Abstract
Background:
Quinazolinon as an important class of heterocycles is attractive in medicinal research areas due to their wide range of biological effects. Cytotoxic activities of the quinazolinone derivatives in various cell lines including: HeLa, L1210 (mouse lymphocytic leukemia) and HT29 (human colon adenocarcinoma) were reported.
Materials and Methods:
In this study, a number of newly made tricycles quinazolinone derivatives such as fused pyridazino-quinazolinones and fused pyrrolo-quinazolinones were evaluated on two cancerous cell lines, melanoma (B16F10) and prostate (PC3) using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide colorimetric assay.
Results:
The results of cytotoxicity evaluations indicated that almost all of the compounds at the concentrations of 10 and 100 μM showed significant differences in viability in comparison with negative control at 48 h exposure (P < 0.05). However, during 24 h exposure some of the compounds showed cytotoxicity activity.
Conclusion:
Results showed that both cell lines were sensitive to synthesized compounds and longer duration of exposure (48 h) had better results compared to that of 24 h screening.
Keywords: Cytotoxic, melanoma and prostate cancer, quinazolinone
Introduction
Of greatest problems in cancer chemotherapy is tumor cells resistance to a wide range of cytotoxic drugs. Major efforts have been carried out to synthesize new anticancer agents with improved efficacy.[1] Some herbal components have also shown promising effects.[2,3] Quinazolinone and its derivatives [Figure 1] are noteworthy due to their wide range of biological effects[4,5] which include antibacterial, antifungal,[6,7,8,9,10,11,12,13,14] anticancer,[15,16,17] anti-inflammatory[18,19,20] and antihypertensive.[21]
Figure 1.
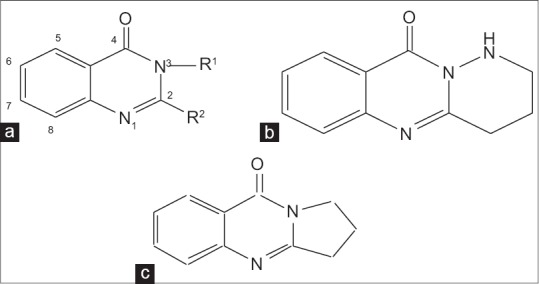
Quinazolinone basic structure (a) fused- pyridazino-quinazolinones (b) and fused- pyrrolo-quinazolinones (c) derivatives
Proposed anticancer mechanisms for quinazolines include: Inhibition of the DNA repair enzyme system,[16] interaction with biological nucleophiles such as L-cysteine and sulfhydryl bearing enzymes in a Michael-type addition reaction as an alkylation agent,[17] inhibition of epidermal growth factor receptor (EGFR) (a cellular trans-membrane tyrosine kinases that is over-expressed in a significant number of human tumors),[16] thymidylate enzyme inhibition[22] and inhibitory effects for tubulin polymerize.[23,24] Raltitrexed [Figure 2] (a thymidylate enzyme inhibitor) and gefitinib (an EGFR inhibitor) are marketed quinazolinone derivatives with anticancer activities.[16]
Figure 2.
Raltitrexed (a) and gefitinib (b)
In the previous works, we synthesized a series of novel quinazolinone derivatives (fused pyridazino-quinazolinones and fused pyrrolo-quinazolinones).[25] During screening for evaluation of cytotoxicity, these molecules exhibited different cytotoxicity in the range of 10–100 μM on HeLa cell line. In this study cytotoxic effects of some of the selected compounds were measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay on melanoma and prostate cell lines after 24 and 48 h exposure.
Materials and Methods
Cell lines and cell culture
Two cancerous cell lines including prostate (PC3) and melanoma (B16F10) were purchased from national cell bank of Iran affiliated to Pasteur Institute, Tehran, Iran, and cultured in Dulbecco's Modified Eagle Medium (DMEM) supplement with 1% antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) plus 10% fetal calf serum till the third passage were performed before evaluating cytotoxicity effects. All the cell culture materials were purchased from Gibco, USA. Cells were grown at the temperature of 37°C and in 5% CO2/air.
Sample preparations
The prepared stock solutions of compounds (10 mM) in dimethyl sulfoxide (DMSO) were diluted with the medium (DMEM) to reach 10, 100, 1000 μM concentrations.
In vitro cytotoxicity assay
In vitro cytotoxicity assay was initiated by separately plating (180 μl) of the melanoma and prostate cells (5 × 104 cells/ml of media) in 96-well micro plates and incubating for 24 h (37°C, air humidified 5% CO2). After 24 h, 20 μl of each dilution of compounds was added to the 96-well micro plate containing 180 μl of the cell suspensions in order to obtain 1, 10, 100 μM concentrations. Wells containing 180 μl of the cell suspension and 20 μl of DMSO (1%) were considered as negative control while the blank wells contained only 200 μl of the DMEM medium. The micro-plates were further incubated for 24 or 48 h at the same condition. Each well was then treated with 20 μl of MTT solution for 3 h. Afterward, the media in each well was replaced with 200 μl DMSO to dissolve the blue insoluble formazan crystals. The metabolic activity in each well was determined by a rapid colorimetric assay using MTT.[26,27] Plates were read using an enzyme-linked immunosorbent assay plate reader at 540 nm. The cell viability was determined by the following formula 1 and was compared with untreated control.
Formula 1:

Results
The cytotoxicity of compounds [Figure 3] were evaluated against melanoma and prostate cell lines at different concentrations (final concentrations 1, 10, and100 μM) after 24 and 48 h using MTT assay [Figures 4–7]. Metabolic reduction of soluble MTT by succinic dehydrogenase enzyme of mitochondria took place when tumor cells were viable. The results are the mean of three triplicate experiments. Analysis of variance carried out by Tukey test and significance differences level was set at P < 0.05. The synthesized target molecules exhibited significant cytotoxicity in the range of 10–100 μM on melanoma and prostate cell lines after 48 h.
Figure 3.
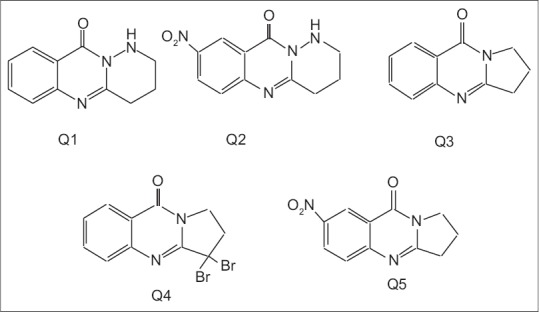
Fused- pyridazino-quinazolinones (Q1 and Q2) and fused- pyrrolo-quinazolinones (Q3, Q4, Q5) derivatives
Figure 4.
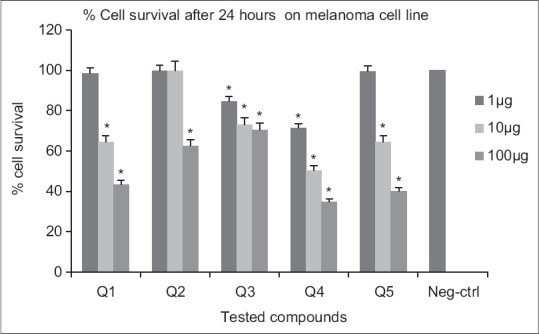
Cytotoxic effects of compounds on melanoma cell line following exposure to different concentrations of compounds (after 24 h). Data are presented as mean ± standard deviation, *P < 0.05, n = 3, Neg-ctrl: Negative control
Figure 7.
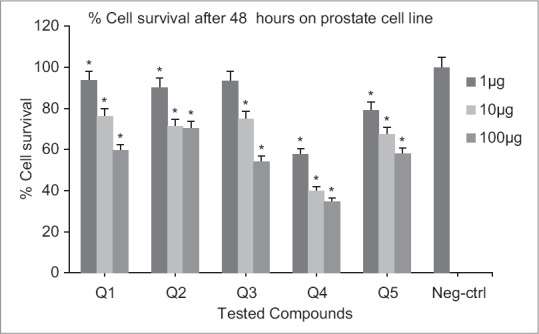
Cytotoxic effects of compounds on prostate cell line following exposure to different concentrations of compounds (after 48 h). Data are presented as mean ± standard deviation, *P < 0.05, n = 3, Neg-ctrl: Negative control
Discussion
Quinazoline derivatives have therapeutic benefit as anticancer agents for activity in early and advanced tumors.[15] Amine or substituted amine on 4th position and either halogens or electron-rich substituents on 6th position of quinazolinone can improve activity against cancer cell lines.[15]
In the previous work, novel quinazolinone derivatives (fused pyridazine-quinazolinones and fused pyrrolo-quinazolinones and other derivatives) were synthesized and screened against Hella cell line by our group.[28] Some of these compounds showed significant cytotoxic activity on HeLa cell line in the range of 10–100 μM and obtained results revealed that the nitro substituted compounds were more cytotoxic than their bromo-containing counterparts also compounds Q3 and Q4 exhibited acceptable cytotoxicity approximately 50% at 10 μM concentration on this cell line. It could be concluded that the existence of a substituent NO2 group on 6th position of the phenyl ring could improve the cytotoxic effects of tested compounds. In this study, a selection of quinazolinone derivatives was screened against melanoma and prostate cell lines, using the MTT colorimetric assay.
Following 48 h exposure of compounds to melanoma cell line, significant differences in viability (P < 0.05) were resulted compared to the negative control at 1, 10 and 100 μM concentrations [Figure 5]. At 24 h exposure, only Q3 and Q4 showed significant activities at all concentrations. For other derivatives (Q1, Q5, Q2) higher concentrations (10 and 100 μM) were necessary [Figure 4]. Significant differences in viability (P < 0.05) at 1, 10, 100 μM concentrations were observed after 24 h exposure of Q4 to prostate cell line. Nitro-derivatives of fused pyrrolo-quinazolinone Q5 and fused pyridazine-quinazolinone Q2 showed significant differences in viability (P < 0.05) at 10, 100 μM concentrations [Figure 6]. A 48 h continuous drug exposure on prostate cell line exhibited a significant difference in viability (P < 0.05) at 1, 10, 100 μM concentrations for all tested compounds except Q3 [Figure 7].
Figure 5.
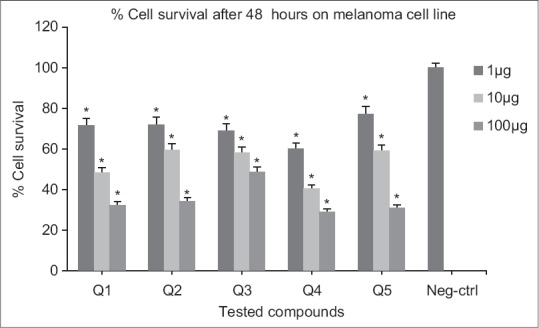
Cytotoxic effects of compounds on melanoma cell line following exposure to different concentrations of compounds (after 48 h). Data are presented as mean ± standard deviation, *P < 0.05, n = 3, Neg-ctrl: Negative control
Figure 6.
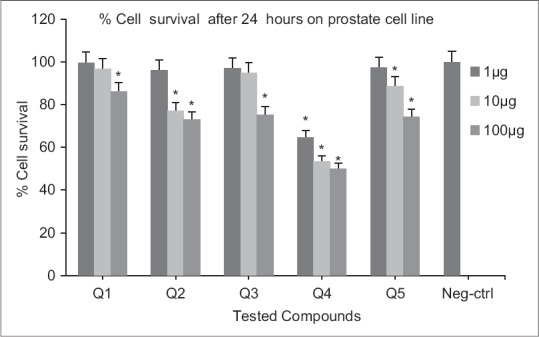
Cytotoxic effects of compounds on prostate cell line following exposure to different concentrations of compounds (after 24 h). Data are presented as mean ± standard deviation, *P < 0.05, n = 3, Neg-ctrl: Negative control
Conclusion
Results showed that both cell lines were sensitive to synthesized compounds, and longer duration of exposure (48 h) had better results compared to that of 24 h screening. Although this compound showed a promising result on these two cell lines, however, its beneficial effects in human cancers and the mechanism of action is not clear and should be established.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Hakimelahi for helping drugs synthesis.
References
- 1.Wang S, Ryder H, Pretswell I, Depledge P, Milton J, Hancox TC, et al. Studies on quinazolinones as dual inhibitors of Pgp and MRP1 in multidrug resistance. Bioorg Med Chem Lett. 2002;12:571–4. doi: 10.1016/s0960-894x(01)00804-6. [DOI] [PubMed] [Google Scholar]
- 2.Shirzad H, Taji F, Rafieian-Kopaei M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J Med Food. 2011;14:969–74. doi: 10.1089/jmf.2011.1594. [DOI] [PubMed] [Google Scholar]
- 3.Shirzad H, Kiani M, Shirzad M. Impacts of tomato extract on the mice fibrosarcoma cells. J Herb Med Pharmacol. 2013;2:13–6. [Google Scholar]
- 4.Khosropour AR, Mohammadpoor-Baltork I, Ghorbankhani H. Bi (TFA) 3-[nbp] Fecl4: A new, efficient and reusable promoter system for the synthesis of 4 (3H)-quinazolinone derivatives. Tetrahedron Lett. 2006;47:3561–4. [Google Scholar]
- 5.Nouira I, Kostakis IK, Dubouilh C, Chosson E, Iannelli M, Besson T. Decomposition of formamide assisted by microwaves, a tool for synthesis of nitrogen-containing heterocycles. Tetrahedron Lett. 2008;49:7033–6. [Google Scholar]
- 6.Nagar AA, Rathi LG, Chugh N, Pise VJ, Bendale A. Microwave assisted one pot synthesis of 2, 3-di-Substituted quinazolin-4-(3H)-ones and their potential biological activity. Pharm Chem. 2010;2:37–43. [Google Scholar]
- 7.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and antimicrobial activities of some novel substituted 2-imidazolyl-N-(4-oxo-quinazolin-3 (4H)-yl)-acetamides. Chem Pharm Bull (Tokyo) 2007;55:1615–9. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 8.Raghavendra NM, Thampi PP, Gurubasavarajaswamy PM. Synthesis and antimicrobial activity of some novel substituted piperazinyle-quinazolin-3 (4H)-ones. Eur J Chem. 2008;5:23–33. [Google Scholar]
- 9.Dave RS, Odedara RJ, Kalaria RI, Upadhyay JJ. Synthesis, characterization and antimicrobial activity of new quinazolin- 4(3H)-one schiff base derivatives. J Chem Pharm Res. 2012;4:4864–9. [Google Scholar]
- 10.Jantova S, Stankovsky S, Spirkova K. In vitro antibacterial activity of ten series of substituted quinazolines. Biol Bratislava. 2004;59:741–52. [Google Scholar]
- 11.Pereira Mde F, Chevrot R, Rosenfeld E, Thiery V, Besson T. Synthesis and evaluation of the antimicrobial activity of novel quinazolinones. J Enzyme Inhib Med Chem. 2007;22:577–83. doi: 10.1080/14756360701425345. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed MS, Kamel MM, Kassem EM, Abotaleb N, Abd El-Moez SI, Ahmed MF. Novel 6,8-dibromo-4 (3H) quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur J Med Chem. 2010;45:3311–9. doi: 10.1016/j.ejmech.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Gursoy A, Unal B, Karali N, Otuk G. Synthesis, characterization and primary antimicrobial activity evaluation of 3-phenyl-6-methyl-4 (3H)-quinazolinone-2-yl-mercaptoacetic acid arylidenehydrazides. Turk J Chem. 2005;29:233–45. [Google Scholar]
- 14.Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4 (3H)-one. Pharm Acta Helv. 1999;74:11–7. doi: 10.1016/s0031-6865(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 15.Chandrika PM, Yakaiah T, Narsaiah B, Sridhar V, Venugopal G, Rao JV, et al. Synthesis leading to novel 2, 4, 6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell line. Indian J Chem. 2009;48B:840–7. [Google Scholar]
- 16.Khalil AA, Abdel-Hamide SG, Al-Obaid AM, El-Subbagh HI. Substituted quinazolines, part 2. Synthesis and in-vitro anticancer evaluation of new 2-substituted mercapto-3H-quinazoline analogs. Arch Pharm (Weinheim) 2003;336:95–103. doi: 10.1002/ardp.200390011. [DOI] [PubMed] [Google Scholar]
- 17.Gürsoy A, Karali N. Synthesis and primary cytotoxicity evaluation of 3-[[(3-phenyl-4 (3H)-quinazolinone-2-yl) mercaptoacetyl] hydrazono]-1H-2-indolinones. Eur J Med Chem. 2003;38:633–43. doi: 10.1016/s0223-5234(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 18.Laddha SS, Kar SG, Meghal SK. Studies on some biologically active substituted 4 (3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory, antimicrobial activity of 6,8-disubstituted2-phenyl-3-[substituted-benzothiazol-2-yl]-4 (3H)-quinazolinone. Arkivoc. 2006;xi:1–20. [Google Scholar]
- 19.Johnson M, Li AR, Liu J, Fu Z, Zhu L, Miao S, et al. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett. 2007;17:3339–43. doi: 10.1016/j.bmcl.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 20.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanda AK, Ganguli S, Chakraborty R. Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4 (3H)-ones: Synthesis and preliminary QSAR studies. Molecules. 2007;12:2413–26. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, et al. Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4 (3H)-quinazolinone analogs. Bioorg Med Chem. 2006;14:8608–21. doi: 10.1016/j.bmc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Jiang JB, Hesson DP, Dusak BA, Dexter DL, Kang GJ, Hamel E. Synthesis and biological evaluation of 2-styrylquinazolin-4 (3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J Med Chem. 1990;33:1721–8. doi: 10.1021/jm00168a029. [DOI] [PubMed] [Google Scholar]
- 24.Raffa D, Edler MC, Daidone G, Maggio B, Merikech M, Plescia S, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem. 2004;39:299–304. doi: 10.1016/j.ejmech.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Jafari E, Khodarahmi GA, Hakimelahi GH, Tsai FY, Hassanzadeh F. Synthesis of some new tricyclic 4 (3H)-quinazolinone derivatives. Res Pharm Sci. 2011;6:93–100. [PMC free article] [PubMed] [Google Scholar]
- 26.Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. 4th ed. United State: Wiley-Liss Press; 1994. pp. 1–5. 9-15, 181-4, 309. [Google Scholar]
- 27.Chandrika PM, Yakaiah T, Rao AR, Narsaiah B, Reddy NC, Sridhar V, et al. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur J Med Chem. 2008;43:846–52. doi: 10.1016/j.ejmech.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Hassanzadeh F, Jafari E, Hakimelahi GH, Khajouei MR, Jalali M, Khodarahmi GA. Antibacterial, antifungal and cytotoxic evaluation of some new quinazolinone derivatives. Res Pharm Sci. 2012;7:87–94. [PMC free article] [PubMed] [Google Scholar]


