Abstract
Background
Prenatal alcohol exposure can result in physical and neurocognitive deficits that are collectively termed ‘Fetal Alcohol Spectrum Disorders’ (FASD). Though FASD is associated with life-long intellectual disability, the mechanisms mediating the emergence of secondary mental-health and physical disabilities are poorly understood. Based on our previous data showing that maternal ethanol exposure in mice resulted in an immediate reduction in cranially directed fetal blood flow, we hypothesized that such exposure would also result in persistent alterations in cranially directed blood flow in the prenatally alcohol exposed (PAE) adult. We also hypothesized that PAE adults exposed to an acute cerebrovascular insult would exhibit more brain damage and neurobehavioral impairment compared to non-PAE adult controls.
Methods
Pregnant C57Bl/6 mice were exposed to ethanol, 3g/kg, or water by intra-gastric gavage. Blood flow in carotid, renal and femoral arteries was assessed by ultrasound imaging in PAE and control adults, at 3, 6, and 12 months of age. To mimic ischemic stroke in young adult populations, 3-month-old PAE and control animals were subject to transient middle cerebral artery occlusion (MCAo) and subsequently assessed for behavioral recovery, stroke infarct volume and brain cytokine profiles.
Results
PAE resulted in a significant age-related decrease in blood acceleration in adult mice, specifically in the carotid artery. A unilateral transient MCAo resulted in equivalent cortico-striatal damage in both PAE and control adults. However, PAE adult mice exhibited significantly decreased post-stroke behavioral recovery compared to controls.
Conclusions
Our data collectively show that PAE adult mice exhibit a persistent, long-term loss of cranially directed blood flow, and decreased capacity to compensate for brain trauma due to acute onset adult diseases like ischemic stroke.
Introduction
Early life experiences, including perturbations during the in utero period, contribute to the etiology of many adult-onset diseases (Barker, 1990), including cardiovascular disease (Barker and Martyn, 1992, Godfrey and Barker, 2000, Tarry-Adkins et al., 2013) hypertension (Barker and Osmond, 1988, Benz and Amann, 2010, Gaillard et al., 2014) and type II diabetes (Barker et al., 2010). Prenatal alcohol exposure (PAE) is an important and common early life perturbation that results in neurodevelopmental disability, along with a collection of craniofacial dysmorphia and growth deficits that are collectively termed the Fetal Alcohol Spectrum Disorders (FASD, (Bertrand et al., 2004, Guerri et al., 2009)). The global prevalence of FASD is estimated at 2–3%, but regional estimates for FASD are as high as 11% of the population (Roozen et al., 2016).
The persistent deficits in brain function associated with FASD, including deficits in cognitive and executive function have been documented (Kodituwakku, 2009). However, though FASD is understood to be an important public health and economic burden (Popova et al., 2012), the long-term health consequences of PAE on health, particularly brain health of the mature and aging FASD adult, are largely unknown, and consequently the costs are unpredictable. It is instructive to note that prenatal exposure to a class of pharmacologically related drugs of abuse, the benzodiazepines, have been previously shown to result in continuing brain oxidative stress in adult and aging animals (Miranda et al., 1990). PAE may result in similar long-term adverse brain health outcomes.
Adequate blood flow is a critical requirement for normal adult brain function, and emerging evidence indicates that PAE may adversely influence vascular hemodynamics. In a previous study, we showed that, in a mouse model, a single episode of ethanol exposure during the 1st trimester-equivalent period of pregnancy preferentially reduced cranially-directed blood flow in developing fetus, and that this effect lasted for at least 24 hours following ethanol exposure (Bake et al., 2012). An intriguing report showed that PAE in a rat model resulted in reduced nephron number at 1 month of age and increased arterial blood pressure at six months of age (Gray et al., 2010). In pre-pubertal human population, a FASD diagnosis was associated with significantly increased risk for hypertension (Cook, 2014). Collectively, these studies identified hemodynamic deficits due to PAE that occurred both in the immediate fetal period, as well as in the more distal, pre-pubertal period of development. It is possible that blood flow deficits due to PAE persist into mature adulthood. In the current study, we assessed the more persistent effects of PAE on cranially directed blood flow in the young to mature adult.
It also remains to be determined if PAE predisposes FASD adults to increased risk for disability following a second adverse life experience in adulthood. In this context, PAE has been shown, in rodent models, to increase the vulnerability of FASD adults to stress (Hellemans et al., 2010, Lee et al., 2000) and to diet-induced metabolic disease (Xia et al., 2014). Two recent studies on human populations linked FASD with increased obesity in adolescence and with eating disorders (Fuglestad et al., 2014, Werts et al., 2014), suggesting a potential link with emerging metabolic disease. Since obesity (Yau et al., 2012) and hypertension (Fujishima et al., 1995) are associated with decreased brain health, PAE is expected to result in increased risk for adverse outcomes following sudden-onset brain disease in the FASD adult. To assess the contribution of PAE to the effects of sudden, adult-onset brain disease, we examined recovery of function following an ischemic stroke in PAE and control mice, using a middle cerebral artery occlusion (MCAo) model (Bake et al., 2014, Balden et al., 2012). We assessed stroke outcomes in young adult animals, because recent data indicates that ischemic stroke is rapidly increasing in this population (George et al., 2011), due in part to cardiovascular and metabolic disease (Ji et al., 2013) that, as pointed out earlier, has also been associated with PAE.
Methods
Multiple Binge Ethanol Exposure Model
All procedures were performed in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines and approval. Timed-pregnant C57Bl/6 female mice (Harlan laboratories, Houston, TX) were intragastrically gavaged with a binge like bolus of ethanol at 3g/Kg bwt. (prepared from 95% Ethanol, ACS grade, Acros Organics, NJ # 61511) twice daily from GD12.5 through GD15.5., a late first trimester and early second trimester equivalent of human pregnancy (Workman et al., 2013). This developmental stage is critical for formation of neurons (Bystron et al., 2008) and blood vessels (Kuban and Gilles, 1985) in the fetal brain, and also encompasses the maturation period for the cardiac electrophysiology (Serrano et al., 2010). Our previous studies showed that this dose of ethanol resulted in a peak maternal blood alcohol concentration of 117mg/dl (Bake et al., 2012), a level equivalent to a binge-like intoxication in humans. In the present study, we chose the same dose and gestational age range because we previously observed effects of maternal ethanol exposure on fetal cranially-directed blood flow, during this period (Bake et al., 2012). Control pregnant dams received an equivalent volume of water by intragastric gavage. The current study utilized a total of 7 control dams and 6 ethanol-exposed dams, and average litter size was 7.5±0.4 (Mean±SEM) pups. Multivariate analysis of outcomes including maternal weight gain at GD15.5 (following the ethanol exposure period), number of male and female pups, total litter number and pup weight at postnatal day (PD) 5 showed no significant overall PAE effects on these pregnancy associated outcomes (MANOVA, Pillai’s trace statistic, F(5,7)=1.729, p<0.246). Post-hoc, univariate analysis (ANOVA) showed that none of these measures were individually influenced by PAE (all F’s(1,11)<1.06, all p’s>0.05, n.s.).
Ultrasound imaging
Animals were anesthetized with isoflurane (3–4%) and maintained with 1% isoflurane on a temperature-controlled mouse platform with sensors for monitoring electrocardiogram, respiration and core body temperature. The neck, abdomen and thigh region was shaved and depilated (using Nair) to improve contact with the transducer. Ultrasound gel (Ecogel, CA), pre-warmed to 37°C, was applied to the body prior to positioning the transducer. Both color and pulse wave Doppler measurements for carotid, renal and femoral arteries were obtained using a high-frequency VEVO2100 ultrasound imaging machine coupled to a MS550D Microscan™ transducer with a center frequency of 40MHz (Visualsonics, Canada). Color Doppler and pulse wave recordings (Fig. 1) were used for measurement of acceleration and velocity time integral using Vevo 2100 analysis software as described previously (Bake et al., 2012). Briefly, acceleration is defined as the change in blood flow velocity from the onset of systolic forward flow to the peak with time (Acc, in mm/sec2). It considered as a measure of cardiac output in peripheral vessels in humans (Chang et al., 2000) and in mice (Phoon et al., 2000). Velocity-Time Integral (VTI, mm3/sec) is the area under the velocity envelope, and is an index of the cardiac stroke volume through a specific vessel (Phoon and Turnbull, 2003). Mean blood-flow values shown in each group represent the average from 5–6 animals, which includes a mean value of 15 waveforms from three separate pulse-wave recordings (5/each recording) for each animal.
Figure 1. Color Doppler images indicate locations for acquisition of blood flow data.
a. Blood flow (in blue, shown in yellow arrows) in the common carotid artery from an anesthetized an 8-month old mouse in supine position. b. Blood flow in the abdominal region in supine position showing the renal artery (in blue, shown in yellow arrows) c. Lower extremity, blood flow in the femoral artery (in blue, shown in yellow arrows). d. Sample pulse wave-form of carotid blood flow in a 6-month old female. i. Acceleration, change in velocity over time. ii. velocity time integral, VTI.
Middle Cerebral Artery Occlusion (MCAo)-induced cerebrovascular ischemic stroke
Animals were anesthetized with isoflurane and maintained at 37°C on heating pads. The neck region was shaved and the skin disinfected with ethanol, following which a ventral midline incision was made on the skin. Superficial fascia on the right side of the neck was dissected and the underlying muscles were blunt dissected to expose the right common carotid (CCA), external (ECA) and internal carotid arteries (ICA). The ECA was separated from the vagus nerve, and tied off distally with silk sutures after cauterizing the small branches. Microsurgical clamps placed on CCA and ICA, a loose tie was placed on the ECA and the free stump of ECA was aligned with the ICA. A size 15 nylon 6.0 suture with a silicone-coated round tip (Doccol Corp., CA) was inserted into ICA lumen through a small nick on the ECA. The suture was advanced along the ICA until it reaches the origin of the MCA (~ 9 mm of suture) and secured in position with nylon ties. The intraluminal suture was maintained for 90 minutes and then withdrawn. Tissue perfusion rate was monitored using Laser-Doppler Flowmetry (Moor Instruments, UK) and the perfusion index was calculated for both ischemic and reperfusion time points. MCAo resulted in a 84.42% ± 2.38% (mean ± SD) reduction of blood flow compared to the pre-occlusion rate and re-perfusion restored the perfusion index back to pre-occlusion levels (there were no effects of PAE or sex on reduction in blood flow, all p’s > 0.16).
Neurological Score
Stroke-induced behavioral changes were assessed by a composite neurological score, equivalent to the NIH stroke and Glasgow coma scales in humans, and consistent with the Stroke Treatment Academic Industry Roundtable (STAIR) recommendations (Albers et al., 2011), because lesion size does not always correlate to post-stroke functional deficits. The neurological score was used to assess various behavioral deficits at 24 h post-stroke. Sub-component tests include tests for fore-paw disability, righting reflex, grip-strength, motility and circling (Zhang et al., 2002, Balkaya et al., 2013). Unilateral damage to the brain (cortex and striatum) can cause asymmetry in the forelimb function on the contralateral side. An animal is scored for every task, and a composite score (based on all 5 features) is used to determine impairment. (a) Fore-paw disability: The animal was placed in a cage and, the ability to use the paws to move around was assessed. The scoring was as follows, placement of ipsilateral paw first followed by the contralateral paw is given a score of 1, incomplete placement of the contralateral paw is scored as 2, and inability to place both paws is scored as 3. (b) Righting reflex: The animal was placed on its back and its ability to right itself to an upright position using all four limbs was tested. Inability to perform this task is scored as 1. (c) Fore-paw Grip-strength: The animal was allowed to grab a metal grid with the fore-limb and was gently pulled backwards from the base of the tail. If the animal loses its grip, it receives a scored of 1. (d) Mobility: The ability of the animal to move around the cage freely was assessed. An animal was scored 1 if it failed to move in a set period of time (2 min). (e) Circling: Animals were observed for circling behavior and scored between 0–5 depending on the severity of the circling behavior: 1- mild inconsistent circling when picked up by the tail, 2- Mild consistent circling towards the contralateral side, 3-strong circling in the cage observed for more than 50% of the time, 4- severe rotation that progressed into barreling or loss of walking, 5- comatose.
Adhesive removal test
This test is a sensitive indicator for sensory motor deficits and was performed using our previously published method (Balden et al., 2012). Two pieces of adhesive tape were attached to the palmar surface of the paw of each forelimb. The time each animal takes to remove the tape was recorded. The trial was terminated at 2 minutes. Three trials were performed pre and post stroke.
Infarct Analysis
Infarct volume analysis was determined using our previous procedures (Bake et al., 2014). Briefly, the brain was removed from the cranium immediately after decapitation and sliced into 1 mm coronal sections using a brain matrix (Roboz, US). Brain slices (1mm thick), between +1.66 (anterior) and −1.46 (posterior) to Bregma, were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, MO) at 37° C. for 20 min and images were captured using an Olympus digital camera attached to a surgical microscope. Images were coded and infarct volume was measured using image analysis software, Image J (NIH, MD) by an experimenter who was blind to the codes. Total brain infarct was calculated from 3 slices (per animal) and is expressed as the ratio of infarct volume in the ischemic hemisphere to the total volume of the non-ischemic hemisphere.
Multiplexed Cytokine profiling
Tissue expression for a panel of cytokines and chemokines was measured in the infarcted tissue from ischemic hemisphere and an equivalent amount of tissue from the similar region of the non-ischemic hemisphere of male and female mice using a multiplexed magnetic bead immunoassay (Millipore Corp. MA) following manufacturer’s instructions. Briefly, the filter plate was blocked with assay buffer for 10 min and decanted. Standards and samples (100 ug) was added into appropriate wells, followed by addition of premixed beads and incubated overnight at 4°C on a plate shaker. Wells were washed twice, 25 μl of detection antibody was added, incubated for 1h at room temperature (RT) and followed by 30 min incubation with 25 μl of streptavidin-phycoerythrin per well. After 2 washes, beads were resuspended in 150 μl of sheath fluid and a minimum of 50 beads per analyte was analysed in a Bio-Plex suspension array system (Bio-Rad Laboratories, CA). Cytokine/chemokine levels were normalized to total protein content. The following cytokines and chemokines were assessed: IL-1α, IL-4, IL-1β, IL-2, IL-5 IL-6, IL-7, IL-9, IL-10, IL-13, IL-15, IL-17, IL-12-p-40, IL-12-p-70, IFN-g, MCP-1, IP-10, KC, TNF-α, RANTES, MIP1-α, MIP1-β, MIP-2, G-CSF, GM-CSF.
Statistical analysis
Blood flow data was analyzed by multivariate (Pillai’s trace statistic, MANOVAPTS) or univariate (ANOVA) analysis of variance. MANOVAs were followed by post hoc univariate ANOVA, using the SPSS statistical package (SPSS v20, IBM). Changes in cytokine profiles in ischemic and non-ischemic hemispheres, as a function of PAE and sex, were assessed using a mixed within- and between-subjects multivariate analysis of variance (MANOVA) model. Infarct volume and behavioral assays were analyzed using two-way ANOVA with sex and treatment as between group measures. For the adhesive removal test, pre- and post-stroke performance was treated as a repeated (within-group) measure. Group differences were considered significant at p<0.05. Results are expressed as mean ± SEM.
Results
Age- and sex-dependent effects of blood flow in adult mice
Blood flow indices of Acceleration (Acc) and Velocity-time integral (VTI) were assessed at 3, 6 and 12 months of age in arteries that were cranially- (carotid), abdominally- (renal) and extremity- (femoral) directed. Multivariate Analysis of Variance showed that there was a significant age effect across all assessed arteries on Acceleration (Acc (MANOVApts, F(6,78)= 11.319, p<4.64E-09) and VTI (MANOVAPTS, F(6,80)=6.97, p< 5.52E-06). Moreover, the effects of age were dependent on sex for Acc (MANOVA,PTS, F(6,78) = 2.871, p<0.014) though not for VTI. Moreover, there was not a significant main effect of sex on blood flow indices.
A follow-up univariate analysis of blood flow in the carotid artery showed that there was a significant difference in Acc (Fig. 2a) with age (F(2,40)= 61.944, p<5.60E-13), with a significantly greater Acc at 6 months of age compared to 3 months and followed by a decline at 12 months of age (all post-hoc t-tests, p<0.05). Furthermore, there was an age by sex interaction effect on carotid flow Acc (F(2,40)= 6.741, p<0.003), with females exhibiting a significant increase in acceleration compared to males at 6 months, but not at 3 or 12 months of age. This suggests that sex is an important modifier of the effect of age on cranially directed blood flow in mature adult, but not middle-aged mice. VTI measurement in the carotid arteries also showed a main effect of age (F(2,41)= 10.529, p<0.0002), and an age by sex interaction, (F(2,41)= 3.585, p<0.037), with higher VTI in 3 and 6 month old females compared to age-matched males. This sex difference was eliminated at 12 months of age.
Figure 2. Age-dependent changes in blood flow from adult male and female mice.
(a.i, b.i, c.i) Ultrasound images showing Carotid artery (a.i.), Renal aretery (b.i) and femoral artery (c.i) pulse wave doppler wave-forms. (a.ii, b.ii, c.ii) Graphs showing average Acceleration and Velocity-time integral estimates at 3, 6 and 12 months of age for Carotid (a.ii), Renal (b.ii) and Femoral Artery (c.ii). n=6 in each group, ***, P<0.001.
Univariate analyses also identified significant main effects of age on blood flow in renal (Acc, F(2,40)=25.165, p<8.41E-08; VTI, F(2,41)=10.529, p<0.0002, Figure 2b) and femoral (Acc, F(2,40)=22.616, p<2.69E-07; VTI, F(2,41)=15.543 p<9.46735E-06, Figure 2c) arteries, but no significant main effects of sex or of age by sex interactions. As with the carotid artery, renal and femoral arterial Acc also showed an age-dependent increase from 3 to 6 months of age, and a decline thereafter at 12 months of age. In contrast to the cranially directed carotid circulation both renal and femoral arteries exhibited a general age-related decline in VTI, with the highest VTI observed at 3-months of age (all post-hoc p-values<0.05).
Effects of prenatal alcohol exposure on blood flow in adult mice
We previously showed that ethanol exposure in pregnant dams reduced cranially-directed arterial blood flow both immediately and persistently in fetal blood vessels (Bake et al., 2012). In this study, we therefore sought to determine whether prenatal ethanol exposure continued to affect cranially-directed arterial blood flow in later adulthood. Overall, by multivariate analysis, we observed a main effect of treatment on Acc (MANOVAPTS, F(3.38)=3.35, p<0.029), and an age by treatment interaction effect for both Acc (MANOVAPTS, F(6,78)=2.513, p<0.028) and VTI (MANOVAPTS, F(6,80)=2.562, p<0.025). However, univariate analysis showed that the main effect of treatment was specific to the carotid artery. More specifically, univariate ANOVA showed a main effect of treatment, prenatal ethanol exposure, on the carotid artery acceleration (F(1,40)=10.442, p<0.002) and an interaction effect of treatment by age for Acc (F(2,40)=5.823, p<0.006) and for VTI (F(2,41)= 8.212, p<0.001). There was no significant sex by treatment interaction effect (F(1,40)=3.12, p<0.082). Ethanol treatment resulted in a small but significant (p<0.038) increase in acceleration in 3 month old PAE-adults but a larger and significant decrease in Acc in 6 (p<0.017) and 12 month (p<2.845E-05)-old PAE groups relative to controls (Fig. 3b and c). Furthermore, qualitative assessment of the ultrasonograms shows decreased systole amplitude and apparent arrhythmias in the inter-systole contractions in PAE-adults compared to control. In contrast, blood flow measurements for both renal and femoral arteries did not exhibit a significant main effect of treatment or an interaction effect of treatment with age (all p’s>0.05, data not shown).
Figure 3. Prenatal alcohol exposure (PAE) alters blood flow in carotid arteries of adult mice.
(a) Ultrasound images of control and PAE females at 12 months of age showing that PAE results in decreased amplitude and increased variability in pulsatile blood flow through the carotid artery. (b) PAE increased acceleration at 3 months and decreased it at 6 and 12 months of age. (c) PAE caused significant increase in VTI at 3 months of age by a significant decrease at 12 months. n=6 in each group, *, P<0.05; **, P<0.02; ***, P<0.001.
Effect of prenatal alcohol exposure on stroke severity in adults
PAE-associated changes in cranially-directed blood flow, indicative of increased vascular resistance, may predispose individuals to increase risk for disability, following a second adverse life experience like stroke. To examine the association between blood flow changes and stroke outcomes, we examined the effects of PAE on cerebral infarct volume and neurological behaviors post-stroke, in a middle-cerebral-artery occlusion model. Moreover, since approximately 10–14% of all ischemic strokes occur in young adults, and angiopathic factors may predispose this population to stroke (Ji et al., 2013), we assessed the effects of PAE on stroke outcomes in 3 month-old male and female mice. Animals were subjected to middle cerebral artery occlusion by suture insertion, for 90 minutes to produce ischemia, followed by suture withdrawal to permit re-perfusion. Twenty-four hours following recovery, animals underwent behavioral testing. PAE resulted in a significant increase in the post-stroke composite neurological score (F(1,22)=27.57, p<0.00005, Figure 4a & b), indicating significant deficits in motor performance. Importantly, four out of five individual components of the neurological score (i.e., grip strength, fore-paw placement, mobility and circling behavior) were collectively (MANOVAPTS, F(4,14), p<0.004), and individually (ANOVA, all F’s(1,17)>5.11, all p’s<0.037, Figure 4c) influenced by PAE. The fifth component, the righting reflex test was failed by only one animal (in the PAE exposure group) and was therefore excluded from component analyses. There was no significant effect of sex, or an interaction between PAE and sex on post-stroke motor performance.
Figure 4. Prenatal alcohol exposure impairs neurological function after stroke in adult mice.
(a&b) Neurological evaluation to assess recovery of function, showed that PAE resulted in significant impairment of motor behavior as indicated by higher composite neurological scores in both females (a) and males (b). (c) Histogram showing performance on individual test components of the composite neurological score (data for both males and females were combined). n=5–6 in each group *, P<0.05; **, P<0.02; ***, P<0.001.
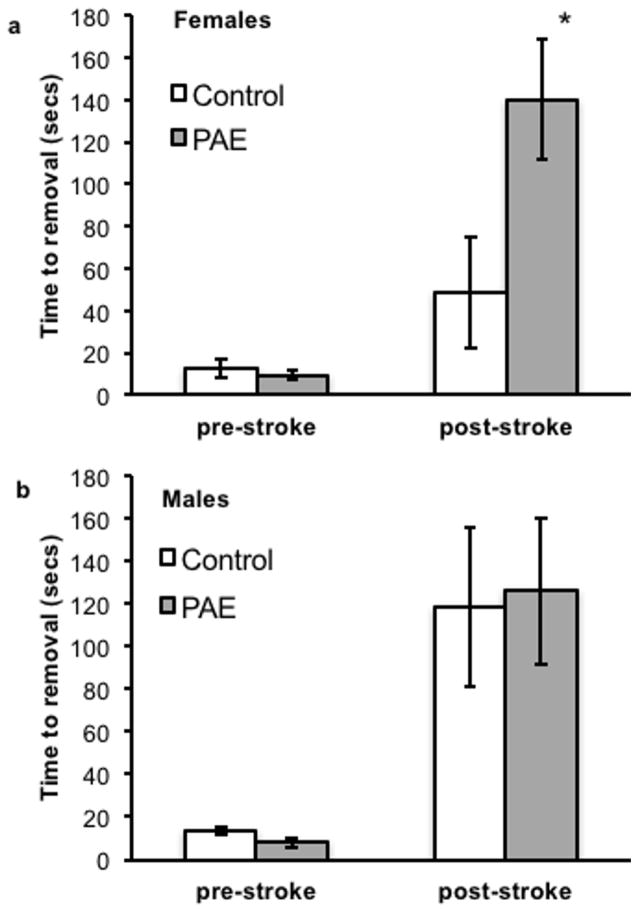
The adhesive removal test assesses sensory-motor integration on the limb contralateral to the stroke hemisphere. A mixed within and between-subjects design was used to assess changes in task performance following stroke, relative to pre-stroke performance control and PAE adults. Male and female animals were assessed in separate models. Stroke resulted in increased time to adhesive removal in both female (F(1,10)=18.83, Greenhouse-Geisser-corrected p<0.001) and male adults (F(1,9)=18.51, Greenhouse-Geisser-corrected p<0.002). Our data also indicate that PAE significantly increased post-stroke time to adhesive removal relative to pre-stroke performance in female PAE (Fig. 5a, F(1,10)=6.04, Greenhouse-Geisser-corrected p<0.034) but not male PAE adults (Fig. 5b, F(1,9)=0.06, Greenhouse-Geisser-corrected p<0.8, n.s.). Qualitative analysis of the data indicate that PAE females performed as poorly on the adhesive removal test as both control and PAE males, whereas control females took ~2/3rd less time to remove the adhesive tape. These data collectively indicate that prenatal ethanol significantly affects post-stroke recovery of function. Following euthanasia, brain sections were stained with TTC to assess infarct volume. Importantly, there was no difference in infarct volume between control and PAE-adults (Fig. 6a &b) indicating that behavioral deficits could not be attributed to increased stroke-related brain damage in PAE adults.
Figure 5. PAE impairs post-stroke sensorimotor function in adult female mice.
(a) Post-stroke performance on the adhesive removal test, a sensorimotor behavioral measure, was significantly worse in PAE adult-females compared to non-PAE, control females. (b) Performance on the adhesive removal test was not further impaired by PAE in adult males. Therefore, PAE females exhibited comparable impairment to both control and PAE males. n=5 in each group *, P<0.05.
Figure 6. Prenatal alcohol exposure and stroke severity in adults.
Brain slices stained with TTC showing stroke-induced damage (white areas as demarcated in adjacent line drawings) in females. Control and PAE animals did not exhibit significant difference in infarct volume. n=5 in each group.
Cytokine profiles following ischemic stroke in PAE and control adults
PAE has been shown to alter brain cytokine profiles in pre-pubertal rats (Bodnar et al., 2016). We therefore hypothesized that PAE would also alter cytokine expression profiles in the adult, following stroke in both ischemic and contralateral control hemispheres. In the multiplex assay, 7 out of 15 assessed cytokines and 6 out of 10 chemokines were detected in brain tissue. A mixed within-(ischemic vs. non-ischemic hemisphere) and between-(sex, PAE) subjects multivariate analysis showed that the stroke hemisphere had significantly elevated cytokine levels compared to the non-stroke hemisphere (MANOVAPTS, F(13,4)=10.88, p<0.017). Univariate analysis showed that the ischemic hemisphere specifically exhibited significantly elevated levels of IL-1a, IL-2, IL-5, IL-6, IL-7, IL-9, IL-10, KC, MCP-1, MIP-2 and G-CSF (all F’s(1,16)>10.8, all Greenhouse-Geisser-corrected p’s<0.002, Figure 7a &b). However, there were no overall between-subjects effects due to PAE (MANOVAPTS, F(13,4)=0.895, p<0.61) or sex ((MANOVAPTS, F(13,4)=0.994, p<0.56)).
Figure 7. Brain cytokine expression was elevated in the ischemic hemisphere, 24 hours post-stroke compared to the non-ischemic hemisphere.
Stroke resulted in increased expression of (a) cytokines and (b) chemokines in the ischemic hemisphere compared to the non-ischemic hemisphere. There were no significant effects of either sex or prenatal alcohol exposure on cytokine/chemokine profiles. Therefore, data were combined for both sex and PAE and composite data comparing ischemic to non-ischemic hemisphere depicted. n=5 in each group *, P<0.05
Discussion
PAE is a well-established causal factor in behavioral and cognitive impairment in children (Mattson and Riley, 1998, Mattson et al., 2011, Roebuck et al., 1999, Hamilton et al., 2003), and in continuing mental health problems in adults (Famy et al., 1998, Streissguth et al., 1991). In the present study, we report that binge-type moderate PAE during the 1st trimester equivalent period additionally results in persistent and specific changes in cranially directed blood flow in adult animals, suggesting that FASD may include a cranial vascular component. These data, in conjunction with evidence that PAE results in eating disorders (Werts et al., 2014) and metabolic disease (Gray et al., 2010, Probyn et al., 2013, Yao and Gregoire Nyomba, 2007, Dobson et al., 2012), support the hypotheses that systemic consequences of PAE may continue to impact the course of adult-onset neurologic disease. Consistent with the above hypothesis, we also report that PAE adults exhibit impaired functional recovery after cerebrovascular ischemic stroke.
The current study used high-resolution ultrasound imaging to evaluate age- and PAE-mediated changes in blood flow patterns in carotid (an elastic artery), renal and femoral arteries (muscular arteries). This non-invasive technique allows for the acquisition and quantification of high quality Doppler signals to measure acceleration. In the present study, we observed a notable age-related increase in acceleration from 3 to 6 months of age, mainly in female animals, followed by a precipitous drop in acceleration at 12 months, i.e., by middle age. The pattern of blood flow in any blood vessel depends on several factors, including cardiac ejection rate, vessel wall distensibility and resistance of downstream blood vessels, and any of these factors could contribute to changes in acceleration. However, these data show that age itself contributes significantly to changes in vascular physiology. In addition, PAE had a very specific effect in that it altered carotid artery acceleration and VTI in an age-specific manner, without affecting flow in renal and femoral arteries. PAE resulted in an increase in both Acc and VTI in the carotid artery at 3 months of age, but a decrease in Acc at both 6 and 12 months of age, and a decrease in VTI at 12 months of age. Several research groups including our own have shown that in animal models, PAE results in immediate effects on fetal blood flow (Parkington et al., 2014, Parnell et al., 2007, Bake et al., 2012), and lead to increased arterial pressure in adults (Gray et al., 2010). Data from the current study indicate that the effects of PAE continue further, into adulthood and even middle-age.
Studies in human populations report that common carotid arterial stiffness increases with age (Kawasaki et al., 1987), reduces carotid blood flow (Scheel et al., 2000, Siennicki-Lantz et al., 2012), and is an important predictor of stroke in hypertensive subjects (Laurent et al., 2003). Cerebral hypoperfusion is shown to cause attention deficits (Duschek et al., 2005) memory loss (de la Torre, 2012) and vascular dementia (Thomas et al., 2015) in humans. Although the mechanisms underlying blood flow changes in the present study remain unknown, these data show that carotid arterial, i.e., cranially directed, blood flow is specifically susceptible to the effects of ethanol, as compared to flow, directed to other distal tissues and organs via renal and femoral arteries. The PAE-related increase in Acc in young animals followed by a decrease in middle-aged animals supports a hypothesis that PAE results in a hypertensive phenotype in young animals, but cerebral hypoperfusion in older, middle-aged animals. The PAE-related decline in Acc at 6 months of age, when Acc peaked in un-treated animals, suggests that PAE results in premature aging of cranially directed vasculature.
Accelerated vascular aging due to PAE is consistent with the hypothesis that developmental perturbations, including adverse intrauterine environments can predispose individuals to disease in adulthood (Barker and Martyn, 1992, Barker, 1990). PAE effects on cranially directed blood flow may not directly result in disease, but may, nevertheless amplify the effect of a second adverse life experience in adulthood. For example, PAE enhances hypersensitivity of hypothalamic pituitary axis to adult-onset stress (Weinberg, 1992), and exacerbates non-alcoholic fatty liver disease (Shen et al., 2014), adrenocorticotrophic hormone (ACTH), serum glucose and insulin resistivity (He et al., 2015), associated with high fat diet in adult rats. PAE has been shown to induce metabolic disease (Chen and Nyomba, 2004, Probyn et al., 2013), which along with changes in cranially directed blood flow are known risk factors for cerebrovascular ischemia/stroke.
The present study focused on stroke outcomes following PAE in 3-month old young adult animals, since recent epidemiological surveys have identified a sharp increase in stroke in young adults of both sexes (Tibaek et al., 2016, Ramirez et al., 2016), caused perhaps by an increase in metabolic disease in this population. In our studies, stroke due to transient middle cerebral artery occlusion resulted in cortical and striatal infarct after 24 h of reperfusion, comparable to the types of damage observed in human populations (Kaufmann et al., 1999) and in rodent studies (Manwani et al., 2013). Interestingly, PAE did not alter the size of the MCAo-induced lesion in cortex and striatum and did not result in increased basal or stroke-induced cytokine expression. However, post-stroke neurological deficits were significantly more severe in PAE animals compared to controls. Moreover, the adhesive removal test, a test for sensory-motor integration showed unexpected sex difference in the effects of PAE on post-stroke outcomes. Control females successfully completed the test in approximately a third of the time required by control and PAE males. This data is consistent with epidemiological data, which shows that stroke outcomes including mortality are significantly worse in young adult men compared to women (Aarnio et al., 2014, Varona, 2010). However, PAE females performed as poorly as both control and PAE males, suggesting that PAE eliminates the resiliency advantage in young females.
PAE did not result in a significant change in brain cytokine levels in either male or female offspring, but stroke itself, as we have previously shown (Bake et al., 2014), resulted in elevated brain cytokine levels. Interestingly, other research groups have shown microglial activation and increased pro-inflammatory brain cytokines immediately following early developmental alcohol exposure (Boschen et al., 2016, Topper et al., 2015). However, these pro-inflammatory brain effects of PAE may be undetectable in the adult. PAE may also elevate systemic immune responses, at least into the adolescent period (Bodnar et al., 2016), which in turn may influence stroke outcomes in the young adult. It is possible that the reduced severity of stroke outcomes in young control female adults is due to the neuroprotection afforded by circulating levels of ovarian hormones like estrogen in this age group (Selvamani and Sohrabji, 2010). In contrast, PAE has been shown to alter the HPA axis (Weinberg et al., 2008) and increase testosterone levels in adolescent females (Carter et al., 2014), perhaps contributing to adverse stroke outcomes in young adulthood, comparable to those seen in males.
To our knowledge, our study presents the first evidence that PAE can adversely affect stroke outcomes. These data also indicate that PAE may not directly synergize with an ischemic event to promote additional damage, but rather, decreases the capacity of the organism to adapt to acute, adult-onset disease, i.e., to recover from a second ‘hit’. Collectively, data from the present study suggest that prenatal ethanol exposure has long-term consequence on adult health, is an important regulator of blood flow in major cranially directed arteries like carotid artery and adversely influences stroke outcome in adults. Clearly, further studies are warranted to delineate molecular mechanisms that mediated these adverse effects of PAE and to identify aging associated risks for adults with an FASD diagnosis. Moreover, while we focused on the 3-month old PAE adults to model stroke outcome in young adults, it will be important, in future studies, to assess stroke outcomes in mature adult and aging PAE animals. Our research in rodent models indicate that whereas a stroke episode results smaller infarcts and better recovery in young females relative to young males, infarct size and stroke outcomes are actually more severe in middle-aged females compared to age-matched males (Bake et al., 2014, Selvamani and Sohrabji, 2010). Therefore, middle-aged FASD adults may well be at increased risk for stroke and may experience increased disability following stroke. However, currently no data exists to inform clinical care for acute-onset neurological disease in the aging FASD adult.
Acknowledgments
Authors would like to thank Gipshu Dave, Tiffany Heard and Jeremy Rawlings for their assistance at different stages of this study. This study was supported by R01AG042189 and R01NS074895 (FS), R01AA013440, R01AA024659 and R01HD086765 (RCM) and the Texas A&M George Chiou pilot grant award (SB).
References
- Aarnio K, Haapaniemi E, Melkas S, Kaste M, Tatlisumak T, Putaala J. Long-term mortality after first-ever and recurrent stroke in young adults. Stroke. 2014;45:2670–2676. doi: 10.1161/STROKEAHA.114.005648. [DOI] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, Hurn P, Liebeskind DS, Nogueira RG, Saver JL. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One. 2014;9:e91427. doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res. 2012;36:748–758. doi: 10.1111/j.1530-0277.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkaya M, Krober JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33:330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail. 2010;12:819–825. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Martyn CN. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health. 1992;46:8–11. doi: 10.1136/jech.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz K, Amann K. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochim Biophys Acta. 2010;1802:1309–1317. doi: 10.1016/j.bbadis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O’Conner M, Johnson KA, Riley EP, Cohen DE National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effect; US DEPARTMENT OF HEALTH AND HUMAN SERVICES, editor. Series Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. CDC; Atlanta, GA: 2004. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. [Google Scholar]
- Bodnar TS, Hill LA, Weinberg J. Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen KE, Ruggiero MJ, Klintsova AY. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience. 2016;324:355–366. doi: 10.1016/j.neuroscience.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nature reviews. Neuroscience. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcohol Clin Exp Res. 2014;38:1671–1679. doi: 10.1111/acer.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Chang FM, Yu CH, Liang RI, Ko HC, Chen HY. Systemic assessment of fetal hemodynamics by Doppler ultrasound. Ultrasound Med Biol. 2000;26:777–785. doi: 10.1016/s0301-5629(00)00207-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Nyomba BL. Whole body insulin resistance in rat offspring of mothers consuming alcohol during pregnancy or lactation: comparing prenatal and postnatal exposure. J Appl Physiol (1985) 2004;96:167–172. doi: 10.1152/japplphysiol.00751.2003. [DOI] [PubMed] [Google Scholar]
- Cook JC. Series Association analysis of fetal alcohol syndrome and hypertension status in children, adolescents, and young adults. MPH, Public Health, Georgia State University; Atlanta, GA: 2014. Association analysis of fetal alcohol syndrome and hypertension status in children, adolescents, and young adults. [Google Scholar]
- de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CC, Mongillo DL, Brien DC, Stepita R, Poklewska-Koziell M, Winterborn A, Holloway AC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases adiposity and disrupts pancreatic morphology in adult guinea pig offspring. Nutr Diabetes. 2012;2:e57. doi: 10.1038/nutd.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med. 2005;30:149–158. doi: 10.3200/BMED.30.4.149-160. [DOI] [PubMed] [Google Scholar]
- Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- Fuglestad AJ, Boys CJ, Chang PN, Miller BS, Eckerle JK, Deling L, Fink BA, Hoecker HL, Hickey MK, Jimenez-Vega JM, Wozniak JR. Overweight and obesity among children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2014;38:2502–2508. doi: 10.1111/acer.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima M, Ibayashi S, Fujii K, Mori S. Cerebral blood flow and brain function in hypertension. Hypertens Res. 1995;18:111–117. doi: 10.1291/hypres.18.111. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Rurangirwa AA, Williams MA, Hofman A, Mackenbach JP, Franco OH, Steegers EA, Jaddoe VW. Maternal parity, fetal and childhood growth, and cardiometabolic risk factors. Hypertension. 2014;64:266–274. doi: 10.1161/HYPERTENSIONAHA.114.03492. [DOI] [PubMed] [Google Scholar]
- George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- Gray SP, Denton KM, Cullen-McEwen L, Bertram JF, Moritz KM. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol. 2010;21:1891–1902. doi: 10.1681/ASN.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- He Z, Li J, Luo H, Zhang L, Ma L, Chen L, Wang H. Sex-specific increase in susceptibility to metabolic syndrome in adult offspring after prenatal ethanol exposure with post-weaning high-fat diet. Sci Rep. 2015;5:17679. doi: 10.1038/srep17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70:51–57. doi: 10.1001/jamaneurol.2013.575. [DOI] [PubMed] [Google Scholar]
- Kaufmann AM, Firlik AD, Fukui MB, Wechsler LR, Jungries CA, Yonas H. Ischemic Core and Penumbra in Human Stroke. Stroke. 1999;30:93–99. doi: 10.1161/01.str.30.1.93. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Neurocognitive Profile In Children With Fetal Alcohol Spectrum Disorders. Developmental disabilities research reviews. 2009;15:218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban KC, Gilles FH. Human telencephalic angiogenesis. Ann Neurol. 1985;17:539–548. doi: 10.1002/ana.410170603. [DOI] [PubMed] [Google Scholar]
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Ceckler T, Guillet R, Kellogg CK. Aging-related changes in brain metabolism are altered by early developmental exposure to diazepam. Neurobiol Aging. 1990;11:117–122. doi: 10.1016/0197-4580(90)90044-z. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Kenna KR, Sozo F, Coleman HA, Bocking A, Brien JF, Harding R, Walker DW, Morley R, Tare M. Maternal alcohol consumption in pregnancy enhances arterial stiffness and alters vasodilator function that varies between vascular beds in fetal sheep. J Physiol. 2014;592:2591–2603. doi: 10.1113/jphysiol.2013.262873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92:933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Aristizabal O, Turnbull DH. 40 MHz Doppler characterization of umbilical and dorsal aortic blood flow in the early mouse embryo. Ultrasound Med Biol. 2000;26:1275–1283. doi: 10.1016/s0301-5629(00)00278-7. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genomics. 2003;14:3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L, Rehm J. Health care burden and cost associated with fetal alcohol syndrome: based on official Canadian data. PLoS One. 2012;7:e43024. doi: 10.1371/journal.pone.0043024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probyn ME, Parsonson KR, Gardebjer EM, Ward LC, Wlodek ME, Anderson ST, Moritz KM. Impact of low dose prenatal ethanol exposure on glucose homeostasis in Sprague-Dawley rats aged up to eight months. PLoS One. 2013;8:e59718. doi: 10.1371/journal.pone.0059718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez L, Kim-Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ, Towfighi A. Trends in Acute Ischemic Stroke Hospitalizations in the United States. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res. 2016;40:18–32. doi: 10.1111/acer.12939. [DOI] [PubMed] [Google Scholar]
- Scheel P, Ruge C, Schoning M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: reference data and the effects of age. Ultrasound Med Biol. 2000;26:1261–1266. doi: 10.1016/s0301-5629(00)00293-3. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu Z, Gong J, Zhang L, Wang L, Magdalou J, Chen L, Wang H. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol Appl Pharmacol. 2014;274:263–273. doi: 10.1016/j.taap.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Siennicki-Lantz A, Wollmer P, Elmstahl S. Carotid velocities determine cerebral blood flow deficits in elderly men with carotid stenosis <50% Int J Vasc Med. 2012;2012:579531. doi: 10.1155/2012/579531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991;265:1961–1967. [PubMed] [Google Scholar]
- Tarry-Adkins JL, Martin-Gronert MS, Fernandez-Twinn DS, Hargreaves I, Alfaradhi MZ, Land JM, Aiken CE, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J. 2013;27:379–390. doi: 10.1096/fj.12-218685. [DOI] [PubMed] [Google Scholar]
- Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain. 2015;138:1059–1069. doi: 10.1093/brain/awv025. [DOI] [PubMed] [Google Scholar]
- Tibaek M, Dehlendorff C, Jorgensen HS, Forchhammer HB, Johnsen SP, Kammersgaard LP. Increasing Incidence of Hospitalization for Stroke and Transient Ischemic Attack in Young Adults: A Registry-Based Study. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. Journal of neuroinflammation. 2015;12:160. doi: 10.1186/s12974-015-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varona JF. Long-term prognosis of ischemic stroke in young adults. Stroke Res Treat. 2010;2011:879817. doi: 10.4061/2011/879817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol. 1992;9:427–432. doi: 10.1016/0741-8329(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts RL, Van Calcar SC, Wargowski DS, Smith SM. Inappropriate feeding behaviors and dietary intakes in children with fetal alcohol spectrum disorder or probable prenatal alcohol exposure. Alcohol Clin Exp Res. 2014;38:871–878. doi: 10.1111/acer.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia LP, Shen L, Kou H, Zhang BJ, Zhang L, Wu Y, Li XJ, Xiong J, Yu Y, Wang H. Prenatal ethanol exposure enhances the susceptibility to metabolic syndrome in offspring rats by HPA axis-associated neuroendocrine metabolic programming. Toxicol Lett. 2014;226:98–105. doi: 10.1016/j.toxlet.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Yao XH, Gregoire Nyomba BL. Abnormal glucose homeostasis in adult female rat offspring after intrauterine ethanol exposure. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1926–1933. doi: 10.1152/ajpregu.00822.2006. [DOI] [PubMed] [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]