Abstract
Background:
A large number of stroke patients are not the perfect candidate for craniotomy and invasive procedures, so providing an alternative and noninvasive method, which is applicable in terms of costs and facilities, is necessary. Thus, the present study aimed to determine the effects of mannitol 20% on outcome of the patients with nontraumatic intracerebral hemorrhage (ICH) in patients admitted to Isfahan's Al-Zahra Hospital during 2012 and 2013.
Materials and Methods:
This is a clinical trial study which is conducted during 2012–2013 in Isfahan's Al-Zahra Hospital. In this study, 41 patients suffering from ICH received mannitol 20% for 3 days, and volume of hemorrhage and Glasgow Coma Scale (GCS) of patients were controlled every 12 h. The collected data were analyzed via SPSS software.
Results:
The mean ICH volume was 22.1 ± 6.3 ml in pre intervention and 38.4 ± 19.3 ml in post intervention, and according to the t-paired test, before and after treatment the difference was significant (P < 0.001). Hemorrhage volume was stable in nine patients (22%), it increased in 25 patients (61%), and decreased in seven patients (17.1%). The mean index of GCS before and after treatment was 11.85 ± 1.6 and 9.37 ± 2.65, respectively. Moreover according to t-paired test, the difference was significant before and after treatment (P < 0.001). During using mannitol, the GCS index was stable in eight patients (19.5%), it increased in eight patients (19.5%) and decreased in 25 patients (61%).
Conclusions:
Mannitol injection was not effective in reducing hemorrhage size, and its use is not recommended, also, further studies in this field have been proposed.
Keywords: Intracerebral hemorrhage, level of consciousness, mannitol
Introduction
Brain strokes are the third leading cause of death and a major cause of morbidity. Nontraumatic intracerebral hemorrhages (ICHs) constitute 15% of strokes in Western societies and 20–30% of strokes in Asia. ICH is the most lethal type of brain stroke and survivors suffer from its severe complications and disabilities. The overall incidence of ICH is approximately 12–15 cases per 100,000 in year. The mortality rate of ICH is 30–40% in the 1st month, but it reaches 50% by the end of the 1st year.[1,2] There is a primary damage as a result of ICH, which manifests in the form of hemorrhage, brain edema, seizure, and/or hydrocephalus, but there adds some more damages.[3,4] These damages are known as secondary damages, including hypoxia, hypercapnia, hypotension, and permanent fever.[3,5]
Although initial damages cause neurological disorders for patients, secondary damages exacerbate them however they can be prevented.[4,6]
Using mannitol treatment for intracranial hemorrhages is difficult. Studies on humans and animals suggest that prescribing common drugs may worsen prognosis,[7,8] and still some of patients may need to craniotomy.[9] Although craniotomy is used in treatment of intracranial hemorrhage, many patients, particularly old patients, cannot tolerate anesthesia or such procedures,[10,11] but theoretically, the existence of hyperosmolar solutions such as mannitol 20% in cerebral arteries can effect the perilesional edema.[12,13] Hence, beside therapeutic effect of conservative treatment the large number of such patients in our community, we decided to do a study method to evaluate the effects of mannitol 20% on outcome of patients with ICH in patients admitted to Isfahan's Al-Zahra Hospital. Because of many controversies in effectiveness of surgery (craniectomy) in treatment of ICH, the surgical trial in intracerebral hemorrhage investigated early surgical removal of hematoma versus medical therapy and failed to show benefit in the surgical arm),[14] medical treatment is an important part of treatment in these patients. In addition, because of many controversies in use of mannitol in text books, this study was done to understand of better treatment in our patients.
Materials and Methods
This is a clinical trial study that conducted on nontraumatic ICH patients who were hospitalized for treatment in 2012 and 2013 in Al-Zahra Hospital, Isfahan.
Inclusion criteria are patients with ICH, patients over 18, lack of sensitivity to mannitol and there is no indication of surgery before intervention. In addition, it was decided to exclude patients who die during the study and need to surgery.
A sample size of the study was determined using sample size calculation formula to estimate the mean. It was estimated 55 patients with consideration of 95% confidence, 80% test power, 1.33 standard deviation of the volume of hemorrhage, and also accepting error rate of 0.5. The sampling method was easy.
The study was done in such a way that, after the approval of the proposal and reception of permission from Medical Ethics Committee of the University, patients with inclusion criteria were interred into the study after being justified and obtaining a written consent from them. At first, a full neurological history and physical examination of patients were taken and their level of consciousness (LOCs) (based on Glasgow Coma Scale [GCS]) was controlled at the time of admission and every 6 h, periodically. Patients treated with mannitol 20% (with loading dose of 1 g/kg, and then 0.5 g/kg every 6 h) for 3 days, and in order for follow-up, serial computed tomography scans were performed every 12 h. In case of indications for surgery, patients underwent craniotomy. Patients remained hospitalized until the stabilization of their general and neurologic conditions and then discharged.
We used standard dose of mannitol in comparison to other studies[15,16,17,18] that used low-dose mannitol 20% (e.g., 100 ml every 4–6 h), because we thought that low dose mannitol cannot had appropriate effect on ICH (textbooks also recommend our routine dose).[19,20,21,22]
The volume of bleeding was measured and recorded with Hounsfield criteria, and this continued until patient's discharge or until they had an indication of surgery. ABC/2 method to estimate the volume of ICH.[20]
Data were input into a computer and were analyzed by SPSS version 22(made by IBM company, USA). The Chi-square test (for comparison of qualitative variables between groups), t-test (for comparison of quantitative data between the two groups), t-paired test (for comparison of quantitative data before and after intervention), and Pearson correlation test (for determining the relation between quantitative data) were used.
Results
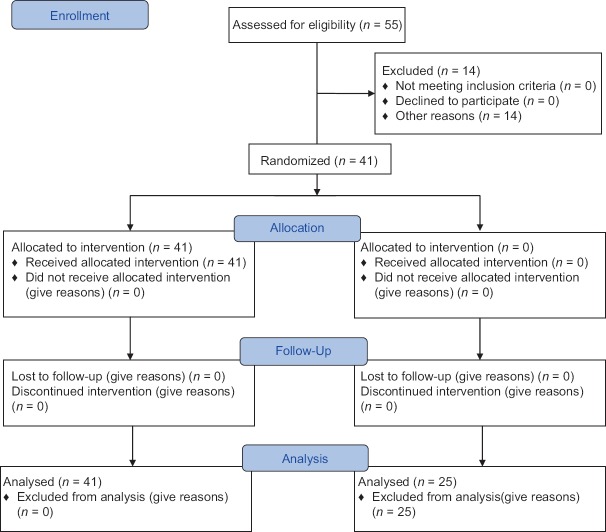
Fifty-five patients with nontraumatic ICH were analyzed in this study, and during treatment, 14 patients were excluded because of death or discharge with consent, and 41 patients were present to the end of the study [Figure 1].
Figure 1.
CONSORT flow diagram (17)
Twenty-five patients (61%), after the injection of mannitol, had increased hemorrhage as well as ICH and followed by that a drop in GCS and Hounsfield Value. Four patients (9.8%) improved and 12 patients (29.3%) had no change in their clinical conditions. Twenty-five patients underwent craniotomy surgery due to the ineffectiveness of mannitol.
The mean age of the so-called patients was 62.5 ± 8.4 with the age range of 49–81 years. Thirty patients (73.2%) of this study were male, and 11 (26.8%) were female.
The mean volume of ICH was 22.1 ± 6.3 ml in preintervention and 38.4 ± 19.3 ml in postintervention. According to the t-paired test, the difference before and after the treatment were significant (P < 0.001). The volume of hemorrhage did not change in nine patients (22%), it increased in 25 patients (61%) and decreased in seven patients (17.1%). In Figure 2, the frequency distribution of hemorrhage is illustrated before and after treatment.
Figure 2.
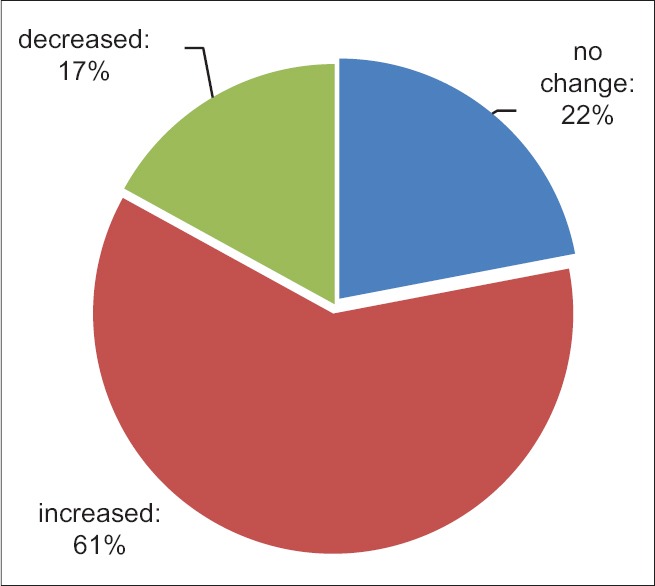
Frequency of hemorrhage before and after intervention
The mean GCS index was 11.85 ± 1.6 and 9.37 ± 2.65, respectively, before and after treatment, and according to the t-paired test, there was no significant difference before and after the treatment (P < 0.001). During treatment, GCS index did not change in eight patients (19.5%), it increased in eight patients (19.5%) and decreased in 25 patients (61%) [Figure 3].
Figure 3.
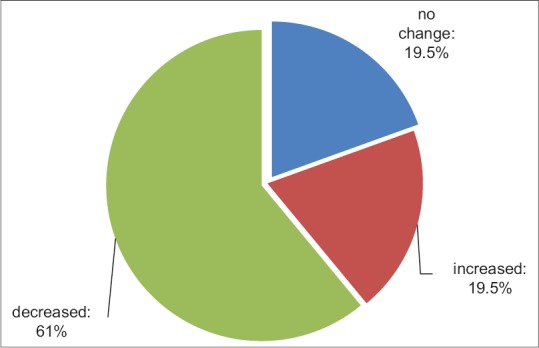
Frequency of Glasgow Coma Scale before and after intervention
In Table 1, the mean hemorrhage and GCS index of patients under craniotomy and those under mannitol treatment is shown. According to the t-test, the mean hemorrhage before and after treatment in two groups with or without indications for craniotomy did not differ significantly, however, hemorrhage volume was significantly higher in the group eligible for craniotomy. The mean volume of hemorrhage increased 28.6 ± 6.5 ml in the group undergoing craniotomy, whereas in the group with no indications for craniotomy, hemorrhage volume decreased 28.8 ± 3.6 ml, and the difference between two groups was significant.
Table 1.
The mean and SD of hemorrhage volume and GCS before and after intervention in two groups with or without craniotomy
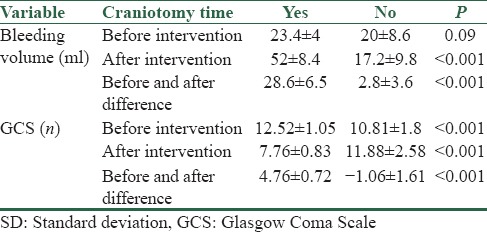
The mean GCS index, both before and during craniotomy, with or without indications for craniotomy, was significantly different between two groups. In addition, GCS index decreased 4.76 ± 0.72 units in the group eligible for craniotomy. Whereas, it increased 1.06 ± 1.61 units in the group without craniotomy, and the difference was significant between two groups.
Pearson correlation analysis on the obtained data showed an inverse correlation (0.31) between initial GCS and initial hemorrhage volume, which was statistically significance (P = 0.045) [Figure 4]. In addition, at the end of treatment, there found to be an inverse correlation (0.83) between hemorrhage volume and GCS, which was statistically significant (P < 0.001) [Figure 5]. In Figures 4 and 5, the correlation between hemorrhage volume and GCS is shown before and after the treatment.
Figure 4.
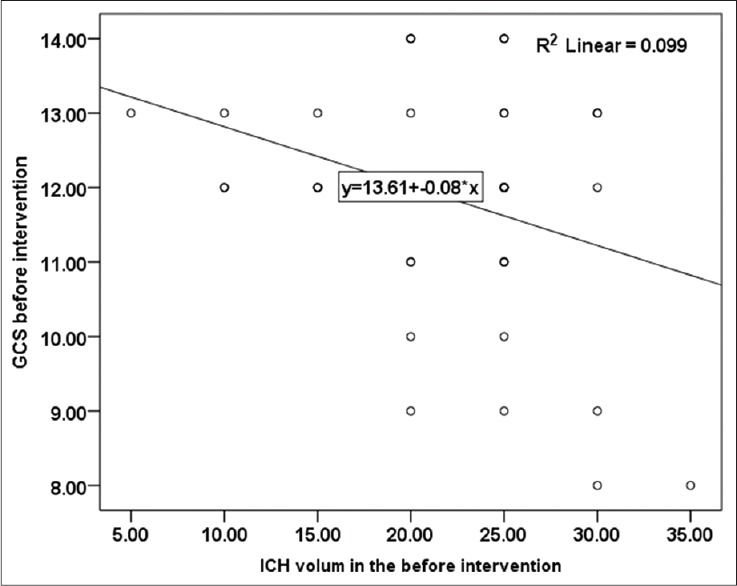
Correlation between hemorrhage volume and initial Glasgow Coma Scale
Figure 5.
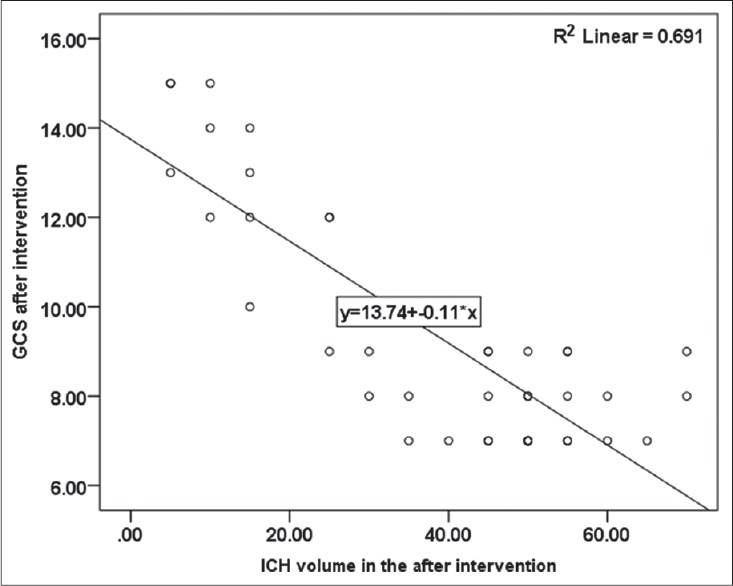
Correlation between hemorrhage volume and Glasgow Coma Scale after intervention
Discussion
Given the high incidence of brain strokes and particularly ICH in Iran, it is necessary to make use of innovative methods to try to improve these patients, because craniotomy surgery is not possible for most of patients; thus, the aim of this study was to determine the effect of mannitol 20% on outcome of patients with nontraumatic ICH, who were admitted to Isfahan's Al-Zahra Hospital during 2012 and 2013, so as to provide noninvasive and inexpensive approaches for treatment of these patients.
According to our results, after the injection of mannitol 20% (with dose of 1 g/kg and then 0.5 g/kg every 6 h), the hemorrhage volume did not change in 22% of patients, it increased in 61% and decreased only in 17.1% of patients, thus, manittol 20% injection is not effective in ICH treatment, and 61% of patients had increased hemorrhage. In a study conducted by Kumar and Badrinath in India, blood pressure and hyperosmolar medications such as mannitol had relative effectiveness in ICH treatment.[13] In the study of Werneck et al. in Portuguese, the use of mannitol 20%, surgical treatment and drugs that lower blood pressure had increased mortality rate.[14] Thus, with respect to studies conducted by other researchers[15] as well as this study, injection of hyperosmolar mannitol 20% indicates that mean volume of ICH only decreased in 17% of patients, and 61% patients had increased in hemorrhage volume, and following the above changes, after the use of mannitol 20%, the CGS is decreased in 19.5% of patients after the first 3 days of hemorrhage. In fact, mannitol does not cross the intact blood-brain barrier in adults. Indeed, a rise in brain mannitol space is evidence of a breach of the integrity of the blood-brain barrier.[23,24] The balance between these two possibilities may, to an extent, explain why a randomized controlled study of mannitol in ICH[25] found no evidence of benefit from administering mannitol, and why the confidence intervals of the odds ratio for case fatality at 30 days and 1 year were wide (with the likelihood of harm as well as benefit in some) in the subgroup of 111 patients with ICH treated with mannitol.[26]
GCS is an indication of the LOCs and LOC is an important factor for assessing the patient. For example, response to medical or surgical treatment is an important indication of surgery.[24]
The LOC is (GCS) in ICH mainly depends on the volume of hematoma plus surrounding edema as a mass that can compress adjacent vital organs of brain and increase intracranial pressure (ICP) and finally decreases the GCS. Hence, any intervention (medical or surgical) for reducing of this mass effect (the volume of hematoma or peripheral edema or both) can improve the GCS.
Conclusions
According to our study, there is a relation between the volume of hemorrhage and GCS level in the before and after intervention. It is obvious that there is a relationship between volume of hemorrhage and GCS level due to increase ICP because of hemorrhage and peripheral edema and mass effect.[17]
Despite in our main textbook, the use of mannitol is ICH is recommended in our practice and we found some negative and adverse effect of mannitol.
The mentioned articles use low dose mannitol in their studies (200 ml/6 h) for all patients. However, we use recommended standard dose of mannitol in text books.
Therefore, the overall conclusion is that although mannitol decreases edema in ICH at first,[16,17] according to the three following mechanisms, it finally widens ICH, thus, its use is not recommended.
Edema decreases in ICH after injecting mannitol because mannitol is a hyperosmolar[14,18]
As edema decreases, the pressure is removed from ICH, and hemorrhage volume increases[18]
Blood-brain barrier collapses in ICH, at this time the injection of mannitol makes this hyperosmolar material to moves to ICH through cerebral arteries and consequently increases ICH.[19,20]
There are some of limitation in our study included, lack of sample size and there is no control group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was registered in medical school of Isfahan and all bodgetes was provided by medical school of Isfahan. We thank the medical school of Isfahan for financial and scientific support.
References
- 1.Pascual AM, López-Mut JV, Benlloch V, Chamarro R, Soler J, Láinez MJ. Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: A longitudinal study. Cerebrovasc Dis. 2007;23:6–13. doi: 10.1159/000095752. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Mayer SA. Epidemiology of intracerebral hemorrhage. In: Feldmann E, editor. Intracerebral Hemorrhage. Armonk, New York: Futura Publishing Co; 1994. pp. 3–23. [Google Scholar]
- 3.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2013;23:1001–9. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thapa LJ, Shrestha A, Pokhrel B, Poudel R, Rana PV. Stroke mortality in intensive care unit from tertiary care neurological center. JNMA J Nepal Med Assoc. 2013;52:332–6. [PubMed] [Google Scholar]
- 5.Ziai WC, Melnychuk E, Thompson CB, Awad I, Lane K, Hanley DF. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Crit Care Med. 2012;40:1601–8. doi: 10.1097/CCM.0b013e318241e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corral L, Ventura JL, Herrero JI, Monfort JL, Juncadella M, Gabarrós A, et al. Improvement in GOS and GOSE scores 6 and 12 months after severe traumatic brain injury. Brain Inj. 2007;21:1225–31. doi: 10.1080/02699050701727460. [DOI] [PubMed] [Google Scholar]
- 7.Khurram A, Kleinig T, Leyden J. Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke. 2014;4:1224–9. doi: 10.1161/STROKEAHA.113.004298. [DOI] [PubMed] [Google Scholar]
- 8.Abrignani MG, Colivicchi F. Thromboembolic and hemorrhagic risk stratification in patients with atrial fibrillation. Part I: The thromboembolic risk. Monaldi Arch Chest Dis. 2013;80:60–5. doi: 10.4081/monaldi.2013.80. [DOI] [PubMed] [Google Scholar]
- 9.Wang HK, Tsai KJ, Huang CY, Wang LC, Lu K, Chen HJ, et al. Newly diagnosed dementia and increased risk of hemorrhagic stroke. Curr Alzheimer Res. 2014;31:45–9. doi: 10.2174/1567205011666140131120351. [DOI] [PubMed] [Google Scholar]
- 10.Caglar NS, Akin T, Erdem IH, Ozgonenel L, Aytekin E, Tutun S, et al. Where are we in terms of poststroke functional outcomes and risk factors. NeuroRehabilitation. 2014;28:58–60. doi: 10.3233/NRE-141060. [DOI] [PubMed] [Google Scholar]
- 11.Ye H, Su Y. Hemodynamic effects of mannitol infusion in patients with acute intracerebral hemorrhage. Acta Cir Bras. 2013;28:106–11. doi: 10.1590/s0102-86502013000200004. [DOI] [PubMed] [Google Scholar]
- 12.Vicenzini E, Ricciardi MC, Zuco C, Sirimarco G, Di Piero V, Lenzi GL. Effects of a single mannitol bolus on cerebral hemodynamics in intracerebral hemorrhage: A transcranial Doppler study. Cerebrovasc Dis. 2011;32:447–53. doi: 10.1159/000330639. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Badrinath HR. Early recombinant factor VIIa therapy in acute intracerebral hemorrhage: Promising approach. Neurol India. 2006;54:24–7. doi: 10.4103/0028-3886.24697. [DOI] [PubMed] [Google Scholar]
- 14.Werneck LC, Scola RH, Ferraz LE. Spontaneous intracerebral hematomas: Study of 121 cases. Arq Neuropsiquiatr. 1991;49:18–26. doi: 10.1590/s0004-282x1991000100003. [DOI] [PubMed] [Google Scholar]
- 15.Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld DM, Trentman T, Patel NP. Pontine hemorrhage following a recently implanted intrathecal drug delivery system. Pain Pract. 2009;9:312–6. doi: 10.1111/j.1533-2500.2009.00290.x. [DOI] [PubMed] [Google Scholar]
- 17.Misra UK, Kalita J, Vajpayee A, Phadke RV, Hadique A, Savlani V. Effect of single mannitol bolus in intracerebral hemorrhage. Eur J Neurol. 2007;14:1118–23. doi: 10.1111/j.1468-1331.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Sun CA, Yang T, Chu CH, Bai CH, You SL, et al. Impact of metabolic syndrome components on incident stroke subtypes. J Hum Hypertens. 2014;16:152–9. doi: 10.1038/jhh.2013.152. [DOI] [PubMed] [Google Scholar]
- 19.Stone JJ, Childs S, Smith LE, Battin M, Papadakos PJ, Huang JH. Hourly neurologic assessments for traumatic brain injury in the ICU. Neurol Res. 2014;36:164–9. doi: 10.1179/1743132813Y.0000000285. [DOI] [PubMed] [Google Scholar]
- 20.Richard W. Youmans Neurological Surgery. 6th ed. Philadelphia: Elzevier Corp; 2011. pp. 3428–70. [Google Scholar]
- 21.Rowland LP. Merits Neurology. 12th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. pp. 278–9. [Google Scholar]
- 22.Greenberg MS. Handbook of Neurosurgery. 7th ed. New York: Theme Corp; 2010. p. 877. [Google Scholar]
- 23.Sharaz S, Ghaziani T. Handbook of Iran Pharma. 2nd ed. Tehran: Teymourzadeh Corp; 2008. p. 373. [Google Scholar]
- 24.Prakash ES. Is the use of hypertonic mannitol appropriate in the management of intracerebral hemorrhage? Stroke. 2008;39:e85. doi: 10.1161/STROKEAHA.108.516435. [DOI] [PubMed] [Google Scholar]
- 25.Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral hemorrhage: A randomized controlled study. J Neurol Sci. 2005;234:41–5. doi: 10.1016/j.jns.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Bereczki D, Liu M, do Prado GF, Fekete I. Mannitol for acute stroke. Stroke. 2008;39:512–3. doi: 10.1002/14651858.CD001153.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]