Abstract
The gut hormone, glucagon like peptide-1 (GLP-1) exerts anti-inflammatory effects. However, its clinical use is limited by its short half-life. Previously, we have shown that GLP-1 as a nanomedicine (GLP-1 in sterically stabilized phospholipid micelles, GLP-1-SSM) has increased in vivo stability. The current study was aimed at testing the efficacy of this GLP-1 nanomedicine in alleviating colonic inflammation and associated diarrhea in dextran sodium sulfate (DSS) induced mouse colitis model. Our results show that GLP-1-SSM treatment markedly alleviated the colitis phenotype by reducing the expression of pro-inflammatory cytokine IL-1β, increasing goblet cells and preserving intestinal epithelial architecture in colitis model. Further, GLP-1-SSM alleviated diarrhea (as assessed by luminal fluid) by increasing protein expression of intestinal chloride transporter DRA (down regulated in adenoma). Our results indicate thatGLP-1 nanomedicine may act as a novel therapeutic tool in alleviating gut inflammation and associated diarrhea in inflammatory bowel disease (IBD).
Keywords: GLP-1 SSM, Diarrhea, IBD, Colitis
Highly debilitating inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis (UC) are chronic relapsing inflammatory disorders of the gastrointestinal (GI) tract. Abnormal immune response coupled to gene–environment interactions is considered to be the major causative factor for the disease,1 however, the exact pathogenesis remains unknown. Current treatments for IBD (anti-inflammatory agents and immunosuppressive drugs) are inadequate in their efficacy, lack specificity, have poor bioavailability, and pose long-term adverse effects.2–4 IBD thus, remains an intractable gastrointestinal disease with no definitive therapies.2 Therefore, there is an urgent need for specific and effective drugs against IBD that can target multiple events in the disease.
Typical symptoms of IBD include mucosal inflammation resulting in mucosal injury, alterations in intestinal epithelial structure and function culminating into repeated episodes of diarrhea and abdominal pain. IBD associated diarrhea is linked to significantly reduced luminal and Na+/H+ exchange activity and/or levels in distal intestine5,6 SLC26A3 (DRA, down regulated in adenoma), is the key Cl−/HCO3− exchanger in mammalian intestine and has recently been identified as a novel risk factor for UC development.8 Disturbances in DRA expression and function have been shown to occur in response to proinflammatory cytokines, in animal models of colitis and in UC patients.7,9–11 DRA has thus emerged as an important and novel therapeutic target for IBD.5
Glucagon like peptide-1 (7–36) (GLP-1) is a peptide hormone secreted by enteroendocrine L-cells in response to nutrient ingestion.12 Apart from its glucoregulatory action, GLP-1 is known to exert neuro- and cardiovascular protection by the virtue of its anti-apoptotic, anti-oxidative and anti-inflammatory properties.13 GLP-1 reduces the expression of proinflammatory cytokines like CXCL-10, STAT-3, Mcp-1 and TNF-α.14 GLP-1 also inhibits NFκB activation and modulates the activity of natural killer cells in pancreas, CNS and endothelial cells.14–16 However, the anti-inflammatory effects of GLP-1 in intestinal inflammation have not been investigated.
GLP-1 use as a potential treatment modality is limited by its short half-life in vivo due to rapid degradation by enzymes such as dipeptidyl peptidase IV (DPP-4). In this regard, DPP-4 resistant GLP-1 analogues including exenatide, liraglutide, albiglutide, dulaglutide are FDA approved and these analogues exhibit increased half-life.17 However, these analogues suffer from reduced bioactivity, increased risk of immunogenicity and high cost of synthesis.18,19 In this regard, we have recently designed a nanocarrier system with sterically stabilized phospholipid micelles (SSM), to which native human GLP-1 self-associates with.18 This carrier system increases the in vivo stability of GLP-1 while maintaining its anti-inflammatory properties.18 Furthermore, due to the nano-size (~14 nm) of the carrier, SSM associated peptide can only extravasate out of the circulation at the leaky vasculatures of the inflamed tissues by EPR (enhanced permeability and retention) effect resulting with passive targeting. This targeted effect should not only increase the peptide activity for a given dose, but also eliminate the peptide toxicity to the healthy tissues. Our previous studies have demonstrated the anti-inflammatory effects of GLP-1-SSM, but not GLP-1 alone, against lipopolysaccharide induced acute lung injury (ALI) in mouse model.18
Based on our previous studies, where GLP-1-SSM exerted beneficial effects in ALI inflammation, we hypothesized that GLP-1 nanomedicine could alleviate the inflammatory and diarrheal phenotype in dextran sodium sulfate (DSS) induced colitis in a mouse model. Our results demonstrated that GLP-1-SSM markedly reduced the inflammation and partially attenuated the diarrheal phenotype in DSS colitis mice. To our knowledge, this is the first report, where GLP-1 has been tested for IBD.
Methods
Preparation of SSM and GLP-1 SSM
GLP-1 in SSM was prepared as described previously by us.18 Briefly, SSM was prepared by mixing 5 mM 1,2-distearyl-snglycero-3-phosphatidylethanolamine-N-[methoxy(polyethyleneglycol)-2000 (DSPE-PEG2000, Ludwigshafen, Germany) in normal saline (Baxter, IL, USA) by vortexing for 2 minutes followed by incubation of the dispersion at 25 °C for 1 hour in the dark. Human GLP-1 (Protein Research Laboratory, University of Illinois at Chicago, Chicago, IL) in normal saline was added to SSM at a constant molar ratio of GLP-1:SSM (1:30) at all times (final concentration 15 nmol/100 µl), and the dispersion was further incubated at 25 °C for 2 hours in the dark. Particle size analysis of aqueous native GLP-1, SSM and GLP-1-SSM was conducted by dynamic light scattering (Agilent 7030 NICOMP DLS, Agilent Technologies, Santa Clara, CA). The size of SSM and GLP-1-SSM was ~13 nm as a single peak while aqueous GLP-1 was heterogenous with dominating peak at ~1700 nm in diameter as previously shown by us.18
Murine DSS colitis model
All the animal studies were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. 6–8 weeks C57BL/6J male mice were obtained from Jackson Laboratories (Bar Harbor, Maine). The experiment included 4 groups (10 animals/group) − SSM control, GLP-1-SSM, DSS and DSS + GLP-1-SSM. Colitis was induced by 3% (wt/vol) DSS (36–50 kDa, MP Biomedicals, Solon, OH) supplemented in drinking water for 7 days. 15 nmol/100 µl of GLP-1-SSM or SSM was administered daily intra peritoneally in respective groups during the 7 days of DSS treatment.
H and E staining
Colon samples were collected from mice and quickly rinsed in PBS. Tissues were embedded in OCT media and immediately snap frozen in liquid nitrogen. 5 µm-thick longitudinal sections were stained with hematoxylin and eosin (H&E) as described in manufacture’s protocol (ScyTek Laboratories, Logan, UT).
PAS staining
Goblet cell staining was carried according to the manufacturer’s instructions (Leica Microsystems, Buffalo Grove, IL). In brief, 5 µm-thick distal colon sections from different groups were fixed in 10% formalin in PBS. Sections were washed three times in 1×-PBS for 5 min followed by a single wash with water. For Alcian blue staining, sections were incubated in 3% acetic acid for 3 min and then in Alcian blue solution (pH 2.5) for 30 minutes with subsequent washing with distilled water. Freshly prepared periodic acid (0.5%) was applied for 5 min at RT, slides washed in distilled water, and then stained with Schiff’s reagent for 15 min, followed by 5 min wash in running tap water. Nuclei were counterstained with nuclear fast red for 30 sec, and then washed in running tap water for 2 min, followed by dehydration (twice in 95% EtOH then twice in 100% EtOH). Slides were mounted using permount (Fisher Scientific).
Western blotting
Tissue lysates were prepared from the scraped colonic mucosa using RIPA lysis buffer (Cell Signaling, Danvers, MA) supplemented with protease inhibitor mixture (Roche, Indianapolis, IN). Lysates were run on a 7.5% gel and then transferred onto nitrocellulose membrane. (1×) PBS and 5% nonfat dry milk was used as a blocking buffer for 1 h. The membranes were then probed with human anti-DRA (1:100 dilution) or GAPDH antibodies (1:3000 dilution) in 1× PBS and 2.5% nonfat dry milk overnight at 4 °C. The membranes were then washed four times with wash buffer containing 1× PBS and 0.1% Tween-20 for 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:2000 dilution) for 1 h and the bands were visualized with enhanced chemiluminiscence (ECL) detection reagents (Biorad, Hercules, CA). Images were acquired by imagelab 5.0 (Biorad, Hercules, CA). Quantification of band intensities was done using Image-J software.
Immunofluorescence
Sections of colonic tissues from different mice groups were snap-frozen in optimal cutting temperature (OCT) embedding medium. Immunostaining was performed as previously described.9 In brief, 5-µm frozen sections were fixed with 4% para-formaldehyde (PFA) in PBS for 10 min at room temperature. Fixed sections were washed in PBS, permeabilized with 0.3% Nonidet P-40 for 5 min, and blocked with 2.5% normal goat serum (NGS) for 1 hour. Tissues were incubated with DRA and villin antibody (1:100) in PBS with 1% NGS for 90 min at room temperature, followed by incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse antibody for 60 min. Sections were then washed and mounted under cover slips using Slowfade Gold antifade with DAPI reagent (Invitrogen, Grand Island, NY). Carl Zeiss LSM 510 laser-scanning confocal microscope equipped with ×20 water immersion objective was used for imaging.
Realtime PCR
RNA was extracted from mouse colonic mucosal samples using Qiagen RNeasy mini kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. The quality and quantity of total RNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). Extracted RNA was amplified by Brilliant SYBR Green qRT-PCR Master Mix kit (Agilent, Santa Clara, CA) utilizing mouse gene specific primers for IL-1β; Forward: 5′-GCAACTGTTCCTGAACTCAACT-3′; reverse: 5′-ATCTTTTGGGGTCCGTCAACT-3′; and GAPDH forward: 5′-TGTGTCCGTCGTGGATCTGA −3′; reverse: 5′- CCTGCTTCACCACCTCTTGAT 3′. The relative mRNA levels of IL-1β were expressed as% of control normalized to GAPDH used as internal control gene.
Statistical analysis
Data are presented as mean ± SEM. Differences between groups were analyzed by Student’s t test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P value of 0.05 or less was considered statistically significant.
Results
GLP-1 nanomedicine
We have previously shown GLP-1 SSM preparation and its characterization (such as size and charge)18 where it was effective against ALI. The focus of the current study was to therefore, examine the efficacy of GLP-1 SSM in ameliorating DSS induced colitis and the associated diarrheal phenotype.
Amelioration of colitis in mice by GLP-1 nanomedicine
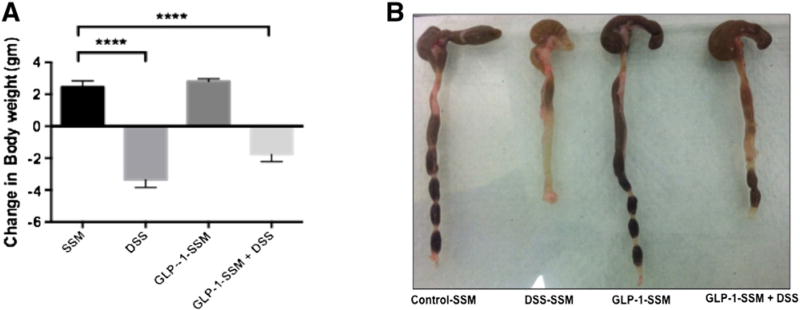
After 7 days of treatment the severity of colitis was evaluated on the basis of weight loss and qualitative assessment of stool consistency. Mice treated with DSS exhibited a marked weight loss of −3.36 ± 0.48 vs a weight gain in SSM control group 2.46 ± 0.38. This decline in weight was partially attenuated by treatment with GLP-1-SSM in DSS + GLP-1-SSM group (loss of 2.05 ± 0.36). The control animals (SSM and GLP-1-SSM groups) exhibited anticipated changes in body weight (Figure 1, A).
Figure 1.
GLP-1 nanomedicine attenuates DSS induced colitis in mice. Change in body weight (A), preservation of stool consistency (B) during DSS treatment. Colitis was induced by 3% (wt/vol) DSS supplemented in drinking water for 7 days to DSS and DSS + GLP1-SSM groups. 15 nmol of GLP1-SSM or SSM was administered by i.p injection everyday to mice in respective groups during the 7 days of DSS treatment. Data were expressed as the mean ± SEM, ****P < 0.0001. n = 10 mice per group.
The stool consistency (Figure 1, B) as examined visually (qualitative measurement) was loose in the colon of DSS treated mice reflecting the diarrheal phenotype. This feature was partially attenuated with GLP1-SSM treatment in DSS + GLP-1-SSM group. Taken together, these results indicate potential antidiarrheal effects of the GLP-1 nanomedicine.
GLP-1 nanomedicine alleviates histologic changes observed in DSS induced colitis
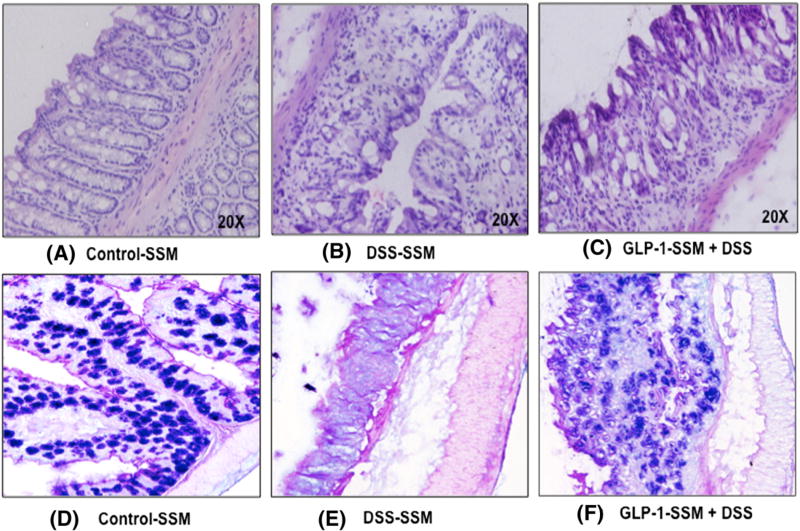
Histologic analyses of distal colonic tissues showed that mucosal structures were significantly damaged in DSS treated mice, as manifested by damage to crypt structures and high levels of neutrophil infiltration (Figure 2, B). DSS treatment caused nearly complete loss of mature goblet cells as revealed by PAS staining of the sections (Figure 2, E). In contrast, colonic tissue from DSS + GLP-1-SSM treated mice showed partial preservation of crypt structures containing some goblet cells (fewer in number as compared to SSM control group) despite the presence of intense inflammatory infiltrate (Figure 2, C & F).
Figure 2.
GLP-1 nanomedicine alleviates histologic alterations observed in DSS induced colitis. After 7 days of treatment, the colon was collected and 5 µm thick sections of distal colon were prepared. Sections were stained by H & E (A–C) and PAS (D–F) and assessed histologically.
GLP-1 nanomedicine suppresses proinflammatory cytokine IL-1β in the intestinal mucosa of mice with DSS-induced colitis
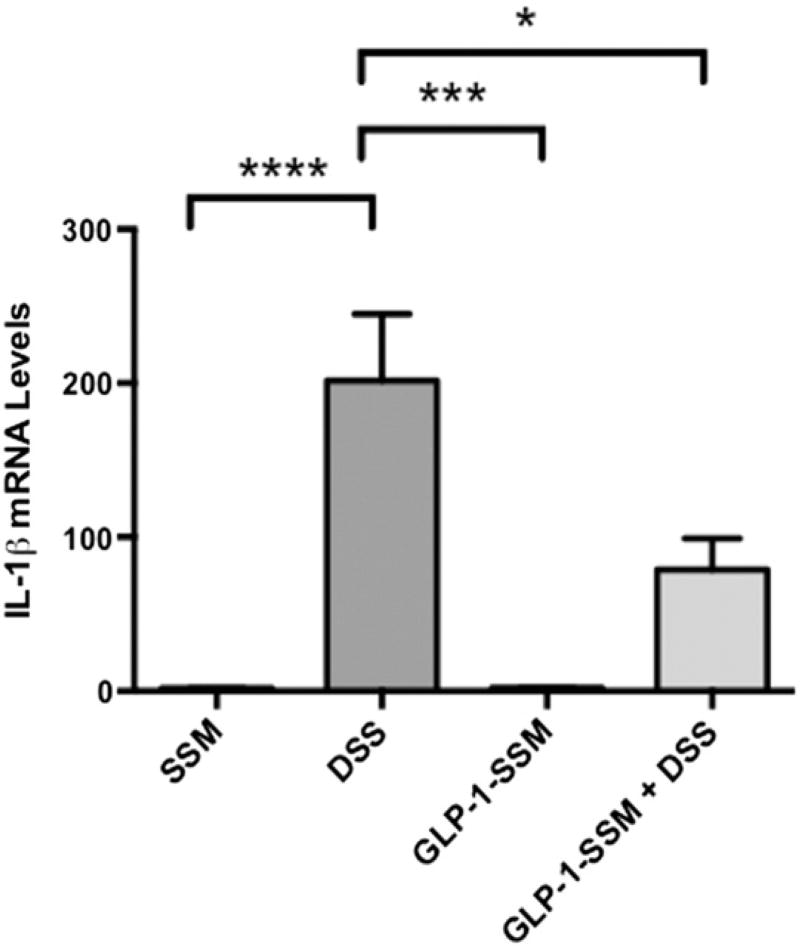
Expression of proinflammatory mediator IL-1β was also determined in the colonic mucosa. DSS treated mice showed a huge increase (~200 fold) in IL-1β mRNA levels, whereas GLP-1-SSM caused a significant decline in the mRNA levels of IL-1β (Figure 3) in DSS + GLP-1-SSM group compared to DSS alone.
Figure 3.
Anti-inflammatory effects of GLP-1 nanomedicine in mice with colitis. After 7 days of treatment RNA from mucosal scrapings from distal colon were prepared, and expression of IL-1β was measured by qRT PCR. Data were expressed as the mean ± SEM, *P < 0.05, ***P < 0.001, ****P < 0.0001. n = 10 mice per group.
GLP-1 nanomedicine inhibits the decline in DRA protein expression by DSS colitis
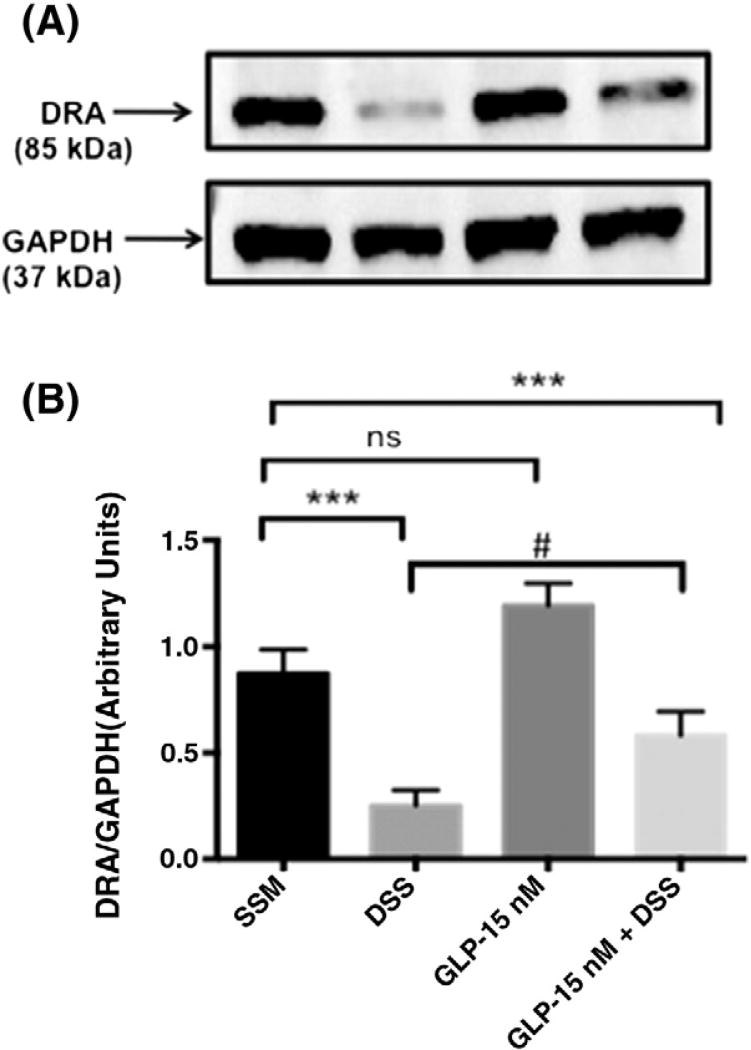
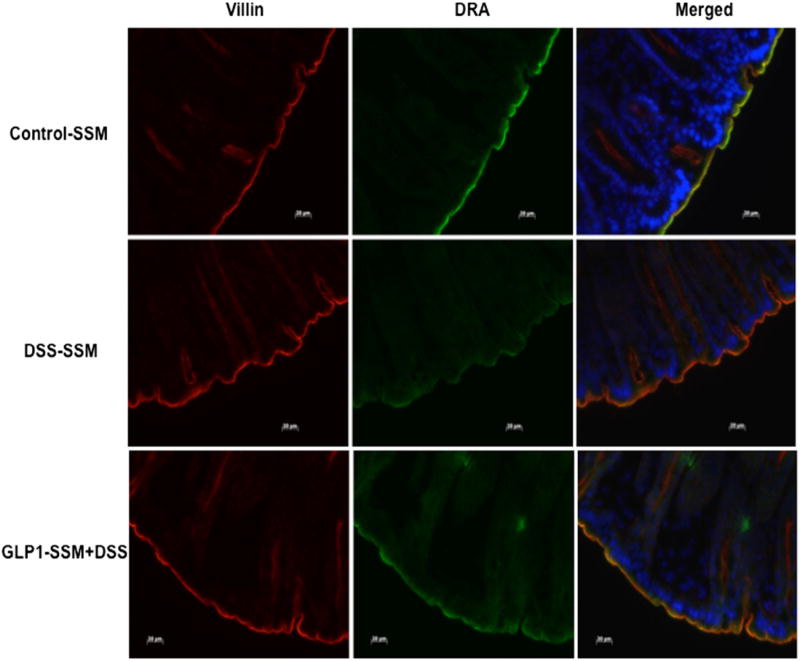
Previous studies from our laboratory have shown that DRA expression declines significantly in the colon of DSS treated mice.9 Results of this study also demonstrated a decrease in colonic DRA expression (~60%) in DSS treated mice (Figure 4). This decrease in DRA protein levels was significantly alleviated by GLP-1-SSM in DSS + GLP-1-SSM group. Immunofluorescence staining of colonic sections of DSS mice showed a noticeable decrease in DRA protein levels on the apical membrane (Figure 5). In contrast, GLP-1-SSM blocked the inhibitory effects of DSS on DRA expression. This attenuation of decreased DRA expression by GLP-1-SSM in DSS colitis mice further substantiates the anti-diarrheal effects of GLP-1 nanomedicine (Figure 1).
Figure 4.
GLP-1 nanomedicine blocks DSS induced decrease in DRA protein expression. DRA protein levels in distal colonic mucosal tissue lysates were measured by Western blot. A representative blot is shown (A). Densitometric analysis of relative band intensities (B). Data were expressed as mean ± SEM ***P < 0.001. n = 10 mice per group, # denotes DSS and DSS + GLP1-SSM groups are not significantly different by one-way (ANOVA) but two groups are significantly different according to Student’s t test (P < 0.05).
Figure 5.
GLP-1 nanomedicine blocks the DSS-induced decrease in DRA expression. Green, DRA; red, villin; blue, nuclei; scale bar = 20 µm.
Discussion
Our results demonstrate that human glucagon-like peptide −1 (7–36) self-associated with PEGylated phospholipid micelles, GLP-1-SSM, is effective against DSS induced colitis in mice. This acute intestinal injury model exhibits symptoms comparable to those of human ulcerative colitis such as diarrhea, body weight loss, mucosal damage and shortening of colon length.20 These features were prominent in our model system where mice were treated with 3% DSS for 7 days. Co-treatment with 15 nmol/100 µl of GLP-1-SSM daily partially abrogated the DSS induced weight loss (Figure 1, A). More prominently, GLP-1-SSM treatment also partly restored stool consistency. It should be noted that GLP-1-SSM administration did not significantly alter ad libitum blood glucose levels in mice in all the groups (data not shown) suggesting that GLP-1 SSM effects were specific to the anti-inflammatory/anti-diarrheal properties, without any adverse side effects likely to result from free GLP-1 peptide. Our results indicate two potential mechanisms of GLP-1-SSM mediated alleviation of the diarrheal phenotype in DSS mice via modulation of: 1) colonic inflammation, and 2) DRA expression.
IBD is associated with severe and chronic inflammatory responses disrupting the colonic epithelium and allowing intense infiltration of neutrophils into the affected tissue site.21 Our results with DSS colitis model mimicked the colonic inflammatory cascade of IBD. Increase in expression of proinflammatory cytokine IL-1β was partly reversed by GLP-1-SSM indicating the potential anti-inflammatory effects of GLP-1 SSM. GLP-1 is known to reduce immune cell infiltration and proinflammatory cytokine secretion by increasing cAMP levels (PKA activation) and inflammasome repression (PKC inhibition).13,18,22 As the GLP-1 nano-formulation retains its biologic activity for longer times, the observed effects may be mediated through GLP-1-R activation.18
GLP-1-SSM treatment helped to maintain the colonic crypt architecture and prevented complete loss of goblet cells as compared to DSS mice, though immune cell infiltration was not blocked significantly (Figure 2, C & F). These distended crypt structures associated with some goblet cells, in the DSS + GLP-1-SSM treated mice are indicative of either better survival or rapid regeneration of colonic epithelial crypt cells in the setting of intestinal inflammation. Our data are consistent with recent studies where GLP-1 or its synthetic analogues (through GLP-1-R) engaged several downstream signaling pathways and exerted pro-survival, proliferative and, anti-apoptotic effects on human/murine islets, endothelial cells and some other tissues.13,23,24 Also, sustained GLP-1-R activation has been shown to increase small intestine and colonic mucosal epithelium mass in rodents.25
One of the major symptoms of IBD is diarrhea, which occurs secondary to inflammation. In this regard, DRA plays an important role in maintaining electrolyte balance and preventing abnormal fluid loss from the intestine.5 Additionally, DRA knockout mice exhibit diarrheal phenotype.26 Studies from our lab and others showed a repression of DRA expression in inflamed intestine.5 Current studies for the first time demonstrate that GLP-1 nanomedicine preserves DRA protein levels in the intestine (Figures 4 & 5). It can be speculated that maintenance of intestinal epithelial cell integrity and preservation of goblet cells by GLP-1-SSM might play an essential role in attenuating inflammation induced decrease in DRA expression in DSS + GLP-1-SSM group (Figures 2, 4 & 5). DRA activity is also crucial to maintenance of proper mucus layer in the colon mainly by regulation of alkaline secretion.27 Preservation of goblet cells in GLP1-SSM treated DSS mice could thus be due to recovered DRA protein levels (Figures 2, 4 & 5).
Moreover, studies have shown that DPP4 inhibition reduces neutrophil recruitment in mouse lung transplantation model.28 However, protective effects of GLP1 against colitis and associated diarrhea have not been studied in detail and have remained undiscovered due to predominant glucoregulatory role of GLP-1. Some of the potential anti-inflammatory effects of free GLP-1 are also offset due to its rapid in vivo degradation or high costs involved in synthesis of DPP-4 (GLP-1 degrading peptidase) resistant analogues18 of GLP-1 also is an important limitation. However, our current approach to use GLP-1 in a nano carrier system has overcome these limitations.
In addition GLP-1-SSM when given as intraperitoneal injection may not leave abdominal cavity as particles, but release GLP-1 locally to show effects as observed in this study. However, real life application of GLP-1 nanomedicine should be performed preferably via an intravenous route, where the nanoparticles may accumulate only in the inflamed tissues through leaky vasculature by passive targeting. This approach not only may show higher efficiency of the peptide but also decrease the possible side effects of GLP-1, such as lowering of blood sugar. Previously, we have shown that the blood pressure-lowering side effects of another anti-inflammatory peptide, VIP, was totally eliminated when administered as a nanomedicine.29
Taken together, our results project GLP-1-nanomedicine to be a novel and potent therapeutic tool against intestinal inflammation and diarrhea associated with IBD. Furthermore, as Crohn’s disease and ulcerative colitis patients are often presented with insulin resistance,30 GLP-1-SSM is also likely to regulate body glucose in such settings in addition to attenuation of inflammation and diarrhea.
Acknowledgments
These studies were supported by NIH (NIDDK): DK-54016 (PKD), DK-81858 (PKD), DK-92441 (PKD), DK96254 (SS) Department of Veterans Affairs Merit Award BX002011 (PKD), TUBITAK AWARD (HO). GLP-1 SSM was prepared in facility constructed with support from Research Facilities Improvement Program Grant Number C06RR15482 from National Center for Research Resources, National Institutes of Health.
Abbreviations
- GLP-1
glucagon like peptide-1
- SSM
sterically stabilized phospholipid micelles
- DSS
dextran sodium sulfate
- DRA
down regulated in adenoma
- IBD
inflammatory bowel diseases
- IL-1β
interleukin-1β
Footnotes
Part of this work has been presented at the Digestive Disease Week-2014 conference, Chicago, Illinois.
Conflict of interest: Authors state no conflict of interest.
References
- 1.Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161(2):231–5. doi: 10.1016/j.imlet.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viscido A, Capannolo A, Latella G, Caprilli R, Frieri G. Nanotechnology in the treatment of inflammatory bowel diseases. J Crohns Colitis. 2014;8(9):903–18. doi: 10.1016/j.crohns.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Pichai MV, Ferguson LR. Potential prospects of nanomedicine for targeted therapeutics in inflammatory bowel diseases. Gastroenterol. 2012;18(23):2895–901. doi: 10.3748/wjg.v18.i23.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143(4):1027–36 [e3]. doi: 10.1053/j.gastro.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms underlying dysregulation of electrolyte absorption in inflammatory bowel disease-associated diarrhea. Inflamm Bowel Dis. 2015;21(12):2926–35. doi: 10.1097/MIB.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447(5):710–21. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, et al. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Phys. 1998;275(6 Pt 1):G1445–53. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 8.Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, et al. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet. 2009;41(12):1325–9. doi: 10.1038/ng.482. [DOI] [PubMed] [Google Scholar]
- 9.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, et al. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Physiol Gastrointest Liver Physiol. 2014;307(6):G623–31. doi: 10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohi H, Makela S, Pulkkinen K, Hoglund P, Karjalainen-Lindsberg ML, Puolakkainen P, et al. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Physiol Gastrointest Liver Physiol. 2002;283(3):G567–75. doi: 10.1152/ajpgi.00356.2001. [DOI] [PubMed] [Google Scholar]
- 11.Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV. Dextran sulfate sodium-induced murine colitis activates NF-kappa B and increases galanin-1 receptor expression. Physiol Gastrointest Liver Physiol. 2000;278(5):G797–804. doi: 10.1152/ajpgi.2000.278.5.G797. [DOI] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Pugazhenthi U, Velmurugan K, Tran A, Mahaffey G, Pugazhenthi S. Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: potential therapeutic benefits in diabetic patients. Diabetologia. 2010;53(11):2357–68. doi: 10.1007/s00125-010-1849-y. [DOI] [PubMed] [Google Scholar]
- 15.Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58(9):2148–61. doi: 10.2337/db09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54(11):2745–54. doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. A novel peptide nanomedicine against acute lung injury: GLP-1 in phospholipid micelles. Pharm Res. 2011;28(3):662–72. doi: 10.1007/s11095-010-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol. 2009;297(1–2):137–40. doi: 10.1016/j.mce.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HS, Oh SM, Kim JK. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2008;151(1):165–73. doi: 10.1111/j.1365-2249.2007.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80(4):802–15. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 22.Dai Y, Dai D, Wang X, Ding Z, Mehta JL. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther. 2014;28(5):425–32. doi: 10.1007/s10557-014-6539-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Bastarrachea RA, Roberts BJ, Voruganti VS, Frost PA, Nava-Gonzalez EJ, et al. Successful beta cells islet regeneration in streptozotocin-induced diabetic baboons using ultrasound-targeted microbubble gene therapy with cyclinD2/CDK4/GLP1. Cell Cycle. 2014;13(7):1145–51. doi: 10.4161/cc.27997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezanno H, Pawlowski V, Abdelli S, Boutry R, Gmyr V, Kerr-Conte J, et al. JNK3 is required for the cytoprotective effect of exendin 4. J Diabetes Res. 2014;2014:814854. doi: 10.1155/2014/814854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen L, Pilgaard S, Orskov C, Rosenkilde MM, Hartmann B, Holst JJ, et al. Exendin-4, but not dipeptidyl peptidase IV inhibition, increases small intestinal mass in GK rats. Physiol Gastrointest Liver Physiol. 2007;293(1):G288–95. doi: 10.1152/ajpgi.00453.2006. [DOI] [PubMed] [Google Scholar]
- 26.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281(49):37962–71. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 27.Xiao F, Yu Q, Li J, Johansson ME, Singh AK, Xia W, et al. Slc26a3 deficiency is associated with loss of colonic HCO3 (−) secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf) 2014;211(1):161–75. doi: 10.1111/apha.12220. [DOI] [PubMed] [Google Scholar]
- 28.Jungraithmayr W, De Meester I, Matheeussen V, Baerts L, Arni S, Weder W. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-Thoracic. Surgery. 2012;41(5):1166–73. doi: 10.1093/ejcts/ezr180. [DOI] [PubMed] [Google Scholar]
- 29.Sethi V, Rubinstein I, Kuzmis A, Kastrissios H, Artwohl J, Onyuksel H. Novel, biocompatible, and disease modifying VIP nanomedicine for rheumatoid arthritis. Mol Pharm. 2013;10(2):728–38. doi: 10.1021/mp300539f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chouliaras G, Panayotou I, Margoni D, Mantzou E, Pervanidou P, Manios Y, et al. Circulating leptin and adiponectin and their relation to glucose metabolism in children with Crohn’s disease and ulcerative colitis. Pediatr Res. 2013;74(4):420–6. doi: 10.1038/pr.2013.114. [DOI] [PubMed] [Google Scholar]