Abstract
Background:
Glioblastoma (GBM) is the most common and aggressive brain tumor, which has a poor prognosis despite the advent of different therapeutic strategies. There are numerous molecular biomarkers to contribute diagnosis, prognosis, and prediction of response to the current therapy in GBM. One of the most important markers that are potentially valuable is immortalization-specific or immortalization-associated marker named “hTERT messenger ribonucleic acid (mRNA)” the key subunit of telomerase enzyme, which is expressed in more than 85% of cancer cells, in spite of the majority of normal somatic cells. In this study, we investigated the effects of resveratrol (RSV) on this mRNA marker level, leading to cancer progression.
Materials and Methods:
U-87MG cell line was obtained from Pasteur Institute of Iran and treated with various concentrations of 0–160 μg/mL of RSV and at different time points (24, 48, and 72 h). To evaluate viability of U-87MG cells, standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed. Real-time polymerase chain reaction (RT-PCR) was used for comparative and quantitative assessment of human telomerase reverse transcriptase (hTERT) mRNA copy number versus control–untreated group.
Results:
The results of our investigation suggested that RSV effectively inhibited cell growth and caused cell death in dose-dependent (P < 0.05) and not in time-dependent manner (P > 0.05), in vitro. Interestingly, quantitative RT-PCR analysis demonstrated that at half inhibition concentration, RSV dramatically decreased mRNA expression of hTERT, the catalytic subunit of telomerase enzyme, which leads to prevention of cell division and tumor progression.
Conclusion:
With regard to downregulation of this immortalization-associated marker, RSV may potentially be used as a therapeutic agent against GBM.
Keywords: Glioblastoma, human telomerase reverse transcriptase messenger ribonucleic acid, resveratrol
Introduction
Glioblastoma (GBM), or astrocytoma Grade IV, is the most common aggressive and malignant primary brain tumor, with a poor prognosis despite the advent of different therapeutic strategies.[1] This tumor has an incidence of 5–8/100,000 population, and patients only have a median survival time of 15 months because of the tumor's resistance to the current therapeutic approaches.[2,3]
GBMs are usually highly malignant (cancerous) because the cells reproduce quickly and they are supported by a large network of blood vessels. This tumor contains approximately 15.4% of all primary brain tumors and about 60–75% of all astrocytomas.[4] As wide ranges of tumors, the explicit cause of GBM remained unknown. GBM can be difficult to treat because the tumors comprise so many different types of cells. Some tumor cells may exhibit a good response to certain therapies while others represent unfavorable results. This is why the treatment strategy for this kind of cancer may deploy several approaches. Molecular experiments have signified that some molecular signing is associated with tumor aggressiveness as well as with disease progression. According to McNamara et al., there are numerous biomarkers, or molecular indicators, including O(6)-methylguanine-deoxyribonucleic acid (DNA)-methyltransferase promoter and DNA methylation, loss of heterozygosity (LOH) of chromosomes 1p and 19q, LOH of 10q, isocitrate dehydrogenase mutations, epidermal growth factor receptor, epidermal growth factor, latrophilin, and 7 transmembrane domain-containing protein 1 on chromosome 1, vascular endothelial growth factor, tumor suppressor protein p53, phosphatase and tensin homolog, p16INK4a gene, cytochrome c oxidase, phospholipid metabolites, telomerase messenger expression (human telomerase reverse transcriptase [hTERT] messenger ribonucleic acid [mRNA]), microRNAs, cancer stem cell markers and imaging modalities which have the potential to contribute to diagnosis, prognosis, and prediction of response to therapy in GBM to help clinical management of GBM.[5]
In this context, markers that are potentially interesting are immortalization-specific or immortalization-associated markers which include activated telomere-lengthening enzyme telomerase, a ribonucleoprotein consisting of a complex of an RNA component and proteins. Advances in the understanding principals of the regulation of telomerase activity and the telomere structure, as well as the identification of telomerase and telomere-associated binding proteins, have opened new avenues for therapeutic intervention. In contrast to normal cells, telomerase activity is found in a high percentage of human tumors. Several subunits of human telomerase have been determined, composed of RNA component, hTR17 and hTERT as catalytic subunit. Too many evidence has confirmed that among these subunits, hTERT is the rate-limiting component of telomerase activity. Introducing hTERT into several cell types is sufficient to induce telomerase activity and prevent telomere shortening in vitro. Expression at the mRNA level is associated strongly with enzyme activity in several tissues and cell cultures, including lung, breast, colon, and GBM cancer tissues. Inhibition of hTERT results in telomere loss and limits the growth of tumor cells that undergo apoptosis when their telomeres reach a critically short length.[6]
Trans-resveratrol (RSV), also known as 3,5,4,′-tri-hydroxystilbene, is one of the promising dietary phytochemicals with chemopreventive and chemotherapeutic potential, which belongs to the stilbene class of polyphenolic compounds.[7] Skin of red grapes is especially rich in RSV and evolves from a common synthesis pathway in plants.[8] Many studies suggest that its biological effects, depend on cell and tissue types, may alter and be nature-specific. It has been shown that RSV has a crucial role in initiation, promotion, and progression in carcinogenesis and may suppress angiogenesis and metastases of cancer cells.[2,9] Other profound investigations confirm that RSV can modulate multiple pathways including cell growth, apoptosis, and inflammation with association of its antioxidant activity;[10,11] however, we need more research indicating other molecular mechanisms which can describe the underlying interventions for the anti-cancer activity of RSV. In this study, we treated U-87MG cancer cell line with RSV in various dosages and specified half inhibition concentration (IC50) of RSV and affected that concentration on cancer cells for evaluating effects of RSV, as a potent anticancer substance, on hTERT mRNA expression which encoded for the catalytic subunit of telomerase in transcriptional level of regulation in Stage IV malignant cancer cell line.
Materials and Methods
Trans-RSV was purchased from Sigma-Aldrich Corp., St. Louis, MO, USA, and dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich Corp., St. Louis, MO, USA) at a concentration of 12.8 mg/mL, stored as a stock solution at −80°C, and diluted in culture medium just before use.
Cell culture
The human GBM cell line U-87MG, derived from metastatic Stage IV GBM, was purchased from National Cell Bank, Pasteur Institute of Iran, and cultured at 37°C in 5% CO2 humidified atmosphere and maintained in Dulbecco's modified Eagle's medium (high GlutaMAX-Gibco, USA), supplemented with inactivated 10% fetal bovine serum (Gibco, USA) in the presence of 100 units/mL penicillin and 100 μg/mL streptomycin to have complete media (CM). At the end of incubation, the cells were harvested by trypsinization and the media were changed two times/week.
Cell viability assay
Confluent U-87MG cells were harvested, counted, spread in CM, and seeded into 96-well cell culture plates (SPL) at a concentration of 2 × 104 cells/well in 200 μl of CM and allowed to adhere overnight. U-87MG cells in logarithmic growth phase were treated with different concentrations of RSV, dissolved in sterile DMSO at a concentration not exceeding 0.1% (v/v), and added to the well in a volume of 5 μL/well. The effects of RSV on cell proliferation were studied in a different dose of 0–160 μg/mL and at different time points (24, 48, and 72 h). To evaluate the viability of U-87MG cells, standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed, and 20 μl of 5 mg/mL MTT solution was added to each well and incubated for 4 h at 37°C, 5% CO2. Three replica wells were used for controls (DMSO 1:400) and each drug concentration. After incubation, media were removed and 200 μL of MTT solubilizer solution including DMSO and isopropanol (1:1) was added to dissolve formazan crystals at the bottom part of each plate. Plates were read at 570 nm wavelength using a microplate reader (BIO-RAD550, USA). Percentage of viability was determined by this formula: Optic density (OD) of the test sample/OD of the control sample.
In the next step, we detected the IC50 of U-87MG cells. All experiments were repeated three times.
Cell morphology
The cells were cultured in 6-well plates at 37°C under a humidified atmosphere of 5% CO2 for 24 h. After overnight incubation, the cells were treated with concentrations of 48.23 μg/mL for evaluating morphology alteration. To specify RSV cytotoxicity effects on noncancerous and normal human cell line, we used human foreskin fibroblast (HFF) and the cells affected by the same concentration of drug and the culture plates were photographed by Olympus-IX71 inverted microscope (Olympus, USA).
Assessment of human telomerase reverse transcriptase messenger ribonucleic acid levels by real-time polymerase chain reaction
RT-PCR was used for detecting the inhibition rate of candidate drugs on hTERT gene expression. Total cellular RNA was extracted with TRIzol (Invitrogen, CA, USA) according to the manufacturer's instructions. For removal of genomic DNA from RNA preparations before complementary DNA (cDNA) synthesis, RNA treated with DNase I, RNase-free (Fermentas, USA). Synthesis of cDNA was carried out in 20 μL reaction on 3 μg of total RNA using first Strand cDNA Synthesis Kit (Fermentas, USA) which was used as a template for quantitative RT-PCR (qRT-PCR). qRT-PCR was carried out using Applied Biosystems® StepOne™ system to determine the expression of hTERT (214 bp) and glyceraldehyde 3-phosphate dehydrogenase (238 bp, GAPDH as a control) using Maxima SYBR Green/qPCR Master Mix ROX 200 (Fermentas, USA). The PCR primer sequences specific for all the variants of hTERT mRNA were 5’-CGTGGTTTCTGTGTGGTGTC-3’ (sense) and 5’-CCTTGTCGCCTGAGGAGTAG-3’ (antisense) and for GAPDH primers were 5’-TGC ACC ACC AAC TGC TTA GC-3’ (sense) and 5’-GGC ATG GAC TGT GGT CAT GAG-3’ (antisense). After an initial denaturation at 95°C for 10 min, the samples were subjected to 40 cycles of RT-PCR (95°C for 15 s, annealing temperature 64°C (hTERT) and 60°C (GAPDH) for 30 s, and extension temperature 72°C for 30 s). RT-PCR data analysis was done, based on using 2−ΔΔCt method by calculation of threshold cycle (Ct) values for target genes and GAPDH, as an endogenous control gene. PCRs were performed in triplicate with a negative control (no DNA).
Statistical analysis
Quantitative results were expressed as mean ± standard error of mean. Statistical analysis was performed using a two-tailed unpaired t-test (between two groups) or a one-way analysis of variance by SPSS for Windows, version 20 (SPSS Inc., Chicago, Illinois, USA) and (GraphPad Prism, version 6.01, California, USA). P < 0.05 was considered statistically significant.
Results
Resveratrol inhibits proliferation of U-87MG cells
The results showed that RSV inhibited cell growth in a dose-dependent manner (P = 0.02). We treated U-87MG cells with RSV at 24, 48, and 72 h and as a consequence, the cell viability level at a concentration of 80 and 160 μg/mL was significantly decreased compared with control [Figure 1a]. The results showed a trend toward growth inhibition, but it was not statistically significant among three time points (P > 0.05) [Figure 1b].
Figure 1.
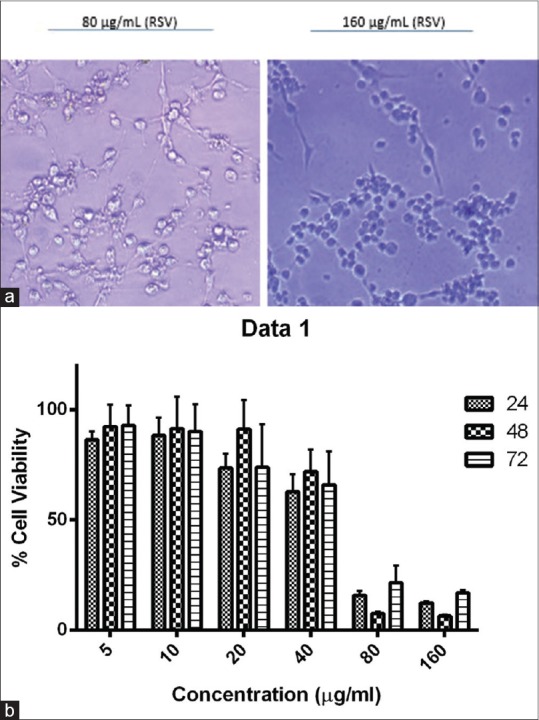
(a) At a concentration of 80 and 160 μg/mL, the number of viable cells was significantly decreased in comparison with control. (b) Cell viability analysis in resveratrol-treated U-87MG cells evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. U-87MG cells were treated with 5, 10, 20, 40, 80, and 160 μg/mL resveratrol and dimethyl sulfoxide 0.1% (v/v) as negative control in a 24, 48, and 72 h. This graph illustrates that time points factor has no effect on trend of growth overall according to GraphPad Prism software analysis (P > 0.05)
This study first investigated the impact of the candidate drug on the proliferation of U-87MG cells and calculated the IC50 after 48 h treatment [Figure 2].
Figure 2.
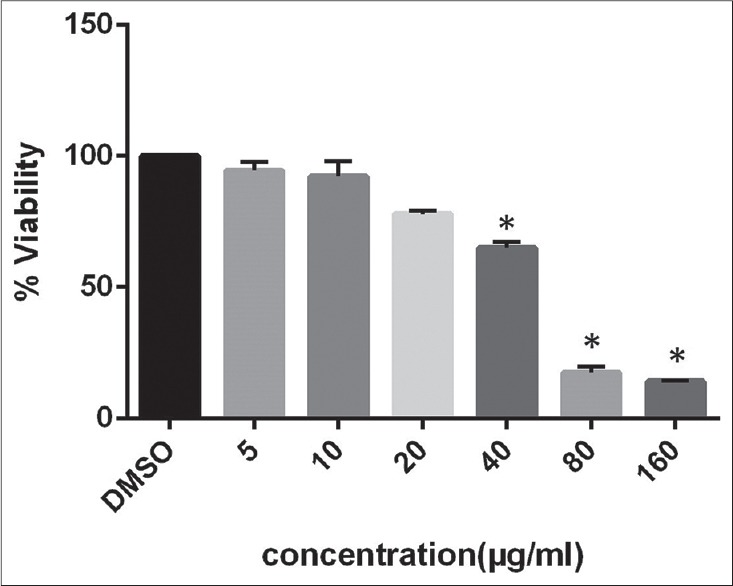
The half inhibition concentration determined after 48 h incubation which is 48.23 μg/mL. Data are mean from three independent experiments which indicate activity in the absence and presence of resveratrol is significantly different (n = 3, *P < 0.05)
Effective role on changing normal morphology to the apoptotic case in cancer cell line and nontoxic manifestation in normal human cell
Morphological changes of U-87MG cell for 48 h incubation by RSV at 48.23 μg/mL dose of agent were monitored and results showed that affected cells changed to round, necrotic and distinct apoptotic structure while the untreated counterparts were well spread and kept natural appearance [Figure 3a]. HFF cell line displayed no eminent change in the presence and absence of RSV in vitro [Figure 3b].
Figure 3.
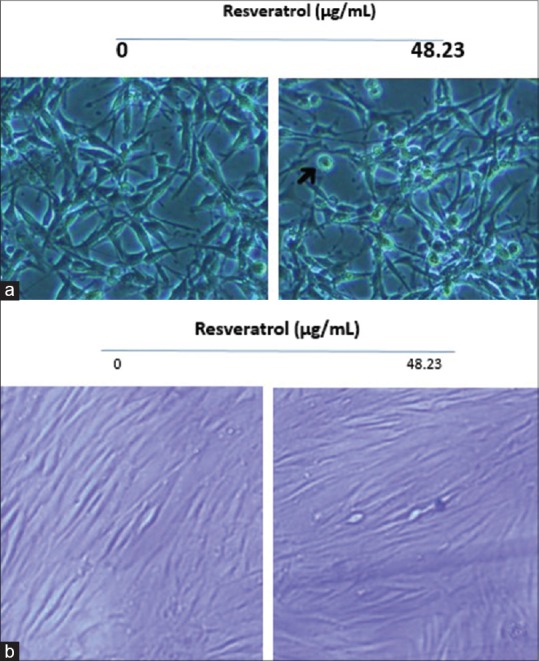
(a) U-87MG cells were grown in 6-well culture dishes to near confluence 50%. Arrows indicate cells with apoptotic morphology. (b) Human fibroblast normal cells have not affected by resveratrol in this concentration; this figure shows that resveratrol effects on cancerous cells and causes apoptosis in abnormal cell types
Downregulation of human telomerase reverse transcriptase messenger ribonucleic acid transcripts affected by resveratrol
In this research, the effect of RSV on the inhibition of the hTERT expression was assessed. Our results demonstrated that RSV is an anticancer agent with regard to decreasing expression of a key subunit of a crucial enzyme named telomerase, which has important role in regulation of cell division and apoptosis of metastatic cancer cells. With our data which illustrated IC50 of 48.23 μg/mL for RSV in cultured cells, this dose of the experiment was chosen for investigating RSV's effect on the hTERT gene marker expression in this population of cells. Our results showed that this gene was significantly down-regulated by RSV in a dose-dependent manner, in comparison to control group as a nontreated cells (P < 0.05) [Figure 4].
Figure 4.
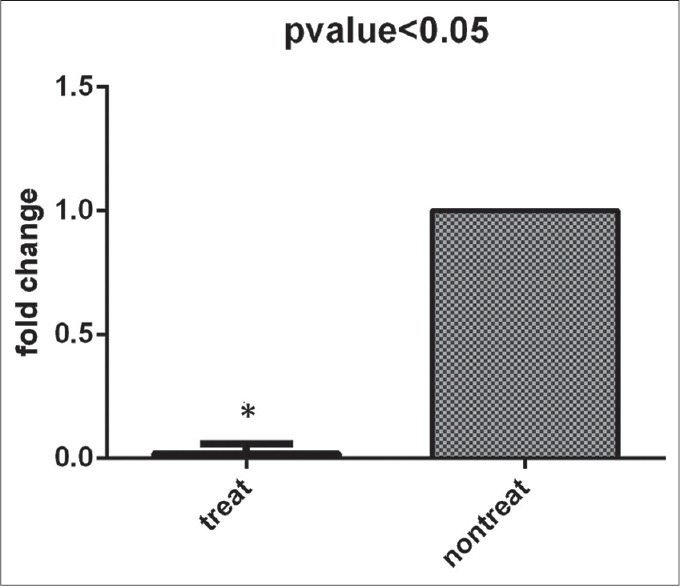
Effects of resveratrol on the level of human telomerase reverse transcriptase transcript. After 48 h resveratrol-treatment, analyzed results showed remarkably decrease expression of this molecular marker in comparison with control group. Expression level was normalized to glyceraldehyde 3-phosphate dehydrogenase as a housekeeping gene. Results are mean ± standard error and indicate three independent experiments (*P < 0.05 vs. control group, Student's t-test analysis)
Discussion
Telomerase and telomeres are attractive targets for anticancer therapy. Many investigations supported evidence depicting that the majority of human cancers express the telomerase enzyme which is essential to maintain telomere and leads to indefinite cell proliferation as a hallmark of cancer including GBM.[12] Telomerase activity typically belongs to limited specific cell types. However, telomerase activation in somatic cells can be considered as a major factor in cell immortalization and cancer. Targeting telomerase by designing different therapeutic and clinical strategies due to cancer treatment is an overriding molecular approach.[13] Telomerase expression occurs in the later phases of multistep tumor progression in some classes of tumors[14] and is especially characteristic of higher-grade neoplasms; thus, in the present study, we chose Grade IV GBM cell line for research to unravel the effects of RSV, natural phytoalexin, and polyphenolic compound, on one of important molecular mechanisms for further application in prognosis, diagnosis, and treatment of GBM.
Recently, the wide range of study has described preventive mechanisms by which interference of RSV with anticancer properties including its effect on regulation of various transcription factors such as AP-1, NF-kB, p53, as well as many apoptosis pathway genes.[15] It regulates the activity of p53, a key tumor suppressor gene, and thus triggers apoptosis processes in cancer cells. In addition to its effects on a number of transcription factors, RSV can also inhibit telomerase activity. A few studies revealed that RSV treatment downregulated telomerase activity and hTERT mRNA expression in breast and colon cell line in vitro.[16,17] However, the role of RSV on telomere and telomerase activity has not been studied in GBM multiform.
Telomerase regulation can be achieved either by the transcriptional regulation of hTERT or by the posttranscriptional alternative splicing of hTERT.[18,19] Specific inhibitors are explored for blockage different components of telomerase enzyme at various levels of regulation, such as AZT, B1BR1532 and vaccine for hTERT (catalytic subunit), 2–5A antisense, GRN163 L, gene-directed enzyme prodrug therapy for hTERC (telomerase RNA component), and 17-AAG, 17-DMAG for telomerase-associated proteins.[20] These findings demonstrate availability of underlying approaches to suppression of carcinogenesis through surmounting high telomerase activity.
Based on a study by Boldrini et al., results confirmed telomerase expression in 26–89% of multiform GBM patients. However, the studies of Langford et al., De Masters et al., and Nakatani et al. show paradoxical finding that a percentage of multiform GBMs and anaplastic astrocytomas were negative or at low-level for telomerase expression in a high degree of malignancy and in contrast to the almost uniform telomerase positivity of high-grade systemic tumors.[14]
Several univariate studies have declared a correlation between the levels of hTERT transcription with telomerase activity in two glioma age groups. hTERT significantly is transcribed at similar copy numbers in young and old groups; however, these mRNAs are translated to telomerase in 100% of the young compared to only 25% of older patients. Evidence has suggested that age is a significant prognostic factor in this cancer.[19]
It is important to notice cancer is not a single disease, and the idea of a “moonshot” cure is misleading and outdated. Overall, our study, irrespective of possible association with telomerase activity, signifies that RSV may have important role to a reduction of expression at the level of transcription in gliomas. However, Western blot analysis for translational evaluation, as well as TRAP assay is necessary to prove whether RSV decreases telomerase activity following downregulation of mRNA variant transcript. It is important to notice that multiform GBMs are highly heterogeneous tumors, and this variability might influence telomerase expression[21,22,23] and it is mostly recommended to do experiments at least on three types of GBM cell lines.
Conclusion
Our results accompany with other experiments which have pointed out the key role of telomerase in carcinogenesis may encourage scientists to consider this factor as a fundamental marker due to diagnoses and treatment of patients with malignant gliomas. With regard to downregulation of this immortalization-associated marker, RSV may potentially be used as a therapeutic agent against GBM.
Financial support and sponsorship
Shahid Sadoughi University of Medical Sciences, Stem Cell Biology Research Center and Recurrent Abortion Research Center, Yazd, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by Stem Cell Biology Research Center and Recurrent Abortion Research Center, Yazd, Iran.
References
- 1.Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27. doi: 10.1007/s11060-011-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, Salbego C, Lenz G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009;100:1655–62. doi: 10.1111/j.1349-7006.2009.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 5.McNamara MG, Sahebjam S, Mason WP. Emerging biomarkers in glioblastoma. Cancers (Basel) 2013;5:1103–19. doi: 10.3390/cancers5031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijders PJ, Breuer RH, Sutedja TG, Egging M, Voorhorst FJ, Steenbergen RD, et al. Elevated hTERT mRNA levels: A potential determinant of bronchial squamous cell carcinoma (in situ) Int J Cancer. 2004;109:412–7. doi: 10.1002/ijc.11732. [DOI] [PubMed] [Google Scholar]
- 7.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kala R, Tollefsbol TO, Li Y. Potential of Resveratrol in Inhibiting Cancer and Slowing Aging. Journal of Nutrition and Food Sciences 2012. 2015 Nov 27; [Google Scholar]
- 9.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–61. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach JP, Weller M. Apoptosis in gliomas: Molecular mechanisms and therapeutic implications. J Neurooncol. 2004;70:245–54. doi: 10.1007/s11060-004-2753-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang YP, Chang YL, Huang PI, Chiou GY, Tseng LM, Chiou SH, et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J Cell Physiol. 2012;227:976–93. doi: 10.1002/jcp.22806. [DOI] [PubMed] [Google Scholar]
- 12.George J, Banik NL, Ray SK. Knockdown of hTERT and concurrent treatment with interferon-gamma inhibited proliferation and invasion of human glioblastoma cell lines. Int J Biochem Cell Biol. 2010;42:1164–73. doi: 10.1016/j.biocel.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprouse AA, Steding CE, Herbert BS. Pharmaceutical regulation of telomerase and its clinical potential. J Cell Mol Med. 2012;16:1–7. doi: 10.1111/j.1582-4934.2011.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Ali G, Pieracci N, et al. Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol. 2006;28:1555–60. doi: 10.3892/ijo.28.6.1555. [DOI] [PubMed] [Google Scholar]
- 15.Pezzuto JM. Resveratrol as an inhibitor of carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 16.Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int J Oncol. 2006;28:641–8. [PubMed] [Google Scholar]
- 17.Fuggetta MP, Lanzilli G, Tricarico M, Cottarelli A, Falchetti R, Ravagnan G, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp Clin Cancer Res. 2006;25:189–93. [PubMed] [Google Scholar]
- 18.Olaussen KA, Dubrana K, Domont J, Spano JP, Sabatier L, Soria JC. Telomeres and telomerase as targets for anticancer drug development. Crit Rev Oncol Hematol. 2006;57:191–214. doi: 10.1016/j.critrevonc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Shervington A, Patel R. Differential hTERT mRNA processing between young and older glioma patients. FEBS Lett. 2008;582:1707–10. doi: 10.1016/j.febslet.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Dang S, Gabrani R. Recent patents on anti-telomerase cancer therapy. Recent Pat Anticancer Drug Discov. 2012;7:102–17. doi: 10.2174/157489212798357958. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara M, Okayasu I, Takemura T, Tanaka I, Masuda R, Furuhata Y, et al. Telomerase activity significantly correlates with chromosome alterations, cell differentiation, and proliferation in lung adenocarcinomas. Mod Pathol. 2000;13:723–9. doi: 10.1038/modpathol.3880125. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani K, Yoshimi N, Mori H, Yoshimura S, Sakai H, Shinoda J, et al. The significant role of telomerase activity in human brain tumors. Cancer. 1997;80:471–6. doi: 10.1002/(sici)1097-0142(19970801)80:3<471::aid-cncr15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Kheirollahi M, Mehrazin M, Kamalian N, Mohammadi-asl J, Mehdipour P. Telomerase Activity in Human Brain Tumors: Astrocytoma and Meningioma. Cell Mol Neurobiol. 2013;33:569–74. doi: 10.1007/s10571-013-9923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


