Abstract
While graphene has substantial commercial promise, numerous aspects regarding its ecological effects such as its potential for bioaccumulation are not well known. 14C-labeled few layer graphene (FLG) was dispersed in artificial freshwater and uptake of FLG by Limnodrilus hoffmeisteri, an oligochaete, was assessed. After exposure for 36 h to a 1 mg/L FLG suspension, the FLG body burden in the organism was nearly 60 ng/mg (on a dry mass basis). Multiple characterization results confirmed that the proteins secreted by the organisms during the exposure period coated the FLG, thus increasing its stability and decreasing its size in suspension. Uptake behaviors of Eisenia foetida exposed to FLG and protein-coated FLG at concentrations of approximately 1 mg/kg or to Daphnia magna at 100 μg/L were also quantified. Protein-coated FLG demonstrated different bioaccumulation behaviors for both organisms compared to uncoated FLG, with the FLG body burden in E. foetida increased but that in D. magna reduced. The data provide the first evidence that the proteins secreted by Limnodrilus hoffmeisteri after exposure to FLG can coat FLG, thus increasing the aqueous stability of FLG, decreasing its size, and changing its bioaccumulation potential.
1. Introduction
Carbon nanomaterials (CNMs), such as nanotubes, fullerene and graphene, are novel manufactured materials with widespread potential applications [1]. In particular, graphene, a CNM with a honeycomb lattice structure composed of planar sp2 bound carbon atoms, has drawn considerable attention in recent years because of its unique properties [1–3]. It is inevitable that graphene will be released into the environment during the production and usage of graphene-enabled consumer products, but the potential risks of graphene in the environment are not yet well understood [4]. To date, the majority of studies have focused on the toxicity of graphene [5–9], with only a limited number of studies on bioaccumulation by ecological receptors [4, 10], a particularly important component of risk assessment. In two bioaccumulation studies conducted using Daphnia magna and few layer graphene (FLG), body burdens up to 1% were recently measured after exposure for 24 h to 250 μg FLG/L [4], while graphene uptake was substantially lower for FLG partly degraded by the Fenton reaction [11]. In addition, the biodistribution of graphene oxide injected into zebrafish embryos has recently been studied [10]. However, no bioaccumulation studies have been conducted with graphene to our knowledge in any other ecologically relevant species.
Numerous studies have addressed how environmental processes such as enzymatic reaction, photodegradation and Fenton reaction impact the physicochemical properties of graphene in natural environments [11–14]. Transformation of the CNMs caused by these environmental processes can significantly affect their transport, fate, bioavailability, and toxicity [13,14]. Recent studies reported the uptake and depuration behaviors of carbon nanotubes and fullerene in various organisms such as daphnia, sediment-dwelling oligochaete and earthworms [15–18]. While organisms have been shown to increase sedimentation of certain CNMs [19], the impacts of these organisms on the surface chemistry and ecological risks of CNMs to other organisms are largely unknown. It is known that, after exposure to metal contamination, worms (e.g. Limnodrilus udekemianus, Eisenia foetida and Limnodrilus hoffmeisteri) secreted proteins to bind the metals and thereby decreased the metal toxicity [20–22]. Whether a similar process could happen with CNMs, such as secreted proteins interacting with the CNMs, is unknown. A previous study has shown that proteins used as dispersants can completely change the pulmonary toxicity of graphene [23]. To the best of our knowledge, the impact of coatings on the bioaccumulation of graphene by ecological receptors has not yet been studied.
In this study, the uptake behaviors of 14C-labeled FLG by fresh water oligochaete worm Limnodrilus hoffmeisteri (L. hoffmeisteri) was investigated. L. hoffmeisteri can be found in many freshwater ecosystems including lakes, ponds, marshes and streams. They prefer shallow water and often build small tubes in the sediment orienting themselves head downward with their posterior end in the water column [24–26]. Assessment of the impacts of L. hoffmeisteri on the physicochemical properties of FLG and its bioaccumulation behaviors by two other organisms, D. magna and E. foetida, were the goals of the study. D. magna and E. foetida are standard test organisms and have a central position in food web dynamics [27]. These results thus provide the first uptake results of FLG with L. hoffmeisteri and E. foetida.
2. Materials and methods
2.1 Materials
All reagents used are of analytical grade without further purification. Synthesis, purification, and characterization of 14C-labeled FLG were described in our previous study [4]. Briefly, FLG were synthesized by graphitization and exfoliation of sandwich-like FePO4/dodecylamine hybrid nanosheets and then purified using hydrochloric acid to remove the iron catalysts. The specific radioactivity of the purified FLG was 16.12 ± 0.59 mCi/g (n=3; uncertainties always indicate standard deviation values). The atomic ratio of C:O in the FLG was determined to be 89:6 (the remaining 5% is 1.4% of H and 3.6% of N) using X-ray photoelectron spectroscopy (XPS), and XPS-peak-fitting analysis of the average oxygen content showed that the percent of oxygen participating in C=O, O-H, C-O, and O=C–O bonds was 1.5%, 1.5%, 2%, and 1% [11]. The FLG mainly consisted of 4 to 6 layers (> 72%) and had a specific surface area of 660 m2/g [4]. Liquid scintillation counting (LSC) and mass spectrometry analyses could not detect the formation of carbon-14 byproducts from the synthesis, purification, or dispersion processes [4].
2.2 FLG uptake by L. hoffmeisteri
Culture conditions of L. hoffmeisteri are described in the Supplementary Materials. A 1.0 mg sample of 14C-FLG was weighed (Mettler Toledo, XP56 Microbalance) and added to a 500-mL beaker containing 250 mL freshwater. The FLG stock suspension was prepared by probe sonication in ice-water bath for 6 h (100 W, JY88-II, Nanjing Immanuel Instrument Equipment Co.) [4]. Probe sonication was performed using a 3 s “on”/2 s “off” pulse sequence with a probe tip that placed approximately 0.4 cm from the bottom of the beaker. The FLG stock suspension was diluted immediately with aerated freshwater to yield initial concentrations of approximately 1000, 500, 250, and 100 μg/L for uptake experiments; all experiments were performed in the absence of sediments. The exact FLG concentrations in each container were measured by mixing 1 mL water sample with 3 mL Gold Star scintillation cocktail (Meridian), followed by radioactivity measurements via LSC. Thirty L. hoffmeisteri were added to each petri dish containing 20 mL of FLG suspension and were kept in dark at (20 ± 2) °C [28, 29]. Triplicate control containers (without L. hoffmeisteri but with FLG) for each FLG concentration with 20 mL of exposure solution were used to monitor FLG settling during the exposure period. At predetermined intervals (1, 6, 12, 24, and 48 h), organisms were removed from each of triplicate containers; organism mortality was not observed for any exposure condition. After removal from the container, L. hoffmeisterii were placed in clean water and were pipetted vigorously to remove FLG particles attached to the skin until the radioactivity in the eluate was not detectable (<100 dpm) by LSC. Thus, the impact of the skin-associated FLG on the total mass of FLG ingested by the organisms is expected to be minimal. Then, the freeze-dried (24 h) worms from each petri dish were weighed (Mettler Toledo) and combusted in a biological oxidizer (BO) (OX-500; Zinsser Analytic, Germany) at 900 °C for 4 min under a stream of oxygen gas running at 360 mL/min. The 14CO2 released during the combustion process was captured in 10 mL alkaline scintillation cocktail (Zinsser Analytic, Germany) and then the radioactivity was quantified by LSC. The radioactivity from control samples (i.e. L. hoffmeisteri unexposed to FLG) was 36.4 ± 9 dpm (n=3; uncertainties always indicate standard deviation values); background values were similarly determined for all sample matrices and were subtracted from all of the radioactivity results. Before worm removal, the radioactivity of water sample was also measured as described above to determine the concentration of FLG remaining in the aqueous phase. Elimination experiments were conducted similarly to the uptake experiments. L. hoffmeisteri were exposed to graphene in aerated freshwater for 48 h with an initial suspended graphene concentration of 1000 μg/L. Depuration occurred in the aerated freshwater. After 12 h, L. hoffmeisteri were sampled from the depuration freshwater and sacrificed to measure graphene concentration in the body.
Bovine serum albumin (BSA) was selected as a protein to test for comparison to the proteins secreted by L. hoffmeisteri. BSA-coated FLG were prepared by mixing FLG (0.1 mg) with 20 mL of solution containing 3.4 mg/L BSA in a 40-mL sealed glass conical bottle for 48 h. This yielded a BSA loading of ~ 400 mg on the FLG (based on the sorption isotherms of BSA on FLG (Fig. S1 of Supplementary Materials)), a loading equal to the quantity of the proteins on the FLG after exposure with L. hoffmeisteri for 48 h. Experiments were also carried out using the same reactor experimental setup and procedure described above (FLG uptake by L. hoffmeisteri) to examine the uptake of BSA-coated FLG (1000 μg/L) by L. hoffmeisteri.
2.3 Characterizations of proteins and protein-coated FLG
Exposure experiments were conducted as described in the FLG uptake by L. hoffmeisteri section with an initial FLG concentration of 1000 μg/L. After the L. hoffmeisteri removal, the absorption spectra of the culture solution at each sampling time (0, 1, 6, 12, 24, and 48 h) was measured using UV-vis spectroscopy (Varian, USA). The hydrodynamic diameter of FLG in the culture solution was analyzed by dynamic light scattering (DLS) (ZetaPlus, Brookhaven Instrument); it should be noted though that the instrument algorithm used for analyzing DLS results is based on spherical nanoparticles and thus results of other shapes (e.g. plate-shaped FLG) should be interpreted with caution.
FLG in the culture solution at each sampling time (0, 1, 6, 12, 24, and 48 h) were collected by centrifugation at 49 000 g (Biofuge Stratos, Kendro Laboratory Products Co., US) for 60 min (4 °C), and were washed with deionized water five additional times. The obtained FLG was dried under vacuum at 40 °C and analyzed by XPS (PHI 5000 VersaProbe with a monochromatic Al Ka X-ray source), Fourier transformed infrared (FT-IR) (Bruker Tensor 27 Spectrophotometer), and Raman (XploRA PLUS system, Horiba Scientific, 532 nm incident radiation) spectrometers. Our preliminary results suggested that the presence of proteins had significant interference on the size analysis by Atomic Force Microscope (AFM). Therefore, the coated proteins on FLG were removed by 1 mg/mL Proteinase K (Sigma) solution (37 °C, 120 min) [30]. After protein digestion, the FLG were collected by centrifugation (49 000 g, 10 min, 4 °C) and then analyzed by AFM (Bruker, German) (details are provided in the Supplementary Materials). The FLG sample from the containers without L. hoffmeisteri were treated by the same procedures and served as a control.
The protein content in the culture solution (at 48 h) before and after the removal of FLG (by centrifugation, 49 000 g, 60 min, 4 °C) was determined by the Coomassie brilliant blue method at 595 nm by a UV-vis spectrophotometer [31]. A calibration curve was constructed using BSA with this assay and used to estimate the amount of secreted proteins by L. hoffmeisteri. However, the amino acid composition of the secreted protein may differ from that of BSA and thus the values determined are only estimates of the secreted protein concentration. An FLG suspension with the same FLG concentration as that after incubation for 48 h with organisms (700 μg/L) was analyzed with the protein assay and the potential bias of the suspended FLG ((8.0 ± 0.5) %; n=3) on the protein assay was subtracted when determining the protein concentration. The centrifugation step caused the FLG concentration to decrease from (700 ± 4.5) μg/L (the FLG concentration in suspension measured after L. hoffmeisteri exposure to 1000 μg/L FLG suspension for 48 h; n=3) to below the detection limit ((5 ± 1) ng/L; n=3). After centrifugation, the supernatant of the culture solution from each triplicate was combined and concentrated using dialysis bags (cutoff, 500D to 1000D; flat width, 31 mm; diameter, 20 mm; 3.1 mL/cm; Sectra/Pro CE, Spectra Technologies Holdings Co. Ltd) to identify the proteins. The proteins in the concentrated supernatant were separated by the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method [32]. The proteins in the SDS-PAGE gels were extracted and identified by LC-MS/MS analysis (detailed description is provided in the Supplementary Materials). Triplicate containers with 20 mL freshwater (without FLG) and 30 L. hoffmeisteri were prepared using the same method to evaluate the extent to which the organisms secrete proteins in the absence of FLG after during a 48 h period.
2.4 Agglomeration kinetics of FLG and protein-coated FLG
DLS was used to measure the intensity-averaged hydrodynamic diameter (Dh) of 0.5 mg/L FLG or protein-coated FLG at varying NaCl concentrations [33]. The capped cuvette containing 0.5 mg/L FLG (or protein-coated FLG) suspension and prescribed concentrations of NaCl were briefly vortexed and placed in the DLS instrument [34]. Agglomeration of the BSA-coated FLG was also tested at the NaCl concentration of 100 mmol/L. All agglomeration experiments were conducted in triplicate at pH 7.0.
2.5 Uptake of FLG and protein-coated FLG by E. foetida
Culture conditions of adult earthworms (E. foetida) are described in the Supplementary Materials. The FLG, which was either protein-coated by exposure to L. hoffmeisteri or uncoated and suspended by sonication as described above, was spiked into the soil to yield an initial concentration of 1 mg FLG/kg dry soil. The exact FLG concentrations were measured by combusting the freeze-dried FLG-spiked soil samples in a BO, and then the radioactivity of 14CO2 was counted by LSC. Three adult E. foetida with combined masses between 0.9 g and 1.2 g were transferred to 30 g (dry mass) moist (60% to 70% water holding capacity) FLG-spiked soils in a 250 mL glass jar, which was loosely closed with a cap to prevent earthworms from escaping and to allow air exchange. The jars were then held in the dark at (20 ± 2) °C. Milli-Q water was added every four days to maintain a relatively constant soil moisture. Three E. foetida were added to each of triplicate containers for each data point. Three jars were removed after exposure for 1, 5, 9, 13, 17, and 21 d. Organism mortality was not observed during these experiments. After removal from the soils, the E. foetida were washed with Milli-Q water, transferred to wet filter paper in petri dishes for 48 h in the dark to allow gut clearance. After rinsing with Milli-Q water (the radioactivity of the rinsed water reached a background level), the E. foetida were then transferred to centrifuge tubes, and the purged gut contents of the three worms from each replicate were combined. Then, the freeze-dried (24 h) earthworms and gut contents were weighed and separately combusted in a BO, and then the radioactivity of the samples was determined via LSC. The minimum detection limit of BO is determined to be 4.15 ng 14C FLG per gram worms (dry mass), corresponding to the signal from blank samples plus three times the standard deviation of the blank samples. After E. foetida removal, the soil was sampled at each sampling time and the FLG concentration was determined.
2.6 Uptake of FLG, BSA-coated FLG and protein-coated FLG by D. magna
BSA-coated FLG were prepared as described above. The BSA-coated FLG were collected, washed using DI water and dispersed in fresh artificial freshwater (AF) (CaCl2·2H2O, 58.8 mg/L; MgSO4·2H2O, 24.7 mg/L; NaHCO3, 13.0 mg/L; KCl, 1.2 mg/L; hardness [Ca2+] + [Mg2+]=0.5 mmol/L) [35]. The protein concentration in the supernatant was measured using UV spectroscopy and the amount of protein adsorbed was calculated from material balance (details are provided in the Supplementary Material) [36].
A certain volume of FLG dispersion (BSA- or protein-coated) was diluted using AF and sonicated for 20 min with the probe tip of ultrasonic processor (50 W) to yield exposure concentration of approximately 100 μg/L for uptake experiments. Uptake experiments of the BSA-coated FLG, protein-coated FLG and uncoated FLG dispersions were conducted using the same method employed in our earlier study [4]; the uncoated FLG dispersion was prepared as described above for the L. hoffmeisteri exposure. In brief, thirty Daphnia neonates (< 24 h) were added to beakers containing 90 mL of exposure solution [4]. While neonates are capable of feeding, they were not fed during these experiments. After the exposure duration, D. magna were placed in beakers containing 30 mL clean water and pipetted vigorously to remove FLG particles attached to their carapaces, and this step repeated 3 times. Then, the D. magna from each container were added to foil boats, dried, weighed, then added to scintillation vials with 3 mL of Gold Star cocktail, ultrasonicated for 20 min, allowed to sit for at least 24 h, and then analyzed using LSC. Before D. magna removal, aqueous-phase radioactivity was also measured. Organism immobilization was not observed during these experiments. Triplicate control containers (without D. magna but with 100 μg/L FLG) with 90 mL of the BSA-coated FLG, protein-coated FLG or uncoated FLG exposure solution were used to quantify FLG settling during the exposure period.
Depuration experiments were conducted similarly to the uptake experiments. Daphnia were exposed to graphene in AF for 24 h with an initial suspended BSA-coated FLG, protein-coated FLG or uncoated FLG concentration of 100 μg/L. Depuration occurred in the AF. After 4 or 10 h, Daphnia were sampled from the depuration AF and sacrificed to measure graphene concentration in the body.
2.7 Statistical analysis
All statistical analyses were performed using SPSS 18.0 (PASW Statistics, IBM Company); differences were considered statistically significant at p < 0.05. Results were analyzed by one-way analysis of variance (ANOVA) and Tukey’s post hoc test for comparisons among multiple conditions or t-tests for comparisons among two conditions.
3. Results
3.1 Uptake of FLG by L. hoffmeisteri
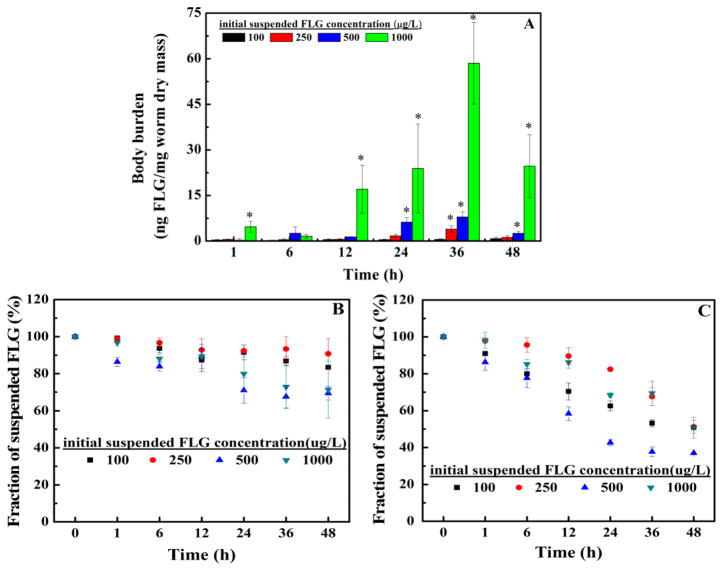
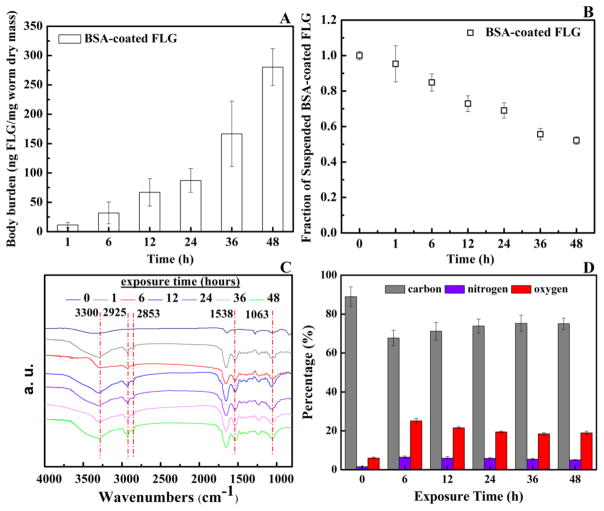
Our preliminary results indicated that (97 ± 1.2) % (n=3) of FLG was recovered after mixing FLG solution with the dried organism, drying the mixture, combusting the sample using BO, and measuring the radioactivity using LSC. As shown in Fig. 1A, statistically significant uptake of FLG was not observed during the exposure time under the tested concentration of 100 μg/L compared to a water-only control. However, the body burden was significantly increased for some time points for FLG exposure concentrations of 250, 500, and 1000 μg/L and was 60 ng/mg dry mass after 36 h exposure to a 1000 μg/L FLG suspension. The graphene concentration remaining in the L. hoffmeisteri (that had been exposed for 48 h to a graphene concentration of 1000 μg/L and then depurated in clean water for 12 h) was 1.54 (±0.64) ng/mg dry mass and statistically greater than 0, thus revealing that the FLG uptake mainly remained in the gut tract and L. hoffmeisteri was able to eliminate most of the accumulation of FLG in clean water. The body burden values of FLG coated with BSA increased with exposure time (Fig. 2A), and the body burdens after 48 h were approximately 10 times higher than for the organisms exposed to FLG (Fig. 1A). These results suggest that the impacts of the BSA and the secreted proteins on the FLG uptake may differ, although the BSA-coated FLG were coated for the duration of the exposure period while those initially added as uncoated FLG became coated during the exposure period. The data in Fig. 2B suggest that the settling behaviors of FLG mixed with BSA and the secreted proteins by L. hoffmeisteri were similar (Fig. 1B). During the exposure period, the radioactivity in the exposure solutions without L. hoffmeisteri was also measured to assess FLG settling (see Fig. 1C). Roughly 50% to 65% of the FLG settled from the exposure solution under the tested concentrations at 48 h. However, the data in Fig. 1B suggest that less settling occurred during the exposure time with the presence of L. hoffmeisteri where the FLG concentration in the dispersion remained at approximately 70% to 90% of the initial concentration after 48 h. As such, the presence of L. hoffmeisteri in the exposure solution enhanced the dispersion of FLG in the suspension. This marks the first time to our knowledge that the presence of an organism has enhanced the aqueous stability of a CNM.
Fig. 1.
(A) FLG uptake by L. hoffmeisteri. L. hoffmeisteri were exposed to FLG in artificial freshwater for 48 h with an initial suspended FLG concentration of 100, 250, 500, or 1000 μg/L. The asterisk in Fig. 1A indicates significantly different from zero. (B) Measured concentration of FLG in the uptake experiment solution after L. hoffmeisteri removal. (C) The fraction of the FLG concentration remaining in the exposure solution with time relative to the initial concentration in containers without L. hoffmeisteri. Mean and standard deviation values were calculated from triplicate samples.
Fig. 2.
(A) Uptake of BSA-coated FLG (1000 μg/L) by L. hoffmeisteri. (B) Measured concentration of BSA-coated FLG in the uptake experiment solution after L. hoffmeisteri removal. (C) and (D) displays the FT-IR spectra and XPS results of the FLG in solution containing L. hoffmeisteri at different times. Mean and standard deviation values were calculated from triplicate samples.
3.2 Protein identification
To explore the mechanism that caused decreased settling, measurements of the proteins released by L. hoffmeisteri at different exposure time with 1000 μg/L FLG were analyzed using UV-vis and LC-MS/MS. UV-vis spectrophotometry results revealed a chromophore at 275 nm and that the absorbance at 275 nm increased with the incubation time (Fig. S2A). In control experiments without added FLG, there was no detectable changes in the absorption spectrum of the culture solution of L. hoffmeisteri after 48 h. The concentration of the total proteins before and after the removal of FLG from the solution was determined to be (0.78 ± 0.08) mg/L and (0.53 ± 0.06) mg/L (n=3), respectively (Fig. S2B). The LC-MS/MS data were used to identify protein types based on sequence by matching tryptic peptide sets using the MASCOT search engine. Twelve types of proteins with scores ≥ 75 were identified and their detailed information is summarized in Table S1. The results suggest that the proteins were produced by L. hoffmeisteri after exposure to FLG.
3.3 Properties of the protein-coated FLG
After incubation for 1, 6, 12, 36, and 48 h, flocs together with FLG were separated from the dispersion by centrifugation and the amount of floc seems to increase with longer incubation times (Fig. S3). The FLG obtained by centrifugation at each sampling time (0, 6, 12, 24, 36, and 48 h) was washed and then analyzed using FT-IR and XPS. FT-IR analysis further revealed several peaks at 3300 cm−1 and 1538 cm−1 (-NH-), 2925 cm−1 and 2853 cm−1 (-C-(CH2)n-C), and 1063 cm−1 (C-O) on the surface of FLG (Fig. 2C) [34, 37, 38]. These results indicate that the proteins were likely associated with the FLG surface. This finding was supported by the XPS analysis results which showed an increase of O and N, and decrease of C element on the surface of FLG after L. hoffmeisteri exposure (Fig. 2D). The protein concentration (see Fig. S2B) in the solution before and after the removal of FLG by centrifugation was 0.78 mg/L and 0.53 mg/L, respectively. As shown in Fig. 1B, about 70% of the 0.02 mg FLG was contained in the solution (20 mL) and thus the loading capacity of proteins to FLG was approximately 357 mg/g.
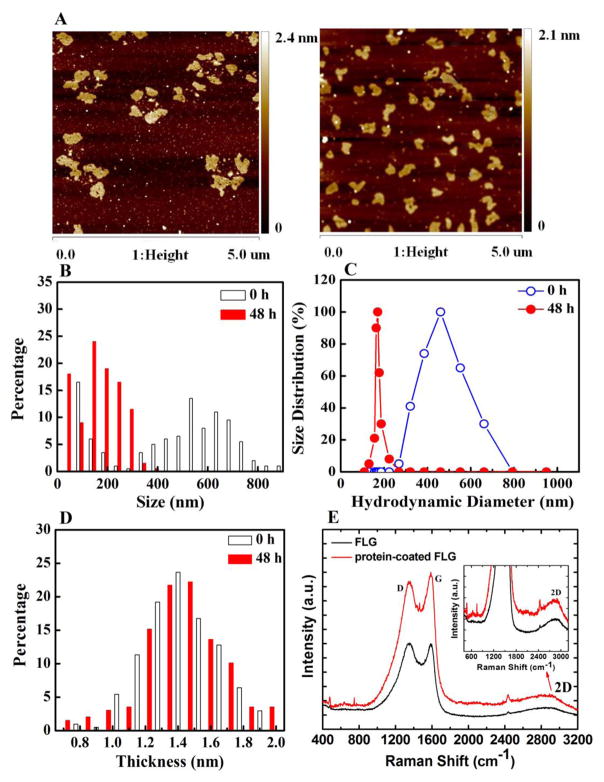
From the AFM characterization (see Fig. 3A and B), the FLG at 0 h had a continuous lateral size distribution from 90 nm to 890 nm with two major modes at 90 nm and 530 nm. However, the size distribution of protein-coated FLG at 48 h was decreased to between 50 nm and 300 nm with one major mode at 150 nm. As such, the size of FLG was decreased after protein coating. This result was corroborated by the DLS results, which also showed a decrease in the hydrodynamic diameter of FLG after incubation with L. hoffmeisteri (Fig. 3C). The decreased FLG size may be due to enhanced dispersion of the FLG by the coating protein causing additional disagglomeration compared to the initial suspension. Conversely, FLG from the control exposure without L. hoffmeisteri increased in size with the main peak at ~ 1500 nm after 48 h (see Fig. S4). The results in Fig. 3A and D suggest that the FLG thickness did not noticeably change, but these measurements were taken after removal of the protein coating. When analyzing graphene with Raman spectroscopy (Figure 3E), the observed D and G bands are distinctive of graphitic materials: the D band represents the disorder present in sp2-hybridized carbon systems, while the G band represents the stretching of C-C bonds. Both G and 2D bands can be used to monitor the number of graphene layers by characterizing the shift of G band and the shapes of 2D spectra [39]. The G and 2D bands (Figure 3E) did not noticeably differ between the pristine FLG and the protein-coated FLG indicating that the FLG was not degraded during exposure with L. hoffmeisteri and that the thickness was not changed.
Fig. 3.
Characterization of protein-coated FLG using AFM, Raman spectroscopy, and DLS. (A) Representative AFM image of protein-coated FLG deposited onto mica. FLG with an initial concentration of 1 mg/L was cultured with L. hoffmeisteri and collected by centrifugation at sampling time (0 and 48 h). Then it was treated by using Proteinase K solution, collected by centrifugation and analyzed using AFM. (B) Histogram of lateral flake size for FLG and protein-coated FLG (n=214). (C) Size distribution of the initial FLG suspension and after incubation with L. hoffmeisteri for 48 h measured using DLS. (D) Histogram of lateral flake thickness for FLG and protein-coated FLG (n=214). (E) Raman spectra of the FLG and protein-coated FLG; the insert figure is the enlarged 2D spectra.
Agglomeration profiles of the pristine FLG and the protein-coated FLG (0.5 mg/L) in NaCl solutions (10 mmol/L to 100 mmol/L) are shown in Fig. S5A. At the tested NaCl concentrations, FLG was unstable as the hydrodynamic diameter (Dh) increased rapidly with faster rates at higher ionic strength (Fig. S5A). However, the protein-coated FLG was stable and the Dh remained constant through the NaCl concentration range studied (up to 100 mmol/L) (Fig. S5B). BSA-coated FLG was also stable at a high NaCl concentration (100 mmol/L; Fig. S5C). It seems that the proteins may have provided the FLG with a combination of steric and electrostatic stabilization after adsorption [40].
3.4 Uptake of FLG and protein-coated FLG by E. foetida
Recoveries for the FLG and protein-coated FLG spiked soils were measured. Our results suggested that >98.7% of the spiked radioactivity was detected (1 mg/kg) and the radioactivity was dispersed uniformly in the soil (Fig. S6 of the Supplementary Materials shows that the coefficient of variation was less than 6.7%). The bioaccumulation factors values (BAF; concentration of the chemical in the worm divided by that in the soil) at 9 d, 13 d, 17 d and 21 d significantly differed for FLG with and without the protein coating (p<0.05) (Fig. 4), revealing that the protein-coated FLG did have higher uptake values compared to the non-modified FLG. Statistical analysis of the protein-coated BAF values indicated that the 1 d and 5 d time points differed from all of the other time points (p < 0.05) while the 9 d, 13 d, 17 d, and 21 d data points did not differ (p > 0.05). Thus, uptake results of the protein-coated FLG showed a general increase during the first 9 d followed by a plateau from 9 d to 21 d. In contrast, statistical analysis of the uncoated FLG BAF values did not indicate statistically different values among any of the time points (p > 0.05), indicating no change during the 21 d accumulation period for the FLG. The increase of the organism mass during the 21 d exposures was less than 15% for both treatments and thus changes in the organism mass cannot account for the changes in the protein-coated FLG concentration increase during the first 9 d. Importantly, it is possible that the protein coatings on the FLG may be modified and degraded by soil microorganisms during the exposure as has been shown in other studies for microbial degradation of nanoparticle coatings [41], but this was not measured in this study.
Fig. 4.
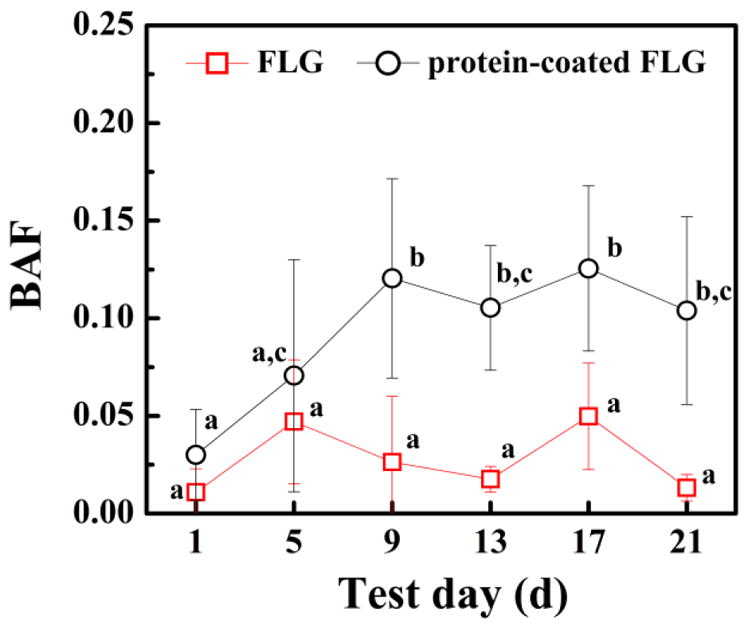
Bioaccumulation factors (BAFs; FLG concentration in organism tissue divided by the FLG soil concentration) of FLG and protein-coated FLG spiked to soil E. foetida. E. foetida were exposed to FLG or protein-coated FLG in soil with an initial suspended FLG concentration of 1 mg/kg. Mean and standard deviation values were calculated from triplicate samples. Data points with the same letter are not significantly different from one another; Tukey’s multiple comparisons test, p ≥ 0.05.
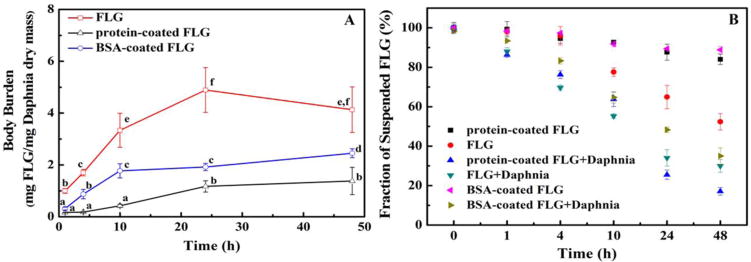
3.5 Uptake of FLG, BSA-adsorbed FLG and protein-coated FLG by D. magna
Uptake of FLG, protein-coated FLG and BSA-coated FLG in D. magna was also tested (Fig. 5A). The protein-coated FLG and BSA-coated FLG, which had better stability in water, showed lower body burden values (Fig. 5A). Substantial uptake (4.8 μg/mg of dry tissue) of FLG was measured in the D. magna exposed to FLG (100 μg/L) after 24 h, while uptake of the protein-coated FLG (100 μg/L) after exposure for 24 h was a quarter of that amount (≈ 1.2 μg/mg) and BSA-coated FLG (100 μg/L) was ≈ 1.9 μg/mg (Fig. 5A). After depuration for 10 h, ~64%, 92% and 94% of the uptake of the FLG, protein-coated FLG and BSA-coated FLG was remained in the Daphnia (that had been exposed for 24 h to a graphene concentration of 100 μg/L and then depurated in clean water for 10 h), respectively (see Figure S7 of Supplementary Materials).
Fig. 5.
(A) Pristine, BSA-coated and protein-coated FLG uptake by D. magna. Daphnia were exposed to FLG in artificial freshwater for 48 h with an initial suspended FLG concentration of 100 μg/L. (B) The fraction of the FLG (FLG), BSA-coated (BSA-coated FLG) and protein-coated FLG (protein-coated FLG) concentration remaining in the exposure solution with time relative to the initial concentration in containers without D. magna; measured concentration of FLG (FLG + Daphnia), BSA-coated FLG (BSA-coated FLG + Daphnia) and protein-coated FLG (protein-coated FLG + Daphnia) in the uptake experiment solution after D. magna removal. Mean and standard deviation values were calculated from triplicate samples. Data points with the same letter are not significantly different from one another; Tukey’s multiple comparisons test, p ≥ 0.05.
During the exposure period, the aqueous-phase radioactivity after D. magna removal and the radioactivity in the exposure solutions without D. magna were also measured, respectively (Fig. 5B). In the absence of D. magna, roughly 10% of the oligochaete protein or BSA-coated FLG and 50% of FLG settled from the exposure solution under the tested concentrations at 48 h. The presence of Daphnia in the exposure solution enhanced the settling rates of graphene; approximately 80% of the protein-coated FLG and 70% of BSA-coated FLG and FLG settled from the exposure solution after 48 h.
4. Discussion
While numerous studies have assessed proteins associating with carbon nanotubes and graphene in cell culture studies [42–44], this is the first study to our knowledge on the interaction of proteins produced by a multi-cellular organism with a CNM and the bioaccumulation behaviors of these protein-coated CNMs. Accumulation results of the three higher tested concentrations (250, 500, and 1000 μg/L) by L. hoffmeisteri showed a general increase during the first 36 h followed by a slight decrease from 36 h to 48 h (Fig. 1A). This reveals that a pseudo-steady-state concentration was reached after 36 h; the increase in body burden during the 48 h exposure period could not be explained by decreasing organism mass because the mass actually decreased by less than 25% (see Fig. S8 of Supplementary Materials) and body burden increased by more than a factor of 10 (Fig. 1A). The decrease in the body burdens from 36 h to 48 h is likely a result of the decreasing aqueous phase concentration during the first 36 h and the body burdens adjusting to the lower suspended FLG concentration (Fig. 1B). While many standard aquatic toxicity methods encourage maintaining relatively constant (within 20%) exposure concentrations, this is often not feasible with nanomaterials as a result of their instability in water [45]. After depuration for 12 h in clean water, L. hoffmeisteri was able to eliminate most of the uptake FLG (>90%). Overall, the uptake concentrations in this organism are orders of magnitude lower than those in previous studies with D. magna which revealed FLG body burdens of 7.8 μg/mg dry mass after 24 h exposure to a 250 μg/L FLG suspension [4].
The FLG detected in the earthworms after exposure may be at least partly accounted for soil remaining in the earthworms’ guts after depuration. The FLG concentration in the soil that purged from the earthworm guts was 94% of the initial FLG concentration in the soil (see Fig. S6). Gut loading (dry weight soil per dry weight worm) for E. foetida was found to be 0.63 ± 0.022 for mineral soil [46]. A 0.05 fraction of gut content remaining after 24 h depuration has been reported for E. foetida, a value similar to the fraction of gut content (0.056 ± 0.021) remaining for Eisenia Andrei after 24 h depuration [47]. However, the slightly higher values observed for the protein coated-FLG treatment compared to the uncoated FLG suggest that soil remaining in the gut cannot fully explain the uptake results (Fig. 4). It is possible that the protein-coated FLG associated with the gut tract to some extent, but additional biodistribution measurements are needed to determine the location of the protein-coated FLG in the earthworms.
The increased settling rate of BSA or protein-coated FLG during the exposures with D. magna in comparison to control experiments without Daphnia is likely attributable to the graphene particles being impacted during passage through the organism gut tract [4]. Increased agglomeration may have occurred during passage through the gut tract. In addition, the Daphnia may have consumed the surface coating as a food source [48], after which point the stabilization provided by the coating would be removed. The increased settling of the coated FLG result from D. magna differs from that observed with the Fenton-treated FLG which did not show a decrease in the aqueous phase concentration during D. magna exposures [11].
The comparable uptake results by D. magna for the FLG coated with BSA or secreted proteins are similar to previous studies which showed similar uptake concentrations for fullerenes dispersed with different types of natural organic matter (NOM) or multiwall carbon nanotubes (MWCNTs) dispersed with different polyethyleneimine functionalizations [49, 50]. The increase of D. magna mass during the FLG, protein-coated FLG or BSA-coated FLG exposure was less than 18%, indicating that the results were not strongly impacted by a change in organism mass because there was at least a four-fold increase in the FLG body burdens from 1 h to 48 h. Statistical analysis of the uptake results for FLG indicated that the 1 h, 4 h and 10 h time points differed from all of the other time points (p < 0.05) while the 24 h and 48 h data points did not differ (Fig. 5A). The uptake results for FLG thus showed a general increase during the first 24 h followed by keeping stable from 24 h to 48 h. The body burden of the protein-coated FLG and BSA-coated FLG at 48 h was ≈ 1.4 and 2.5 μg/mg, respectively, which was less than that for the pristine FLG uptake results (≈ 4.2 μg/mg). These results are similar to those previously obtained for FLG transformed by oxidative coupling or the Fenton reaction. FLG transformed by both reactions resulted in higher aqueous stability but lower D. magna uptake compared to the unmodified FLG [11, 14]. Overall, processes which make FLG more stable in water tend to cause a decrease in the body burdens with D. magna. This results likely stems from decreased agglomeration in the Daphnia gut tract which has a substantial impact on the body burdens given that most FLG is located in the gut tract. The depuration rates of the three types of FLG were significantly different (Fig. S7). Additional research is needed to quantify the impacts of exposure conditions (e.g., feeding with algae, no feeding) on the depuration rates and explore the possible mechanisms. Uptake of FLG by D. magna may thus differ from that by E. foetida: the agglomeration potential of FLG strongly impacts the body burdens of D. magna while other factors such as the concentration of soil remaining in the gut tract and interactions between the FLG and gut microvilli may be more critical for FLG bioaccumulation with earthworms. The impact of aqueous stability of the carbon nanomaterial on D. magna uptake also explains why uncoated MWCNTs and MWCNTs with polyethyleneimine coatings had similar uptake behaviors in a previous study [50]: their similar aqueous stabilities in the culture medium led to similar results in contrast to this study where FLG was unstable in suspension in the absence of a protein coating. Thus, D. magna uptake studies not including NOM, which has been shown to enhance graphene oxide stability [51], may overestimate D. magna uptake in the natural environment where NOM is ubiquitous.
5. Conclusion
During exposure to FLG, L. hoffmeisteri secreted proteins which coated the FLG and impacted the size distribution of FLG in suspension. After exposure to organisms that secrete proteins which coat FLG such as L. hoffmeisteri, these protein-coated FLG particles may be transported within water and sediment and be encountered by other organisms. Our results indicate that the protein-coated FLG have higher uptake by E. foetida yet lower uptake by D. magna compared to the uncoated FLG. Thus, when assessing the potential environmental fate and effects of nanomaterials, it is important to consider that interactions with one organism may impact the nanomaterial’s effects on and uptake by other organisms. Overall, these results provide key information about the bioaccumulation potential of FLG by multiple organisms, information that was previously unavailable partly as a result of the significant difficulty in making quantitative measurements of graphene family materials in organism tissues. This data can be valuable in comparisons of the bioaccumulation behaviors among different types of carbon nanomaterials (e.g., carbon nanotubes, fullerenes, nanocellulose, and graphene) and it can inform risk assessment of graphene materials thereby supporting the sustainable development of graphene-enabled commercial products.
Supplementary Material
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (21377049 and 21237001) and a Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201355). Certain commercial equipment, instruments and materials are identified to specify experimental procedures as completely as possible. In no case does such identification imply a recommendation or endorsement by the National Institute of Standards and Technology nor does it imply that any of the materials, instruments or equipment identified are necessarily the best available for the purpose.
Appendix A. Supplementary data
Additional description of experimental procedures; Figure S1–S8 and Table S1.
References
- 1.Petersen EJ, Henry TB, Zhao J, MacCuspie RI, Kirschling TL, Dobrovolskaia MA, et al. Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ Sci Technol. 2014;48(8):4226–46. doi: 10.1021/es4052999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen MJ, Tung VC, Kaner RB. Honeycomb carbon: A review of graphene. Chem Rev. 2010;110(1):132–45. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Kim F, Huang J. Seeing graphene-based sheets. Mater Today. 2010;13(3):28–38. [Google Scholar]
- 4.Guo X, Dong S, Petersen EJ, Gao S, Huang Q, Mao L. Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environ Sci Technol. 2013;47(21):12524–31. doi: 10.1021/es403230u. [DOI] [PubMed] [Google Scholar]
- 5.Ma Hock L, Strauss V, Treumann S, Küttler K, Wohlleben W, Hofmann T, et al. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Part Fibre Toxicol. 2013;10(12):1–20. doi: 10.1186/1743-8977-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan G, Kang SG, Tian X, Garate JA, Zhao L, Ge C, et al. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale. 2015;7:15214–24. doi: 10.1039/c5nr01839k. [DOI] [PubMed] [Google Scholar]
- 7.Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4(10):5731–6. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 8.Yang K, Li Y, Tan X, Peng R, Liu Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small. 2013;9(9–10):1492–503. doi: 10.1002/smll.201201417. [DOI] [PubMed] [Google Scholar]
- 9.Bianco A. Graphene: Safe or toxic? The two faces of the medal. Angew Chem Int Edit. 2013;52(19):4986–97. doi: 10.1002/anie.201209099. [DOI] [PubMed] [Google Scholar]
- 10.Jeong Jo, Cho HJ, Choi M, Lee WS, Chung BH, Lee JS. In vivo toxicity assessment of angiogenesis and the live distribution of nano-graphene oxide and its PEGylated derivatives using the developing zebrafish embryo. Carbon. 2015;93:431–40. [Google Scholar]
- 11.Feng Y, Lu K, Mao L, Guo X, Gao S, Petersen EJ. Degradation of 14C-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects. Water Res. 2015;84:49–57. doi: 10.1016/j.watres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Hou W, Chowdhury I, Goodwin DG, Henderson WM, Fairbrother DH, Bouchard D, et al. Photochemical transformation of graphene oxide in sunlight. Environ Sci Technol. 2015;49(6):3435–43. doi: 10.1021/es5047155. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Zhou M, Zhou Q. Ambient water and visible-light irradiation drive changes in graphene morphology, structure, surface chemistry, aggregation, and toxicity. Environ Sci Technol. 2015;49(6):3410–8. doi: 10.1021/es503003y. [DOI] [PubMed] [Google Scholar]
- 14.Lu K, Huang Q, Wang P, Mao L. Physicochemical changes of few-layer graphene in peroxidase-catalyzed reactions: Characterization and potential ecological effects. Environ Sci Technol. 2015;49(14):8558–65. doi: 10.1021/acs.est.5b02261. [DOI] [PubMed] [Google Scholar]
- 15.Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JVK. Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ Toxicol Chem. 2010;29(5):1072–8. doi: 10.1002/etc.124. [DOI] [PubMed] [Google Scholar]
- 16.Petersen EJ, Huang Q, Weber WJ. Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus. Environ Health Perspect. 2008;116(4):496–500. doi: 10.1289/ehp.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Fortner JD, Johnson DR, Chen C, Li Q, Alvarez PJJ. Bioaccumulation of C-14(60) by the Earthworm Eisenia fetida. Environ Sci Technol. 2010;44:9170–5. doi: 10.1021/es1024405. [DOI] [PubMed] [Google Scholar]
- 18.Petersen EJ, Pinto RA, Zhang L, Huang Q, Landrum PF, Weber WJ. Effects of polyethyleneimine-mediated functionalization of multi-walled carbon nanotubes on earthworm bioaccumulation and sorption by soils. Environ Sci Technol. 2011;45(8):3718–24. doi: 10.1021/es103004r. [DOI] [PubMed] [Google Scholar]
- 19.Patra M, Xin M, Isaacson C, Bouchard D, Poynton H, Lazorchak JM, et al. Changes in agglomeration of fullerenes during ingestion and excretion in Thamnocephalus platyurus. Environ Toxicol Chem. 2011;30(4):828–35. doi: 10.1002/etc.468. [DOI] [PubMed] [Google Scholar]
- 20.Deeds JR, Klerks PL. Metallothionein-like proteins in the freshwater oligochaete Limnodrilus udekemianus and their role as a homeostatic mechanism against cadmium toxicity. Environ Pollut. 1999;106(3):381–9. doi: 10.1016/s0269-7491(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki KT, Yamamura M, Mori T. Cadmium-binding proteins induced in the earthworm. Arch Environ Contam Toxicol. 1980;9(4):415–24. doi: 10.1007/BF01055293. [DOI] [PubMed] [Google Scholar]
- 22.Ek H, Bengtsson G, Rundgren S. Evolutionary response of earthworms to long-term metal exposure. Oikos. 1992;63(2):289–97. [Google Scholar]
- 23.Wang X, Duch MC, Mansukhani N, Ji Z, Liao Y, Wang M, et al. Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano. 2015;9(3):3032–43. doi: 10.1021/nn507243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klerks PL, Bartholomew PR. Cadmium accumulation and detoxification in a Cd-resistant population of the oligochaete Limnodrilus hoffmeisteri. Aquat Toxicol. 1991;19(2):97–112. [Google Scholar]
- 25.Matisoff G, Wang X, Mccall PL. Biological redistribution of lake sediments by Tubificid Oligochaetes: Branchiura sowerbyi and Limnodrilus hoffmeisteri/Tubifex tubifex. J Great Lakes Res. 1999;25(1):205–19. [Google Scholar]
- 26.Dias RJP, Cabra AF, Martins RT, Stephan NNC, Alves RdG, D’Agosto M. Occurrence of peritrich ciliates on the limnic oligochaete Limnodrilus hoffmeisteri (Oligochaeta, Tubificidae) in the neotropics. J Nat Hist. 2009;43(1–2):1–15. [Google Scholar]
- 27.Petersen EJ, Huang Q, Weber JWJ. Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida. Environ Sci Technol. 2008;42(8):3090–5. doi: 10.1021/es071366f. [DOI] [PubMed] [Google Scholar]
- 28.Martinez DE, Levinton J. Adaptation to heavy metals in the aquatic oligochaete Limnodrilus hoffmeisteri: Evidence for control by one gene. Evolution. 1996;50(3):1339–43. doi: 10.1111/j.1558-5646.1996.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 29.Volpers M, Neumann D. Tolerance of two tubificid species (Tubifex tubifex and Limnodrilus hoffmeisteri) to hypoxic and sulfidic conditions in novel, long-term experiments. Arch Hydrobiol. 2005;164:13–38. [Google Scholar]
- 30.Raussens V, Ruysschaert JM, Goormaghtigh E. Fourier transform infrared spectroscopy study of the secondary structure of the gastric H+,K+-ATPase and of its membrane-associated proteolytic peptides. J Biol Chem. 1997;272(1):262–70. doi: 10.1074/jbc.272.1.262. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Li K, Tong S. A linear regression method for the study of the Coomassie brilliant blue protein assay. Talanta. 1997;44:923–30. doi: 10.1016/s0039-9140(96)02140-6. [DOI] [PubMed] [Google Scholar]
- 32.Schägger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1(1):16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 33.Lanphere JD, Luth CJ, Walker SL. Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ Sci Technol. 2013;47(9):4255–61. doi: 10.1021/es400138c. [DOI] [PubMed] [Google Scholar]
- 34.Hwang YS, Li Q. Characterizing photochemical transformation of aqueous nC60 under environmentally relevant conditions. Environ Sci Technol. 2010;44(8):3008–13. doi: 10.1021/es903713j. [DOI] [PubMed] [Google Scholar]
- 35.OECD. OECD guideline for testing of chemicals: 202 daphnia straus acute immobilization test. 2004 [Google Scholar]
- 36.Norde W, Giacomelli CE. BSA structural changes during homomolecular exchange between the adsorbed and the dissolved states. J Biotec. 2000;79(3):259–68. doi: 10.1016/s0168-1656(00)00242-x. [DOI] [PubMed] [Google Scholar]
- 37.Zielke U, Hüttinger KJ, Hoffman WP. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon. 1996;34(8):983–98. [Google Scholar]
- 38.Sun Y, Wang Q, Chen C, Tan X, Wang X. Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol. 2012;46(11):6020–7. doi: 10.1021/es300720f. [DOI] [PubMed] [Google Scholar]
- 39.Calizo I, Balandin AA, Bao W, Miao F, Lau CN. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007;7(9):2645–9. doi: 10.1021/nl071033g. [DOI] [PubMed] [Google Scholar]
- 40.Hyung H, Kim JH. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ Sci Technol. 2008;42(12):4416–21. doi: 10.1021/es702916h. [DOI] [PubMed] [Google Scholar]
- 41.Kirschling TL, Golas PL, Unrine JM, Matyjaszewski K, Gregorgy KB, Lowry GV, et al. Microbial bioavailability of covalently bound polymer coatings on model engineered nanomaterials. Environ Sci Technol. 2011;45(12):5253–5259. doi: 10.1021/es200770z. [DOI] [PubMed] [Google Scholar]
- 42.Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, et al. Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl Mater Inter. 2012;4(4):2259–66. doi: 10.1021/am300253c. [DOI] [PubMed] [Google Scholar]
- 43.Shannahan JH, Brown JM, Chen R, Ke PC, Lai X, Mitra S, et al. Comparison of nanotube-protein corona composition in cell culture media. Small. 2013;9(12):2171–81. doi: 10.1002/smll.201202243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Wu C, Guo S, Zhang J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol Rev. 2013;2(1):27–45. [Google Scholar]
- 45.Petersen EJ, Diamond S, Kennedy AJ, Goss G, Ho K, Lead JR, et al. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: Key issues and consensus recommendations. Environ Sci Technol. 2015;49(16):9532–47. doi: 10.1021/acs.est.5b00997. [DOI] [PubMed] [Google Scholar]
- 46.Hartenstein F, Hartenstein E, Hartenstein R. Gut load and transit-time in the earthworm Eisenia foetida. Pedobiologia. 1981;22:5–20. [Google Scholar]
- 47.Jager T, Rhlj F, Roelofs W, de Groot AC. Feeding activity of the earthworm Eisenia andrei in artificial soil. Soil Biol Biochem. 2003;35(2):313–22. [Google Scholar]
- 48.Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin S, et al. In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol. 2007;41(8):3025–9. doi: 10.1021/es062572a. [DOI] [PubMed] [Google Scholar]
- 49.Pakarinen K, Petersen EJ, Alvila L, Waissi-Leinonen GC, Akkanen J, Leppänen MT, et al. A screening study on the fate of fullerenes (nC60) and their toxic implications in natural freshwaters. Environ Toxicol Chem. 2013;32(6):1224–32. doi: 10.1002/etc.2175. [DOI] [PubMed] [Google Scholar]
- 50.Petersen EJ, Pinto RA, Mai DJ, Landrum PF, Weber WJ. Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna. Environ Sci Technol. 2011;45(3):1133–8. doi: 10.1021/es1030239. [DOI] [PubMed] [Google Scholar]
- 51.Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ Sci Technol. 2013;47(12):6288–96. doi: 10.1021/es400483k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.