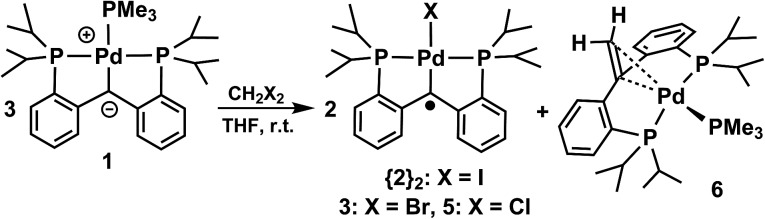
A series of palladium(ii) persistent radical carbene complexes, [PC˙(sp2)P]PdX (X = Cl, Br, I), was synthesized from the nucleophilic carbene [PC(sp2)P]PdPMe3.
A series of palladium(ii) persistent radical carbene complexes, [PC˙(sp2)P]PdX (X = Cl, Br, I), was synthesized from the nucleophilic carbene [PC(sp2)P]PdPMe3.
Abstract
A series of palladium(ii) radical carbene complexes, [PC˙(sp2)P]PdI, [PC˙(sp2)P]PdBr, and [PC˙(sp2)P]PdCl (PC(sp3)H2P = bis[2-(di-iso-propylphosphino)-phenyl]methane), is described. Compound [PC˙(sp2)P]PdI dimerizes to {[PC(sp2)P]PdI}2 in the solid state, akin to the formation of Gomberg's dimer. While the bromo and the iodo derivatives could be obtained from the oxidation of [PC(sp2)P]Pd(PMe3) by the respective dihalogens, a halogen transfer reaction from CH2Cl2 was used for the formation of [PC˙(sp2)P]PdCl. The halogen transfer from CH2X2 (X = Cl, Br, I) could be used to obtain all three radical carbene palladium complexes and also allowed the isolation of [PC(CH2)P]Pd(PMe3), which is the result of methylene group transfer from CH2X2. Compound [PC(CH2)P]Pd(PMe3) was independently synthesized from [PC(CH3)HP]PdCl2, which contains a supporting ligand analogous to that of the radical carbene complexes but has one of the hydrogen atoms replaced by a methyl group. All three carbene radical species abstract a hydrogen from 9,10-dihydroanthracene or n Bu3SnH.
Introduction
Radicals have long fascinated scientists in general and chemists in particular.1 Controlling the reactivity of such species has been challenging but rewarding; the isolation of stable radicals opens new avenues for finding interesting reactions.2 One way to control these species is by coordination to transition metals, which can impart selectivity to the reactions of these radicals via metal control and/or auxiliary ligands.3
Although transition metal carbene complexes, which can display electrophilic or nucleophilic character, have been studied for some time,4 the corresponding radical species have been known mostly for electrophilic carbenes (Fischer type) of late transition metals. They are obtained by the reduction of the corresponding complexes,5 and are mostly observed as transient species with intricate reactivity.6 However, these radicals are very reactive and their characterization proved to be challenging. 5a,7 A few examples of two-coordinate metal complexes containing cyclic alkylamino carbene ligands with singlet biradicaloid character were recently reported.8 Herein, we report the synthesis of such a series, i.e., palladium(ii) carbenes as persistent radicals originating from a nucleophilic carbene.
Results and discussion
Synthesis and characterization of palladium radical carbene complexes
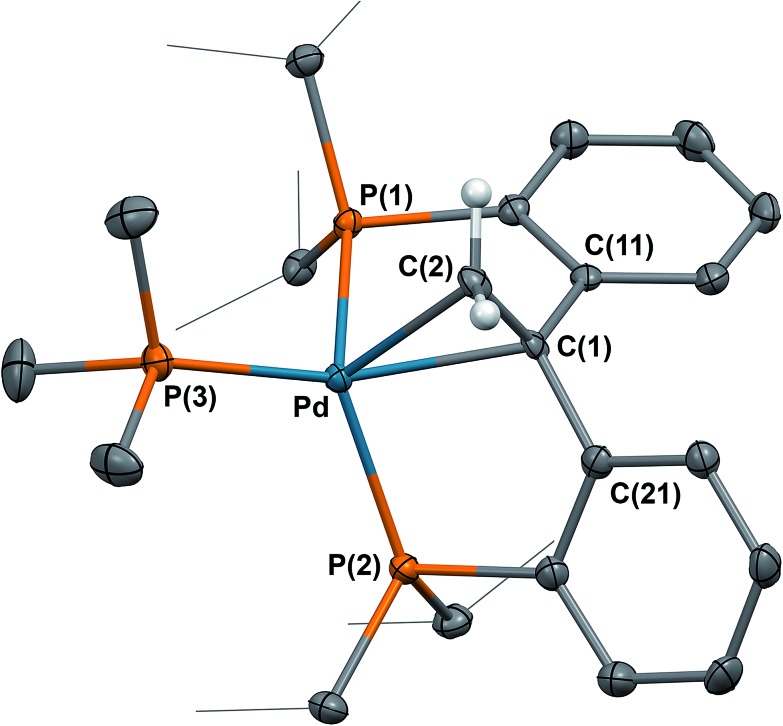
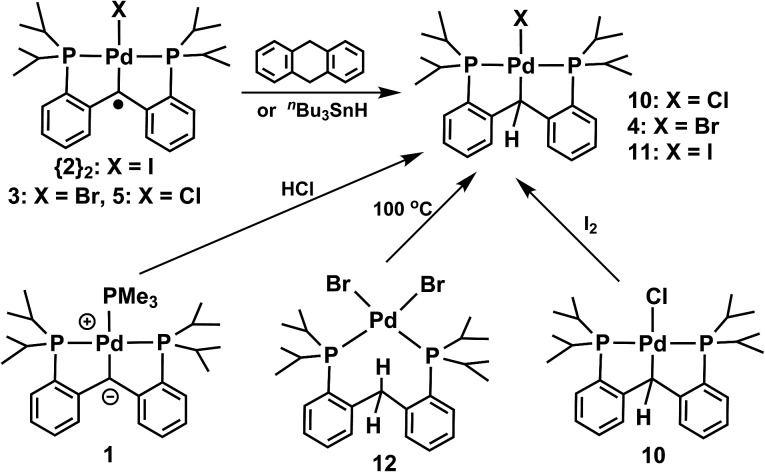
We previously established that the carbene carbon in [PC(sp2)P]Pd(PMe3) (1, PC(sp3)H2P = bis[2-(di-iso-propylphosphino)-phenyl]methane)9 has nucleophilic character.10 DFT calculations indicated that the HOMO of 1 is localized on the carbene carbon atom, therefore, the loss of an electron might occur from the same orbital. Compound 1 shows a reversible oxidation wave at –0.15 V vs. Cp2Fe/Cp2Fe+ by cyclic voltammetry (ESI: Fig. S2†). Although the chemical oxidation of 1 with [Cp2Fe]+ did not result in an isolable product, the analogous reaction with I2 (Scheme 1) allowed the observation in solution of a green paramagnetic species, [PC˙(sp2)P]PdI (2), in good yield (70%). Compound 2 represents, to the best of our knowledge, the first example of a palladium radical carbene complex. The radical nature of 2 in solution is supported by the value of the magnetic moment of 1.76 μ B, corresponding to one unpaired electron. Interestingly, 2 dimerizes in the solid state to form {2}2 (Fig. 1), the result of radical coupling.
Scheme 1. Synthesis of radical carbene palladium complexes.
Fig. 1. Molecular structure of {2}2, 3, and 5 with thermal ellipsoids at 50% probability. Most hydrogen atoms were omitted for clarity. Selected distances (Å) and angles (°) for {2}2: Pd(1)–C(1) = 2.127(3), Pd(2)–C(2) = 2.043(3), C(2)–C(51) = 1.380(4), C(2)–C(61) = 1.481(4); for 3: Pd–C = 2.020(3), Pd–Br = 2.5117(4), C(11)–C–C(21) = 123.0(3), Pd–C–C(11) = 118.1(2), Pd–C–C(21) = 118.7(2); for 5: Pd–C = 2.005(2), Pd–Cl = 2.3884(6), C(11)–C–C(21) = 122.2(2), Pd–C–C(11) = 118.78(17), Pd–C–C(21) = 118.87(17).
The formation of {2}2 from 2 (Scheme 1) is analogous to the formation of Gomberg's dimer.11 Metrical parameters for {2}2 agree with this interpretation. For example, the C–C distances C(51)–C(52), C(52)–C(53), C(53)–C(54), C(54)–C(55), C(55)–C(56), and C(56)–C(51) of 1.465(4), 1.345(4), 1.496(4), 1.508(4), 1.339(4), and 1.465(4) Å, respectively, in the dearomatized phenyl ring show bond alternation. In addition, the C(2)–C(51) distance of 1.380(4) Å indicates double bond character, while the C(2)–C(61) distance of 1.481(4) Å is consistent with a single bond. Moreover the C(1)–C(54) distance of 1.588(4) Å indicates an elongated C–C bond, in agreement with a weak interaction between the two monomers in the solid state. The Pd(1)–C(1) distance of 2.127(3) Å is slightly longer than the corresponding value in 1 (2.086(4) Å). To investigate the dimerization process further, a variable temperature magnetization study in solution indicated that by lowering the temperature, the magnetic moment of 2 decreases from 1.76μ B at room temperature to 1.39μ B at 220 K, in agreement with the formation of the dimer at lower temperatures.
The dimerization of 2 is the consequence of radical coupling of one of its resonance structure (radical on the para position of the phenyl ring, Scheme 1). The resonance structures possible for 2 show that the radical creates an increased electron density on the ortho and para positions of the phenyl ring; both the central carbene carbon and the two ortho positions are sterically hindered, therefore, the contribution of the negatively charged para resonance structure is significant in determining the coupling position.
We also pursued the synthesis of the chloro and bromo analogues of 2. In a similar manner, the reaction between 1 and Br2 (Scheme 1) generated a new paramagnetic species, [PC˙(sp2)P]PdBr (3). In the reaction mixture we also observed [PC(sp3)HP]PdBr (4), likely due to the presence of small amounts of HBr in Br2. We previously reported the protonation of carbene 1 with HCl10a and, in a similar reaction, the formation of [PC(sp3)HP]PdBr (4) could be accomplished from 1 and HBr. Interestingly, in this case, the radical carbene 3 is monomeric in both solution and the solid state. The solution magnetic moment (2.19μ B) confirms a one electron radical species. While mononuclear Pd(iii) complexes are known,12 in this case the oxidation takes place on the ligand, similar to the oxidation of PNP or MePNP (PNP = (o-PiPr2-C6H4)2N; MePNP = (2-PiPr2-4-MeC6H3)2N) complexes that generate a nitrogen-based radical.13 In the solid state (Fig. 1), the Pd(ii) metal center is distorted square planar, with a Pd–C distance of 2.020(3) Å, slightly shorter than that observed for 1 (2.086(4) Å) or [PC(sp3)HP]PdBr (4, 2.071(4) Å). The carbene carbon is planar with a sum of angles of 359.8°.
The last complex of the series, the chloro derivative, [PC˙(sp2)P]PdCl (5) was synthesized by halogen atom abstraction from dichloromethane in an analogous manner with the synthesis of [F(PNP)PtCl][BArF 4] (F(PNP) = (4-F-2-(iPr2P)C6H3)2N; ArF = 3,5-(CF3)2C6H3) to generate a nitrogen based radical on the PNP ligand.14 The reaction proceeds slowly at room temperature and, after 2.5 days, the product was obtained in 75% of the theoretical yield (Scheme 2).
Scheme 2. Reactions of 1 with dihalogenomethanes.
Similarly to the bromo derivative, the chloro substituted complex 5 is monomeric in both solution (1.86μ B) and the solid state. The molecular structure of 5 (Fig. 1) indicates that the carbene carbon is planar and found at 2.005(2) Å from the metal center. Like the bromo and the iodo derivatives, 5 is best described as a Pd(ii) metal center attached to a radical carbon, interpretation supported by DFT calculations (see below and ESI: Fig. S27†).
EPR spectroscopy indicates that all three radical species, [PC˙(sp2)P]PdI (2), [PC˙(sp2)P]PdBr (3), and [PC˙(sp2)P]PdCl (5) display g-factors close to 2, supporting the radical state of the backbone. 5b,15 However, the g-factor increases slightly from 5 (g = 2.0100) to 3 (g = 2.0105), and to 2 (g = 2.0110). No hyperfine structure was resolved for [PC˙(sp2)P]PdBr and [PC˙(sp2)P]PdI, but dilute solutions of [PC˙(sp2)P]PdCl display a well-resolved hyperfine splitting (Fig. 2) attributed to 8 phenyl ring protons: a 1(2H) = 4.5 G; a 2(2H) = 2.6 G; a 3(2H) = 2.1 G; a 4(2H) = 1.2 G. The computed hyperfine interactions for 5, a(2H, 4,4′) = 4.54 G; a(2H, 6,6′) = 4.18 G; a(2H, 5,5′) = 2.00 G; a(2H, 3,3′) = 1.90 G, are in agreement with experimental values. Hyperfine coupling to 105Pd nucleus (nuclear spin 5/2, natural abundance 22.33%) gives rise to broad satellites on either side of the central multiplet (Fig. 2). In 2 and 3, hyperfine coupling to ring protons contributes to the inhomogeneous line width (ESI: Fig. S3–S5† for details).
Fig. 2. EPR spectrum for 5 (298 K, 100 μM solution in toluene, X-band). The blue line represents the experimental data and the red line the simulated spectrum. The small satellite peak on the right comes from the quartz sample tube.
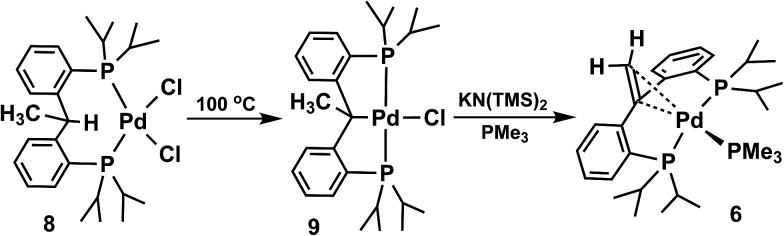
The halogen atom transfer reaction from CH2X2 to 1 also proved a good way to synthesize {2}2 (X = I) and 3 (X = Br). In all cases, the identity of the paramagnetic species was confirmed by X-ray crystallography, solution magnetic moment, and subsequent reactivity studies (see below). A new diamagnetic complex was observed in all three crude reaction mixtures (Scheme 2). The corresponding 1H NMR spectra show a new resonance as a doublet at 3.81 ppm in the olefinic region, while the 31P NMR spectra display an AX2 spin system, δ (A) is –31.55 ppm (t, 2 J PP = 21.6 Hz) and δ (X2) is 33.85 ppm (d, 2 J PP = 21.7 Hz), consistent with the presence of PMe3 in the molecule. The 13C NMR spectra show the backbone carbon atom resonating at 112.16 ppm as a doublet of triplets, due to coupling to both types of phosphorus nuclei present in the molecule (J CP = 17.6 Hz, J CP = 2.3 Hz), and a methylenic carbon is found at 64.4 ppm as a triplet of doublets due to long range phosphorus coupling (J CP = 7.5 Hz, J CP = 5.1 Hz). X-ray crystallography indicates that the new product, [PC(CH2)P]Pd(PMe3) (6), is the result of coupling of the CH2 group of dihalogenomethane with the carbene carbon of 1 to generate a new carbon–carbon double bond. The C C bond formation in 6 is somewhat reminiscent of the formation of the C O bond in the iridaepoxide [P′C(O)P′]IrCl (P′C(sp3)H2P′ = bis[2-(di-iso-propylphosphino)-benzothiophene]methane), isolated from the reaction of the iridium PCcarbeneP complex [P'CcarbeneP′]IrCl and N2O.16
To the best of our knowledge, a similar “CH2” transfer reaction as that described above has not been previously characterized, although examples of nickel17 or iron18 catalysed cross-coupling reactions of CH2Cl2 with Grignard reagents are known. It has also been reported that Kharasch addition reactions of perhalogenated reagents to olefins involve Pd(0)/Pd(i) or Pd(ii)/Pd(iii) oxidations and halogen transfer, but the intermediate metal species have not been characterized.19 It is important to note that in the reactions of 1 with CH2X2 (X = Cl, Br, I), palladium is not oxidized and the electron transfer takes place at the carbene ligand.
In 6 (Fig. 3), the metal centre is coordinated in a side-bound fashion to the new C C double bond.20 The metrical parameters for 6 point to a distorted tetrahedral palladium(0) metal center (P(1)–Pd–P(3) = 109.332(19)°, P(2)–Pd–P(3) = 118.946(19)°, P(1)–Pd–P(2) = 115.868(19)°). The C(1)–C(2) distance of 1.398(3) Å is slightly longer than 1.34 Å (C(sp2)–C(sp2)), likely due to π-backbonding.
Fig. 3. Molecular structure of 6 with thermal ellipsoids at 50% probability. Most hydrogen atoms were omitted for clarity. Selected distances (Å) and angles (°): C(1)–C(2) = 1.398(3), Pd–C(1) = 2.2242(18), Pd–C(2) = 2.2613(19), C(1)–Pd–C(2) = 36.30(7).
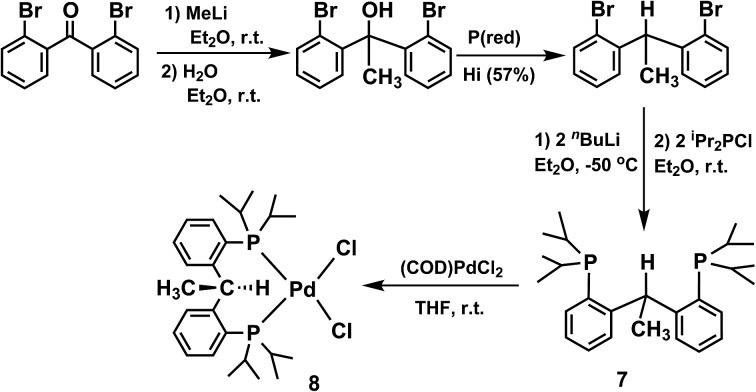
The formation of 6 in the above reactions (Scheme 2) occurs in a relatively low yield since two thirds of 1 convert to the radical species, while the rest converts to 6 (75%, 38%, and 64% conversion to 6 observed besides the formation of {2}2, 3, and 5, respectively, isolated yield). Therefore, an independent synthesis of compound 6 was designed. We reasoned that the deprotonation of a methyl group that is a substituent of the carbon atom that connects the two phosphine phenyl rings in [PC(CH3)HP] (7) would lead to the isolation of 6. Compound 7 was synthesized in three steps from bis(2-bromophenyl)methanone (Scheme 3). Reaction of bis(2-bromophenyl)methanone with methyl lithium led to the isolation of the 1,1-bis(2-bromophenyl)ethan-1-ol. Reduction of this carbinol in the presence of red phosphorous and hydroiodic acid generated 1,1-bis(2-bromophenyl)ethane, the precursor for 7. [PC(CH3)HP] (7) was synthesized from this precursor by double lithiation with n BuLi followed by metathesis with iPr2PCl in 75% yield, as a clear oil. From 7, a palladium(ii) complex could be isolated in 76% yield by mixing (COD)PdCl2 and [PC(CH3)HP] at room temperature in THF to give [PC(CH3)HP]PdCl2 (8).
Scheme 3. Synthesis of PC(CH3)HP (7) and [PC(CH3)HP]PdCl2 (8).
Heating [PC(CH3)HP]PdCl2 (8) at 100 °C in toluene resulted in the C–H activation of the backbone (Scheme 4); a subsequent dehydrohalogenation generates the square planar complex [PC(CH3)P]PdCl (9).10a A second dehydrohalogenation, using KN(SiMe3)2 in the presence of PMe3, achieved the second C–H activation, now at the methyl group, and led to the isolation of 6 (Scheme 4) in high yield (75%).
Scheme 4. Independent synthesis of 6.
DFT calculations
DFT calculations were carried out using Gaussian09 on model complexes of the three carbene radical species, 2′, 3′, and 5′, in which the iso-propyls on phosphines were replaced by methyl groups. Geometry optimization results indicate a good agreement between the calculated structures of 3′ and 5′ and the corresponding experimental structures (Table 1).
Table 1. Selected metrical parameters for the calculated and experimental structures of 3 and 5 .
| 3′ (DFT) | 3 (X-ray) | 5′ (DFT) | 5 (X-ray) | |
| Pd–C (Å) | 2.047 | 2.020(3) | 2.042 | 2.005(2) |
| Pd–X (Å) | 2.604 | 2.5117(4) | 2.463 | 2.3884(6) |
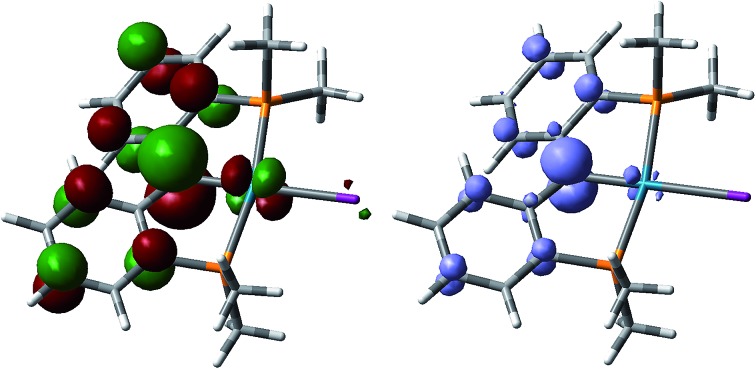
DFT calculations indicate that the unpaired electron is localized mostly on the carbene carbon atom and slightly delocalized over the two phenyl rings in 2′, 3′, and 5′ (Fig. 4). These results together with the fact that ca. 64% of the spin density was found on the former carbene carbon atom for all three radical carbene complexes support the interpretation that most of the spin density rests on this atom (Fig. 4). Furthermore, the composition of the SOMO for the three radical species indicates that 63.9%, 63.7%, and 63.4% of the p orbital of the carbon atom and 2.2%, 2.3%, and 2.4% of the palladium d orbital contribute in 2′, 3′, and 5′, respectively. Since the three radical species are formed by the oxidation of 1 and the electron is removed from an antibonding orbital, the order of the Pd–C bond increases for 2′, 3′, and 5′, respectively, as also shown by the decrease of the Pd–C distance in the radical species (see above).
Fig. 4. Left: SOMO for 2′; right: spin density for 2′.
In agreement with experimental results, the corresponding coupling product {2}2 is less stable than 2 by 4.96 kcal mol–1, consistent with the observation of the monomeric species in solution. Consequently, the difference between the respective dimers, {3}2 and {5}2, and the radical species increases from the iodo to the bromo (6.45 kcal mol–1) and chloro (8.03 kcal mol–1) derivative. The fact that 3 and 5 do not dimerize in the solid state is also supported by a slight decrease of the spin density on the para carbon involved in the coupling reaction (18.23% in 2, 18.06% in 3, and 18.03% in 5).
Reactivity studies of palladium radical carbene complexes
The radical nature of all three carbene radical species was probed by their reactions with hydrogen atom donors, 9,10-dihydroanthracene and n Bu3SnH (Scheme 5). In all cases, the reactions were slow and it took a few hours in order to achieve moderate conversions; consequently, the isolated yields were relatively low: 14% for 10, 16% for 4, and 17% for 11 for the reaction with 9,10-dihydroanthracene and 48% for 10, 24% for 4, and 78% for 11 for the reaction with n Bu3SnH. In order to confirm the identity of the respective products of these reactions, [PC(sp3)HP]PdCl (10),10a [PC(sp3)HP]PdBr (4), and [PC(sp3)HP]PdI (11) were compared with samples synthesized by independent methods (Scheme 5). Compound [PC(sp3)HP]PdCl (10) was previously reported.10a Compound [PC(sp3)HP]PdBr (4) was synthesized using a method analogous to that used for the synthesis of [PC(sp3)HP]PdCl: [PC(sp3)H2P]PdBr2 (12) could be isolated by reacting PC(sp3)H2P (13) with (COD)PdBr2 at ambient temperature21 in 91% yield. This complex undergoes dehydrohalogenation through ligand C–H activation by heating it at 100 °C in toluene to generate [PC(sp3)HP]PdBr (4) in high yield (83%). On the other hand, compound [PC(sp3)HP]PdI was synthesized from the reaction of [PC(sp3)HP]PdCl with one equivalent of I2 in THF. A usual workup with Et2O allowed the isolation of 11 as light yellow crystals in 68% yield.
Scheme 5. Reactions of the radical carbene palladium complexes with hydrogen donors and alternate synthesis of these products.
Conclusions
In conclusion, we described the formation of a series of palladium(ii) radical carbene complexes, [PC˙(sp2)P]PdI (2), [PC˙(sp2)P]PdBr (3), and [PC˙(sp2)P]PdCl (5). These radical species are persistent in solution and, for 3 and 5, also in the solid state as indicated by X-ray crystallography. Metrical parameters for 3 and 5 indicate that Pd–C distances are slightly shorter than the corresponding values observed for [PC(sp2)P]Pd(PMe3) (1) and [PC(sp3)HP]PdX (X = Cl, Br). The carbene carbon is planar and palladium shows a distorted square planar geometry in both metal complexes. Compound 2 dimerizes in the solid state to {2}2, akin to the formation of Gomberg's dimer.
While 2 and 3 could be obtained from the oxidation of [PC(sp2)P]Pd(PMe3) by the respective dihalogens, a halogen transfer reaction from CH2Cl2 was used for the formation of 5. The halogen transfer from CH2Br2 and CH2I2 also led to the isolation of the corresponding radical carbene palladium complexes; in addition, this reaction allowed the isolation of [PC(CH2)P]Pd(PMe3) (6), the result of methylene group transfer. Compound 6 was independently synthesized from [PC(CH3)HP]PdCl2, which contains a supporting ligand analogous to that of the radical carbene complexes but has one of the hydrogen atoms replaced by a methyl group.
The radical nature of the carbene carbon was also confirmed by the results of DFT calculations and EPR spectroscopy. All three radical species, [PC˙(sp2)P]PdI (2), [PC˙(sp2)P]PdBr (3), and [PC˙(sp2)P]PdCl (5), display g factors close to 2, supporting the radical state of the backbone. In addition, although no hyperfine structure was resolved for [PC˙(sp2)P]PdBr and [PC˙(sp2)P]PdI, dilute solutions of [PC˙(sp2)P]PdCl display a well-resolved hyperfine splitting attributed to 8 phenyl ring protons and 105Pd nucleus.
Reactivity studies with 2, 3, and 5 showed that all three compounds abstract a hydrogen from 9,10-dihydroanthracene or n Bu3SnH supporting their radical nature. In addition to hydrogen-abstraction reactions, the radical carbene species discussed herein may be involved in redox reactions, conferring the supporting ligand a non-innocent behavior.22 We are currently exploring these possibilities.
Experimental
All experiments are performed under an inert atmosphere of N2 using standard glovebox techniques. Solvents, hexane, n-pentane, CH2Cl2, and diethylether, were dried by passing through a column of activated alumina and stored in the glovebox. THF was dried over LiAlH4 followed by vacuum transfer and stored in the glovebox. Deuterated solvents, CDCl3 and CD2Cl2, were dried over 4 Å molecular sieves under N2, while C6D6 and C6D5CD3 were dried over CaH2 followed by vacuum transfer, and stored in the glovebox. Bis(2-bromophenyl)methanone,23 [PC(sp2)P]Pd(PMe3) (1),10a [PC(sp3)HP]PdCl (10),10a and PC(sp3)H2P (13)9b were prepared according to literature procedures. All other materials were used as received. 1H, 13C{1H} and 31P{1H} NMR spectra were recorded on a Bruker DRX 400 or 500 spectrometer. All chemical shifts are reported in δ units with references to the residual solvent resonance of the deuterated solvents for proton and carbon chemical shifts or to external H3PO4 for 31P. Magnetic moments were determined by the Evans method24 using capillaries containing hexamethylsiloxane in C6D6 as a reference and hexamethylsiloxane in the sample solution. EPR spectra were recorded on a Bruker EMXplus EPR spectrometer with a standard X-band EMXplus resonator and an EMX premiumX microwave bridge. Electrochemical data was collected on a Metrohm Autolab PGSTAT-128N instrument. CHN analyses were performed on a CE-440 Elemental Analyzer or by Midwest Microlab, LLC. Gaussian 03 (revision D.02) was used for all reported calculations.25 The B3LYP (DFT) method was used to carry out the geometry optimizations on model compounds specified in text using the LANL2DZ basis set. The validity of the true minima was checked by the absence of negative frequencies in the energy Hessian.
Synthesis of {[PC(sp2)P]PdI}2 ({2}2)
58.1 mg of [PC(sp2)P]Pd(PMe3) (1, 0.1 mmol) was dissolved in THF and cooled at –50 °C. Then, 0.5 mL of a chilled I2 solution in THF (0.1 M, 0.05 mmol, –50 °C) was added dropwise and a rapid colour change was observed from dark brown to very dark green-brown. The solution was allowed to react for two additional hours at –50 °C, then warmed up to room temperature and the volatiles were removed under reduced pressure. The residual powder was triturated with n-pentane. The residue was dissolved in Et2O, filtered through a Celite plug and set to crystallize at –35 °C in the glovebox to yield analytically pure {2}2 (44.2 mg, 70%). For {2}2: 1H NMR (400 MHz, C7D8, 250 K) δ = 7.77 (d, J = 6.1 Hz, 1H, ArH), 7.27 (d, J = 8.0 Hz, 1H, ArH), 7.17 (d, J = 5.6 Hz, 1H, ArH), 6.92 (m, 9H, ArH), 6.58 (d, J = 9.3 Hz, 1H, ArH), 6.36 (d, J = 10.7 Hz, 1H, ArH), 5.30 (d, J = 9.0 Hz, 1H, ArH), 4.95 (s, 1H, ArH), 2.56 (m, 2H, CH(CH3)2), 2.44 (m, 2H, CH(CH3)2), 2.38 (m, 2H, CH(CH3)2), 2.34 (m, 2H, CH(CH3)2), 1.48 (m, 12H, CH(CH 3)2), 1.29 (m, 21H, CH(CH 3)2), 0.87 (m, 12H, CH(CH 3)2), 0.67 (m, 3H, CH(CH 3)2). 31P{1H} NMR (162 MHz, C7D8, 250 K) δ = 60.60 (d, J = 350.4 Hz), 30.68 (d, J = 350.5 Hz), 62.20 (d, J = 363.3 Hz), 58.11 (d, J = 363.1 Hz). 1H NMR (400 MHz, C6D6, 300 K) δ = 5.26 (br, Δ 1/2 = 666.67 Hz, 8H, ArH), 2.96 (br, Δ 1/2 = 414.81 Hz, 24H, CH(CH 3)2), 1.36 (br, Δ 1/2 = 88.88 Hz, 2H, CH(CH3)2), 1.00 (br, Δ 1/2 = 103.70 Hz, 2H, CH(CH3)2). Magnetic moment (298 K): μ eff = 1.76μ B. EPR: g = 2.0110. Anal. calcd for C50H72I2P4Pd2: C, 47.52; H, 5.74. Found: C, 47.50; H, 5.70.
Synthesis of [PC˙(sp2)P]PdBr (3)
A cold solution of 58.1 mg [PC(sp2)P]Pd(PMe3) (1, 0.1 mmol) in 5 mL THF at –35 °C was stirred for 5 min prior to the addition of 0.05 mL solution of Br2 (0.1 M in n-pentane). The colour changed rapidly to green. In the crude mixture, the presence of [PC(sp3)HP]PdBr (4) was determined by 1H NMR spectroscopy but was not quantified. The volatiles were removed under reduced pressure and the residue extracted in Et2O, concentrated, and filtered over Celite. Analytically pure 3 crystallized at –35 °C (36.8 mg, 63%). For 3: 1H NMR, 31P{1H} NMR and 13C{1H} NMR spectra show no signals. Magnetic moment (298 K): μ eff = 2.19μ B. EPR: g = 2.0105. Anal. calcd for C25H36BrP2Pd: C, 51.34; H, 6.20. Found: C, 50.99; H, 6.15.
Synthesis of [PC(sp3)H2P]PdBr2 (12) (Scheme 6)
Scheme 6. Synthesis of [PC(sp3)H2P]PdBr2 (12).
A mixture of 420.5 mg of PC(sp3)H2P (13, 1.05 mmol) and 374.4 mg (COD)PdBr2 (1 mmol) in 10 mL THF was stirred for 3 hours. Over this period of time, the mixture changed colour from cream to pale orange/light yellow. The volatiles were removed under reduced pressure and the residue was triturated 3 times with 5 mL of n-pentane. The resulted orange powder was dried under reduced pressure and was analytically pure by 1H and 31P{1H} NMR spectroscopy. Yield: 340.7 mg, 91%. For 12: 1H NMR (500 MHz, CD2Cl2, 248 K) δ = 7.63 (t, J = 7.9 Hz, 1H, ArH), 7.60–7.55 (m, 1H, ArH), 7.51 (t, J = 7.2 Hz, 1H, ArH), 7.42 (t, J = 7.5 Hz, 1H, ArH), 7.37 (dt, J = 14.8, 6.3 Hz, 3H, ArH), 7.28 (t, J = 7.0 Hz, 1H, ArH), 6.69 (ddd, J = 14.8, 4.8, 4.8 Hz, 1H, –CHendo), 4.03 (d, J = 14.8 Hz, 1H, –CHexo), 3.96 (dt, J = 13.3, 6.9 Hz, 1H, CH(CH3)2), 3.67 (m, 1H, CH(CH3)2), 2.64 (m, 1H, CH(CH3)2), 1.77 (dd, J = 20.3, 7.4 Hz, 3H, CH(CH 3)2), 1.62 (ddd, J = 18.2, 13.6, 6.9 Hz, 6H, CH(CH 3)2), 1.45 (dd, J = 19.7, 6.8 Hz, 3H, CH(CH 3)2), 1.34 (dd, J = 18.3, 7.0 Hz, 3H, CH(CH 3)2), 1.07 (dd, J = 15.7, 7.2 Hz, 3H, CH(CH 3)2), 0.89 (dd, J = 14.2, 6.8 Hz, 3H, CH(CH 3)2), 0.69 (m, 1H, CH(CH3)2), 0.04 (dd, J = 13.8, 7.3 Hz, 3H, CH(CH 3)2). 31P{1H} NMR (202 MHz, CD2Cl2, 248 K) δ = 44.66 (d, J = 15.0 Hz), 30.65 (d, J = 15.1 Hz). 13C{1H} NMR (126 MHz, CD2Cl2, 248 K) δ = 144.99 (d, J = 12.2 Hz, ArC), 141.15 (d, J = 11.2 Hz, ArC), 133.12 (d, J = 7.2 Hz, ArC), 132.82 (s, ArC), 132.49 (d, J = 8.8 Hz, ArC), 132.02 (s, ArC), 131.46 (s, ArC), 130.62 (s, ArC), 130.52 (s, ArC), 126.61 (d, J = 3.5 Hz, ArC), 126.41 (m, ArC), 43.17 (t, J = 10.5 Hz, –CH2–), 31.30 (d, J = 29.4 Hz, CH(CH3)2), 30.36 (d, J = 32.3 Hz, CH(CH3)2), 25.34 (dd, J = 18.1, 4.9 Hz, CH(CH3)2), 24.61 (d, J = 23.9 Hz, CH(CH3)2), 24.38 (d, J = 7.1 Hz, CH(CH3)2), 22.47 (d, J = 5.0 Hz, CH(CH3)2), 21.95 (d, J = 7.6 Hz, CH(CH3)2), 21.21 (d, J = 6.9 Hz, CH(CH3)2), 20.50 (s, CH(CH3)2), 19.75 (s, CH(CH3)2), 19.58 (s, CH(CH3)2), 17.38 (d, J = 5.8 Hz, CH(CH3)2). Anal. calcd for C25H38Br2P2Pd: C, 45.04; H, 5.74. Found: C, 44.92; H, 5.71.
Synthesis of [PC(sp3)HP]PdBr (4)
130 mg of [PC(sp3)H2P]PdBr2 (12, 0.195 mmol) was stirred in 10 mL toluene and added as a slurry in a Schlenk flask, under nitrogen. The suspension was heated at 100 °C for 2 days, resulting in a light cream solution. Aliquots were taken from the reaction mixture at intermediate times and analysed by 1H and 31P{1H} NMR spectroscopy to monitor the reaction progress. After 48 hours, only the desired product 4 was observed by 31P{1H} NMR spectroscopy. The volatiles were removed under reduced pressure and the residue was triturated twice with 10 mL of n-pentane. The resulted cream powder was dried under reduced pressure and was analytically pure by 1H NMR spectroscopy. Yield: 83.4 mg, 73%. For 4: 1H NMR (500 MHz, CDCl3) δ = 7.43 (dtd, J = 5.0, 3.9, 1.1 Hz, 2H, ArH), 7.33–7.28 (m, 2H, ArH), 7.26 (d, J = 7.9 Hz, 2H, ArH), 7.17 (t, J = 7.2 Hz, 2H, ArH), 6.15 (s, 1H, –C(H)Pd), 2.78–2.70 (m, 2H, CH(CH3)2), 2.69–2.62 (m, 2H, CH(CH3)2), 1.43 (td, J = 8.4, 7.2 Hz, 6H, CH(CH 3)2), 1.34 (td, J = 8.4, 7.1 Hz, 6H, CH(CH 3)2), 1.28 (q, J = 7.3 Hz, 6H, CH(CH 3)2), 1.17 (dd, J = 14.8, 7.6 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (202 MHz, CDCl3) δ = 50.33 (s). 13C{1H} NMR (126 MHz, CDCl3) δ = 157.75 (t, J = 14.7 Hz, ArC), 133.56 (t, J = 17.0 Hz, ArC), 131.90 (s, ArC), 130.23 (s, ArC), 127.09 (t, J = 9.2 Hz, ArC), 125.45 (t, J = 3.2 Hz, ArC), 54.23 (t, J = 3.8 Hz, –C(H)Pd), 25.90 (t, J = 10.2 Hz, CH(CH3)2), 25.54 (t, J = 12.0 Hz, CH(CH3)2), 19.47 (t, J = 2.8 Hz, CH(CH3)2), 18.80 (t, J = 2.1 Hz, CH(CH3)2), 18.78 (s, CH(CH3)2), 18.38 (s, CH(CH3)2). Anal. calcd for C25H37BrP2Pd: C, 51.26; H, 6.37. Found: C, 51.20; H, 5.99.
Reaction of [PC(sp2)P]Pd(PMe3) (1) with CH2Cl2
To a solution of 58.1 mg of [PC(sp2)P]Pd(PMe3) (1, 0.1 mmol) in 5 mL of THF, was added 1 mL solution of CH2Cl2 (0.1 M) in THF and the reaction mixture was stirred at room temperature for 2.5 days. The color gradually changed from dark brown to green. The reaction was monitored by 1H NMR spectroscopy. The volatiles were removed under reduced pressure. [PC(CH2)P]Pd(PMe3) (6) was present in the crude mixture by 1H NMR spectroscopy. The residue was dissolved in Et2O. Analytically pure 5 crystallized from this Et2O solution layered with n-pentane at –35 °C. Yield for 5: 29 mg, 80%. The supernatant contained mostly 6. The volatiles were removed under reduced pressure and the residue was dissolved in n-pentane, filtered over Celite. 6 crystallized from this n-pentane solution at –35 °C. Yield: 15 mg, 75%. Due to the low theoretical yield and difficulties encountered in separation, an analytically pure sample of 6 was obtained by a different method (vide infra). For 5: 1H, 31P{1H} and 13C{1H} NMR are silent. Magnetic moment (298 K): μ eff = 1.86μ B. EPR: g = 2.0100. Anal. calcd for C25H36ClP2Pd: C, 55.57; H, 6.72. Found: C, 55.71; H, 6.33.
Reaction of [PC(sp2)P]Pd(PMe3) (1) with CH2Br2
To a solution of 58.1 mg of [PC(sp2)P]Pd(PMe3) (1, 1 mmol) in 5 mL of THF, was added 1 mL solution of CH2Br2 (0.1 M) in THF and the reaction mixture was stirred at room temperature. The color changed within 10 minutes from dark brown to greyish-green. The volatiles were removed under reduced pressure. [PC(CH2)P]Pd(PMe3) (6) was present in the crude mixture by 1H and 31P{1H} NMR spectroscopy. The residue was dissolved in Et2O and filtered. Analytically pure 3 crystallized from this Et2O solution layered with n-pentane at –35 °C. Yield for 3: 21.1 mg, 54%. The identity of the product 3 was confirmed by converting it to [PC(sp3)HP]PdBr (4) in a subsequent step (vide infra), and by X-ray crystallography. The supernatant contained mostly 6. The volatiles were removed under reduced pressure, the residue was dissolved in n-pentane, and the resulting solution filtered over Celite. Compound 6 crystallized from this n-pentane solution at –35 °C. Yield: 7.5 mg, 38%. The spectroscopic data for the crystallized sample was identical to the one obtained by a different method (vide infra).
Reaction of [PC(sp2)P]Pd(PMe3) (1) with CH2I2
To a solution of 58.1 mg of [PC(sp2)P]Pd(PMe3) (1, 0.1 mmol) in 5 mL of THF was added 1 mL solution of CH2I2 (0.1 M) in THF and the reaction mixture was stirred at room temperature. The color gradually changed from dark brown to greyish-green. The volatiles were removed under reduced pressure. [PC(CH2)P]Pd(PMe3) (6) was present in the crude mixture by 1H and 31P{1H} NMR spectroscopy. The residue was dissolved in Et2O. Analytically pure {2}2 crystallized from this Et2O solution layered with n-pentane at –35 °C. Yield for {2}2: 29.6 mg, 64%. The identity of the product {2}2 was confirmed by converting it to [PC(sp3)HP]PdI (7) in a subsequent step (vide infra), and by X-ray crystallography. The supernatant contained mostly 6. The volatiles were removed under reduced pressure and the residue was dissolved in n-pentane. 6 crystallized from this n-pentane solution at –35 °C. Yield: 9.3 mg, 47%. The spectroscopic data for the crystallized sample was identical to data for a sample obtained by a different method (vide infra).
Synthesis of 1,1-bis(2-bromophenyl)ethan-1-ol
To a suspension of 5.4 g of bis(2-bromophenyl)methanone (16 mmol) in 50 mL of Et2O, 10 mL of MeLi (1.6 M in Et2O) was added dropwise at room temperature with stirring over a period of 10 minutes. The reaction mixture was stirred at room temperature for 12 hours. 50 mL of H2O was carefully added to the reaction mixture. The aqueous layer was extracted 3 times with 50 mL of Et2O. The combined organic layers were washed with brine (3 times, 24 mL) and dried over anhydrous sodium sulfate. After filtration, the volatiles were removed under reduced pressure. The crude product was isolated as a clear oil. Yield: 5.5 g, 96%. The crude product was used in the next step without further purification. An analytically pure sample of 1,1-bis(2-bromophenyl)ethan-1-ol was obtained through separation by column chromatography (silica gel, hexanes : ethyl acetate = 95 : 5). For 1,1-bis(2-bromophenyl)ethan-1-ol: 1H NMR (500 MHz, CDCl3) δ = 7.92 (dd, J = 7.9, 1.2 Hz, 2H, ArH), 7.50 (dd, J = 8.0, 0.8 Hz, 2H, ArH), 7.42–7.34 (m, 2H, ArH), 7.20–7.11 (m, 2H, ArH), 3.57 (s, 1H, –C(OH)–CH3), 2.05 (s, 1H, –C(OH)–CH 3). 13C{1H} NMR (126 MHz, CDCl3) δ = 144.94 (s, ArC), 134.79 (s, ArC), 129.70 (s, ArC), 129.09 (s, ArC), 127.34 (s, ArC), 124.86 (s, ArC), 77.94 (s, Ar2 C(OH)–CH3), 28.35 (s, Ar2C(OH)–CH3).
Synthesis of 1,1-bis(2-bromophenyl)ethane
A mixture of 5 g of 1,1-bis(2-bromophenyl)ethan-1-ol (14 mmol), 15 g of red phosphorous (483 mmol) and 15 mL of HI (57%) was refluxed for 12 hours. The reaction mixture was diluted with 100 mL H2O, and extracted multiple times with CH2Cl2. The combined organic extract was washed with diluted NaOH, water, brine and dried over anhydrous sodium sulfate. After filtration, the volatiles were removed under reduced pressure to generate the crude product as an oil. The product was purified on a silica gel column using hexanes : ethyl acetate 95 : 5. Yield: 4 g, 84%. For 1,1-bis(2-bromophenyl)ethane: 1H NMR (500 MHz, CDCl3) δ = 7.60 (d, J = 7.3 Hz, 2H, ArH), 7.29 (t, J = 7.5 Hz, 2H, ArH), 7.19–7.09 (m, 4H, ArH), 4.86 (q, J = 7 Hz, 1H, Ar2C(H)–CH3), 1.62 (d, J = 7 Hz, 3H, Ar2C(H)–CH 3). 13C{1H} NMR (126 MHz, CDCl3) δ = 144.15 (s, ArC), 133.19 (s, ArC), 128.57 (s, ArC), 127.95 (s, ArC), 127.54 (s, ArC), 125.60 (s, ArC), 77.16 (s, Ar2 C(H)–CH3), 20.35 (s, Ar2C(H)–CH3).
Synthesis of PC(CH3)HP (7)
To a solution of 1,1-bis(2-bromophenyl)ethane (4 g, 11.8 mmol) in 50 mL of Et2O, 15 mL of a n BuLi solution (1.6 M in hexanes, 24 mmol) was added dropwise at –50 °C in a nitrogen-filled glovebox. The reaction mixture was warmed up to room temperature and stirred for an additional hour. To this mixture, a solution of 3.66 g of iPr2PCl (24 mmol) in Et2O was added dropwise over a period of 30 minutes and stirred overnight at room temperature. The reaction mixture was quenched with 5 mL of a degassed, saturated NH4Cl solution in water. The volatiles were removed under reduced pressure and the residue was dissolved in n-pentane. The pentane solution was dried over anhydrous sodium sulfate, filtered over Celite and concentrated under reduced pressure. The product, 7, crystallized as a white solid from this solution at –35 °C. Yield: 3.8 g, 78%. For 7: 1H NMR (500 MHz, C6D6) δ = 7.39–7.33 (m, 2H, ArH), 7.19 (ddd, J = 7.8, 3.8, 1.3 Hz, 2H, ArH), 7.08 (td, J = 7.5, 1.4 Hz, 2H, ArH), 7.03 (td, J = 7.3, 1.4 Hz, 2H, ArH), 6.25 (m, J = 6.9 Hz, 1H, –C(H)CH3), 2.05 (m, 2H, CH(CH3)2), 1.90 (m, 2H, CH(CH3)2), 1.76 (d, J = 7.1 Hz, 3H, –C(H)CH 3), 1.17 (dd, J = 14.2, 6.9 Hz, 6H, CH(CH 3)2), 1.09 (dd, J = 13.6, 6.9 Hz, 6H, CH(CH 3)2), 0.91 (dd, J = 11.3, 7.0 Hz, 6H, CH(CH 3)2), 0.86 (dd, J = 13.5, 7.1 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (202 MHz, C6D6) δ = –8.28. 13C{1H} NMR (126 MHz, C6D6) δ = 153.55 (d, J = 25.8 Hz, ArC), 136.71 (d, J = 19.9 Hz, ArC), 132.97 (s, ArC), 128.81 (s, ArC), 128.57 (t, J = 3.5 Hz, ArC), 125.72 (s, ArC), 40.71 (t, J = 23.2 Hz, –C(H)CH3), 26.34 (d, J = 14.8 Hz, CH(CH3)2), 25.39 (d, J = 11.7 Hz, CH(CH3)2), 24.33 (t, J = 3.6 Hz, –C(H)CH3), 22.02–21.50 (m, CH(CH3)2), 21.32–20.82 (m, CH(CH3)2), 20.37 (m, CH(CH3)2).
Synthesis of [PC(CH3)HP]PdCl2 (8)
A mixture of 85 mg of PC(CH3)HP (7) (0.206 mmol) and 57 mg of (COD)PdCl2 (0.2 mmol) was stirred in 5 mL of THF for 3 hours at room temperature. The solution remained cloudy yellow throughout the reaction. The volatiles were removed under reduced pressure and the residue was triturated 3 times with 5 mL of n-pentane. The resulting yellow powder (91 mg, 76%) was analytically pure based on 1H and 31P{1H} NMR spectroscopy. Because the room temperature 1H NMR spectrum was broad, additional NMR data was recorded at 300, 310, and 320 K. For 8: 1H NMR (400 MHz, CDCl3, 320 K) δ = 7.60–7.52 (m, 4H, ArH), 7.44 (t, J = 7.6 Hz, 2H, ArH), 7.27 (t, J = 7.6 Hz, 2H, ArH), 7.00–6.91 (m, 1H, –C(H)CH3), 3.76–3.50 (m, 2H, CH(CH3)2), 1.80 (d, J = 6.7 Hz, 3H, –C(H)CH 3), 1.71 (br s, 2H, CH(CH3)2), 1.66 (dd, J = 15.9, 7.1 Hz, 6H, CH(CH 3)2), 1.46 (ddd, J = 17.4, 15.1, 7.2 Hz, 12H, CH(CH 3)2), 0.94 (dd, J = 13.5, 6.8 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (162 MHz, CDCl3, 320 K) δ = 39.96 (s). 13C{1H} NMR (101 MHz, CDCl3, 290 K) δ = 148.92 (s, ArC), 131.82 (s, ArC), 131.31 (d, J = 2.0 Hz, ArC), 129.67 (s, ArC), 129.42 (d, J = 7.9 Hz, ArC), 126.14 (d, J = 6.7 Hz, ArC), 40.69 (t, J = 10.4 Hz, –C(H)CH3), 29.02 (d, J = 30.1 Hz, CH(CH3)2), 26.80 (d, J = 23.9 Hz, CH(CH3)2), 25.67 (s, –C(H)CH3), 22.26 (s, CH(CH3)2), 22.11 (s, CH(CH3)2), 21.85 (d, J = 2.0 Hz, CH(CH3)2), 20.96 (d, J = 3.7 Hz, CH(CH3)2). Anal. calcd for C26H40Cl2P2Pd: C, 52.76; H, 6.81. Found: C, 53.01; H, 6.79.
Synthesis of [PC(CH3)P]PdCl (9)
A solution of 100 mg of [PC(CH3)HP]PdCl2 (8, 0.169 mmol) in 10 mL of dioxane was heated at 100 °C for 36 hours in a Schlenk tube. The volatiles were removed under reduced pressure and the residue was triturated 3 times with 10 mL of n-pentane. After recrystallization from a concentrated Et2O solution at –35 °C, analytically pure 9 was isolated in high yield (92%, 86.3 mg). For 9: 1H NMR (400 MHz, C6D6) δ = 7.45 (dd, J = 8.0, 0.8 Hz, 2H, ArH), 7.14 (ddd, J = 7.5, 3.7, 1.2 Hz, 2H, ArH), 7.09–7.04 (m, 2H, ArH), 6.94 (t, J = 7.3 Hz, 2H, ArH), 2.58–2.38 (m, 4H, CH(CH3)2), 2.05 (t, J = 3.9 Hz, 3H, Pd–C(CH 3)), 1.44 (dd, J = 13.9, 5.1 Hz, 6H, CH(CH 3)2), 1.39 (dd, J = 13.6, 5.3 Hz, 6H, CH(CH 3)2), 1.14 (dd, J = 15.1, 7.4 Hz, 6H, CH(CH 3)2), 0.98 (dd, J = 15.2, 7.2 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (162 MHz, C6D6) δ = 46.67 (s). 13C{1H} NMR (101 MHz, C6D6) δ = 164.21 (t, J = 14.7 Hz, ArC), 135.49 (t, J = 15.6 Hz, ArC), 132.72 (s, ArC), 129.94 (s, ArC), 127.27 (t, J = 9.3 Hz, ArC), 126.06 (t, J = 3.0 Hz, ArC), 65.94 (t, J = 4.7 Hz, Pd–C(CH3)), 40.74 (t, J = 2.2 Hz, Pd–C(CH3)), 26.96 (t, J = 11.7 Hz, CH(CH3)2), 26.40 (t, J = 9.9 Hz, CH(CH3)2), 19.56 (t, J = 1.7 Hz, CH(CH3)2), 19.30 (t, J = 2.5 Hz, CH(CH3)2), 19.13 (t, J = 2.2 Hz, CH(CH3)2), 19.01 (t, J = 1.4 Hz, CH(CH3)2). Anal. calcd for C26H39ClP2Pd: C, 56.23; H, 7.08. Found: C, 56.31; H, 6.72.
Synthesis of [PC(CH2)P]Pd(PMe3) (6)
To a solution of 50 mg of [PC(CH3)P]PdCl (9, 0.090 mmol) in 5 mL of THF, one equivalent of PMe3 (0.9 mL of a 0.1 M solution in THF) was added and the mixture was cooled to –35 °C. To this cold mixture, 1.36 mL KN(TMS)2 (0.066 M in toluene) was added. The solution rapidly changed color to orange, and the reaction was warmed up to room temperature, and stirred for one additional hour. After removal of volatiles under reduced pressure, the residue was extracted with n-pentane and the solution filtered over Celite. Analytically pure product was isolated by crystallization at –35 °C from n-pentane. Yield: 40 mg (75%). For 6: 1H NMR (400 MHz, C6D6) δ = 7.80 (dd, J = 7.7, 1.4 Hz, 2H, ArH), 7.30 (ddd, J = 7.6, 3.6, 1.4 Hz, 2H, ArH), 7.07 (t, J = 7.4 Hz, 2H, ArH), 7.00 (td, J = 7.3, 1.4 Hz, 2H, ArH), 3.81 (d, J = 4.4 Hz, 2H, –Pd(C CH2)), 2.24 (m, 2H, CH(CH3)2), 1.99 (m, 2H, CH(CH3)2), 1.36 (d, J = 4.6 Hz, 9H, –P(CH 3)3), 1.21 (dd, J = 15.3, 6.9 Hz, 6H, CH(CH 3)2), 1.04 (ddd, J = 13.0, 10.2, 7.1 Hz, 12H, CH(CH 3)2), 0.95 (dd, J = 12.4, 7.0 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (162 MHz, C6D6) δ = 33.85 (d, J = 21.7 Hz, P iPr2), –31.55 (t, J = 21.6 Hz, PMe3). 13C{1H} NMR (101 MHz, C6D6) δ 155.08–154.28 (m, ArC), 143.19 (dt, J = 10.5, 8.0 Hz, ArC), 131.38 (d, J = 1.3 Hz, ArC), 129.80 (dd, J = 9.9, 5.1 Hz, ArC), 127.58 (s, ArC), 125.40 (s, ArC), 112.16 (dt, J = 17.6, 2.3 Hz, –Pd(C CH2)), 64.40 (td, J = 7.5, 5.1 Hz, –Pd(C CH2)), 28.38–28.15 (m, CH(CH3)2), 26.49 (t, J = 4.9 Hz, CH(CH3)2), 25.02 (t, J = 5.7 Hz, P(CH3)3), 24.90 (t, J = 5.8 Hz, CH(CH3)2), 21.07 (t, J = 6.0 Hz, CH(CH3)2), 20.77 (t, J = 8.2 Hz, CH(CH3)2), 19.96–19.66 (m, CH(CH3)2). Anal. calcd for C29H47P3Pd: C, 58.54; H, 7.96. Found: C, 58.50; H, 8.01.
Reaction of 5, 3 or {2}2 with 9,10-dihydroanthracene
In a typical experiment a 20 mL scintillation vial, the radical (5, 54 mg, 0.1 mmol; 3, 59 mg, 0.1 mmol; {2}2, 64 mg, 0.05 mmol) were mixed with 36 mg of 9,10-dihydroantracene (0.4 mmol) in 5 mL of THF and stirred at room temperature. After about 12 hours, the color changed to a lighter shade of green. The volatiles were then removed under reduced pressure and the residue was extracted in Et2O. The reaction was monitored by 1H and 31P{1H} NMR. The product was isolated by crystallization from this concentrated Et2O solution at –35 °C. The 1H and 31P{1H} NMR spectra matched the spectra previously obtained for the products. Isolated yield: 14% for 10, 16% for 4 and 17% for 11.
Reaction of 5, 3 or {2}2 with n Bu3SnH
In a typical experiment a 20 mL scintillation vial, the radical (5, 54 mg, 0.1 mmol; 3, 59 mg, 0.1 mmol; {2}2, 64 mg, 0.05 mmol) were mixed with 4 mL solution of n Bu3SnH (0.05 M in THF, 0.2 mmol) and stirred at room temperature. After about 12 hours the color changed to light green. The reaction was monitored by 1H and 31P{1H} NMR. The volatiles were then removed under reduced pressure and the residue was extracted in Et2O. The products were isolated by crystallization from this concentrated Et2O solution at –35 °C. The 1H and 31P{1H} NMR spectra matched the spectra previously obtained for these compounds. Yield 48% for 10, 24% for 4 and 78% for 11.
Synthesis of [PC(sp3)HP]PdI (11)
54.1 mg of [PC(sp3)HP]PdCl (10, 0.1 mmol) were stirred in 5 mL of THF in a 20 mL scintillation vial, at –50 °C for 30 minutes. To this mixture, 1 mL solution of I2 (0.1 M in THF) was added dropwise. The color gradually changed from cream to bright yellow. After warming up to room temperature, the reaction mixture was stirred for an additional 1 hour. The volatiles were removed under reduced pressure, and the yellow residue was extracted with Et2O and filtered over Celite. This Et2O solution was concentrated under reduced pressure and set to crystallize at –35 °C. After 2 days, analytically pure 11 (43 mg, 68%) was isolated as light yellow crystals. For 11: 1H NMR (400 MHz, C6D6) δ = 7.29 (ddd, J = 7.8, 1.4, 1.0 Hz, 2H, ArH), 7.14 (ddd, J = 7.5, 3.9, 1.5 Hz, 2H, ArH), 7.09–7.04 (m, 2H, ArH), 6.90 (t, J = 7.4 Hz, 2H, ArH), 6.37 (s, 1H, Pd–CH), 2.66–2.55 (m, 2H, CH(CH3)2), 2.55–2.44 (m, 2H, CH(CH3)2), 1.38 (qd, J = 8.3, 7.1 Hz, 12H, CH(CH 3)2), 1.06 (dd, J = 14.7, 7.4 Hz, 6H, CH(CH 3)2), 1.02 (q, J = 7.5 Hz, 6H, CH(CH 3)2). 31P{1H} NMR (162 MHz, C6D6) δ = 51.43 (s). 13C{1H} NMR (101 MHz, C6D6) δ = 158.14 (t, J = 14.6 Hz, ArC), 135.04 (t, J = 16.7 Hz, ArC), 132.27 (s, ArC), 130.40 (s, ArC), 127.40 (t, J = 9.2 Hz, ArC), 125.47 (t, J = 3.2 Hz, ArC), 59.31 (t, J = 4.4 Hz Pd–CH), 26.77 (t, J = 10.4 Hz, CH(CH3)2), 26.74 (t, J = 12.6 Hz, CH(CH3)2), 19.88 (t, J = 2.6 Hz, CH(CH3)2), 19.26 (t, J = 1.9 Hz, CH(CH3)2), 18.47 (s, CH(CH3)2). Anal. calcd for C25H37IP2Pd: C, 47.45; H, 5.89. Found: C, 47.48; H, 5.75.
Acknowledgments
We thank Dr Allen Oliver for crystallographic assistance. This work was partially supported by the University of Notre Dame. We thank the ND Energy Materials characterization facility for the use of the potentiostat. Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research (ACS PRF # 53536-DNI3). MV acknowledges support from the U.S. Department of Energy, Office of Science, and Office of Basic Energy Sciences under Award Number DOE DE-FC02-04ER15533DE-FC02-04ER15533. Notre Dame Radiation Laboratory document number: NDRL-5061.
Footnotes
References
- (a) Meunier B., de Visser S. P., Shaik S. Chem. Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]; (b) Baik M.-H., Newcomb M., Friesner R. A., Lippard S. J. Chem. Rev. 2003;103:2385–2420. doi: 10.1021/cr950244f. [DOI] [PubMed] [Google Scholar]; (c) Stubbe J., Nocera D. G., Yee C. S., Chang M. C. Y. Chem. Rev. 2003;103:2167–2202. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- (a) Buckel W. Angew. Chem., Int. Ed. 2009;48:6779–6787. doi: 10.1002/anie.200900771. [DOI] [PubMed] [Google Scholar]; (b) Pintauer T., Matyjaszewski K. Chem. Soc. Rev. 2008;37:1087–1097. doi: 10.1039/b714578k. [DOI] [PubMed] [Google Scholar]; (c) Shi Y., in Modern Oxidation Methods, Wiley-VCH Verlag GmbH & Co. KGaA, 2005, pp. 51–82. [Google Scholar]
- Torraca K. E., McElwee-White L. Coord. Chem. Rev. 2000;206–207:469–491. [Google Scholar]
- (a) Dorwald F. Z., Metal carbenes in organic synthesis, Wiley-VCH, Weinheim, New York, 1999. [Google Scholar]; (b) Glorius F., in Topics in organometallic chemistry 21, Springer, Berlin, New York, 2007. [Google Scholar]; (c) Scott J., Mindiola D. J. Dalton Trans. 2009:8463–8472. doi: 10.1039/b908684f. [DOI] [PubMed] [Google Scholar]
- (a) Dzik W. I., Zhang X. P., de Bruin B. Inorg. Chem. 2011;50:9896–9903. doi: 10.1021/ic200043a. [DOI] [PubMed] [Google Scholar]; (b) Krusic P. J., Klabunde U., Casey C. P., Block T. F. J. Am. Chem. Soc. 1976;98:2015–2018. [Google Scholar]
- (a) Otte M., Kuijpers P. F., Troeppner O., Ivanović-Burmazović I., Reek J. N. H., de Bruin B. Chem.–Eur. J. 2014;20:4880–4884. doi: 10.1002/chem.201400055. [DOI] [PubMed] [Google Scholar]; (b) Paul N. D., Mandal S., Otte M., Cui X., Zhang X. P., de Bruin B. J. Am. Chem. Soc. 2014;136:1090–1096. doi: 10.1021/ja4111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Dzik W. I., Reek J. N. H., de Bruin B. Chem.–Eur. J. 2008;14:7594–7599. doi: 10.1002/chem.200800262. [DOI] [PubMed] [Google Scholar]; (b) Russell S. K., Hoyt J. M., Bart S. C., Milsmann C., Stieber S. C. E., Semproni S. P., DeBeer S., Chirik P. J. Chem. Sci. 2014;5:1168–1174. [Google Scholar]
- (a) Samuel P. P., Mondal K. C., Roesky H. W., Hermann M., Frenking G., Demeshko S., Meyer F., Stückl A. C., Christian J. H., Dalal N. S., Ungur L., Chibotaru L. F., Pröpper K., Meents A., Dittrich B. Angew. Chem., Int. Ed. 2013;52:11817–11821. doi: 10.1002/anie.201304642. [DOI] [PubMed] [Google Scholar]; (b) Ung G., Rittle J., Soleilhavoup M., Bertrand G., Peters J. C. Angew. Chem., Int. Ed. 2014;53:8427–8431. doi: 10.1002/anie.201404078. [DOI] [PubMed] [Google Scholar]; (c) Mondal K. C., Samuel P. P., Li Y., Roesky H. W., Roy S., Ackermann L., Sidhu N. S., Sheldrick G. M., Carl E., Demeshko S., De S., Parameswaran P., Ungur L., Chibotaru L. F., Andrada D. M. Eur. J. Inorg. Chem. 2014;2014:818–823. [Google Scholar]; (d) Weinberger D. S., Melaimi M., Moore C. E., Rheingold A. L., Frenking G., Jerabek P., Bertrand G. Angew. Chem., Int. Ed. 2013;52:8964–8967. doi: 10.1002/anie.201304820. [DOI] [PubMed] [Google Scholar]; (e) Singh A. P., Samuel P. P., Roesky H. W., Schwarzer M. C., Frenking G., Sidhu N. S., Dittrich B. J. Am. Chem. Soc. 2013;135:7324–7329. doi: 10.1021/ja402351x. [DOI] [PubMed] [Google Scholar]
- (a) Burford R. J., Piers W. E., Ess D. H., Parvez M. J. Am. Chem. Soc. 2014;136:3256–3263. doi: 10.1021/ja412650s. [DOI] [PubMed] [Google Scholar]; (b) Burford R. J., Piers W. E., Parvez M. Organometallics. 2012;31:2949–2952. [Google Scholar]; (c) Gutsulyak D. V., Piers W. E., Borau-Garcia J., Parvez M. J. Am. Chem. Soc. 2013;135:11776–11779. doi: 10.1021/ja406742n. [DOI] [PubMed] [Google Scholar]
- (a) Comanescu C. C., Iluc V. M. Organometallics. 2014;33:6059–6064. [Google Scholar]; (b) Cui P., Comanescu C. C., Iluc V. Chem. Commun. 2015;51:6206–6209. doi: 10.1039/c5cc00868a. [DOI] [PubMed] [Google Scholar]
- (a) Gomberg M. J. Am. Chem. Soc. 1900;22:757–771. [Google Scholar]; (b) Lankamp H., Nauta W. T., MacLean C. Tetrahedron Lett. 1968;9:249–254. [Google Scholar]
- (a) Khusnutdinova J. R., Rath N. P., Mirica L. M. J. Am. Chem. Soc. 2010;132:7303–7305. doi: 10.1021/ja103001g. [DOI] [PubMed] [Google Scholar]; (b) Tang F., Zhang Y., Rath N. P., Mirica L. M. Organometallics. 2012;31:6690–6696. [Google Scholar]
- (a) Adhikari D., Mossin S., Basuli F., Huffman J. C., Szilagyi R. K., Meyer K., Mindiola D. J. J. Am. Chem. Soc. 2008;130:3676–3682. doi: 10.1021/ja7108486. [DOI] [PubMed] [Google Scholar]; (b) Radosevich A. T., Melnick J. G., Stoian S. A., Bacciu D., Chen C.-H., Foxman B. M., Ozerov O. V., Nocera D. G. Inorg. Chem. 2009;48:9214–9221. doi: 10.1021/ic9010218. [DOI] [PubMed] [Google Scholar]
- DeMott J. C., Bhuvanesh N., Ozerov O. V. Chem. Sci. 2013;4:642–649. [Google Scholar]
- (a) Tumanskii B., Sheberla D., Molev G., Apeloig Y. Angew. Chem., Int. Ed. 2007;46:7408–7411. doi: 10.1002/anie.200702297. [DOI] [PubMed] [Google Scholar]; (b) Mayer P. M., Glukhovtsev M. N., Gauld J. W., Radom L. J. Am. Chem. Soc. 1997;119:12889–12895. [Google Scholar]; (c) Fischer H., in Magnetic Properties of Free Radicals, ed. K. H. Hellwege and A. M. Hellwege, Springer Berlin Heidelberg, 1965, vol. 1, pp. 34–39. [Google Scholar]; (d) Barloy L., Ku S. Y., Osborn J. A., De Clan A., Fischer J. Polyhedron. 1997;16:291–295. [Google Scholar]
- Doyle L. E., Piers W. E., Borau-Garcia J. J. Am. Chem. Soc. 2015;137:2187–2190. doi: 10.1021/ja512602m. [DOI] [PubMed] [Google Scholar]
- (a) Liang L. C., Chien P. S., Lin J. M., Huang M. H., Huang Y. L., Liao J. H. Organometallics. 2006;25:1399–1411. [Google Scholar]; (b) Csok Z., Vechorkin O., Harkins S. B., Scopelliti R., Hu X. J. Am. Chem. Soc. 2008;130:8156–8157. doi: 10.1021/ja8025938. [DOI] [PubMed] [Google Scholar]; (c) Vechorkin O., Csok Z., Scopelliti R., Hu X. Chem.–Eur. J. 2009;15:3889–3899. doi: 10.1002/chem.200802059. [DOI] [PubMed] [Google Scholar]; (d) Gartia Y., Biswas A., Stadler M., Nasini U. B., Ghosh A. J. Mol. Catal. A: Chem. 2012;363–364:322–327. [Google Scholar]
- (a) Qian X., Kozak C. M. Synlett. 2011;2011:852–856. [Google Scholar]; (b) Gartia Y., Pulla S., Ramidi P., Farris C., Nima Z., Jones D., Biris A., Ghosh A. Catal. Lett. 2012;142:1397–1404. [Google Scholar]
- Jahn U., in Radicals in Synthesis III, ed. M. Heinrich and A. Gansäuer, Springer Berlin Heidelberg, 2012, vol. 320, pp. 323–451. [Google Scholar]
- (a) Werner H., Crisp G. T., Jolly P. W., Kraus H. J., Krueger C. Organometallics. 1983;2:1369–1377. [Google Scholar]; (b) Kim H.-J., Choi N.-S., Lee S. W. J. Organomet. Chem. 2000;616:67–73. [Google Scholar]; (c) Poverenov E., Leitus G., Milstein D. J. Am. Chem. Soc. 2006;128:16450–16451. doi: 10.1021/ja067298z. [DOI] [PubMed] [Google Scholar]; (d) Barrett B. J., Iluc V. M. Organometallics. 2014;33:2565–2574. [Google Scholar]
- Comanescu C. C., Iluc V. M. Inorg. Chem. 2014;53:8517–8528. doi: 10.1021/ic5010566. [DOI] [PubMed] [Google Scholar]
- Luca O. R., Crabtree R. H. Chem. Soc. Rev. 2013;42:1440–1459. doi: 10.1039/c2cs35228a. [DOI] [PubMed] [Google Scholar]
- Bickelhaupt F., Jongsma C., de Koe P., Lourens R., Mast N. R., van Mourik G. L., Vermeer H., Weustink R. J. M. Tetrahedron. 1976;32:1921–1930. [Google Scholar]
- (a) Bain G. A., Berry J. F. J. Chem. Educ. 2008;85:532–536. [Google Scholar]; (b) Sur S. K. J. Magn. Reson. 1989;82:169–173. [Google Scholar]; (c) Evans D. F. J. Chem. Soc. 1959:2003–2005. [Google Scholar]
- Frisch M. J., in Gaussian 03, revision D.02, Gaussian, Inc., Wallingford CT, 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.