]
- 55.Bergstrom J, Lindholm B, Lacson E, Jr, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial. 2000;13:163–175. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 56.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 59.Kalantar-Zadeh K, Block G, McAllister CJ, et al. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 60.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 61.Ling PR, Smith RJ, Kie S, et al. Effects of protein malnutrition on IL-6-mediated signaling in the liver and the systemic acute-phase response in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R801–R808. doi: 10.1152/ajpregu.00715.2003. [DOI] [PubMed] [Google Scholar]
- 62.Kalantar-Zadeh K, Ikizler TA, Block G, et al. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Dolson GM. Do potassium deficient diets and K+ removal by dialysis contribute to the cardiovascular morbidity and mortality of patients with end stage renal disease? Int J Artif Organs. 1997;20:134–135. [PubMed] [Google Scholar]
- 64.Kalantar-Zadeh K, Kopple JD, Deepak S, et al. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler TR, Evans ME, Fernandez-Estivariz C, et al. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 66.Jandacek RJ, Anderson N, Liu M, et al. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol. 2005;288:G292–G299. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- 67.Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 68.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 69.Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 70.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008 doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 71.Kalantar-Zadeh K, Streja E, Kopple JD, McAllister CJ, Kovesdy CP. A possible explanation for hemoglobin-survival paradox? J Am Soc Nephrol. 2007;18:81A. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 73.Pupim LB, Flakoll PJ, Ikizler TA. Nutritional supplementation acutely increases albumin fractional synthetic rate in chronic hemodialysis patients. J Am Soc Nephrol. 2004;15:1920–1926. doi: 10.1097/01.asn.0000128969.86268.c0. [DOI] [PubMed] [Google Scholar]
- 74.Pupim LB, Majchrzak KM, Flakoll PJ, et al. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17:3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 75.Stratton RJ, Bircher G, Fouque D, et al. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2005;46:387–405. doi: 10.1053/j.ajkd.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 76.Czekalski S, Hozejowski R. Intradialytic amino acids supplementation in hemodialysis patients with malnutrition: results of a multicenter cohort study. J Ren Nutr. 2004;14:82–88. doi: 10.1053/j.jrn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Feldt-Rasmussen B, Lange M, Sulowicz W, et al. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol. 2007;18:2161–2171. doi: 10.1681/ASN.2006111207. [DOI] [PubMed] [Google Scholar]
- 78.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. 1999;281:1275–1281. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 79.Barton PA, Chretien C, Lau AH. The effects of nandrolone decanoate on nutritional parameters in hemodialysis patients. Clin Nephrol. 2002;58:38–46. doi: 10.5414/cnp58038. [DOI] [PubMed] [Google Scholar]
- 80.Navarro JF, Mora C, Macia M, et al. Randomized prospective comparison between erythropoietin and androgens in CAPD patients. Kidney Int. 2002;61:1537–1544. doi: 10.1046/j.1523-1755.2002.00271.x. [DOI] [PubMed] [Google Scholar]
- 81.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis rapidly improves nutritional status in hemodialysis patients. Kidney Int. 2001;60:1555–1560. doi: 10.1046/j.1523-1755.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 82.Galland R, Traeger J, Arkouche W, et al. Short daily hemodialysis and nutritional status. Am J Kidney Dis. 2001;37:S95–S98. doi: 10.1053/ajkd.2001.20758. [DOI] [PubMed] [Google Scholar]
- 83.Cano NJ, Fouque D, Roth H, et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol. 2007;18:2583–2591. doi: 10.1681/ASN.2007020184. [DOI] [PubMed] [Google Scholar]
- 84.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 85.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 86.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]


