Abstract
In patients with muscle injury or muscle disease, assessment of muscle damage is typically limited to clinical signs, such as tenderness, strength, range of motion, and more recently, imaging studies. Animal models provide unmitigated access to histological samples, which provide a “direct measure” of damage. However, even with unconstrained access to tissue morphology and biochemistry assays, the findings typically do not account for loss of muscle function. Thus, the most comprehensive measure of the overall health of the muscle is assessment of its primary function, which is to produce contractile force. The majority of animal models testing contractile force have been limited to the muscle groups moving the ankle, with advantages and disadvantages depending on the equipment. Here, we describe in vivo methods to measure torque, to produce a reliable muscle injury, and to follow muscle function within the same animal over time. We also describe in vivo methods to measure tension in the leg and thigh muscles.
Keywords: Skeletal muscle, Lengthening contraction, Contractile function, Torque, Specific force, Injury
1 Introduction
Muscle damage in humans, with muscle injury or muscle disease, is typically assessed with clinical presentations of pain, impaired strength, decreased range of motion, tenderness, and imaging techniques such as MRI and ultrasound. However, muscle damage is difficult to assess because the clinical presentation varies greatly depending on age, gender, underlying genetic mutations and factors that can affect comorbidities, such as diet and level of activity. Furthermore, as the incidence of injury is a random event that is difficult to predict, it is difficult to study injury-induced muscle damage in humans. To obtain biological markers of injury for studying the mechanisms of injury and repair, animal studies have been used [1–9]. These studies have generated much of the data regarding muscle damage and repair, as they provide control over many of the variables. However, histological markers, which provide a “direct measure” of injury and biological markers, such as serum creatine kinase levels that are typically elevated with muscle injury, do not correlate with loss of function [10]. Thus, contractile force can be used for obtaining a more comprehensive measure of muscle health.
Several animal models exist to measure muscle contractility and to induce muscle injury via eccentric contractions. These were initially limited to “hanging” small, thin muscles in a bath mixture for field stimulation (in vitro), but there is strong interest in using in vivo methods [11], as evidenced by the growing number of publications using such methods [4, 5, 12–15].
Measuring muscle strength in vivo can be performed using one of two systems, with one system measuring muscle torque and the other system measuring muscle tension. The first is a system to measure muscle torque around a joint, which is a method for assessing contractile function without dissecting the muscle. The benefits of this system are that the muscle anatomy and biomechanics are not altered, and that the procedure is not terminal. Thus, one can measure contractility in the same animal over time, and compare results to in vivo imaging, such as MRI. Other advantages are that the nerve is not bypassed for stimulation (such as for in vitro preparations) and that the muscle remains in its normal environment, so effects of inflammation, hormones, or other factors can be studied. However, there are some limitations compared to measuring muscle function in isolated muscles. For example, length changes that occur during isometric or lengthening contractions must be estimated, and the muscle mass cannot be measured until it is harvested (although it can be estimated based on the volume measured via MRI) [16].
The second method to measure muscle function in vivo involves releasing the distal tendon and attaching it to a load cell. One advantage of this model is that the contractility of only one muscle with a known length and mass is measured. This allows one to determine the “ specific force ” (force per unit of cross-sectional area) of an individual muscle and avoids force transmission from nearby muscles. Although it provides more experimental control when measuring the force of an individual muscle, the experiment becomes less physiological. Surgical release of the muscle can also alter the anatomy and affect force transmission. The experiment is also terminal, so any changes in muscle contractility cannot be monitored over time.
Our custom-designed injury model is based on the same principles used by others to establish contraction-induced injury in animals [7, 9, 17, 18]. Despite the availability of models in the marketplace, there is little instruction beyond the use of the hardware. Our model has specifications in terms of available range of motion and angular velocity that are advantageous [19, 20], but our main goal is to share the methods. Here, we describe the procedures from start to finish for measuring muscle contractility, specifically torque and tension in vivo. We also describe the technique used to induce muscle injury, as susceptibility to injury is a very useful marker in the study of muscle disease.
2 Materials
2.1 Hardware/Software for Muscle Physiology
BUD value line cabinet (Newark, 06M4718).
Multifunction data acquisition device I/O USB-6221M (National Instruments, 779808-01).
Stepper motor controller (Newark, 16M4189).
Stepper motor (Newark, 16M4198).
Strain gauge amplifier (Honeywell, Sensotec, DV-05).
Torque sensor (Honeywell, QWLC-8M).
Foot plate and stabilization device (custom designed).
Lever arm for quadriceps testing (custom designed).
DC amplifier (model P122, Grass Instruments, Warwick, RI).
Force Displacement Transducer (model FT03, Grass Instruments, Warwick, RI for in vivo muscle tension).
Acquisition Software (PolyVIEW™ 16, Grass Instruments, Warwick, RI).
Software for synchronization of contractile activation, joint rotation and torque data collection (LabView version 2013, National Instruments, Austin, TX).
2.2 Muscle Attachment/Harvest
Precision vaporizer (Vet Equip, Inc., Pleasanton, CA).
Electrodes (J05 Needle Electrode Needles, 36BTP).
Micromanipulator (Kite Manipulator, World Precision Instruments Inc.).
Warmed mineral oil or DMEM.
Sterile ophthalmic cream (Paralube Vet Ointment, PharmaDerm, Floham Park, NJ, if animal is anesthetized for a long period of time).
Microdissection tools (if performing tendon release to obtain specific tension).
4.0 Ethicon suture or customized muscle clamp.
3 Methods
All experimental procedures were approved by the University of Maryland Institutional Animal Care & Use Committee. These procedures can be used for mice, rats, and even larger animals such as rabbits. To begin, place the animal supine under inhalation anesthesia (~4–5 % isoflurane for induction in an induction chamber, then ~2 % isoflurane via a nosecone for maintenance) using a precision vaporizer. If the animal is to be anesthetized for a long period of time, apply sterile ophthalmic cream to each eye to protect the corneas from drying. During the procedure, the animal is kept warm by use of a heat lamp. Animals lose body heat while under inhalation anesthesia, so they need to be kept warm. However, the heat lamp should be kept at a safe distance of at least 12 in. away from the animal. Confirm proper anesthesia by lack of a deep tendon reflex (no foot withdrawal in response to strong pinching the foot). Prepare the skin around the leg and ankle by removing hair, and by cleaning with alternating scrubs of Betadine and 70 % alcohol to prevent seeding skin bacteria into the soft tissue or bone.
3.1 Ankle (Testing Dorsiflexors)
3.1.1 In Vivo Torque
Similar methods for measuring in vivo dorsiflexion and plantarflexion contractility are also described in Chapter 1 by Call and Lowe [21].
A needle (25 G for rat or 27 G for mouse) is manually placed through the proximal tibia by hand. The needle will be used to stabilize the tibia onto the rig (see Notes 1 and 2).
Lock the needle (and consequently, the tibia) into a fixed position with the animal supine on a raised bed such that the tibia lies horizontal to the floor. The ankle should be free to rotate through a full range of motion. We use a custom-made device to secure the needle, and thereby stabilize the leg (Fig. 1a). Other methods for stabilizing the leg can be used instead of the custom- made device, such as a clamp (see Note 3).
Subcutaneous electrodes are used to stimulate the fibular nerve at the outside of the knee where it lies in a superficial position. You should visually confirm isolated dorsiflexion by performing a series of twitches (0.1 ms pulse for the mouse and 1 ms pulse for the rat) before the foot is secured into the foot plate (see Note 4).
Secure the foot onto the custom made footplate with adjustable lever arm using adhesive tape. This footplate is attached to a torque sensor and a stepper motor. Align the axis of rotation of the ankle joint with the axis of motor rotation.
The voltage is adjusted to optimize twitch tension. An increase in fused amplitude in response to an increase in voltage confirms that opposing muscles are not being simultaneously stimulated by excessive current.
The optimal position of the joint is determined by measuring twitches at different lengths of the muscle. This is done by sequentially rotating the foot plate at 5° increments from start position through the full range of motion. The optimal length (Lo or “resting length”) is the angle at which peak twitch amplitude occurs in the torque-angle curve (this is typically at the mid-range of motion).
The optimal frequency is obtained by measuring the torque with progressively increasing frequency of pulses during a tetanic contractions (typically ~200 ms duration) (see Note 5).
The maximal force-producing capacity is recorded as the “maximal isometric torque ” (measured in N mm) with the optimized voltage, the optimized joint angle, and the optimized frequency of pulses. Three separate twitches and tetanic contractions are recorded and saved for further analysis. Representative maximal isometric torque trace is as shown in Fig. 3b
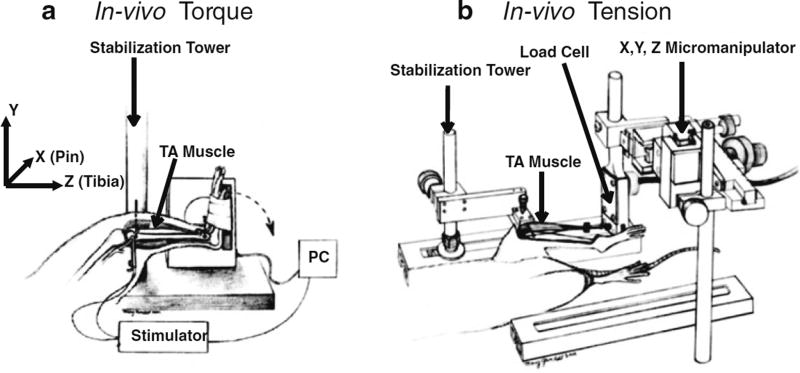
Fig. 1.
Testing ankle dorsiflexor muscles. (a) In vivo torque apparatus. To measure dorsiflexion isometric torque, the tibia is stabilized and the foot is attached to a footplate that is attached to a torque cell. The ankle dorsiflexors are stimulated by subcutaneous electrodes via the fibular nerve. To induce injury, the ankle dorsiflexors are maximally stimulated while a motor, attached to the footplate, forces the foot into plantar flexion (dotted arrow). The magnitude of injury can be regulated by modifying variables such as angular velocity, timing of muscle activation, range of motion, and the number of lengthening contractions. (Image from Lovering et al., J Biomech 2005, used with permission.) (b) In vivo tension apparatus. The tibia is stabilized, and the tendon of the primary dorsiflexor, the tibialis anterior (TA), is released and attached to the load cell. The load cell is mounted to the X, Y, Z micromanipulator, which is used for aligning the muscle and for adjusting the TA to its resting length. The TA muscle is stimulated by subcutaneous electrodes via the fibular nerve (Image reproduced from Lovering et al., J Vis. Exp. 2011, used with permission)
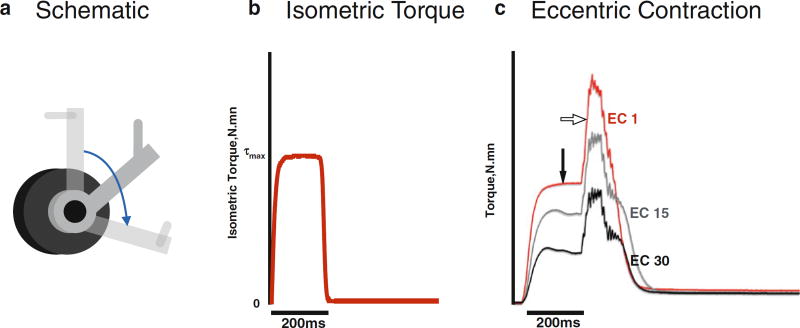
Fig. 3.
Representative data from the apparatus used to obtain muscle torque. (a) Positioning of the joint. For measurement of isometric torque at resting length (optimal length of the muscle), the lever arm is placed midrange (solid grey lever arm in schematic). For eccentric contractions used to induce injury, movement typically starts with the lever arm in a position shorter the optimal length of the muscle, so the joint can be moved through a large range of motion (blue arrow). (b) Isometric Torque. Representative trace data of maximal isometric torque obtained at mid-range (optimal length). (c) Eccentric Contractions. Representative data of torque r ecorded from eccentric contractions (ECs). The muscle is placed at the start position (in this example shorter than optimal length) and stimulated for 200 ms to induce maximal isometric torque (closed arrow) before lengthening (open arrow) using the lever arm. Repeated maximal ECs result in a quantifiable muscle injury. In this example of 30 ECs, the red tracing represents the first EC and the black tracing represents the last EC
3.1.2 In Vivo Tension
The limb is stabilized, and the nerve is stimulated as described above in Subheading 3.1.1 using the same apparatus (Fig. 1b). All instrumentation is turned on at least 30 min prior to testing for proper calibration and to minimize thermal drift of the force transducer. In contrast to the in vivo torque measurements in Subheading 3.1.1, this technique involves a surgical procedure (releasing the muscle insertion) and is therefore terminal. Results will no longer be in units of torque (typically N mm), but instead in units of force (typically grams, or Newtons) (see Note 6).
Incise the skin anterior to the ankle, and sever the tendon of the TA muscle as distally as possible (see Note 7).
Carefully tie 4.0 Ethicon silk non-absorbable suture to the tendon and attach the vicryl suture to the load cell (Fig. 1b) (force displacement transducer) via an S-shaped hook (weight = 0.1 g). Alternatively, a custom clamp (weight = 0.5 g) can be used to attach the tendon to the vicryl suture.
The load cell is mounted to a micromanipulator (Fig. 1b) so that the muscle can be adjusted to resting length and aligned properly (a straight line of pull between the origin and insertion of the muscle and the load cell).
The muscle is protected from cooling by a heat lamp. If the user wishes to expose more than just the tendon, dehydration of the exposed muscle can be minimized by application of mineral oil, as needed (to which extent the TA muscle is released is up to the user, as long as it is performed consistently). Alternatively, a continuous drip of 37 °C culture medium (DMEM) over the muscle can be used.
The signals from the load cell (calibrated before each test) are fed via a DC amplifier to an A/D board to be collected and stored by acquisition software.
Measure single twitches (rectangular pulse of 1 ms) at different muscle lengths in order to determine resting length (Lo). Muscle resting length is defined as the length at which maximal contractile force is produced.
At this length, gradually increase the voltage until maximal force is obtained and then incrementally increase the pulse frequency to establish a force–frequency relationship. A maximally fused tetanic contraction is obtained at approximately 80–150 Hz (200 ms train duration comprising 1 ms pulses) (see Note 5). A representative force–frequency experiment, with various tension traces generated by increasing frequency stimulation, is shown in Fig. 4a (see Note 8).
Use 150 % of the maximum stimulation intensity (pulse voltage) to activate the TA muscle in order to induce maximal contractile tension (Po). Three separate twitches and tetanic contractions are recorded and saved for further analysis.
Maximal tetanic contractions can be performed repeatedly (typically every 0.5–1 s) with the final tension expressed as percentage of Po, providing an index of fatigue at any desired point in time. Representative trace of muscle fatigue assessment using repeated maximal tetanic contractions is shown in Fig. 4b.
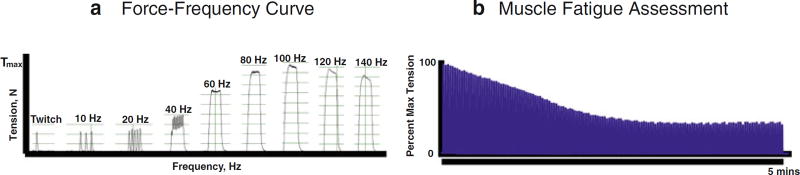
Fig. 4.
Representative data from the apparatus used to obtain muscle tension. (a) Force Frequency Curve. Representative tracings of maximal tension of a muscle with progressively increasing frequency of pulses during a 200 ms pulse train. The maximal contractile tension typically occurs ~ 80–150 Hz, depending on the muscle, electrode placement, etc. In this example, occurs at the optimal frequency (in this example, 100 Hz). (b) Muscle Fatigue Assessment. Representative muscle fatigue assessment, which can be calculated at any point time with repeated maximal tetanic contractions as a percentage of initial maximal contractile tension. In this example, stimulation occurred every 2 s over a period of 5 min. Both these measures (force–frequency curve and muscle fatigue assessment) can also be assessed using the torque apparatus instead of in an isolated muscle, as performed here
3.2 Knee (Testing Quadriceps)
3.2.1 In Vivo Torque
A needle (25 G for rat or 27 G for mouse) is manually placed through the distal femur (instead of the proximal tibia for testing dorsiflexors) by hand. The needle will be used to stabilize the femur onto the rig (see Notes 1 and 2).
Lock the needle (and consequently, the femur) into a fixed position with the animal supine, such that the femur lies horizontal to the floor. The knee should be free to rotate through a full range of motion. We use a custom-made device to secure the needle and thereby stabilize the thigh (Fig. 2a) (see Note 3). Subcutaneous electrodes are used to stimulate the femoral nerve anterior to the hip joint, where it lies in a relatively superficial position. You should visually confirm isolated knee extension by performing a series of twitches (0.1 ms pulse width) before the limb is secured (see Note 4).
Secure the distal leg to a custom-machined adjustable lever arm with adhesive tape. The quadriceps lever arm is attached to a stepper motor and a torque sensor. Align the axis of rotation of the knee joint with the axis of motor rotation.
The optimized voltage, optimized joint angle and the optimized frequency of pulses is obtained similar to that of the ankle, described in Subheading 3.1.1. At the optimized parameters, three separate twitches and tetanic contractions are recorded and saved for further analysis.
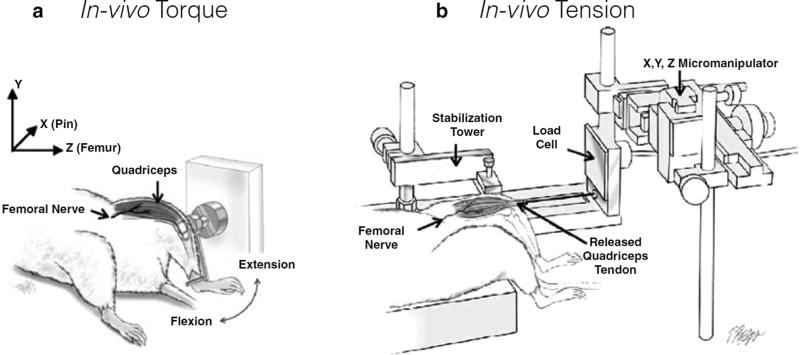
Fig. 2.
Testing knee quadriceps muscles. (a) In vivo torque apparatus. To measure quadriceps isometric torque, the femur is stabilized, and the distal leg is taped (not shown) to a lever arm, which is attached to a torque cell to measure knee extension. The quadriceps are stimulated by subcutaneous electrodes through the femoral nerve, producing knee extension. To induce injury, the quadriceps muscles are stimulated while the lever arm forces the knee into flexion. As with dorsiflexion, the magnitude of injury can be regulated by modifying angular velocity, timing of muscle activation, range of motion, and the number of lengthening contractions. (Image adapted from Pratt et al., J Physiol. 2012, used with permission.) (b) In vivo tension apparatus. The femur is stabilized, and the quadriceps tendon is released and attached to the load cell. The load cell is mounted to the X, Y, Z micromanipulator, which is used for aligning the muscle and for adjusting the quadriceps to their resting length. The quadriceps muscle is stimulated by subcutaneous electrodes through the femoral nerve (Image adapted from Pratt and Lovering, J Biol. Methods 2014, used with permission)
3.2.2 In Vivo Tension
The limb is stabilized, and the nerve is stimulated as described above in Subheading 3.2.1 using the same apparatus. Similar to the measurement of in vivo tension in the TA muscle, all instrumentation is turned on at least 30 min prior to testing.
Incise the skin anterior to the knee, and sever the tendon of the quadriceps muscle as distally as possible (see Note 7).
Carefully tie 4.0 Ethicon silk non-absorbable suture to the tendon and attach the vicryl suture to the load cell (Fig. 2b) via an S-shaped hook (weight = 0.1 g). Alternatively, a custom clamp (weight = 0.5 g) can be used to attach the tendon to the vicryl suture. The load cell, DC amplifier, A/D board and the acquisition software are described in Subheading 3.1.2 for measuring in vivo tension in the TA muscle.
The load cell is mounted to a micromanipulator (Fig. 2b) so that the muscle can be adjusted to resting length and aligned properly (a straight line of pull between the origin and insertion of the muscle and the load cell).
Similar to the TA muscle in Subheading 3.1.2, the quadriceps muscle must be protected from cooling with use of a heat lamp. If the quadriceps muscle is exposed, dehydration must be minimized similar to the TA muscle, using mineral oil or 37 °C culture medium.
Similar to the TA muscle in Subheading 3.1.2, the muscle resting length, the optimized voltage and the optimized pulse frequency is measured for the quadriceps muscle.
Use 150 % of the maximum stimulation intensity (pulse voltage) to activate the quadriceps muscle in order to induce maximal contractile tension (Po). Three separate twitches and tetanic contractions are recorded and saved for further analysis at the optimized parameters. Index of fatigue can be calculated for the quadriceps muscle similar to the method used for the TA muscle.
3.3 Induction of Muscle Injury
Similar methods for in vivo ankle dorsiflexion and plantarflexion eccentric contraction induced injury are also described in Chapter 1 by Call and Lowe [21].
The limb immobilization and the apparatus used for muscle injury induction are identical to the setup used to record maximal isometric torque of the dorsiflexors or quadriceps (Figs. 1a and 2a).
We use commercial software to synchronize contractile activation, onset of joint rotation, and torque data collection. For lengthening contractions, stimulation of the muscles occurs while the computer-controlled motor simultaneously moves the foot plate or lever arm against the direction of the muscle contraction, thus leading to a lengthening contraction (also called “eccentric” contraction which, if maximal and repeated, causes injury of the muscle). The specific protocol depends on the desired magnitude of injury and is designed by the investigator. The magnitude of injury, or tissue damage, can be regulated by manipulation of variables such as angular velocity, timing of muscle activation, range of motion, and the number of lengthening contractions.
To induce injury, superimpose a lengthening contraction onto a maximal isometric contraction (a contraction during which muscle length is held constant), varying the range of motion, velocity of lengthening, and timing of stimulation as needed (see Notes 9 and 10). For example, a maximal isometric contraction for a given muscle is obtained before the onset of motor movement and the muscle is immediately lengthened at a selected velocity to approximate normal movement (900°/s) resulting in a high force peak (Fig. 3c). We have shown that activation before and during motor movement, and the degree of lengthening are important factors in obtaining an injury [19]. As reported previously, this model results in injury to muscle [19, 22–26]. The muscle remains stimulated throughout lengthening.
After injury, the animal is removed from the apparatus. The pin is removed, the leg is cleaned again with Betadine, and over-the-counter antibiotic cream is applied to the needle placement site. The animal is returned to the cage (placed on a temperature-controlled heating block at 37 °C) and monitored until recovery. If follow-up measurements are desired, a new sterile needle is placed into the same location.
3.4 In Vivo Testing of Other Muscles
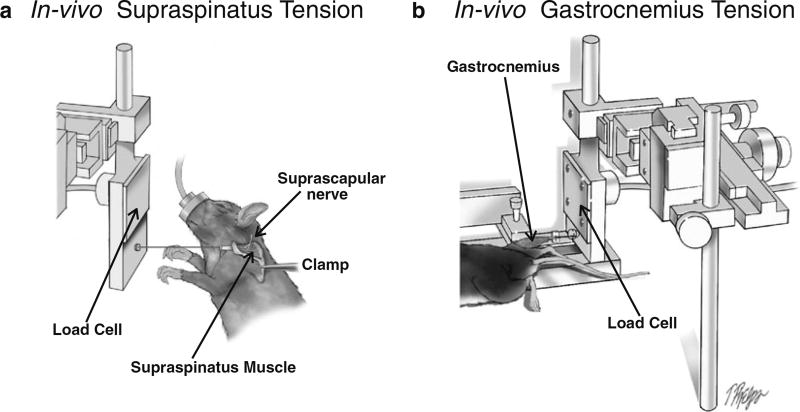
For testing force in isolated muscles, almost any limb muscle can be tested. We provide two examples here. The device can also be used for measuring in vivo tension of other muscles such as supraspinatus (Fig. 5a), a rotator cuff muscle, and the gastrocnemius (Fig. 5b), a hindlimb muscle.
For measuring in vivo tension of supraspinatus muscle, the scapula is stabilized using a clamp. The tendon of the supraspinatus is released and attached to a load cell that is mounted to a micromanipulator, similar to the technique seen in Subheading 3.1.2 for the TA muscle and Subheading 3.2.2 for the quadriceps muscle. The suprascapular nerve is stimulated near the suprascapular notch using subcutaneous electrodes. The maximal contractile tension and the muscle fatigue index can be obtained using the same procedures described in Subheadings 3.1.2 and 3.2.2 for the TA muscle and the quadriceps muscles, respectively.
For measuring in vivo tension of the gastrocnemius muscle, the tibia is stabilized using similar techniques described in Subheading 3.1.1. The Achilles tendon is released and attached to a load cell that is mounted to a micromanipulator, similar to the technique seen in Subheading 3.1.2 for the TA muscle and Subheading 3.2.2 for the quadriceps muscles. The tibial nerve is stimulated in the popliteal fossa using subcutaneous electrodes. The maximal contractile tension and the muscle fatigue index can be obtained using the same procedures described in Subheadings 3.1.2 and 3.2.2 for the TA muscle and the quadriceps muscles, respectively.
Fig. 5.
In vivo testing of other muscles. (a) In vivo supraspinatus tension. The scapula is stabilized and supraspinatus tendon is released and attached to the load cell. Similar to the hind limb experiments, the load cell is mounted to the X, Y, Z micromanipulator, which is used for aligning the muscle and for adjusting the supraspinatus to its resting length. The supraspinatus muscle is stimulated by subcutaneous electrodes via the suprascapular nerve. (b) In vivo gastrocnemius tension. The tibia is stabilized, and the Achilles tendon is released and attached to the load cell (the soleus muscle can be detached to test isolated gastrocnemius). The load cell is mounted to the X, Y, Z micromanipulator, which is used for aligning the muscle and for adjusting the gastrocnemius to its resting length. The gastrocnemius muscle is stimulated by subcutaneous electrodes via the tibial nerve (not shown)
3.5 Harvesting and Storing Muscles
At the end of experiments, animals are euthanized per institutional animal care guidelines and the muscles are harvested, weighed, snap frozen in liquid nitrogen, and then stored at −80 °C. This can be performed at any point in time after the in vivo experiments. Muscles are always harvested immediately after the in vivo tension experiments, as this is a terminal procedure. For detailed morphological studies, the animal is fixed with 4 % paraformaldehyde via perfusion through the left ventricle.
Acknowledgments
This work was supported by grants from the National Institutes of Health by grants to APV (T32AG000268-15S1), SRI (AR07592-20), and to RML (1R01AR059179).
Footnotes
The device we use to stabilize the limb allows three degrees of freedom for positioning, but this is for convenience; the method used to stabilize the trans-osseous needle is not important, as long as the femur or the tibia is immobilized and the position is accurate. We have tried many methods to stabilize the limb without using a needle, but the trans-osseous needle proved superior in terms of stability. For tests of the same limb over time, in our experience, it is relatively easy to place the tip of the needle under the skin and slide along the bone to find the same location used for earlier procedures.
When testing the ankle muscles, the needle should be through the proximal tibia and posterior enough such that the TA muscle is not penetrated by the needle. When testing the quadriceps, the needle should not enter the anterior compartment of the thigh, the quadriceps, or knee joint (he widest aspect of the knee is formed by the femoral epicondyles, which sit just superficial to the condyles and are good landmarks to palpate for needle placement). Needle placement is easy, but can take practice. Correct needle placement can only be confirmed by opening the skin, which can be done during muscle harvesting, whenever the investigator decides to sacrifice the animal.
For all testing, the needle can be immobilized in a variety of methods; one could simply use a vise that is positioned correctly. If the needle is placed correctly in the coronal plane (parallel to the floor), the foot will be aligned so the toes are pointing anteriorly and not to the left or to the right.
One can obtain dorsiflexion or knee extension with the electrodes in approximately correct position. Be sure to try several locations, and also adjust the electrodes superficial to deep, in order to determine which electrode placement results in maximal torque. This type of trial and error is not necessary after you have an idea of what values animals of a specific gender/age/species generate on your device. The fibular nerve used to activate dorsiflexion is located at the lateral knee and is quite superficial. The femoral nerve is “superficial” to the hip joint and surrounding musculature, but it is comparatively deeper.
There are a variety of stimulation parameters in the literature. Very high stimulation frequency (250–300 Hz) is typical for in vitro procedures (removing a muscle from the animal and “hanging it” in a bath for field stimulation). However, for in vivo procedures (stimulating through the motor nerve), maximal contractile tension occurs at a much lower frequency. In our hands, a maximal fused tetanic contraction in vivo for the TA is obtained at ~80–90 Hz, and in the quadriceps at ~100–150 Hz. The frequency, and voltage, should be optimized for each experiment.
Muscle torque is defined mathematically as the cross-product of the force vector and the moment arm vector. For ankle dorsiflexion, the tibia is fixed and the foot is attached to the foot plate. For knee extension, the femur is fixed and the leg is attached to the lever arm. Thus, the length of the moment arm is fixed and the only variable that changes is the magnitude of the muscle force.
Muscles can transmit some of their contractile force laterally, such that muscles in a group (e.g., dorsiflexors) can contribute to force measurements even when only one of the muscle (e.g., tibialis anterior) is tied to the load cell for assessment. Thus, for single muscle experiments (where the distal tendon is released for attachment to the load cell), it can be important to release the muscle entirely to its origin to avoid lateral transmission of force from nearby muscles [27]. For the quadriceps, since there are no additional knee extensor muscles, one can release just the tendon. Releasing the quadriceps completely up to its origin is more difficult than with other muscles due to its more complex attachments.
For single muscle terminal experiments, once the muscle is attached to the load cell there is a small amount of passive force present when the muscle is stretched to resting length. This passive tension can “drift” after the first few contractions. Having moist suture and performing a series of “warm up” twitches can minimize this drift, but the investigator should adjust resting length as needed.
When performing in vivo torque experiments Lo, or resting length, is typically midway through the available range of motion for most joints, but the precise location can be determined by seeing where maximal torque occurs. For inducing injury, the isometric torque that occurs before the onset of lengthening will be lower than the maximal isometric torque if the joint angle start position is not at where Lo was obtained mid-range (Fig. 3a, schematic).
As for injury (repeated eccentric contractions), many variables affect the magnitude of injury (operationally defined as a loss in maximal isometric torque). Assuming the velocity is above 100°/s, increasing velocity of lengthening is not likely to matter. Obtaining a full isometric contraction before (at least 200 ms long) lengthening occurs, as well as using a large range motion (but within physiological limits), will assist in producing a reliable injury that does not require an excessive number of contractions.
References
- 1.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol. 2011;111:1768–1777. doi: 10.1152/japplphysiol.00942.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingalls CP, Warren GL, Zhang JZ, Hamilton SL, Armstrong RB. Dihydropyridine and ryanodine receptor binding after eccentric contractions in mouse skeletal muscle. J Appl Physiol. 2004;96:1619–1625. doi: 10.1152/japplphysiol.00084.2003. [DOI] [PubMed] [Google Scholar]
- 4.Rathbone CR, Wenke JC, Warren GL, Armstrong RB. Importance of satellite cells in the strength recovery after eccentric contractioninduced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1490–R1495. doi: 10.1152/ajpregu.00032.2003. [DOI] [PubMed] [Google Scholar]
- 5.Lieber RL, Shah S, Friden J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop. 2002;(403 Suppl):S90–S99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- 6.Warren GL, Hermann KM, Ingalls CP, Masselli MR, Armstrong RB. Decreased EMG median frequency during a second bout of eccentric contractions. Med Sci Sports Exerc. 2000;32:820–829. doi: 10.1097/00005768-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- 8.Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner JA, Jones DA, Round JM. Injury to skeletal muscles of mice by forced lengthening during contractions. Q J Exp Physiol. 1989;74:661–670. doi: 10.1113/expphysiol.1989.sp003318. [DOI] [PubMed] [Google Scholar]
- 10.Friden J, Lieber RL. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand J Med Sci Sports. 2001;11:126–127. doi: 10.1034/j.1600-0838.2001.011002126.x. [DOI] [PubMed] [Google Scholar]
- 11.Matarese G, La CA, Horvath TL. In vivo veritas, in vitro artificia. Trends Mol Med. 2012;18:439–442. doi: 10.1016/j.molmed.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best TM, McCabe RP, Corr D, Vanderby R., Jr Evaluation of a new method to create a standardized muscle stretch injury. Med Sci Sports Exerc. 1998;30:200–205. doi: 10.1097/00005768-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- 14.Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contractioninduced injury. J Gerontol A Biol Sci Med Sci. 2001;56:B163–B171. doi: 10.1093/gerona/56.4.b163. [DOI] [PubMed] [Google Scholar]
- 15.Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281:R155–R161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- 16.Heemskerk AM, Strijkers GJ, Vilanova A, Drost MR, Nicolay K. Determination of mouse skeletal muscle architecture using three-dimensional diffusion tensor imaging. Magn Reson Med. 2005;53:1333–1340. doi: 10.1002/mrm.20476. [DOI] [PubMed] [Google Scholar]
- 17.Lieber RL, Friden J. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988;133:587–588. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 18.Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol. 1993;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovering RM, Hakim M, Moorman CT, III, De Deyne PG. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J Biomech. 2005;38:1501–1507. doi: 10.1016/j.jbiomech.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dipasquale DM, Bloch RJ, Lovering RM. Determinants of the repeated-bout effect after lengthening contractions. Am J Phys Med Rehabil. 2011;90:816–824. doi: 10.1097/PHM.0b013e3182240b30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Call JA, Lowe DA. Eccentric contraction-induced muscle injury: reproducible, quantitative, physiological models to impair skeletal muscle's capacity to generate force. 2016 doi: 10.1007/978-1-4939-3810-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakim M, Hage W, Lovering RM, Moorman CT, III, Curl LA, De Deyne PG. Dexamethasone and recovery of contractile tension after a muscle injury. Clin Orthop Relat Res. 2005;439:235–242. doi: 10.1097/01.blo.0000177716.70404.f9. [DOI] [PubMed] [Google Scholar]
- 23.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contractioninduced injuries. Arch Phys Med Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Lovering RM, McMillan AB, Gullapalli RP. Location of myofiber damage in skeletal muscle after lengthening contractions. Muscle Nerve. 2009;40:589–594. doi: 10.1002/mus.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratt SJ, Lawlor MW, Shah SB, Lovering RM. An in vivo rodent model of contraction-induced injury in the quadriceps muscle. Injury. 2011;43(6):788–793. doi: 10.1016/j.injury.2011.09.015. 2012; doi: 10.1016/j.injury.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt SJ, Lovering RM. A stepwise procedure to test contractility and susceptibility to injury for the rodent quadriceps muscle. J Biol Methods. 2014 doi: 10.14440/jbm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huijing PA, Baan GC. Myofascial force transmission causes interaction between adjacent muscles and connective tissue: effects of blunt dissection and compartmental fasciotomy on length force characteristics of rat extensor digitorum longus muscle. Arch Physiol Biochem. 2001;109:97–109. doi: 10.1076/apab.109.2.97.4269. [DOI] [PubMed] [Google Scholar]