INTRODUCTION
Ischemic heart disease and stroke have remained the world's leading cause of death over the past 15 years. Our understanding of the pathogenesis of coronary heart disease has been slow and was not elucidated until the beginning of the 20th century. This article will review how the diagnosis and treatment of coronary heart disease was viewed from ancient times to the present day.
MUMMIES AND ANCIENT EGYPT
Coronary heart disease was initially thought to be a disease of modern humans, with the cause being attributed to contemporary lifestyles. However, the disease is not as new as we thought. An article in the Lancet[1] in 2013 with whole body computed tomography (CT) scans of mummies from four different geographical regions (ancient Egypt, ancient Peru, Ancestral Puebloan of Southwest, and the Unangan of the Aleutian Islands) showed that atherosclerosis may be very ancient. The time period spanned more than 4000 years. The investigators found probable or definite atherosclerosis in 34% of the 137 mummies studied. The authors conclude that the disease was common in premodern humans.

Medicine in ancient Egypt
CT scan – a 20th century technology – enabled investigators to see the coronaries of the mummies without performing dissection. Autopsy was not performed in ancient times and recording of clinical observations was not regularly practiced. The first to perform human dissections were the Greek physicians Herophilus and Erasistratus in Alexandria, Egypt.[2] Later, Roman law prohibited dissection and autopsy of the human body, and therefore no new dissection studies were done until the 14th century. When regular anatomical dissection of humans during the Renaissance took place in Europe, descriptions of coronary artery disease (CAD) came to light. Anatomic and clinical correlation was, however, nonexistent.
How do we know if a particular disease was identified in antiquity? From ancient writings of course, Homer (800 BC) remarked in the Odyssey: “In Egypt, the men are more skilled in medicine than any of human kind” and “The Egyptians were skilled in medicine more than any other art.”[3] The medicine of the ancient Egyptians is very old, dating from the beginnings of civilization and was considered highly advanced for its time. Egyptian medical skills and knowledge later influenced medical traditions, including that of the Greeks. Galen acknowledged the contribution of ancient Egyptian medicine to Greek medicine.
Was ischemic heart disease known in ancient Egypt? Information relating to medicine comes from several sets of extensive ancient medical documents dating as far back as 3000 BC. One of these medical documents is known as the Ebers Papyrus, wherein appears the following paragraph:
“Shouldst though examine a patient with stomach disease suffering from pain in the arms, the breast, and on the side of the stomach, say: 'Death threatens.” And if though examinst a man for illness in his cardia, and he has pains in his arm, in his breast, and in side of his cardia, and it is said of him: It is [w3d] illness, then thou shalt say thereof: It is due to something entering the mouth it is death that threatens him. Thou shalt prepare for him: Stimulating herbal remedies…”[4]”
Hence, it would seem that the clinical syndrome of angina pectoris existed in ancient Egypt. Unfortunately, there is no record of any anatomic and clinical correlation. Even though there are reports of atherosclerosis in mummies, there was no attempt to correlate clinical symptoms with pathology. The symptoms in a particular mummy simply were not available. For example, the Pharaoh Merneptah[5] had large bone-like plaques in his aorta which was submitted for analysis in 1909 to Shattock, a British pathologist. The historical records are silent whether the pharaoh suffered from any symptoms. Shattock made frozen sections and confirmed that the aorta was affected with calcification.[6] Even though calcifications have been found in the arterial tree of mummies before, pathologists thought these calcifications were “normal and incidental” findings due to age, and therefore natural and not a disease process.[7]
ARABIA
There is also an accurate but incidental description of angina pectoris in old Arabic love literature revealed in a poem written by Qais ibn Al-Mulawah. It comes from the love story Majnoon Lila. The story is famous in Arabic literature as well as in Arab folk stories. A madman in Arabic is called “Majnoon,” so Majnoon Lila, means “Crazy about Lila.” The poet's name is Qais who lived in the 7th century.

A Mughal miniature illustration of Majnoon Lila
In a nutshell, Majnoon Lila is about a young poet, Qais ibn Al-Mulawah, who fell in love with a girl named Lila. The father of Lila refused to consent to their marriage even though Lila also loved Qais.[8] Lila was forced to marry another man and moved out of town. Qais ran away to live in the desert, alone, losing interest in family, friends, and society. He was considered sick with “love madness.” He wrote poems about his love to Lila. In his poems, he described his tears, sleeplessness, lack of appetite, racing heart beats or palpitations, and fainting episodes when Lila left.[8]
He died immediately after writing a poem to his beloved Lila saying:
"My heart is firmly seized
By a bird's claws;
My heart is tightly squeezed,
When Lila's name flows.
My body is tightly bound,
My body is tightly bound,
Is like a finger ring around."
[8]
Graphic illustration of angina pectoris[8]
An Arab cardiologist, Dr. H. A. Hajar Albinali, the author of an Arabic book Majnoon Lila: Between Medicine and Literature, translated the above poem into English. He claimed that it was the first clear and best description of angina in the history of medicine. He concluded in his book from that poem and other symptoms that the poet had CAD and died with myocardial infarction.[8]
17TH–19TH CENTURY EUROPE
In Europe, it is customary to reference angina pectoris to William Heberden, but he was not the first to describe atherosclerosis. The medical literature is replete with narratives of the first descriptions of a particular disease by different physicians.
Among the first to describe atherosclerosis was Leonardo da Vinci, who reportedly stated that “vessels in the elderly restrict the transit of blood through thickening of the tunics.”[9] Leonardo was not a physician, but he was a great artist and leading intellectual of the Italian Renaissance; he is known as the embodiment of a “Renaissance man.” He believed that studying science made him a better artist. He is renowned for painting the Mona Lisa. Leonardo's experiments, anatomical drawings, and notes (often in mirror writing) provide early descriptions of the structure and function of the heart and circulation. His interest in anatomy was inspired by the anatomist Marcantonio della Torre (1473–1511) who was professor of anatomy in Pisa and then Padua and who commissioned da Vinci to provide the illustrations for his text on anatomy based on dissection.[10]
It was, however, William Heberden who brought angina pectoris to the attention of the medical profession when he presented his paper, “Some Account of a Disorder of the Breast,” at the Royal College of Physicians in London in 1768.[11] Many aspects of his description are true to this day. He describes both typical exertional angina as well as variant angina which affected a patient only when he/she was in bed and was relieved by sitting up. He also points out the influence of mental stress. Although it is a classic, it is not the first description of angina. Heberden wrote:
“Those who are afflicted with it are seized, while they are walking, and more particularly when they walk soon after eating, with a painful and most disagreeable sensation in the breast, which seems as if it would take their life away, if it were to increase or to continue: The moment they stand still, all this uneasiness vanishes.”[9]

William Heberden (1710–1801)
Heberden coined the term “angina pectoris” from Greek ankhonē which means “strangling” and Latin pectoris, meaning “chest.” That historical term – “angina pectoris” – is still used in this modern era of medicine.
Of interest, John Hunter, the 18th century Scottish surgeon and anatomist, suffered from angina pectoris and he was the first in Europe to mention the effect of emotions in precipitating an attack of angina. Hunter unintentionally proved that when he suddenly collapsed after a dispute with a colleague and died. Marked atheroma (presumably in his coronary) was found on autopsy.[12] John Hunter's death occurred during a period of emerging understanding of the relation between angina pectoris and CAD. However, physicians continued to describe the coronary lesions on pathology specimens without correlating them to clinical signs.

Rudolph Virchow (1821–1902)
In 1761, the Italian anatomist Giovanni Morgagni described the lesions as “hardening of the arteries” for the first time. Edward Jenner (1729–1823), a British physician and pioneer of smallpox vaccine, and his contemporary colleague, Caleb Parry (1755–1822), linked the excruciating “disorder of the breast” to the “hardening of the arteries.” However, the disease was looked on, as still, only of pathologic interest.[7]
In 1856, Rudolf Virchow, the “father of pathology,” defined the physiological elements in thrombosis within the vascular system and the risk factors that predispose arteries and veins to thrombus formation. Virchow's concepts on thrombosis remained relevant to the current medicine, especially in cardiology.[13] Only after Virchow postulated the features of thrombosis did scientists begin to consider the clinical implications of coronary heart disease seriously.
Near the end of the 19th century, cardiovascular physiologists noted that occlusion of a coronary artery in the dog caused “quivering” of the ventricle which was rapidly fatal. In 1879, the pathologist Ludvig Hektoen concluded that myocardial infarction is caused by coronary thrombosis “secondary to sclerotic changes in the coronaries.” In 1910, two Russian clinicians described five patients with the clinical picture of acute myocardial infarction (AMI), which was confirmed at postmortem examination. Two years later, James Herrick established the importance of bed rest and used electrocardiography (ECG) to diagnose the condition.[14]
The diagnosis and treatment of CAD underwent many milestones since the publication of William Harvey's De Motu Cordis in 1628 wherein he described the circulation and the function of the heart.[14] These milestones stimulated physicians in successive centuries to explore and put forth theories on the pathogenesis of coronary heart disease, and in the process, made discoveries on how to improve diagnostic accuracy and treatment.
In the 19th century, Claude Bernard catheterized animals, measuring the pressures in the great vessels and cardiac chambers. Werner Forssman, in 1929, performed cardiac catheterization on himself which led to the exploration of cardiac hemodynamics by Andre Frederic Cournand and Dickinson Richards. These three investigators were awarded the Nobel Prize in Physiology or Medicine in 1956.[14]
CORONARY ARTERIOGRAPHY
The coronary arteriogram truly revolutionized our understanding and management of cardiac patients. Dr. Mason Sones of Cleveland Clinic introduced the selective injection of contrast media into the coronary arteries in 1958.[15] In the catheterization laboratory in Cleveland Clinic that day, a 26-year-old patient was being evaluated for rheumatic mitral and aortic valve disease when the catheter whiplashed into the ostium of the right coronary artery. Sones was reportedly in the catheterization laboratory at the time and reportedly exclaimed, “we've killed him!”[15]
However, there was no fatal ventricular arrhythmia; the monitor showed only prolonged asystole after sinus arrest that promptly responded to repeated deep coughs. Two days later, Sones proceeded to a planned selective injection of the coronary arteries.[15] The expected ventricular arrhythmias failed to occur and the technique of selective coronary arteriography was born. The traditional thinking before the introduction of the technique was that, if you injected dye into one coronary artery at a time, the resultant asymmetrical hypoxia of the coronary circulation would create an electrical imbalance and fatal ventricular arrhythmia would ensue.[15]
The images of the coronary arteries obtained with arteriography provided objective evidence to support or refute the clinical diagnosis of angina pectoris.
Two radiologists, Drs. Judkins and Amplatz, designed catheters and used the Seldinger percutaneous technique to gain access to the femoral artery and engaged the ostia of either the left or right coronary artery. Their technique required less training than the Sones' technique which facilitated the widespread use of coronary angiography in cardiology as a diagnostic technique.
The coronary angiogram continues to play an integral role in diagnosis, management, and planning future treatment of CAD. It was the first reliable in vivo marker for the presence of obstructing coronary lesions. It provided objective evidence to support or refute the clinical diagnosis of angina pectoris. It became the standard diagnostic tool for defining vessel anatomy and led to the first studies of the natural history of patients with CAD. It also led to studies confirming the benefit of coronary artery bypass grafting (CABG) over medical treatment in subsets of patients. It was instrumental in the introduction of percutaneous transluminal coronary angioplasty and delineation of restenosis. It has the ability to compare percutaneous coronary intervention (PCI) versus CABG for revascularization outcomes.[15]
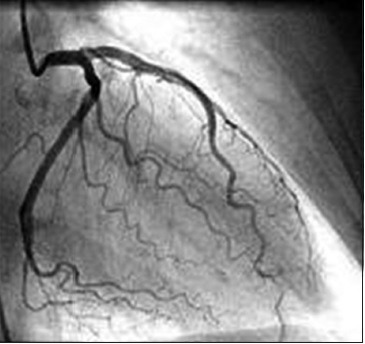
Normal coronary angiogram
THE TREATMENT OF CORONARY ARTERY DISEASE
In our time, much progress has been learned about the pathogenesis and treatment of ischemic heart disease. Once CAD is diagnosed, the findings from coronary angiography guide the strategy for the best treatment. The options of medical therapy, angioplasty, stenting, or CABG depend largely on the severity of disease.
In general, at present, patients with coronary narrowings that do not limit coronary artery blood flow receive medications and lifestyle modification to help prevent progression. If a patient has coronary atherosclerosis that limits blood flow in the coronary arteries, balloon angioplasty and stenting can be offered. In patients with multiple areas of coronary artery narrowing or blockage, CABG surgery is generally recommended.
Below is a brief summary of the modern advances in the therapy of CAD and AMI.
CORONARY CARE UNIT
Before 1961, patients with AMI were placed in nonmonitored beds in the hospital and far away from nurses' stations, so the patients would not be disturbed. Patients were found dead in their beds. The risk of death occurring in the hospital was approximately 30%.[14] Development of the coronary care unit (CCU)[16] took place in 1961. Establishment of the CCU provided continuous ECG monitoring of the patient, closed chest cardiac resuscitation, external defibrillation, and reduced in-hospital mortality by half among patients admitted with AMI. Other influences that reduced mortality were prompt and early diagnosis with sensitive and specific biomarkers[13] and development of surgical methods for revascularization.
SURGERY
The coronary arteriogram provided the foundation for surgical treatment of CAD by means of coronary revascularization. The development and refinement CABG for the treatment of CAD required close collaborations among surgeons, engineers, cardiologists, anesthesiologists, and hematologists. The heart–lung machine developed by Gibbon[17] was originally introduced into cardiac surgery for the repair of intracardiac defects but was soon adopted by cardiac surgeons for adult coronary revascularizations because of its ability to create a motionless, bloodless operative field. Tens of millions of patients benefitted from coronary revascularization on cardiopulmonary bypass.
However, advances in medical therapy and PCI have led to shrinking numbers of CABG being performed.
DRUG THERAPY
There have been many advances in the medical therapy of CAD and AMI. Since the 1970s, large-scale trials have shown that the risk of death is lowered with aspirin, cholesterol-lowering drugs, β-blockers, and angiotensin-converting enzyme inhibitors. However, life-threatening heart failure still occurs late in patients with large infarcts. Prognosis in such patients has been improved with an implantable defibrillator, cardiac resynchronization therapy, pacemakers, and left ventricular assist devices.
Fibrinolytic therapy has been a major advance in the treatment of AMI, leading to improved early survival, less heart failure, less ventricular remodeling, and fewer arrhythmias.[18] Streptokinase (SK) was the first thrombolytic drug to be used in myocardial infarction. Researchers have known for some time of SK' ability to dissolve clots. Fibrinolysis induced by SK resulted in the breakdown of fibrin. SK was used initially for fibrinous pleural exudates, hemothorax, and tuberculous meningitis. Some researchers started using SK in patients with AMI, offering hope that CAD could be “cured.” Experimental intracoronary infusion of SK produced conflicting results initially. Hence, the Italian Group for the Study of Streptokinase in Myocardial Infarction (Gruppo Italiano per la Sperimentazione della Streptochinasi nell'Infarto Miocardico) (GISSI) trial in 1986 addressed this issue by recruiting more than 10,000 patients and proved that SK reduced early mortality in patients with AMI.[19]
The thrombolytic era was found on a fundamental concept that most cases of AMI are the result of sudden obstruction of an epicardial coronary artery by intracoronary thrombus superimposed on a ruptured or fissured atherosclerotic plaque. The GISSI study validated SK as an effective therapeutic method, and therefore fixed protocols for its use in AMI were established. SK has been supplanted by tissue plasminogen activator in developed nations, but SK remains essential to the management of AMI in developing nations.[18,19] The Second International Study of Infarct Survival showed that the addition of aspirin (an antiplatelet drug) led to further reductions in mortality.[20]
MEDICATIONS AND LIFESTYLE MODIFICATIONS
Medications are prescribed to reduce the risk of death by reducing the risk of heart attack, stroke, and heart failure. Lifestyle changes help prevent the continuing buildup of fatty deposits in the coronary arteries. These changes include smoking cessation, a diet low in fat and cholesterol, weight loss, regular exercise, stress management, diabetes control, and blood pressure control. These medications and lifestyle changes are equally important for those patients who also undergo coronary revascularization with PCI, with or without stents or CABG.
ANGIOPLASTY
In the recent years, PCI treatment (introduced by the German radiologist Andreas Gruentzig in 1977) for CAD is oftentimes preferred over CABG because the comparative effects of these two revascularization methods on long-term mortality are still unclear. PCI is also less invasive. However, the journal JAMA Inter Med[21] in 2014 published a meta-analysis of randomized clinical trials comparing CABG versus PCI. The study found that, in patients with multivessel coronary disease, CABG leads to an unequivocal reduction in long-term mortality and myocardial infarctions and to reductions in repeat revascularizations, regardless of whether patients are diabetic or not.[21]
Coronary angioplasty and stenting together with newer, more potent platelet inhibitors such as P2Y and glycoprotein IIb/IIIa platelet receptor blockers further reduced in-hospital mortality from AMI to about 7%.[14] The efficacy of these treatments depends on a short interval between the onset of symptoms and the patient arrival at the hospital.
CONCLUSION
CAD and AMI have been with us since antiquity. We understand now that coronary ischemia and AMI are the result of a sudden obstruction of a coronary artery by intracoronary thrombus superimposed on a ruptured atherosclerotic plaque. Advances in modern therapy are based on this concept. However, the clinical problem of CAD and AMI is still being actively investigated in an effort to refine management and hopefully find a cure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Thompson RC, Allam AH, Lombardi GP, Wann LS, Sutherland ML, Sutherland JD, et al. Atherosclerosis across 4000 years of human history: The Horus study of four ancient populations. Lancet. 2013;381:1211–22. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- 2.von Staden H. The discovery of the body: Human dissection and its cultural contexts in ancient Greece. Yale J Biol Med. 1992;65:223–41. [PMC free article] [PubMed] [Google Scholar]
- 3. [Last accessed on 2017 May 01]. Available from: http://www.crystalinks.com/egyptmedicine.html .
- 4. [Last accessed on 2017 May 01]. Available from: http://www.arabworldbooks.com/articles8b.htm .
- 5.Sandison AT. Degenerative vascular disease in the Egyptian mummy. Med Hist. 1962;6:77–81. doi: 10.1017/s0025727300026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shattock SG. Microscopic sections of the aorta of king Merneptah. Lancet. 1909;1:319. [Google Scholar]
- 7.Leary T. Atherosclerosis, the important form of arteriosclerosis, a metabolic disease. Eleventh Ludvig Hektoen lecture of the Frank Billings Foundation of the Institute of Medicine of Chicago. J Am Med Assoc. 1935;105:475–81. [Google Scholar]
- 8.Hajar HA. Majnoon Lila. Chairman's reflections. Heart Views. 2003;4:127–33. [Google Scholar]
- 9.Slijkhuis W, Mali W, Appelman Y. A historical perspective towards a non-invasive treatment for patients with atherosclerosis. Neth Heart J. 2009;17:140–4. doi: 10.1007/BF03086236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MK, Eollman A. Leonardo da Vinci (1452-1519) Heart. 1996;76:464. doi: 10.1136/hrt.76.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Tellingen C. Chest pain and angina pectoris-Or the ugly swan and the beautiful duckling. Neth Heart J. 2010;18:561–4. doi: 10.1007/s12471-010-0835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leach A. History of Angina. Res Medica 1967, Special Issue. Lauder Brunton Centenary Symposium on Angina Pectoris. 1967. [Last accessed on 2017 May 01]. Available from: http://www.doi. 10.2218/resmedica.v5i3-4.478 .
- 13.Hajar R. Evolution of myocardial infarction and its biomarkers: A historical perspective. Heart Views. 2016;17:167–72. doi: 10.4103/1995-705X.201786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 15.Ryan TJ. The coronary angiogram and its seminal contributions to cardiovascular medicine over five decades. Circulation. 2002;106:752–6. doi: 10.1161/01.cir.0000024109.12658.d4. [DOI] [PubMed] [Google Scholar]
- 16.Julian DG. Treatment of cardiac arrest in acute myocardial ischaemia and infarction. Lancet. 1961;2:840–4. doi: 10.1016/s0140-6736(61)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–85. [PubMed] [Google Scholar]
- 18.Yusuf S, Collins R, Peto R, Furberg C, Stampfer MJ, Goldhaber SZ, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: Overview of results on mortality, reinfarction and side-effects from 33 randomized controlled trials. Eur Heart J. 1985;6:556–85. doi: 10.1093/oxfordjournals.eurheartj.a061905. [DOI] [PubMed] [Google Scholar]
- 19.Sikri N, Bardia A. A history of streptokinase use in acute myocardial infarction. Tex Heart Inst J. 2007;34:318–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–60. [PubMed] [Google Scholar]
- 21.Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs. percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: Meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174:223–30. doi: 10.1001/jamainternmed.2013.12844. [DOI] [PubMed] [Google Scholar]


